Abstract
Adult hippocampal neurogenesis is a critical form of cellular plasticity that is greatly influenced by neural activity. Among the neurotransmitters that are widely implicated in regulating this process are serotonin and norepinephrine, levels of which are modulated by stress, depression and clinical antidepressants. However, studies to date have failed to address a direct role for either neurotransmitter in regulating hippocampal precursor activity. Here we show that norepinephrine but not serotonin directly activates self-renewing and multipotent neural precursors, including stem cells, from the hippocampus of adult mice. Mechanistically, we provide evidence that β3-adrenergic receptors, which are preferentially expressed on a Hes5-expressing precursor population in the subgranular zone (SGZ), mediate this norepinephrine-dependent activation. Moreover, intrahippocampal injection of a selective β3-adrenergic receptor agonist in vivo increases the number of proliferating cells in the SGZ. Similarly, systemic injection of the β-adrenergic receptor agonist isoproterenol not only results in enhancement of proliferation in the SGZ but also leads to an increase in the percentage of nestin/glial fibrillary acidic protein double-positive neural precursors in vivo. Finally, using a novel ex vivo “slice-sphere” assay that maintains an intact neurogenic niche, we demonstrate that antidepressants that selectively block the reuptake of norepinephrine, but not serotonin, robustly increase hippocampal precursor activity via β-adrenergic receptors. These findings suggest that the activation of neurogenic precursors and stem cells via β3-adrenergic receptors could be a potent mechanism to increase neuronal production, providing a putative target for the development of novel antidepressants.
Introduction
The adult mammalian hippocampus harbors neural precursors that reside and proliferate in the milieu of the neurogenic niche (Ming and Song, 2005) to generate neurons that functionally integrate into the hippocampal neurocircuitry, thereby influencing functions such as learning and memory (Lledo et al., 2006). Accumulating evidence has suggested an important role for synaptic activity in regulating this process (Ming and Song, 2005; Zhao et al., 2008). Neural excitation has been shown to activate a latent stem cell pool (Walker et al., 2008), to promote the commitment of precursors to a neurogenic fate (Deisseroth et al., 2004), as well as to enhance the survival and integration of newly born neurons in the adult hippocampus (Ge et al., 2006; Tashiro et al., 2006). Among the factors that are released following synaptic activity are the neurotransmitters, trophic roles for which are increasingly being appreciated in the regulation of neurogenesis (Vaidya et al., 2007; Hagg, 2009). Recent studies have also shown that glutamate and GABA receptors are present on a subset of adult hippocampal precursors and regulate their proliferation (Ge et al., 2007; Nácher et al., 2007).
Within the monoaminergic neurotransmitter family, a large number of in vivo studies have focused on the roles of serotonin and norepinephrine (NE), revealing a strong correlation between their levels and the extent of hippocampal neurogenesis (Brezun and Daszuta, 1999, 2000; Kulkarni et al., 2002) Furthermore, impaired neurogenesis has been demonstrated in animal models of stress and depression (Malberg and Duman, 2003; Vollmayr et al., 2007), where a significant reduction in the levels of serotonin and norepinephrine is also commonly observed (Charney, 1998; Vaidya et al., 2007). In agreement with these lines of evidence, pharmacological agents, such as antidepressants that act by elevating levels of serotonin and norepinephrine, have been shown to enhance hippocampal neurogenesis (Malberg et al., 2000). To date, a proliferative role has been proposed for norepinephrine (Kulkarni et al., 2002), whereas controversy still exists regarding the role of serotonin in regulating the proliferation of hippocampal precursors (Santarelli et al., 2003; Encinas et al., 2006; Holick et al., 2008; Huang et al., 2008). However, one of the limitations of the current in vivo approaches is the inability to dissect out direct versus non-cell-autonomous effects of these neurotransmitters on the precursor population. Whether serotonin or norepinephrine has a direct effect on adult hippocampal precursors, and the cellular and molecular identity of such a precursor population, therefore remains unknown.
In the present study, we investigated the effects of serotonin and norepinephrine on adult hippocampal precursors in vitro using the neurosphere assay. We report that norepinephrine but not serotonin directly activates a self-renewing and multipotent population of stem and precursor cells. We then demonstrate that this effect is mediated by β3-adrenergic receptors both in vitro and in vivo. Finally, we examine the effects of two major classes of widely prescribed antidepressants in a novel slice-sphere assay and show that norepinephrine-selective reuptake inhibitors (NRIs) but not serotonin-selective reuptake inhibitors (SSRIs) significantly enhance hippocampal neural precursor activity via β-adrenergic receptors.
Materials and Methods
Animals.
Adult male (8–12 weeks old) mice were used for the majority of the experiments in this study except for the slice-sphere assay where postnatal day 7 Wistar pups were used. Mice expressing enhanced green fluorescent protein (GFP) under the control of the Hes5 promoter were generated by the Gene Expression and Nervous System Atlas Consortium (Gensat) and were obtained from the Mutant Mouse Regional Resource Center (University of Missouri). Adult male Nestin-GFP mice, maintained on a C57BL/6 background were generated as previously described (Yu et al., 2005). Animals were treated in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and ethics approval was obtained for all experiments from the appropriate institutional Animal Ethics Committee.
Adult hippocampal neurosphere culture.
Adult male C57BL/6 mice were killed by cervical dislocation and their brains removed. Brains were bisected along the midline in the sagittal plane. The hippocampi were isolated from the overlying cortex and minced using a scalpel blade. Minced tissue was digested in 0.1% papain (Invitrogen) for 20 min at 37°C, after which an excess of NeuroCult NSC basal medium (StemCell Technologies) was added to halt the digestion. Tissue was then centrifuged at 100 relative centrifugal force for 5 min, the resulting pellet was resuspended in 1 ml of complete neurosphere medium, and a single-cell suspension was achieved by gentle trituration. The cells were filtered through a 40 μm cell sieve (BD Biosciences) and resuspended in NeuroCult NSC basal medium containing NeuroCult proliferation supplements (StemCell Technologies), 2% bovine serum albumin (Invitrogen) and 2 μg/ml heparin (Sigma-Aldrich). The growth factors added were 20 ng/ml epidermal growth factor (EGF; receptor grade, BD Biosciences) and 10 ng/ml basic fibroblast growth factor (bFGF; recombinant bovine, Roche). The cells were then plated in a 96-well plate and cultured in complete neurosphere medium containing EGF and bFGF, in the presence or absence of 5-hydroxytryptamine hydrochloride (serotonin; 100 nm, 1 μm, 10 μm), l-(−)-noradrenaline (+)-bitartrate salt monohydrate (norepinephrine; 100 nm, 1 μm, 10 μm) or KCl (15 mm). The concentrations of norepinephrine and serotonin used were based on previous reports (Segal, 1980; Lacaille and Harley, 1985). The adrenergic receptor antagonists used were prazosin (100 nm), yohimbine (1 μm), propranolol (1 μm), CGP20712 ([2-(3-carbamoyl-4-hydroxyphenoxy)-ethylamino]- 3-[4-(1-methyl-4-trifluormethyl-2-imidazolyl)-phenoxy]-2-propanolmethanesulfonate) (10 nm), ICI118,551 (3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol) (10 nm), and SR59230A [(3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanol oxalate)] (10 nm). BRL37344 [(±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]acetic acid sodium hydrate] was used as a selective β3-adrenergic receptor agonist (1 and 10 μm). All the compounds were purchased from Sigma-Aldrich. The number of primary neurospheres was counted on days 10–13 and expressed as a percentage relative to the control. Passaging of single primary neurospheres was done essentially as described by Walker et al. (2008).
Immunocytochemistry.
Control or norepinephrine-stimulated neurospheres were plated onto poly-ornithine-coated coverslips or poly-d-lysine-coated BioCoat eight-well culture slides (BD Biosciences) in serum-free basal medium without any mitogens. The neurospheres were allowed to flatten and adhere for 4–6 d in a humidified, 5% CO2 incubator. They were then fixed with 4% paraformaldehyde in 0.1 m PBS at 4°C for 40 min, and immunocytochemistry was performed as described previously (Bull and Bartlett, 2005) using antibodies to the neuronal marker βIII tubulin (1:2000; Promega), the astrocytic marker glial fibrillary acidic protein (GFAP; 1:500; Dako Cytomation) and the oligodendrocyte marker myelin basic protein (MBP; 1:500; Millipore). 4′,6′-Diamidino-2-phenylindole (DAPI; 1:5000; Sigma-Aldrich) was used as a nuclear stain. Slides were mounted using fluoromount (Dako Cytomation) and viewed on a Zeiss-Axio Imager microscope. Images were captured using a digital camera linked to a computer using Zeiss software.
Surgery and drug treatments.
Adult male C57BL/6 mice were anesthetized with a mixture of ketamine (130 mg/kg; Apex Laboratories) and the muscle relaxant xylazine (6 mg/kg; Bayer). The mouse was then placed in a stereotaxic frame (David Kopf Instruments), with the incisor bar maintained at ∼3.3 mm below horizontal to achieve a flat skull position. Bilateral injections were performed using a glass needle (exterior diameter 20 μm) fashioned from a borosilicate glass capillary (World Precision Instruments) and attached to a 5 μl Hamilton syringe. The needle was lowered into the hilus region of the hippocampus (anteroposterior, −1.3 mm; mediolateral, ±0.9 mm; dorsoventrial, −2.0 mm from bregma). The hilus from one hemisphere received an injection (0.5 μl) of the selective β3-adrenergic receptor agonist BRL37344 (10 μm); with the contralateral hemisphere receiving a control injection of 0.9% saline. Infusions were conducted over 5 min and the needle was left in place for a further 10 min to allow for diffusion. At 1 and 24 h following surgery all mice received injections (75 mg/kg, i.p.) of bromodeoxyuridine (BrdU). Three days after the initial BRL37344 infusion, the animals were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 4% paraformaldehyde in 0.1 m PBS. Brains were blocked using a matrix (Stoelting) aligned to the mouse brain atlas (Paxinos and Franklin, 2001), and 40 μm coronal sections were cut through the hippocampus using a sliding microtome (Leica, SM2000r) in four serially adjacent sets and stored in 0.1% sodium azide in 0.1 m PBS. One set of sections (160 μm apart) was processed for BrdU immunohistochemistry, while the second set of sections was mounted on a chrome alum/gelatinized glass slide and stained with cresyl violet. Injection placements were verified under the microscope using the boundaries defined by Paxinos and Franklin (2001).
To study the influence of β-adrenergic receptor stimulation on adult hippocampal progenitors, the β-adrenergic receptor agonist, isoproterenol (2 mg/kg, Sigma) was used. The choice of drug dose was based on previous studies (Ozawa et al., 1969; Yuan et al., 2000), with 0.9% saline being used as the vehicle. Nestin-GFP mice received the drug via intraperitoneal injection, once daily for seven d. All mice received BrdU (100 mg/kg, i.p.) 2 h following the last injection and were killed 24 h later by transcardial perfusion using 4% paraformaldehyde.
Immunohistochemistry.
Hes5-GFP mice were perfused transcardially using ice-cold 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde for 24 h, after which 50 μm sections were cut using a freezing microtome. The sections were blocked in PBS containing 0.1% Triton X-100 (0.1% PBTX) and 10% normal goat serum for 1 h and then labeled with primary antibodies: anti-GFAP (1:500; Dako Cytomation), anti-doublecortin (1:500; Sapphire Bioscience) and anti-nestin (1:100, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). The sections were washed three times using 0.1% PBTX and incubated for 2 h at room temperature with the secondary antibodies goat anti-mouse Alexa 568 or goat anti-rabbit Alexa 568 (1:2000, Invitrogen), and DAPI (1:1000). BrdU immunohistochemistry was performed essentially as described previously (Kulkarni et al., 2002; Jha et al., 2006). In brief, this involved DNA denaturation and acid hydrolysis followed by overnight incubation with mouse anti-BrdU antibody (1:500; Roche). The secondary antibody goat anti-mouse Alexa 488 (Invitrogen) was used at 1:2000. After several washes, the sections were mounted using fluoromount (Dako Cytomation) and viewed on a Zeiss-Axio Imager microscope. Optical sectioning was achieved using ApoTome and images were captured using a digital camera linked to a computer using Zeiss software.
For double-label immunofluorescence for GFP and GFAP in nestin-GFP mice, 4 sections (50 μm) were selected per animal. The choice of sections was such that they were from comparable bregma points across all experimental animals. The sections were incubated for 2 h with 10% horse serum (Invitrogen) before an overnight incubation at room temperature with a mixture of the primary antibodies, rabbit anti-GFP (1:500, Invitrogen) and mouse anti-GFAP (1:1000, Sigma). Sections were then incubated with the secondary antibodies, donkey anti-rabbit IgG (1:250, Invitrogen) and donkey anti-mouse IgG (1:250, Invitrogen) for 4 h at room temperature.
Cell counting analysis.
Analysis was performed on coded sections by an experimenter blind to the study code. To address the effects of β-adrenergic receptor stimulation on nestin/GFAP double-positive quiescent progenitors, the percentage of GFP-positive cells that colocalized with GFAP was determined by confocal microscopy using an Olympus FV1000 confocal microscope. Between 30 and 40 GFP-positive cells from four sections (250 μm apart) per animal were analyzed using z-plane confocal sectioning with 1 μm steps to confirm colocalization of GFP with GFAP.
Fluorescence-activated cell sorting.
Brains from adult male Hes5-GFP mice were removed and hippocampi were isolated as described earlier. A live-cell suspension was prepared from the hippocampus using 0.1% papain, and the dead cells were labeled with propidium iodide (1 μg/ml). GFP-positive and -negative cells were purified by fluorescence-activated cell sorting (FACS). Cells were sorted on a FACS Vantage (Becton Dickinson) with DIVA software. The GFP-negative populations was set relative to the basal fluorescence levels obtained from GFP-negative wild-type littermate controls and a conservative approach was used in selecting only high GFP-expressing cells. The cells were collected in basal medium and plated into 96-well tissue culture plates in medium containing EGF and bFGF with or without norepinephrine (10 μm).
RNA extraction and cDNA synthesis.
RNA was extracted from sorted Hes5-GFP-positive and -negative cells using the RNeasy Mini Kit (Qiagen). Genomic DNA was removed by DNase digestion using a DNA-free kit (Ambion). cDNA was generated using SuperScript III (Invitrogen) with oligo-dT primers.
PCR.
The complete list of primer sequences used for the PCR is detailed in Table 1. Program 1 involved initial denaturation at 95°C for 2 min, followed by 35 cycles of 95°C for 1 min and 70°C for 2 min, with a final elongation step of 72°C for 5 min as described by (Cikos et al., 2005). Program 2 began with initial denaturation at 95°C for 2 min, followed by 32 cycles of 95°C for 30 s, 64°C for 30 s, and 72°C for 30 s, essentially as described by Evans et al. (1999). A total of 45 cycles were used to amplify the β3-adrenergic receptor (Adrb3).
Table 1.
Gene-specific primer sequences for reverse transcriptase-PCR
| Target | Forward | Reverse | Product(bp) | Program |
|---|---|---|---|---|
| Adrb1 | ggagctccctcggacgac | agcctggctctctacaccttg | 173 | 1 |
| Adrb2 | gtactgtgcctagccttagcgt | ggttagtgtcctgtcaaggagg | 115 | 1 |
| Adrb3 | tctagttcccagcggagttttcatcg | cgcgcaccttcatagccatcaaacc | 234 | 2 |
| Hes5 | aagtaccgtggcggtggagatgc | cgctggaagtggtaaagcagctt | 354 | 2 |
| Enhanced GFP | cctacggcgtgcagtgcttcagc | cggcgagctgcacgctgcgtcctc | 300 | 2 |
| Actin | agaagagctatgagctgcctgacg | tacttgcgctcaggaggagcaatg | 301 | 2 |
Generation of hippocampal organotypic slices.
Seven-day-old Wistar pups were killed under isoflurane-induced anesthesia, and the brains were isolated and placed in ice-cold Ringer's solution (containing, in mm: 118 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2.5 CaCl2, 1.3 MgCl2, 25 NaHCO3, and 10 glucose, pH 7.2). The brain was bisected along the sagittal plane and the hippocampi were separated from the overlying cortex. The hippocampi were cut into transverse slices of 300 μm thickness using a tissue slicer (Stoelting). Six to seven slices were then transferred onto a single 0.4 μm Millicell-CM membrane filter (Millipore), and the filters were placed in a 6-well plate containing 1 ml of serum-free NeuroCult NSC basal medium with NeuroCult proliferation supplements (StemCell Technologies) and 2% bovine serum albumin (Invitrogen). d-Glucose (Sigma-Aldrich) was added to the medium to a final concentration of 5 mm. Four filters, each containing 6–7 hippocampal slices, were generated from a single animal. Plates were incubated at 37°C in a humidified 5% CO2 incubator and the slices were cultured for 6 d.
Pharmacological treatment of hippocampal slices.
To assess the influence of specific compounds on hippocampal precursor proliferation in the slice culture, compounds were added to the complete medium at the doses outlined below. Two filters were treated with the compounds for each dose per experiment. On every alternate day half the medium was removed and replaced with fresh medium containing the compounds. Slices were treated with serotonin at 10 and 100 μm and norepinephrine at 1 μm, 10 and 100 μm. The antidepressants used were fluoxetine (1 and 10 μm), citalopram (10 and 100 μm), reboxetine (1 μm, 10 and 100 μm), atomoxetine (1 μm) and maprotiline (1 μm). Propranolol (10 μm) was used to block β-adrenergic receptors in the slices.
Derivation of neurospheres from hippocampal organotypic slices.
On the sixth day of culture, the hippocampal slices from each treatment group were pooled and the tissue was minced using a scalpel blade. Minced tissue was then treated with 0.1% trypsin-EDTA (Invitrogen) for 5 min at 37°C. The digestion was stopped by adding 0.014% w/v trypsin inhibitor (Sigma-Aldrich). A single-cell suspension was achieved by gentle trituration. The total number of viable cells in an aliquot was counted on a hemocytometer based on the exclusion of 0.08% trypan blue (Sigma-Aldrich). The cells were then cultured in complete neurosphere medium containing EGF and bFGF. A 200 μl cell suspension was plated at 2500 cells/ml in a 96-well plate, resulting in a cell density of 500 cells/well. For each experiment there were 20 wells plated for each of the doses per treatment group. The plates were incubated at 37°C in a humidified 5% CO2 incubator. The number of neurospheres obtained per well was counted after 10 d in culture and expressed as a percentage of the control.
Statistical analysis.
Experiments were repeated three times unless otherwise stated and the values expressed as mean ± SEM. Results were subjected to statistical analysis using the statistical software Prism (GraphPad) and analyzed using either Student's t tests or one-way ANOVA with significance determined at p < 0.05 followed by the Bonferroni post hoc test.
Results
Norepinephrine but not serotonin activates adult hippocampal stem and precursor cells and promotes neurogenesis in vitro
To examine the effect of serotonin and norepinephrine in regulating adult hippocampal precursor activity, we used the classical neurosphere assay in the presence of the conventional mitogens EGF and bFGF. The addition of serotonin at 100 nm, 1 or 10 μm produced no change in neurosphere numbers (Fig. 1A) or size (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) compared with the control. In contrast, a significant increase was obtained in the presence of 100 nm and 1 μm norepinephrine, with a twofold increase in neurosphere numbers observed in the presence of 10 μm norepinephrine (p < 0.001, unpaired t test; Fig. 1A). The average size of the neurospheres derived in the presence of 1 and 10 μm norepinephrine (diameter of neurosphere in μm; control: 83.47 ± 2.5; 100 nm NE: 78.2 ± 1.5; 1 μm NE: 99.33 ± 2.2; 10 μm NE: 113.95 ± 5.4) was also significantly greater than that of the control neurospheres (p < 0.01 in both cases; unpaired t test). Importantly, there was also the emergence of a population of very large neurospheres >200 μm in diameter in the presence of 10 μm norepinephrine (Fig. 1B,C). Furthermore, a systematic categorization of neurospheres according to their size revealed not only a significant increase in the percentage of neurospheres measuring 100–150 μm (p = 0.012), 150–200 μm (p = 0.007) and >200 μm (p = 0.004) but a concomitant reduction in the smaller neurospheres measuring <100 μm (p < 0.001) in norepinephrine (10 μm) compared with the control (Fig. 1D). The large (>200 μm in diameter) norepinephrine-derived neurospheres resembled those described previously (Walker et al., 2008) following treatment with depolarizing levels of KCl, suggesting activation of a latent stem cell pool.
Figure 1.
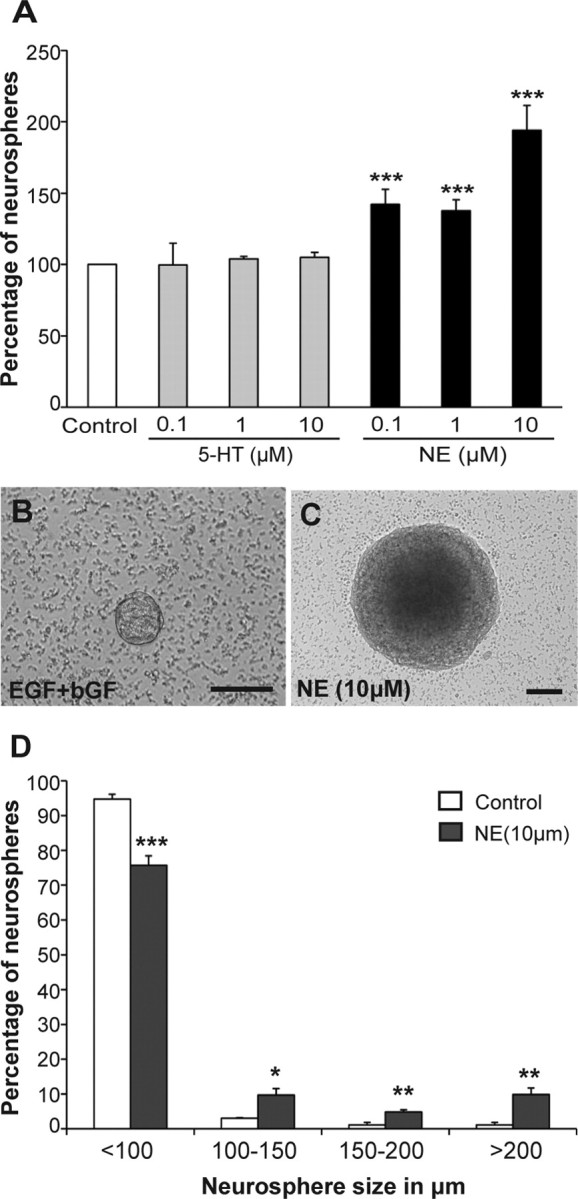
Norepinephrine but not serotonin activates a precursor cell population from the adult hippocampus. A–C, Treatment of adult hippocampal cells with NE but not serotonin (5-HT) in the presence of EGF and bFGF significantly enhanced neurosphere formation with up to a twofold increase observed at 10 μm (mean ± SEM; ***p < 0.001) (A). In addition, norepinephrine treatment generated a number of very large neurospheres, an example of which is shown in C, compared with smaller neurospheres generated in the control (B). Scale bars, 100 μm. D, Neurospheres obtained in the presence of 10 μm norepinephrine were significantly larger than the control neurospheres. Note an increase in the percentage of norepinephrine-derived neurospheres measuring 100–150 μm, 150–200 μm, and >200 μm, but a reduction in the smaller neurospheres measuring <100 μm compared with the control neurospheres (mean ± SEM; *p < 0.05, **p < 0.01***p < 0.001).
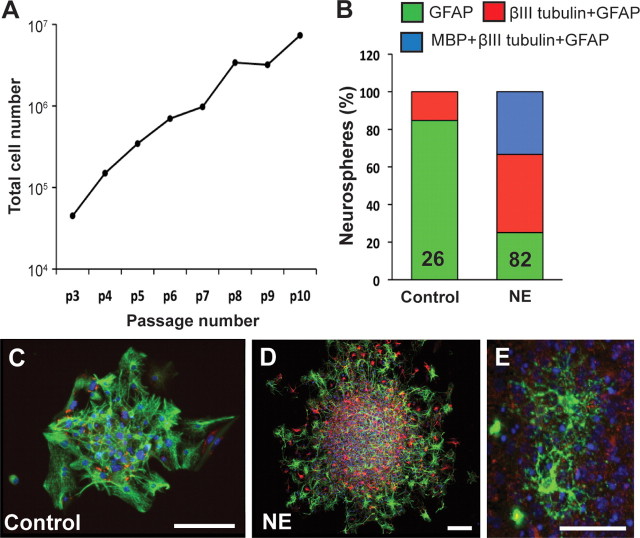
To ascertain whether the norepinephrine-stimulated large neurospheres indeed reflected the activation of stem cells, individual neurospheres were selected and subjected to long-term passaging to assess their self-renewal capacity. A significant proportion (71.4%, 15 of 21) of norepinephrine (10 μm) stimulated large neurospheres (>200 μm in diameter) could be passaged over 10 times (Fig. 2A) compared to none of the smaller neurospheres (<200 μm in diameter) from the control or norepinephrine-treated groups. Similarly, none of the neurospheres (0 of 16) stimulated with either 1 or 10 μm serotonin could be passaged.
Figure 2.
Hippocampal precursors activated by norepinephrine are self-renewing and multipotent. A, A large increase in cell numbers was observed when a single norepinephrine-derived large neurosphere was passaged up to 10 times. B, Relative percentage of the primary neurospheres expressing markers of astrocytes, neurons and oligodendrocytes in control versus NE-treated cultures. Note that all neurospheres examined contained GFAP-positive astrocytes. However, a significantly larger proportion of neurospheres expressed the neuronal marker, βIII tubulin, in the norepinephrine-treated vs the control group. MBP-positive oligodendrocytes were only present in norepinephrine-stimulated neurospheres. C, D, An example of control (C) and norepinephrine-derived (D) neurospheres showing immunofluorescence for GFAP (green) and βIII tubulin (red). Nuclei were stained with DAPI (blue). Scale bars, 100 μm. Note the presence of a large number of βIII tubulin-positive neurons in the norepinephrine-derived sphere. E, MBP-expressing oligodendrocytes (green) were also present in norepinephrine-stimulated neurospheres. Scale bar, 30 μm.
We next determined the multipotentiality of the cells present within the neurospheres generated in the presence or absence of norepinephrine (Fig. 2B–D). All the neurospheres examined contained GFAP-expressing astrocytes. However, only a small proportion (4 of 26 neurospheres examined) of the control neurospheres contained βIII tubulin-positive neurons, as opposed to the majority (62 of 82 neurospheres examined) of the norepinephrine-stimulated neurospheres. One third of the norepinephrine-stimulated neurospheres contained MBP-positive oligodendrocytes (Fig. 2E), whereas none of the control neurospheres expressed the oligodendrocytic marker. Notably, all the norepinephrine-stimulated large neurospheres examined (n = 9) contained >50 neurons. Together, these findings indicate that norepinephrine but not serotonin can activate a self-renewing and multipotent stem cell population in the adult hippocampus.
To examine whether norepinephrine and KCl activate the same latent pool of precursors, we added both KCl (15 mm) and norepinephrine (10 μm) to the cultures. This led to a 4.5-fold increase in total neurosphere numbers compared with the twofold increase observed in the presence of either norepinephrine or KCl alone (p < 0.05, unpaired t test), suggesting that separate populations of precursors were being activated (Fig. 3A). More importantly, there was a fivefold increase in the number of large neurospheres (>200 μm in diameter) in the combined treatment group (control: 0; NE: 7.0 ± 0; KCl: 6.5 ± 5.5; NE+KCl: 34.5 ± 7.5; n = 2 experiments), indicative of activation a much larger population of latent stem cells than previously thought (Fig. 3B) (Walker et al., 2008).
Figure 3.
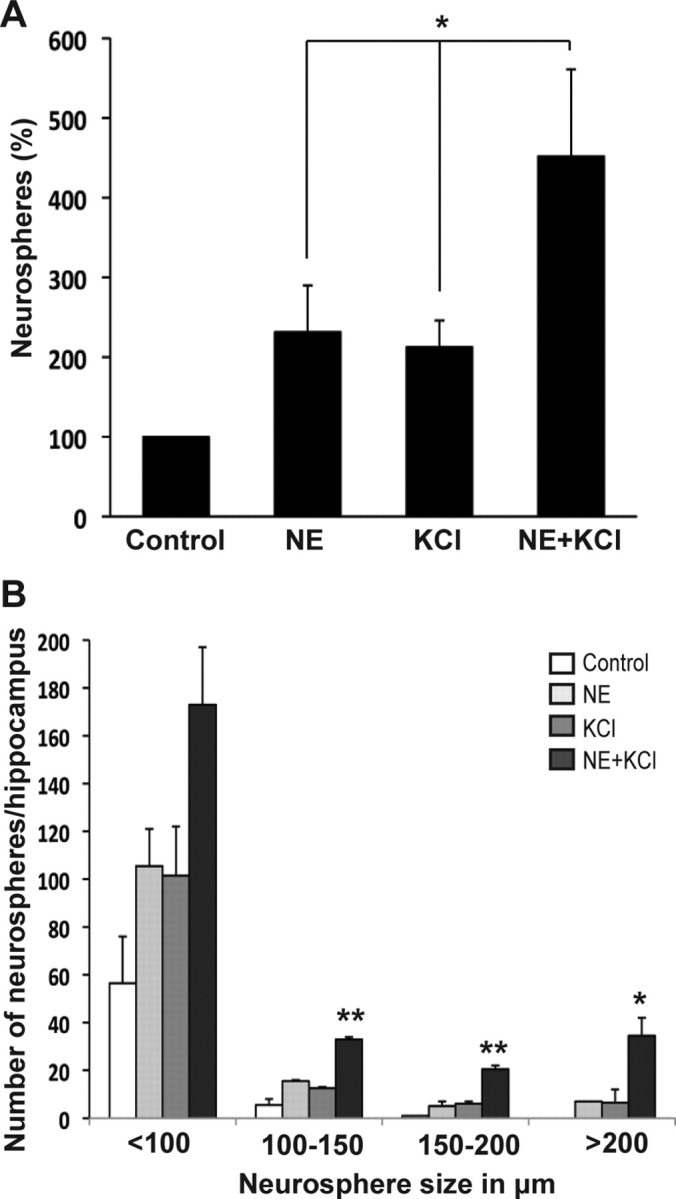
Norepinephrine and KCl activate different populations of hippocampal precursors. A, Culturing adult hippocampal cells in the presence of NE and KCl resulted in over a 4.5-fold increase in neurosphere numbers compared with a twofold increase in the number of neurospheres observed in the presence of either norepinephrine or KCl alone (mean ± SEM; *p < 0.05). B, Distribution of neurospheres according to size showing approximately a fivefold increase in the number of large neurospheres, measuring >200 μm, obtained in the presence of NE+KCl (mean ± SEM; *p < 0.05, **p < 0.01).
Norepinephrine directly stimulates proliferation of aHes5-expressing stem and precursor cell population
To determine whether norepinephrine can directly activate hippocampal precursors, it was necessary to examine the effect of norepinephrine at a clonal density. Given the very low frequency of neurosphere formation from the adult hippocampus (Bull and Bartlett, 2005), we needed to enrich cells with neurosphere-forming activity based on the use of an appropriate marker. Notch signaling has been implicated in the maintenance of neural precursors, and transgenic mice expressing GFP driven by the promoter of one of the downstream effectors of Notch, Hes5, have been used previously to isolate and enrich multipotential stem cells in the embryonic nervous system (Ohtsuka et al., 2006; Basak and Taylor, 2007). We therefore investigated whether Hes5 could also be used as a marker to isolate and enrich for hippocampal precursors in the adult. Using Hes5-GFP transgenic mice, we examined the expression pattern in the adult hippocampus and found that GFP-expressing cells were predominantly located along the subgranular zone (SGZ) of the dentate gyrus and had a radial glia-like morphology (Fig. 4A). The restricted expression and the characteristic morphology of the Hes5-GFP-positive cells prompted us to further examine whether this population represented stem/precursor cells. When colabeling with known stem/precursor cell markers was analyzed in 3–5 sections per hippocampus (n = 4 hippocampi) we found that 39% of the Hes5-GFP-positive cells in the SGZ (total of 927 cells examined) expressed GFAP, a marker for quiescent neural precursor or neural stem-like cells (Fig. 4B,B′), whereas 40% expressed nestin, another marker for the precursor population (total of 477 cells examined; Fig. 4C,C′), suggesting that the Hes5-GFP-positive cells were part of a stem/precursor cell population. No colabeling was observed between doublecortin- and GFP-positive cells (Fig. 4D), indicating that Hes5-GFP does not label neuronal progenitors or newly born neurons, although several doublecortin-positive cells were found in juxtaposition with GFP-expressing cells in the SGZ (Fig. 4D′).
Figure 4.
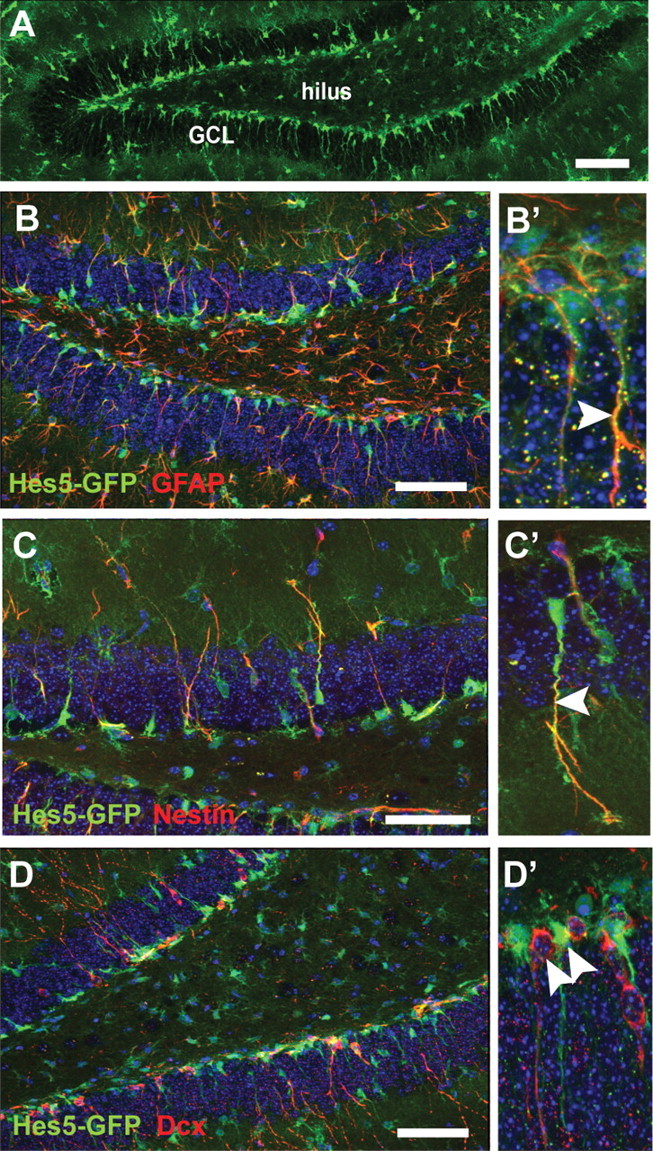
Hes5-GFP-positive cells coexpress markers of stem cells in the adult dentate gyrus. A, Hes5-GFP-positive cells are predominantly present along the subgranular zone and extend radial-glia like processes through the granule cell layer (GCL) in the adult dentate gyrus. B–D′, Hes5-GFP-positive cells coexpress markers of stem cells such as GFAP (red; B) and nestin (red; C). The coexpression is seen predominantly along the processes of the Hes5-GFP-positive cells (arrowheads; B′, C′). No coexpression was seen with doublecortin (red; D), a marker of newly born neurons. However, doublecortin-positive cells were mainly found in juxtaposition with Hes5-GFP-positive cells (arrowheads; D′). Nuclei were labeled with DAPI (blue). Scale bars, 100 μm.
Next, to examine the stem cell potential of the Hes5-GFP-expressing cells, cells were sorted from the adult hippocampus based on GFP expression (Fig. 5A). Reverse transcriptase-PCR analysis of the sorted cells showed the presence of Hes5 mRNA only in the GFP-positive population (Fig. 5B), which represented 5.6 ± 0.5% (n = 5 experiments) of the total viable hippocampal cell population. Subsequently, GFP-positive and -negative cells purified using flow cytometry were cultured for neurosphere generation. On average we observed that 1 of 65.5 ± 15.2 GFP-positive cells formed a neurosphere in control medium containing EGF and bFGF, with no neurospheres being obtained from the GFP-negative fraction (Fig. 5C). More importantly, the addition of norepinephrine resulted in a twofold increase in total neurosphere numbers only in the GFP-positive population (Fig. 5C), with the appearance of very large neurospheres (>200 μm in diameter) as described above (control: 1.0 ± 0.5 neurospheres vs NE: 7.3 ± 0.8 neurospheres per hippocampus). Together, these findings identify Hes5 as a marker of a stem and precursor cell population in the adult hippocampus, including those cells responsive to norepinephrine, and rule out the influence of Hes5-GFP-negative cells on neurosphere formation.
Figure 5.
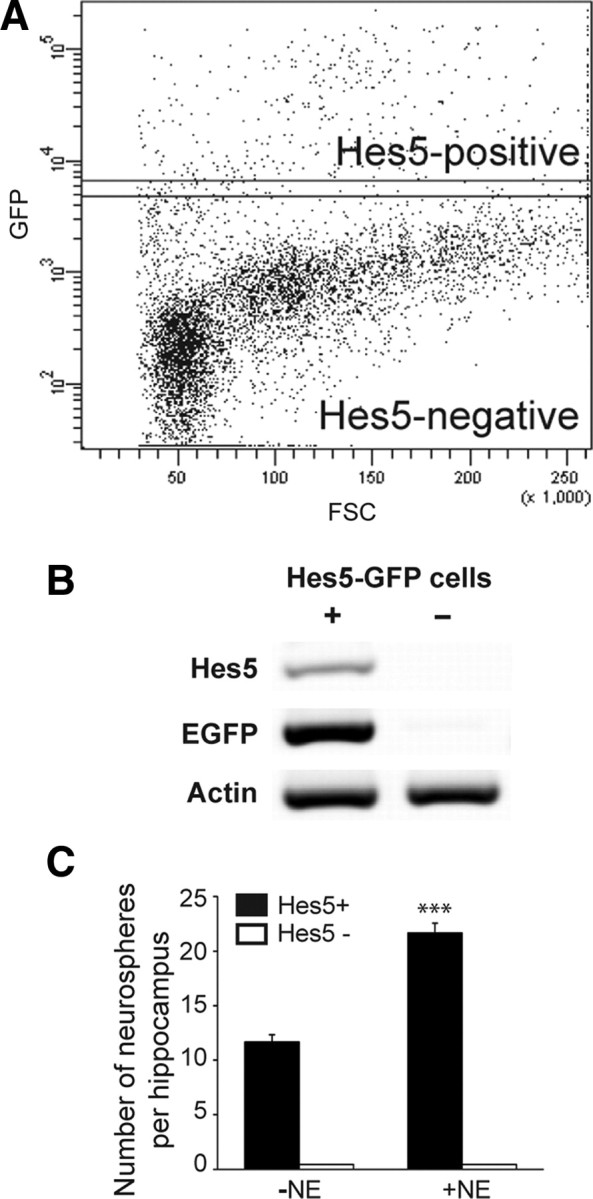
Norepinephrine activates a Hes5-expressing precursor population. A, Hes5-GFP- positive and -negative cells were sorted using flow cytometry based on their GFP expression. FSC, Forward scatter. B, Reverse transcriptase-PCR analysis revealed the presence of Hes5 mRNA only in the GFP-positive population. C, The Hes5-GFP-positive population contained all the neurosphere-forming cells. Note that in the presence of norepinephrine almost twice as many neurospheres were obtained from the Hes5-GFP-positive population. No neurospheres were generated from the Hes5-GFP-negative population (mean ± SEM; ***p < 0.001).
Finally, to determine whether norepinephrine activated the stem cell population directly and not via release of other factors in a paracrine manner in the bulk cultures, Hes5-GFP-positive cells were plated at a clonal density in 96-well plates. Whereas 1 of 32.5 ± 3.1 GFP-positive cells formed a neurosphere in the control medium, the frequency in the medium containing norepinephrine was 1 of 15.5 ± 1.6, resulting in a 215.4 ± 23.5% increase in neurosphere numbers, similar to that obtained in the bulk cultures. Moreover, even at clonal density a number of large neurospheres (>200 μm) expressing GFP were observed in the presence of norepinephrine (data not shown). Together, these findings unequivocally demonstrate that the effect of norepinephrine is direct and specific to hippocampal neural precursors.
β3-Adrenergic receptors mediate the effects of norepinephrine
Given that norepinephrine directly activated hippocampal precursors, we next sought to identify the adrenergic receptor(s) mediating this effect. Adrenergic receptors are a diverse family of receptors divided into two major subclasses, α and β, with six members of the α family and three members of the β family identified to date. The hippocampal cells were treated with specific antagonists to α1-adrenergic receptors (prazosin), α2-adrenergic receptors (yohimbine), or β-adrenergic receptors (propranolol) in the presence or absence of norepinephrine (Fig. 6A). One-way ANOVA revealed a significant difference between groups [F(7,20) = 8.0, p = 0.0001]. Both prazosin (100 nm) and yohimbine (1 μm) failed to inhibit the increase in neurosphere numbers observed in the presence of norepinephrine, whereas propranolol (1 μm) reduced the norepinephrine-mediated response back to control levels (p < 0.01; Bonferroni post hoc test), suggesting that β-adrenergic receptors are required for norepinephrine-dependent activation of precursors. Interestingly, treatment with yohimbine in the absence of norepinephrine resulted in a significant 40% increase (p < 0.05) in the neurosphere numbers compared with the control.
Figure 6.
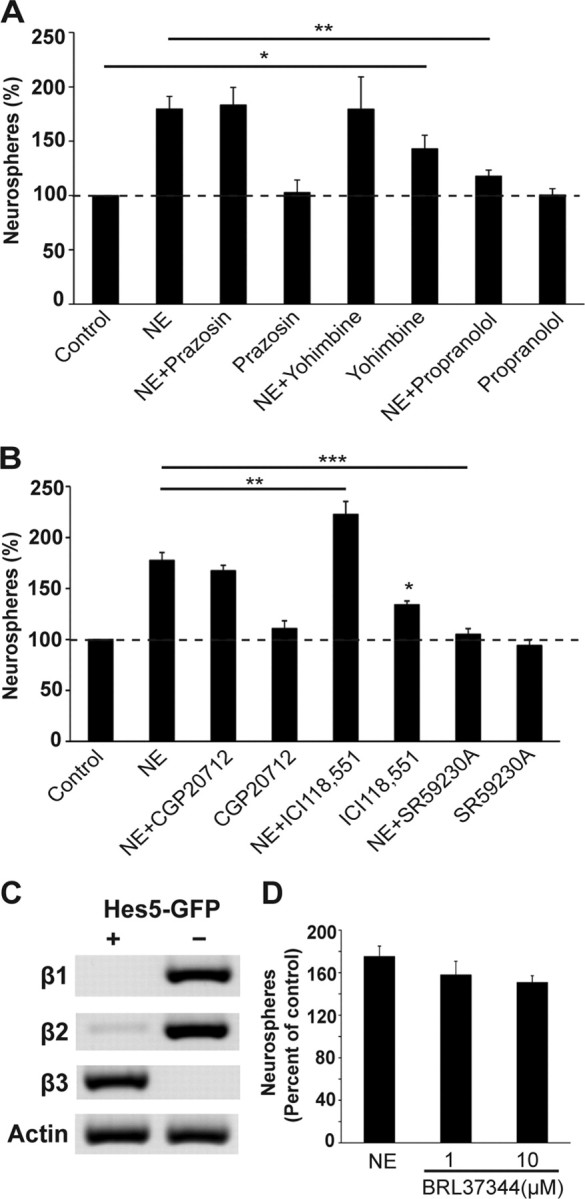
β3 receptors are expressed on neural precursors, and mediate the norepinephrine-dependent activation. A, Neither the α1-adrenergic receptor antagonist prazosin nor the α2-adrenergic receptor blocker yohimbine had any effect on the norepinephrine-stimulated increase in neurosphere numbers. Only the β-adrenergic receptor blocker, propranolol, completely inhibited the norepinephrine-stimulated increase in neurosphere numbers. Note that treatment with propranolol alone had no toxic effect on neurosphere production. A slight but significant increase in the number of neurospheres was also observed in the presence of yohimbine alone. B, The selective β3 blocker SR59230A completely inhibited the norepinephrine-mediated increase in neurosphere numbers. In contrast, the β1 receptor antagonist CGP20712 had no effect, whereas the β2 receptor antagonist ICI118,551 significantly increased neurosphere numbers both in the presence and absence of norepinephrine. C, Expression of β-adrenergic receptors in the sorted population of Hes5-GFP-positive and -negative cells by reverse transcriptase-PCR showed the presence of the β3-adrenergic receptor exclusively in the Hes5-positive population, whereas β1- and β2-adrenergic receptor transcripts were expressed predominantly in the Hes5-negative population. Note that a small amount of β2 receptor mRNA was also detected in the Hes5-positive population. D, A similar increase in neurosphere numbers was observed in the presence of a selective β3-adrenergic receptor agonist BRL37344 at 1 and 10 μm, compared with treatment with norepinephrine (mean ± SEM; *p < 0.05; **p < 0.01; ***p < 0.001).
Next, to identify the subtype of β-adrenergic receptor involved, we tested selective antagonists to β1-, β2-, and β3-adrenergic receptors (Fig. 6B). One-way ANOVA revealed a significant difference between groups [F(7,16) = 42.5, p < 0.0001]. CGP20712 (10 nm), a β1-adrenergic receptor antagonist, had no effect (p > 0.05; Bonferroni post hoc test), whereas the β2-adrenergic receptor blocker ICI118,551 (10 nm) significantly enhanced (p < 0.05; Bonferroni post hoc test) the norepinephrine-mediated response. Moreover, ICI118,551 in the absence of norepinephrine also increased neurosphere generation by ∼34% (p < 0.05) compared with the control. Only SR59230A (10 nm), a specific β3-adrenergic receptor antagonist, completely blocked the norepinephrine-mediated activation of precursors (p < 0.001), and also significantly reduced the generation of very large neurospheres (NE: 12 ± 2.08 neurospheres vs NE+SR59230A: 3.33 ± 0.88 neurospheres; p = 0.018; unpaired t test). Similarly, a significant block in norepinephrine-mediated activation was observed when the purified Hes5-GFP-positive precursor population was treated with SR59230A (control: 28 ± 1.0 neurospheres, NE: 45.5 ± 2.5 neurospheres, NE+SR59230A: 26.5 ± 2.5 neurospheres; n = 2 experiments; p = 0.016, unpaired t test).
Importantly, reverse transcriptase-PCR analysis showed the presence of β3-adrenergic receptors exclusively in the Hes5-positive population, whereas β1- and β2-adrenergic receptors were expressed predominantly in the Hes5-negative population (Fig. 6C). A small amount of β2-adrenergic receptor was also detected in the Hes5-positive cells.
Together the above findings led us to examine the effect of a selective β3-adrenergic receptor agonist BRL37344 on neural precursor activity (Fig. 6D). One-way ANOVA revealed a significant difference between groups [F(3,20) = 16.14, p < 0.0001]. Addition of BRL37344 (1 or 10 μm) or norepinephrine led to a significant increase in neurosphere numbers compared with the control (p < 0.001; Bonferroni post hoc test). Importantly, BRL37344 (either 1 μm or10 μm) treatment increased the neurosphere numbers to a similar extent to that obtained by norepinephrine treatment. Moreover, several very large neurospheres (>200 μm in diameter), indicative of activation of stem cells, were observed in BRL37344-treated cultures (1 μm BRL37344: 5.5 ± 0.5 neurospheres and 10 μm BRL37344: 7.5 ± 0.5 neurospheres) compared to none in the control medium.
Stimulation of β3-adrenergic receptors increases proliferation of hippocampal precursors in vivo
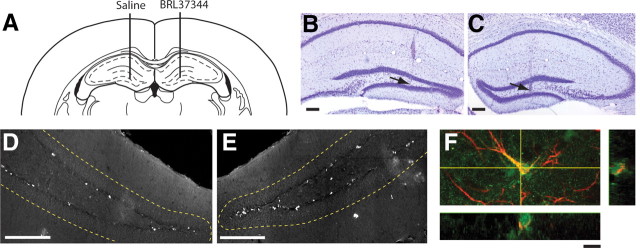
To determine whether similar enhancement of neural precursor activity occurs following stimulation of β3-adrenergic receptors in vivo, we injected BRL37344 directly into the hippocampus (Fig. 7A–C), given the absence of any direct evidence that BRL37344 is systemically active and crosses the blood–brain barrier. A single dose of BRL37344 (0.5 μl of 10 μm) was injected directly into the hilus region on the ipsilateral side, with saline (vehicle control) being injected into the contralateral side; dividing cells were then labeled with BrdU. A significant increase in the linear density of BrdU-positive cells (expressed as number of BrdU-labeled cells per mm of SGZ) was observed in the SGZ of the BRL37344-injected hippocampus compared with the contralateral saline-injected hippocampus (Fig. 7D,E; saline: 9.59 ± 1.3 cells vs BRL37344: 13.36 ± 2.0 cells; n = 5; p = 0.023; paired t test). This finding demonstrates that β3-adrenergic receptor stimulation leads to proliferation of neural precursors in vivo.
Figure 7.
Stimulation of β3-adrenergic receptors increases proliferation of hippocampal precursors in vivo. A–C, Bilateral intrahippocampal microinfusion was verified on Nissl-stained sections. A, A representative coronal section showing the injection track terminating in the hilus region of the hippocampus (Paxinos and Franklin, 2001). The hilus from one hemisphere received a 0.5 μl injection of 10 μm BRL37344 with the contralateral hemisphere receiving a control injection of 0.9% saline. B, C, Nissl-stained sections showing the most ventral point of the microinfusion track (arrows) following infusion of 0.9% saline (B) or BRL37344 (C). Scale bars, 200 μm. D, E, A representative micrograph showing BrdU-labeled cells along the SGZ in saline-treated (D) versus BRL37344-treated (E) hippocampus. The granule cell layer is delineated by the dashed lines. Scale bars, 200 μm. F, A confocal section showing colabeling of a nestin-GFP-positive cell (green) with GFAP (red) in the SGZ of isoproterenol-treated mice. Scale bar, 10 μm.
We also examined the effect of systemic treatment with the β-adrenergic receptor agonist isoproterenol on hippocampal neural precursor activity in mice expressing GFP under the control of nestin. Mice were treated once daily for 7 d with either saline (vehicle control) or isoproterenol, and dividing cells were again labeled with BrdU. Systemic stimulation of β-adrenergic receptors resulted in a significant increase in the total number of proliferating cells in the SGZ (saline: 485.48 ± 43.3 cells vs isoproterenol: 769.15 ± 55.4 cells; n = 5; p = 0.0038; unpaired t test). More interestingly, it also led to a significant increase in the percentage of nestin-GFP/GFAP double-positive cells, considered to be quiescent neural precursors (for review, see Kempermann et al., 2004) in the hippocampus (Fig. 7F; saline: 31.00 ± 2.9% vs isoproterenol: 48.84 ± 3.9%; p = 0.011; unpaired t test), confirming the activation of a latent neural precursor population in vivo.
Norepinephrine but not serotonin increases hippocampal precursor activity in a novel “slice-sphere” assay ex vivo
The above finding that norepinephrine but not serotonin directly activates hippocampal precursors prompted us to examine whether agents such as antidepressants, which act primarily by modulating levels of these neurotransmitters, exert their neurogenic effects by directly regulating hippocampal precursor activity. Given that the primary target of actions for these drugs requires the presence of monoaminergic terminals, which can be maintained only in an intact neurogenic niche, we reasoned that the neurosphere assay, where such a niche would be lost, was less suitable for this purpose. We therefore developed a two-step slice-sphere assay (outlined in Fig. 8A–D), essentially combining the advantages of organotypic slices, which retain the neurogenic milieu, and the neurosphere assay, which serves as a measure of quantifying precursor numbers. Hippocampal organotypic slices prepared from 7-d-old neonatal rats retained a healthy appearance after 6 d ex vivo in a serum-free culture medium. Although a significant reduction of ∼34% in cell number (n = 4 experiments, p = 0.003; unpaired t test) was observed from the slices cultured for 6 d (1.43 × 105 ± 1.57 × 104 cells/ml) compared with acute slices (day 0: 2.17 × 105 ± 1.12 × 104 cells/ml), the frequency of neurosphere formation was remarkably similar at day 0 (28.46 ± 0.6 neurospheres per 500 cells) and day 6 (25.01 ± 2.1 neurospheres per 500 cells; p = 0.216; unpaired t test), indicating not only that the precursors were maintained in the slices but also that they retained their normal proliferative capacity.
Figure 8.
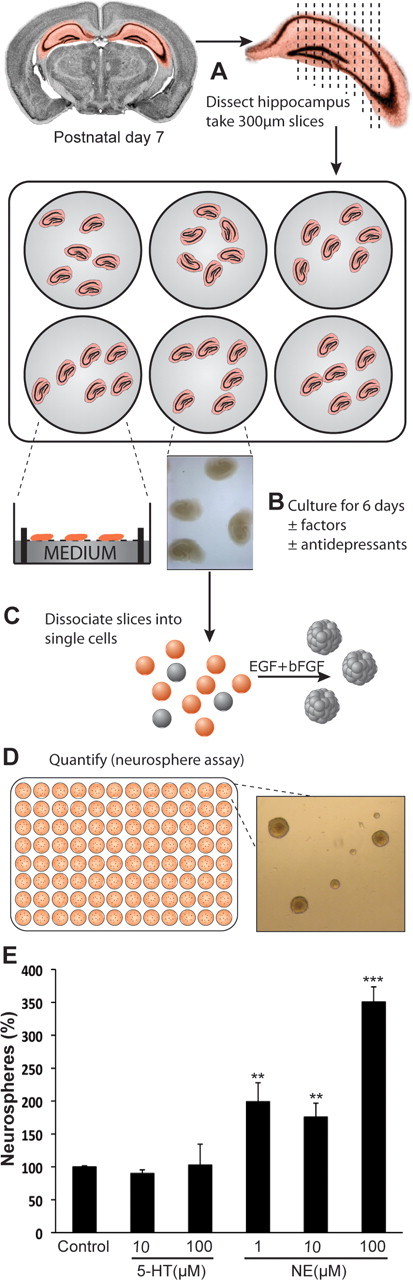
Direct application of norepinephrine but not serotonin enhances hippocampal precursor activity in the slice-sphere assay. A, The hippocampus from a postnatal day 7 Wistar rat was dissected and cut transversely into 300 μm slices. The slices were placed on a 0.4 μm membrane filter that was bathed in 1 ml of complete serum-free NeuroCult medium in a 6-well plate. Four filters, each containing 6–7 slices, were generated from a single animal. B, The organotypic slices were cultured at a liquid-air interphase for a period of 6 d. Antidepressants or neurotransmitters were added to the medium on day 1 and half the medium was replaced with fresh medium every alternate day. C, On the sixth day, the hippocampal slices were enzymatically dissociated and cells were plated in a 96-well plate and cultured in the presence of EGF and bFGF to obtain neurospheres. D, The number of neurospheres generated was quantified after 10–12 d in culture, this being representative of the number of proliferating hippocampal precursors present in the slices. E, Serotonin (5-HT) treatment had no effect on the frequency of neurosphere formation either at 10 or 100 μm. However, direct application of NE to the slices resulted in an ∼2-fold increase in the neurosphere frequency at 1 and 10 μm, and a 3.5-fold increase at 100 μm (mean ± SEM; **p < 0.01; ***p < 0.001).
To determine the influence of an intact neurogenic niche and validate the usefulness of the slice-sphere assay in mediating the effects of serotonin or norepinephrine on precursor activity, hippocampal slices were treated with various concentrations of these neurotransmitters. The neurosphere frequency remained unchanged in the slices treated with serotonin (10 μm: 90.07 ± 5.4% and 100 μm: 102.81 ± 31.7%) compared with the control (Fig. 8E). However, a twofold increase in precursor numbers was obtained in the slice-sphere assay from slices treated with either 1 μm (p = 0.026; unpaired t test) or 10 μm norepinephrine (p = 0.023), consistent with our finding of an enhanced neurosphere frequency in the conventional neurosphere assay. Notably, addition of 100 μm norepinephrine to the slices led to a 3.5-fold increase (p < 0.001) in the precursor activity compared with the control. This suggests that serotonin is not able to modulate precursor activity even when the neurogenic niche is maintained. Moreover, it highlights the importance of an intact neurogenic niche in revealing a significantly larger increase in norepinephrine-dependent precursor activation than observed in the standard neurosphere assay.
Antidepressants that block reuptake of norepinephrine but not serotonin stimulate hippocampal precursor activity inthe slice-sphere assay
Finally, the above findings led us to examine the effect of two major classes of antidepressants on hippocampal precursor activity. Fluoxetine, a prototypical SSRI, had no effect on precursor numbers when added to the slices at a concentration of 1 μm but significantly reduced the neurosphere frequency at 10 μm (Fig. 9A; p = 0.04; unpaired t test). Although fluoxetine is a potent uptake inhibitor of serotonin, it is also known to affect the activity of muscarinic, histaminergic and α-adrenergic receptors (Hyttel, 1994). Hence, the effect of another potent and more specific SSRI, citalopram, was also examined. No significant change in the hippocampal precursor frequency was observed at either 10 μm (113.8 ± 9.9%) or 100 μm (103.8 ± 3.8%) citalopram compared with the control (Fig. 9A).
Figure 9.
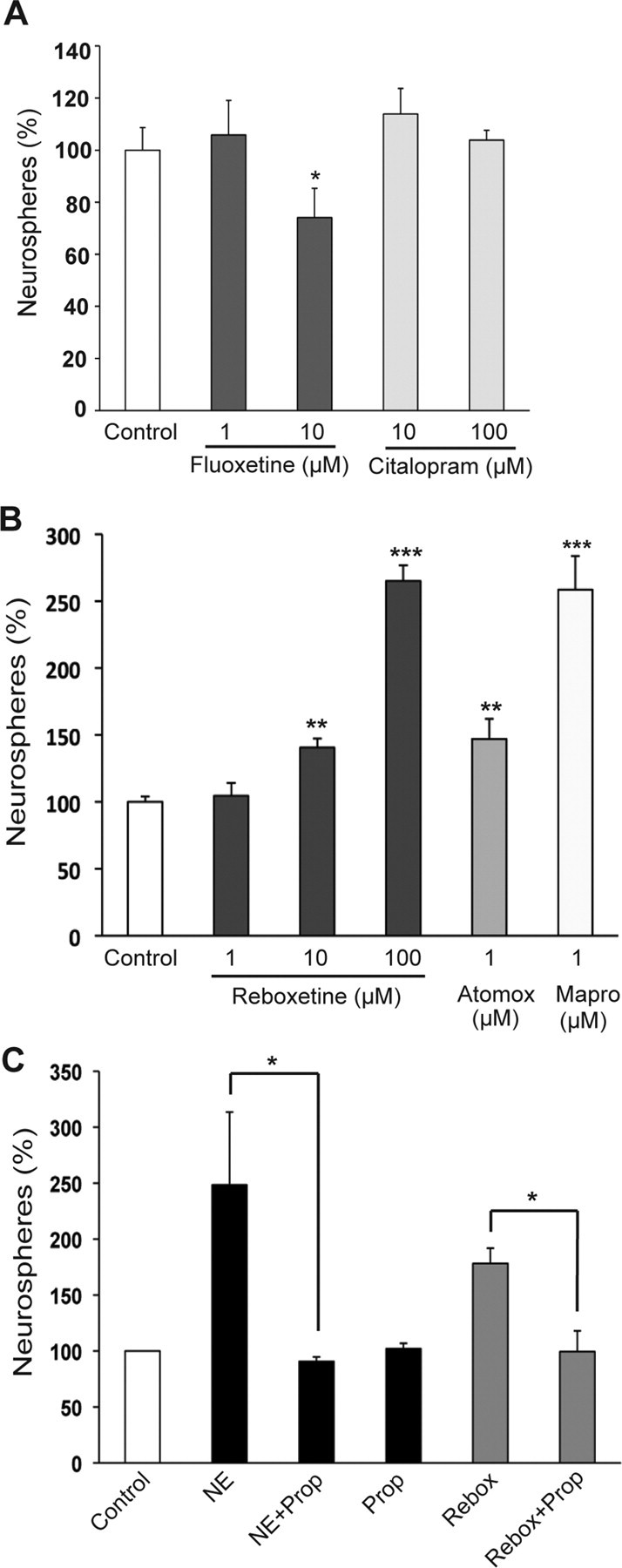
NRIs but not SSRIs increase the activity of hippocampal precursors in the slice-sphere assay. A, Slices treated with the SSRIs fluoxetine (1 μm) or citalopram (10 and 100 μm) showed no significant change in the frequency of neurosphere generation compared with the untreated slices (control). Treatment with 10 μm fluoxetine decreased neurosphere frequency. B, Reboxetine, a prototypical NRI, significantly enhanced the frequency of neurosphere formation at 10 and 100 μm. Treatment of slices with atomoxetine and maprotiline also resulted in a significant increase in neurosphere frequency. C, Blockade of β-adrenergic receptors by propranolol (10 μm) abolished both the norepinephrine- and the reboxetine-mediated increase in neurosphere frequency (mean ± SEM; *p < 0.05; **p < 0.01; ***p < 0.001).
In contrast, reboxetine, a widely used NRI, produced a dose-dependent increase in neurosphere numbers. While the frequency of neurosphere formation remained unchanged at 1 μm (104.5 ± 9.6%), a significant 40% increase was observed at 10 μm (p = 0.0026; unpaired t test), and more than a 2.5-fold increase (265.0 ± 11.7%; p < 0.001) was observed at 100 μm (Fig. 9B). This stimulatory effect on hippocampal precursors was not exclusive to reboxetine, being observed in the presence of other members of the NRI family, namely atomoxetine and maprotiline (Fig. 9B). Atomoxetine, at a concentration of 1 μm, increased the precursor frequency to 147.0 ± 15.0% (n = 2 experiments), comparable to the result obtained with 10 μm reboxetine treatment. Treatment of slices with 1 μm maprotiline produced an even greater increase (258.5 ± 25.5%) in the frequency of neurosphere formation compared with the control (n = 2 experiments). Together, these results suggest that antidepressants that specifically block the reuptake of norepinephrine may exert their neurogenic effects in the hippocampus primarily through activation of a precursor population. In contrast, serotonin and the antidepressants that modulate its levels appear to have no direct role in regulating hippocampal precursor activity.
Finally, to determine whether the norepinephrine- and reboxetine-mediated increase in hippocampal precursor activity involved β-adrenergic receptors, slices were treated with 10 μm propranolol in the presence of either 10 μm norepinephrine or 10 μm reboxetine (Fig. 9C). The ability of propranolol to completely inhibit the norepinephrine-mediated (p = 0.036; unpaired t test) as well as the reboxetine-mediated (p = 0.013) increase in precursor activity indicated the involvement of β-adrenergic receptors.
Discussion
In the present study we have demonstrated that norepinephrine, but not serotonin, activates a stem and precursor cell pool in the adult hippocampus, and have provided evidence for a direct action of norepinephrine on these precursors. Importantly, we have uncovered a novel role for β3-adrenergic receptors in mediating the norepinephrine-dependent activation of the hippocampal precursors both in vitro and in vivo. Consistent with these results, our findings from the slice-sphere assay demonstrate that antidepressants that selectively block the reuptake of norepinephrine but not serotonin enhance hippocampal neurogenesis, primarily by targeting the activity of stem and precursor cells.
The norepinephrine-responsive precursor population appears remarkably similar to the previously identified latent population of stem and precursor cells activated by depolarizing levels of KCl (Walker et al., 2008). The most striking common feature between norepinephrine- and KCl-mediated activation is the emergence of a small number of very large neurospheres (measuring >200 μm in diameter), displaying the classic properties of stem cells, including self-renewal over multiple passages and generation of multipotential lineages. However, the additive effect in neurosphere numbers observed in the presence of both norepinephrine and KCl is indicative of activation of different pools of latent precursors. In fact, 5-fold increase in the number of large neurospheres generated in the presence of both norepinephrine and KCl, and the inability of a selective β3-adrenergic receptor antagonist to block the KCl-dependent activation of the precursors (data not shown), suggest the existence of a much larger pool of latent precursors within the adult hippocampus than previously considered. Also noteworthy is the ability of norepinephrine to support neuronal production, with the majority of primary neurospheres grown in the presence of norepinephrine containing βIII tubulin-positive neurons, and the larger neurospheres containing in excess of 50 neurons. This highlights the neurogenic potential of norepinephrine, which may underlie the enhanced neurogenesis observed in vivo in response to NRIs (Malberg et al., 2000). Thus, it appears that neural activity could regulate hippocampal neurogenesis in multiple ways. First, neural excitation could transiently stimulate neurogenic activity through the release of paracrine factors from the neurogenic niche that could lead to a non-cell-autonomous activation of the latent pool of neural precursors (Walker et al., 2008). Alternatively, neural activity could lead to long-term changes in gene expression of the factors associated with the neurogenic niche, such as brain-derived neurotrophic factor and FGF-1b, by epigenetic mechanisms (Ma et al., 2009). In contrast to these mechanisms, we propose a novel mechanism whereby norepinephrine-mediated neural activity could directly and locally enhance neural precursor activity via β3-adrenergic receptors, a possibility supported by the finding that norepinephrine-containing afferents are found in close proximity to proliferating precursors in the SGZ (Rizk et al., 2006). Whether new neurons generated in the hippocampus in response to firing of noradrenergic neurons in the locus ceruleus or neural activity arising from other brain regions have similar or different electrophysiological properties, and the behavioral consequences of this, remains to be determined.
To date, the identity of the latent precursor population in the adult hippocampus has remained elusive. In the present study, we provide evidence that the Hes5-expressing population in the adult hippocampal SGZ contains a subset of such latent precursors. More importantly, we find that the Hes5-expressing precursor population comprises all the neurosphere-forming cells from the adult hippocampus, including the stem cell population that responds to norepinephrine. Expression of Hes5 in such a latent precursor population is not surprising, given the essential role that Notch signaling and Hes genes play in the maintenance of neural stem and precursor cells in both the developing and the adult nervous system (Kageyama et al., 2005). Moreover, similar neurosphere frequency in bulk as well as clonal density (1 cell/well) cultures of purified Hes5-positive precursors confirmed the direct effect of norepinephrine on adult hippocampal precursors and ruled out the involvement of paracrine factors secreted from other cells. This direct effect may underlie the twofold increase in precursor activity observed in our slice-sphere assay in the presence of either norepinephrine or antidepressants that block the reuptake of norepinephrine. Thus, we propose that Hes5 provides a valuable and robust marker for a subpopulation of stem and precursor cells in the adult hippocampus and could be used to examine the effects of other factors, including those released by neural excitation (Walker et al., 2008).
A striking and unexpected finding of the current study was the expression of β3-adrenergic receptors exclusively on the Hes5-positive precursor population. Until now, predominant expression of β3 receptors has only been reported in brown and white adipose tissue (Strosberg, 1997), with a few reports examining their expression in various brain tissues, including the hippocampus (Summers et al., 1995; Claustre et al., 2008). Our evidence that the nonselective β-adrenergic receptor blocker propranolol, as well as the selective β3-adrenergic receptor antagonist SR59230A, completely inhibit the norepinephrine-dependent stimulation of stem and precursor cells underpins a critical and novel role for β3-adrenergic receptors in regulating adult hippocampal precursor activity. This was further strengthened by our finding that pharmacological stimulation of β3-adrenergic receptors both in vitro and in vivo by the selective β3-adrenergic receptor agonist BRL37344 led to enhanced proliferation of hippocampal precursors. On the other hand, expression of β1 and β2 receptors in the Hes5-negative population was consistent with the inability of their selective antagonists to block the norepinephrine-mediated activation of precursors. In fact, a marginal but significant potentiation effect was observed in the presence of the β2 antagonist ICI118,551, suggesting an inhibitory role for β2-adrenergic receptors in regulating precursor proliferation. Such an inhibitory role has recently been proposed for another member of the adrenergic receptor family, namely α2-adrenergic receptors (Yanpallewar et al., 2010), suggesting that antagonism of α2-adrenergic receptors could also activate neural precursors and result in an increase in neurosphere numbers as observed in this study. Alternatively, α2-adrenergic receptor antagonism may enhance survival of neural precursors, a possibility raised in a previous report (Rizk et al., 2006) that demonstrated an α2-adrenergic receptor antagonist-mediated increase in the survival of newborn neurons. Interestingly, β3-adrenergic receptor blockade had no effect on the baseline proliferation of precursors in the absence of norepinephrine. This raises the possibility of a specific requirement of these receptors in mediating norepinephrine-dependent regulation of hippocampal precursors, including that mediated by NRIs.
How β3-adrenergic receptors mediate activation and proliferation of neural precursors is currently unknown. However, given that β3-adrenergic receptors are seven transmembrane receptors coupled to heterotrimeric G-proteins that signal via multiple intracellular pathways, including activation of adenylate cyclase and cAMP-dependent phosphorylation (for review, see Ursino et al., 2009), and that increases in the intracellular levels of cAMP regulate the proliferation of hippocampal precursors in vivo (Nakagawa et al., 2002), it is possible that β3-adrenergic receptor-driven activation of neural precursors may also use this cAMP-mediated signaling mechanism.
Also relevant to our findings are several reports describing an antidepressant-like profile for the specific and potent β3-adrenergic receptor agonist, SR58611A (amibegron) (Simiand et al., 1992; Consoli et al., 2007; Stemmelin et al., 2008). Interestingly, a study by Claustre et al. (2008) demonstrated that an intraperitoneal injection of SR58611A produced an up to fivefold increase in the level of hippocampal norepinephrine, consistent with enhancement of the firing rate of noradrenergic neurons present within the locus ceruleus. Given the dense innervation of noradrenergic fibers in the dentate gyrus from the locus ceruleus, together with our results demonstrating that norepinephrine can directly activate multipotent precursors, it is tempting to speculate that the antidepressant-like effect of SR58611A observed in animal models may occur via β3-adrenergic receptor-mediated enhancement of hippocampal neurogenesis.
Finally, our data from both the standard neurosphere and slice-sphere assays, together with the lack of any evidence for the expression of serotonin receptors on neural precursors suggest that serotonin and antidepressants that modulate levels of serotonin neither directly nor via release of paracrine factors from the neurogenic niche regulate hippocampal precursor activity. It is possible that the previously reported neurogenic effects of SSRIs on precursor activity (Gould, 1999; Malberg et al., 2000) require more than just the local hippocampal niche. Given reports that the effects of SSRIs, including fluoxetine, are lost in norepinephrine-deficient mice, it has been suggested that some of these effects may involve a role for norepinephrine (Cryan et al., 2004). Alternatively, it has been proposed that the neurogenic effects of fluoxetine require circadian rhythm-associated changes in the level of corticosterone (Huang and Herbert, 2006). It must also be noted that the slice-sphere assay used postnatal day 7 animals, primarily due to the ease of maintaining healthy hippocampal slices ex vivo. Although many of the factors that regulate adult neurogenesis are present in the developing hippocampus, there may be a lack of specific inputs or other factors in the early postnatal brain which preclude the proliferating effects of SSRIs. Nonetheless, recent studies have shown that neither selective depletion or enhancement of serotonin (Jha et al., 2006) nor chronic treatment with fluoxetine have any effect on hippocampal precursor proliferation in vivo (Couillard-Despres et al., 2009; David et al., 2009). Instead, improved survival and morphological changes in doublecortin-positive cells suggest a role for fluoxetine during maturation of newly born neurons (Wang et al., 2008; David et al., 2009).
In summary, our findings suggest that stimulation of β3-adrenergic receptors can directly activate a distinct population of precursors, including stem cells, to generate a large number of neurons in the adult hippocampus. This opens up the possibility of developing novel pharmaceutical agents to enhance neurogenesis as a means of treating a variety of psychiatric diseases, including depression.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia. P.F.B. was the recipient of an Australian Research Council Federation Fellowship, D.J.J. was the recipient of a Human Frontiers Science Program Long-term Fellowship, and V.A.V. was the recipient of a Wellcome Trust Senior Overseas Fellowship in Biomedical Sciences (04082003114133). We are grateful to Dr. Steven Kernie for providing nestin-GFP mice and Rowan Tweedale for editorial assistance.
References
- Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci. 2007;25:1006–1022. doi: 10.1111/j.1460-9568.2007.05370.x. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10:37–46. doi: 10.1002/(SICI)1098-1063(2000)10:1<37::AID-HIPO4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59(Suppl 14):11–14. [PubMed] [Google Scholar]
- Cikos S, Veselá J, Il'ková G, Rehák P, Czikková S, Koppel J. Expression of β adrenergic receptors in mouse oocytes and preimplantation embryos. Mol Reprod Dev. 2005;71:145–153. doi: 10.1002/mrd.20256. [DOI] [PubMed] [Google Scholar]
- Claustre Y, Leonetti M, Santucci V, Bougault I, Desvignes C, Rouquier L, Aubin N, Keane P, Busch S, Chen Y, Palejwala V, Tocci M, Yamdagni P, Didier M, Avenet P, Le Fur G, Oury-Donat F, Scatton B, Steinberg R. Effects of the β3-adrenoceptor (Adrb3) agonist SR58611A (amibegron) on serotonergic and noradrenergic transmission in the rodent: relevance to its antidepressant/anxiolytic-like profile. Neuroscience. 2008;156:353–364. doi: 10.1016/j.neuroscience.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Consoli D, Leggio GM, Mazzola C, Micale V, Drago F. Behavioral effects of the β3 adrenoceptor agonist SR58611A: is it the putative prototype of a new class of antidepressant/anxiolytic drugs? Eur J Pharmacol. 2007;573:139–147. doi: 10.1016/j.ejphar.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, Bogdahn U, Aigner L. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry. 2009;14:856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BA, Papaioannou M, Hamilton S, Summers RJ. Alternative splicing generates two isoforms of the β3-adrenoceptor which are differentially expressed in mouse tissues. Br J Pharmacol. 1999;127:1525–1531. doi: 10.1038/sj.bjp.0702688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Hagg T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist. 2009;15:20–27. doi: 10.1177/1073858408324789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Bannerman D, Flint J. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis, in BALB/cJ mice. Mol Psychiatry. 2008;13:119–121. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Jha S, Rajendran R, Davda J, Vaidya VA. Selective serotonin depletion does not regulate hippocampal neurogenesis in the adult rat brain: differential effects of p-chlorophenylalanine and 5,7-dihydroxytryptamine. Brain Res. 2006;1075:48–59. doi: 10.1016/j.brainres.2005.12.110. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kulkarni VA, Jha S, Vaidya VA. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur J Neurosci. 2002;16:2008–2012. doi: 10.1046/j.1460-9568.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Harley CW. The action of norepinephrine in the dentate gyrus: β-mediated facilitation of evoked potentials in vitro. Brain Res. 1985;358:210–220. doi: 10.1016/0006-8993(85)90965-5. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Nácher J, Varea E, Miguel Blasco-Ibáñez J, Gómez-Climent MA, Castillo-Gómez E, Crespo C, Martínez-Guijarro FJ, McEwen BS. N-Methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Imayoshi I, Shimojo H, Nishi E, Kageyama R, McConnell SK. Visualization of embryonic neural stem cells using Hes promoters in transgenic mice. Mol Cell Neurosci. 2006;31:109–122. doi: 10.1016/j.mcn.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Otsuki I, Shiroishi Y. Supersensitivity to isoproterenol and a new adrenergic β-mimetic agent trimethoquinol on the blood pressure of the reserpine treated rat. Jpn J Pharmacol. 1969;19:110–114. doi: 10.1254/jjp.19.110. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Ed 2. London: Academic; 2001. [Google Scholar]
- Rizk P, Salazar J, Raisman-Vozari R, Marien M, Ruberg M, Colpaert F, Debeir T. The α2-adrenoceptor antagonist dexefaroxan enhances hippocampal neurogenesis by increasing the survival and differentiation of new granule cells. Neuropsychopharmacology. 2006;31:1146–1157. doi: 10.1038/sj.npp.1300954. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of serotonin in the rat hippocampal slice preparation. J Physiol. 1980;303:423–439. doi: 10.1113/jphysiol.1980.sp013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiand J, Keane PE, Guitard J, Langlois X, Gonalons N, Martin P, Bianchetti A, Le Fur G, Soubrié P. Antidepressant profile in rodents of SR 58611A, a new selective agonist for atypical β-adrenoceptors. Eur J Pharmacol. 1992;219:193–201. doi: 10.1016/0014-2999(92)90296-g. [DOI] [PubMed] [Google Scholar]
- Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O, Decobert M, Santucci V, Françon D, Alonso R, Stahl SM, Keane P, Avenet P, Scatton B, le Fur G, Griebel G. Stimulation of the β3-adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology. 2008;33:574–587. doi: 10.1038/sj.npp.1301424. [DOI] [PubMed] [Google Scholar]
- Strosberg AD. Association of β3-adrenoceptor polymorphism with obesity and diabetes: current status. Trends Pharmacol Sci. 1997;18:449–454. doi: 10.1016/s0165-6147(97)01133-4. [DOI] [PubMed] [Google Scholar]
- Summers RJ, Papaioannou M, Harris S, Evans BA. Expression of β3-adrenoceptor mRNA in rat brain. Br J Pharmacol. 1995;116:2547–2548. doi: 10.1111/j.1476-5381.1995.tb17205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Ursino MG, Vasina V, Raschi E, Crema F, De Ponti F. The β3-adrenoceptor as a therapeutic target: current perspectives. Pharmacol Res. 2009;59:221–234. doi: 10.1016/j.phrs.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Vadodaria KC, Jha S. Neurotransmitter regulation of adult neurogenesis: putative therapeutic targets. CNS Neurol Disord Drug Targets. 2007;6:358–374. doi: 10.2174/187152707783220910. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Mahlstedt MM, Henn FA. Neurogenesis and depression: what animal models tell us about the link. Eur Arch Psychiatry Clin Neurosci. 2007;257:300–303. doi: 10.1007/s00406-007-0734-2. [DOI] [PubMed] [Google Scholar]
- Walker TL, White A, Black DM, Wallace RH, Sah P, Bartlett PF. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J Neurosci. 2008;28:5240–5247. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanpallewar SU, Fernandes K, Marathe SV, Vadodaria KC, Jhaveri D, Rommelfanger K, Ladiwala U, Jha S, Muthig V, Hein L, Bartlett P, Weinshenker D, Vaidya VA. α2-Adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci. 2010;30:1096–1109. doi: 10.1523/JNEUROSCI.2309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Dandekar M, Monteggia LM, Parada LF, Kernie SG. Temporally regulated expression of Cre recombinase in neural stem cells. Genesis. 2005;41:147–153. doi: 10.1002/gene.20110. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Bruce JC, Darby-King A, McLean JH. Isoproterenol increases CREB phosphorylation and olfactory nerve-evoked potentials in normal and 5-HT-depleted olfactory bulbs in rat pups only at doses that produce odor preference learning. Learn Mem. 2000;7:413–421. doi: 10.1101/lm.35900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]