Abstract
The effects of tetracycline on the subgingival streptococcal flora of periodontal patients were examined. Before antibiotic treatment, tetracycline-resistant isolates were obtained from 24 to 25 patients. In most patients, the proportion of the subgingival flora resistant to tetracycline increased after 2 weeks of therapy (1,000 mg of tetracycline/day) and then decreased after the cessation of treatment. Cultural conditions used for primary isolation were designed to favor the growth of facultative streptococci. Consequently, the majority (99%) of resistant isolates were identified as streptococci. Among 407 tetracycline-resistant Streptococcus isolates chosen for further classification, 9 species were identified, with S. sanguis (63%) and S. mitis (19%) predominating. There were no significant differences in the distribution of species isolated before and after treatment and after the cessation of tetracycline treatment. Plasmids were isolated from only 23 of 121 resistant streptococcal strains examined, suggesting that tetracycline resistance is not plasmid mediated in the majority of these oral streptococci.
Full text
PDF

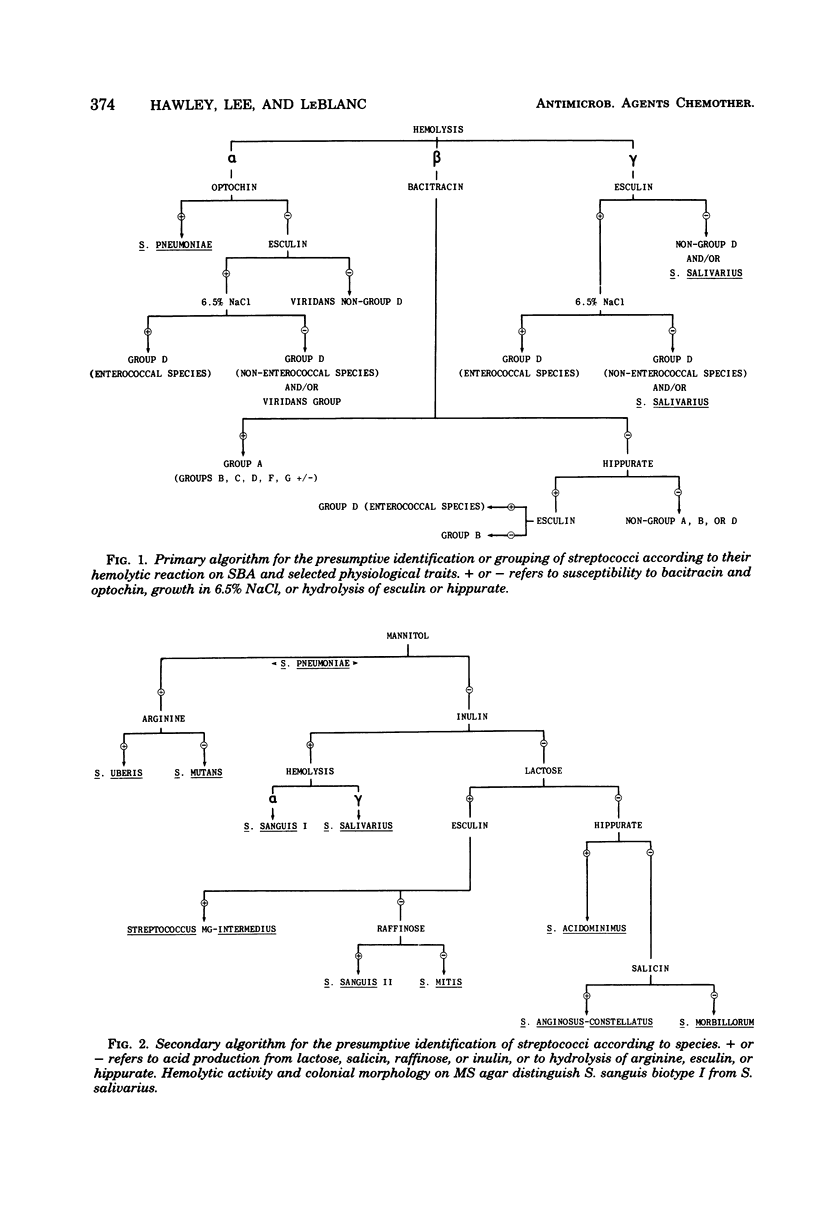

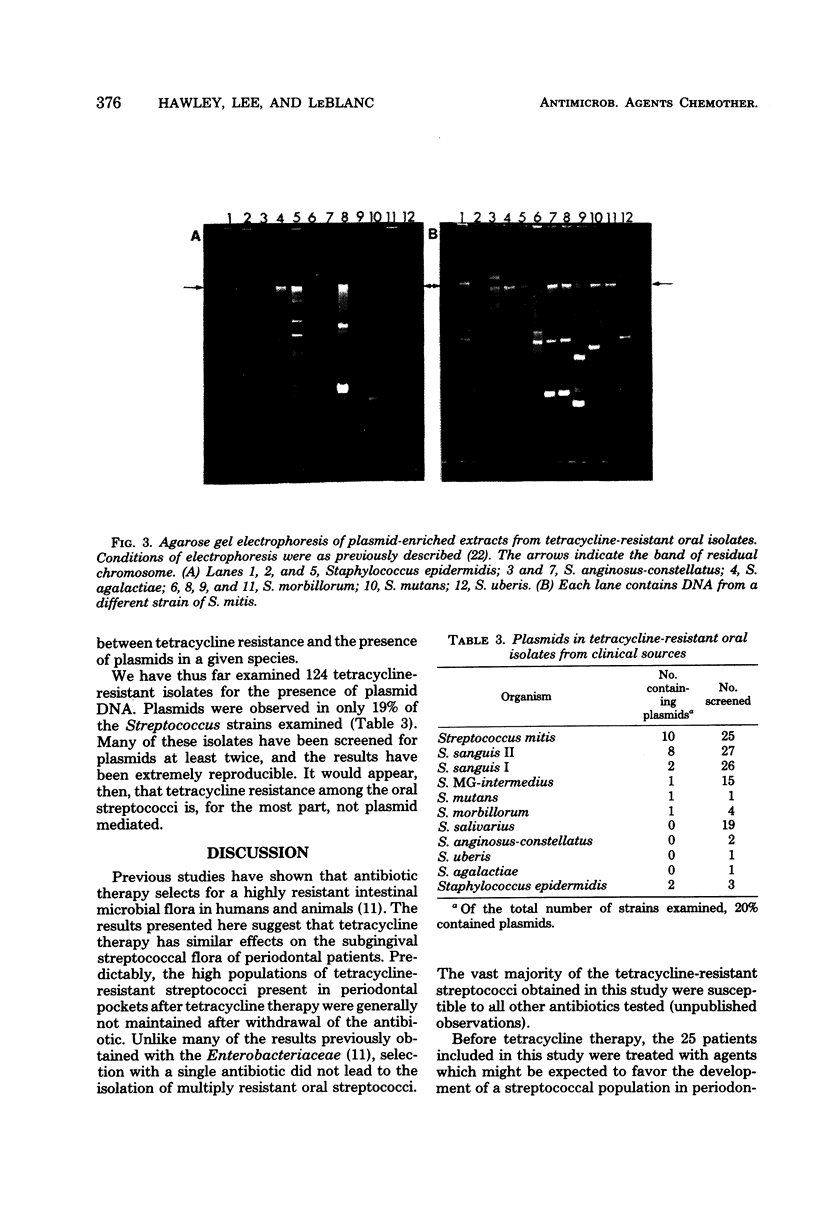


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Croissant O., Blangy D. Identification of two plasmids determining resistance to tetracycline and to erythromycin in group D streptococcus. Mol Gen Genet. 1974;132(3):181–192. doi: 10.1007/BF00269391. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., Lipinski A. E. Infections with beta-Hemolytic Streptococcus resistant to lincomycin and erythromycin and observations on zonal-pattern resistance to lincomycin. J Infect Dis. 1974 Oct;130(4):351–356. doi: 10.1093/infdis/130.4.351. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., Lipinski A. E. Resistance of group A beta-hemolytic streptococci to lincomycin and erythromycin. Antimicrob Agents Chemother. 1972 Apr;1(4):333–339. doi: 10.1128/aac.1.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Solh N., Bouanchaud D. H., Horodniceanu T., Roussel A., Chabbert Y. A. Molecular studies and possible relatedness between R plasmids from groups B and D streptococci. Antimicrob Agents Chemother. 1978 Jul;14(1):19–23. doi: 10.1128/aac.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Padula J. F., Thacker L. G., Wortham E. C., Sconyers B. J. Presumptive identification of group A, B, and D streptococci. Appl Microbiol. 1974 Jan;27(1):107–113. doi: 10.1128/am.27.1.107-113.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972 Jun;23(6):1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J. M., Marsh P. D. Streptococci and the human oral flora. Soc Appl Bacteriol Symp Ser. 1978;7:157–206. [PubMed] [Google Scholar]
- Hershfield V. Plasmids mediating multiple drug resistance in group B streptococcus: transferability and molecular properties. Plasmid. 1979 Jan;2(1):137–149. doi: 10.1016/0147-619x(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bouanchaud D. H., Bieth G., Chabbert Y. A. R plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1976 Nov;10(5):795–801. doi: 10.1128/aac.10.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes P. H., Wright W. E., Howard S. A. The use of phase-contrast microscopy and chemotherapy in the diagnosis and treatment of periodontal lesions--an initial report (I). Quintessence Int Dent Dig. 1978 Jan;9(1):51–56. [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Crow V. L., Lee L. N., Garon C. F. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J Bacteriol. 1979 Feb;137(2):878–884. doi: 10.1128/jb.137.2.878-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hawley R. J., Lee L. N., St Martin E. J. "Conjugal" transfer of plasmid DNA among oral streptococci. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3484–3487. doi: 10.1073/pnas.75.7.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc D. J., Cohen L., Jensen L. Transformation of group F streptococci by plasmid DNA. J Gen Microbiol. 1978 May;106(1):49–54. doi: 10.1099/00221287-106-1-49. [DOI] [PubMed] [Google Scholar]
- Leblanc D. J., Lee L. N. Rapid screening procedure for detection of plasmids in streptococci. J Bacteriol. 1979 Dec;140(3):1112–1115. doi: 10.1128/jb.140.3.1112-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H. Genetics of resistance to macrolide antibiotics and lincomycin in natural isolates of Streptococcus pyogenes. Mol Gen Genet. 1974;135(4):349–367. doi: 10.1007/BF00271149. [DOI] [PubMed] [Google Scholar]
- Malke H., Jacob H. E., Störl K. Characterization of the antibiotic resistance plasmid ERL1 from Streptococcus pyogenes. Mol Gen Genet. 1976 Mar 30;144(3):333–338. doi: 10.1007/BF00341732. [DOI] [PubMed] [Google Scholar]
- Marder H. P., Kayser F. H. Transferable plasmids mediating multiple-antibiotic resistance in Streptococcus faecalis subsp. liquefaciens. Antimicrob Agents Chemother. 1977 Aug;12(2):261–269. doi: 10.1128/aac.12.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Inoue M., Fuse A., Kaneko Y., Oba T. Drug resistance in Streptococcus pyogenes. Jpn J Microbiol. 1974 Jan;18(1):98–99. doi: 10.1111/j.1348-0421.1974.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Niven C. F., Smiley K. L., Sherman J. M. The Hydrolysis of Arginine by Streptococci. J Bacteriol. 1942 Jun;43(6):651–660. doi: 10.1128/jb.43.6.651-660.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg S. K., Williams B. L., Jorgensen J. Long-term effects of tetracycline on the subgingival microflora. J Clin Periodontol. 1979 Jun;6(3):133–140. [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Williams B. L., Osterberg S. K., Jorgensen J. Subgingival microflora of periodontal patients on tetracycline therapy. J Clin Periodontol. 1979 Aug;6(4):210–221. doi: 10.1111/j.1600-051x.1979.tb01923.x. [DOI] [PubMed] [Google Scholar]
- van Embden J. D., Engel H. W., van Klingeren B. Drug resistance in group D streptococci of clinical and nonclinical origin: prevalence, transferability, and plasmid properties. Antimicrob Agents Chemother. 1977 Jun;11(6):925–932. doi: 10.1128/aac.11.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]



