Abstract
To survive, food deprived animals may be forced to forage under hostile conditions. We attempt to use genetically tractable D. melanogaster as a model to elucidate molecular and neural mechanisms that drive a forager to engage in risk-prone food acquisition. Here we describe a paradigm for assessing hunger-driven food acquisition by fly larvae at a deleteriously cold temperature. Genetic analyses reveal that the neural activity of NPFR1, a receptor of neuropeptide F (NPF, the sole fly homolog of neuropetide Y or NPY), was required for cold-resistant feeding behavior of fasted larvae. Conversely, NPFR1 overexpression in fed larvae was sufficient to trigger cold-resistant feeding activity normally associated with fasted larvae. Furthermore, the fly insulin-like system, implicated in the transduction of hunger signals to the central nervous system, regulated negatively larval cold-resistant food acquisition. The results from this and our previous studies suggest that the fly NPY-like system is a central mediator of hunger-elicited resistance to diverse stressors that can be of thermal, gustatory or mechanical form.
Keywords: NPF, food motivation, foraging behavior, stress response, neruopeptides, cold temperature
Natural selection strongly impacts foraging behavior. Animals, when well nourished, tend to forage selectively in favorable habitats. However, they become more risk-prone under prolonged food deprivation, displaying increased willingness to forage in inhospitable or even life-threatening conditions (Charnov, 1976; Cabanac and Johnson, 1983; Bateson, 2002). Despite extensive research efforts, our understanding of how a foraging animal balances rewards and risks under different energy states remains rather limited.
D. melanogaster larvae have provided a useful model for delineating the molecular and neural basis of hunger signaling and its regulation of feeding behavior. Food-deprived larvae display motivated feeding responses including a significant increase in ingestion rate and decreased discrimination against non-preferred foods (Wu et al., 2003). Two evolutionarily conserved neural signaling systems have been identified that are crucial for different aspects of larval food response. Downregulation of the signaling activity of Drosophila insulin-like peptides (dILPs) and their receptor, dInR, in fed larvae triggers diverse behaviors normally associated with fasted larvae including excessive food ingestion and indiscriminative food selection (e.g., unregulated intake of less accessible or quinine-adulterated food). On the other hand, the signaling activity of NPY-like neuropeptide F selectively promotes indiscriminative food selection, but not ingestion of rich, palatable foods (Oldham and Hafen, 2003; Wu et al., 2005a; Wu et al., 2005b). These findings suggest that regulatory mechanisms for hunger response are highly conserved between mammals and insects.
The selective promotion of hunger-driven feeding response to lower-quality foods by the fly NPY-like system has led us to postulate that the conserved NPY/NPF system may be a central motivator that drives all stress-resistant behaviors associated with food acquisition under unfavorable conditions. To provide evidence for this hypothesis, we examined how the signaling activity of NPF and its receptor, NPFR1, affects larval feeding response to rich and palatable foods under an adverse environment. In this report, we developed a behavioral paradigm for quantifying the influence of deleteriously cold temperatures on larval feeding activity. We show that the NPF/NPFR1 pathway is indeed crucial for larval cold-resistant food acquisition. Moreover, dInR signaling activity also plays a critical role in cold-resistant feeding. Based on this and our previous studies, we conclude that the fly NPY-like system appears to be a central mediator of hunger-elicited resistance to diverse stressors that can be in thermal, gustatory or mechanical form (Wu et al., 2005a; Wu et al., 2005b).
Experimental procedures
Flies, media and larval growth
Conditions for fly rearing and the collection of synchronized eggs and larvae were described previously (Wu et al., 2005b). The npfr1-gal4 and UAS-npfr1 lines are in a y w background. The UAS-npfr1dsRNA, UAS-dInRDN and UAS-dInRACT are in the w1118 background.
Behavioral Assays
To measure the cold resistance of foraging larvae, 3% apple juice-agar plates (35-mm) containing 0.3 ml colored yeast paste at the center surface were pre-chilled for at least 45 minutes to desired temperatures in a water bath. The assay plates were chilled and maintained at a constant temperature during the test by exposing directly to the surface of water inside a large insulated container. The yeast paste was made of 1.2 g granular yeast powder, 1.2 ml of water and 130 μl of green food dye. Larvae were rinsed repeatedly with copious amounts of water before the assay. For food deprivation, rinsed larvae were transferred to water-saturated paper for 40 or 120 minutes. At the beginning of the assay, larvae (n = 25) were added to the edge of the yeast paste and allowed to forage freely for 20 minutes. The feeding response was scored based on the presence or absence of green-dyed food in the larval gut as described before (Wu et al., 2005b). The data was analyzed using one-way ANOVA, followed by the Student-Newman-Keuls post hoc analysis.
Results
A Paradigm for Cold-resistant Food Acquisition
D. melanogaster larvae display strongly thermotactic behavior, with a preference for the environmental temperature of 24 °C that is optimal for growth (Wen et al., 2005; Wu et al., 2005a). We designed a quantitative assay to access how a rapid drop of the environmental temperature affects food acquisition of foraging larvae in different energy states. Younger third-instar larvae, which are voracious feeders, were staged to be about 74 hrs after egg laying (74h AEL). For each assay, about 25 synchronized larvae were placed to the edge of dyed yeast paste at the center surface of an apple juice-agar plate. The initial feeding response of larvae on pre-chilled food media was measured within a 20-min test period (Fig. 1A). The temperature of 11 °C was chosen because prolonged exposure to this temperature is deleterious to feeding larvae (data not shown). A majority of larvae fed ad libitum displayed feeding response (> 80% larvae containing green-colored yeast paste in the gut) at 20 °C (Wu et al., 2005b). However, their feeding activity became increasingly attenuated at 15 and 11 °C (ca. 35 and 20% larvae with green food). On the other hand, larvae fasted for 40 or 120 minutes displayed increased feeding response at 11 °C (Fig. 1B). In fact, two hour fasted larvae displayed similarly strong feeding responses at all three temperatures. These results indicate that the rapid reduction of the environmental temperature differentially impacts the feeding activities of fed and fasted larvae and acute food deprivation induces cold resistance.
Fig. 1.
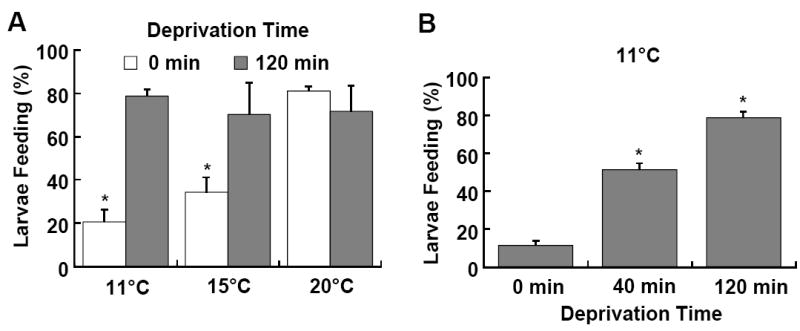
A behavioral paradigm for assessing cold resistance by foraging Drosophila larvae. Larvae were reared at 23 °C with a rich yeast paste/ apple juice diet (Wu et al., 2005b). The feeding response was scored as the percent of larvae that contain green-colored yeast paste in the gut. Prolonged exposure to 11 °C was lethal to larvae fed ad libitum (data not shown). (A) The feeding activity of fed larvae was attenuated at 15 and 11 °C. (B) Larvae fasted for 2 hrs showed cold-resistant feeding at the reduced temperatures. P < 0.001; n = 25 per each of at least three separate trials. Statistic analyses in all figures were performed using a one-way ANOVA, followed by the Student-Newman-Keuls analysis.
Regulation of Cold-resistant Food Acquisition by NPY-like Signaling
NPF has been shown to promote hunger-driven intake of foods that are adulterated with noxious chemicals or difficult to access (Wu et al., 2005a; Wu et al., 2005b). We tested whether the NPF/NPFR1 pathway is limited to processing food rewards or acts as a general motivator that also drives cold-resistant feeding response in fasted larvae. To this end, NPFR1 signaling in feeding larvae (74h AEL) was either downregulated by expressing npfr1 double-stranded RNA (UAS-npfr1dsRNA) or upregulated by expressing a functional npfr1cDNA (UAS-npfr1) under the direction of a npfr1 promoter (npfr1-gal4) (Wu et al., 2005b). The resulting transgenic larvae were assayed for their feeding activities at 11 °C. Fed larvae overexpressing npfr1 (npfr1-gal4 X UAS-npfr1) displayed significantly higher feeding activity than controls (Fig. 2A). Conversely, 2h-fasted larvae expressing npfr1 dsRNA (UAS-npfr1dsRNA) directed by a pan-neural elav-gal4 driver or npfr1-gal4 showed greatly attenuated feeding activity at the low temperature (Fig. 2B). These findings demonstrate that the neural activity of the NPF/NPFR1 pathway is both necessary and sufficient to elicit cold-resistant feeding behavior in foraging animals. Importantly, the feeding response of elav-gal4 and npfr1-gal4 X UAS-npfr1dsRNA larvae remained normal at ambient temperature, indicating that the NPF/NPFR1 system is dispensable for feeding in the absence of the thermal stressor (Wu et al., 2005b).
Fig. 2.
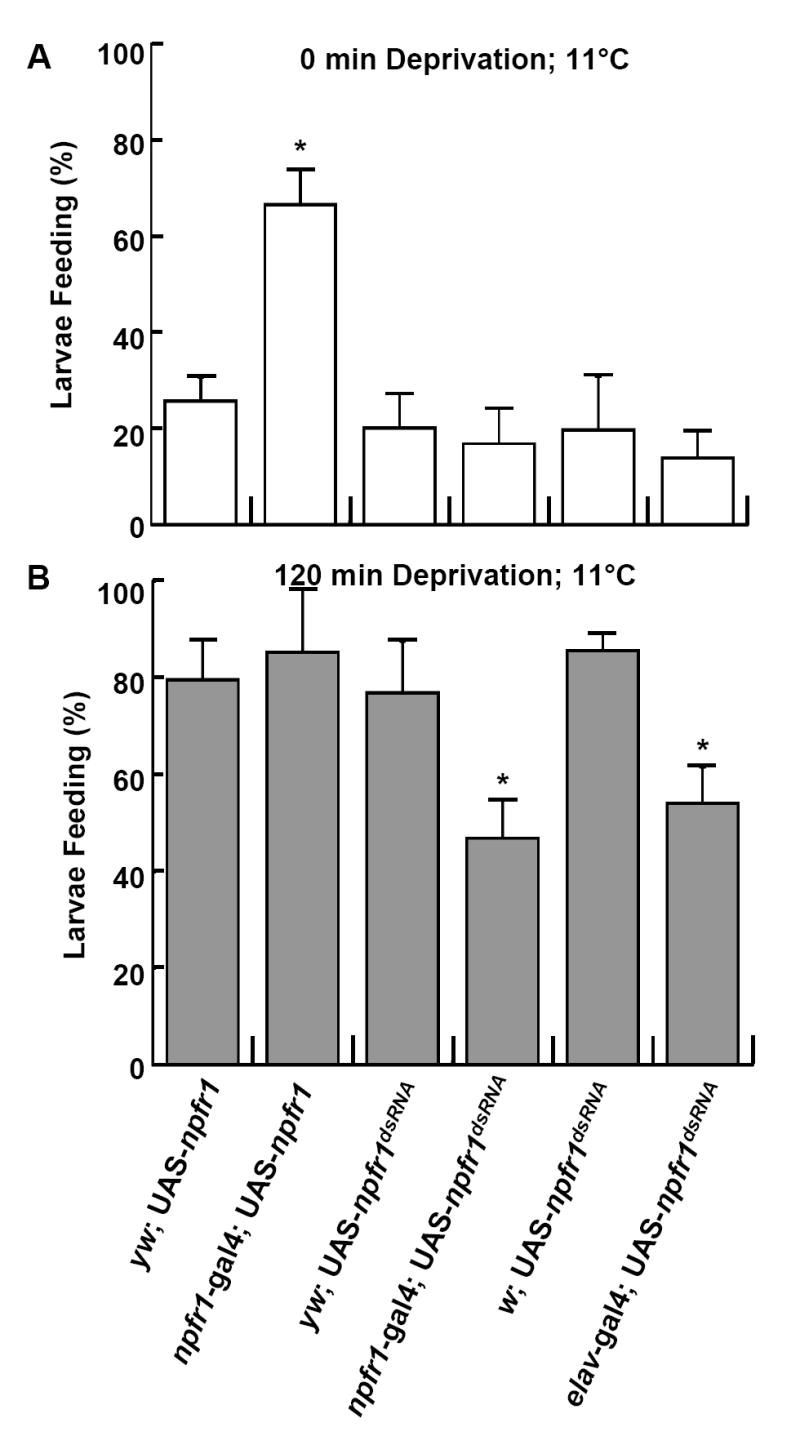
Genetic regulation of cold-resistant food acquisition by the NPY-like system. elav-gal4 is a pan-neural driver. The npfr1-gal4 driver is derived from a promoter sequence of the npfr1 gene. UAS-npfr1 and UAS-npfr1dsRNA encodes a functional npfr1cDNA and a sequence for npfr1 double-stranded RNA, respectively (Wu et al., 2005b). (A) The increased npfr1 expression driven by npfr1-gal4 was sufficient to induce a cold-resistant feeding response in fed larvae fed (P < 0.001). (B) Conversely, reduced NPFR1 signaling caused attenuated cold resistant food acquisition in 2-hr fasted larvae (P < 0.01).
Regulation of Cold-resistant Food Acquisition by Insulin-like Signaling
Our previous studies suggest that reduced insulin-like signaling in fed larvae causes feeding behaviors normally associated with fasted larvae, and dILPs may directly act on NPFR1 neurons (Wu et al., 2005a). Therefore, dILP signaling was also expected to impact cold-resistant food acquisition. To this end, we tested the cold resistance of larvae expressing a dominant-negative or constitutively active form of the insulin-like receptor (UAS-dInRDN and UAS-dInRACT) under the npfr1-gal4 driver. At 11 °C, fed larvae with reduced dInR singaling (npfr1-gal4 X UAS-dInRDN) showed significantly higher feeding activity than fed control larvae (e.g., UAS-dInRDN alone; Fig. 3A). Conversely, 2h-fasted larvae overexpressing dInR activity (npfr1-gal4 X UAS-dInRACT) displayed attenuated feeding response relative to controls (Fig. 3B). These data suggest the dILP/dInR pathway negatively regulates the cold resistance of foraging larvae by targeting NPFR1 neurons.
Fig. 3.
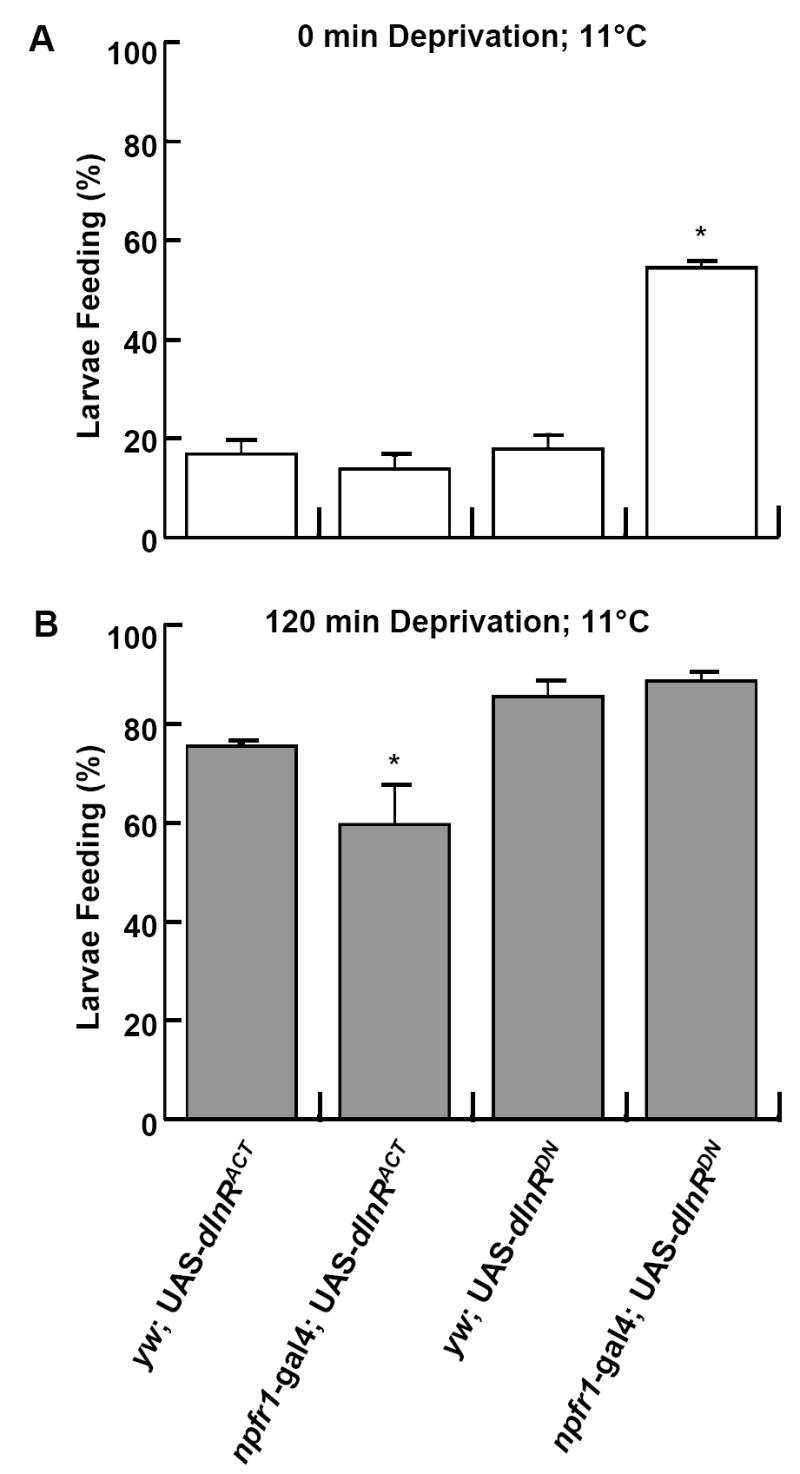
Genetic regulation of cold-resistant food aquisition by the insulin-like system. UAS-dInRDN and UAS-dInRACT encode a dominant-negative and constitutively active form of the insulin-like receptor, respectively. (A) The decreased expression of dInR driven by npfr1-gal4 was sufficient to induce a cold-resistant feeding response by larvae fed ad libitum. (B) Conversely, enhanced dInR signaling caused attenuated cold-resistant feeding in 2-hr fasted larvae. P < 0.001;
Discussion
Our study has shown that an acute cold stress has a strong inhibitory effect on the feeding activity of fed but not fasted larvae, and the cold-resistant food acquisition of fasted larvae requires the functional coordination between a negatively acting insulin-like system and positively acting NPY-like system (Fig. 4). These findings suggest that the insulin- and NPY-like systems may be central to hunger-induced resistance to environmental stressors.
Fig. 4.
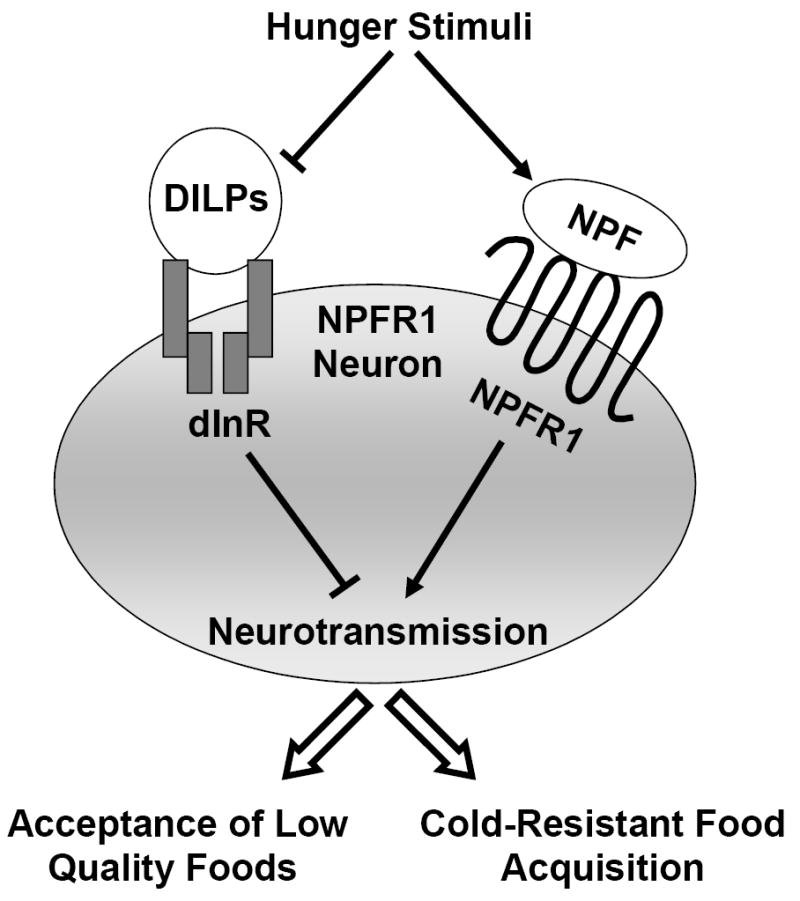
A model for the regulation of cold-resistant food acquisition by insulin- and NPY-like signaling pathways in larvae. Hunger stimuli leads to increased NPF/NPFR1 signaling and decreased dILPs/dInR signaling to NPFR1 neurons, leading to diverse hunger-driven behaviors including cold-resistant food acquisition and acceptance of lower quality food.
Dietary restriction (DR) and intermittent food deprivation have been shown to enhance stress resistance, which may contribute significantly to the improved health and longevity of different experimental animals (Wan et al., 2003; Bross et al., 2005). Recent studies show that factors other than caloric value are likely to be responsible for the life-span extension of DR animals (Mair et al., 2005). Here we provide evidence that the conserved insulin-like system mediates hunger-elicited resistance to a stressful foraging environment. This finding also raises the possibility that the insulin pathway may play a conserved role in hunger-induced stress resistance in insects and mammals. We postulate that reduced insulin signaling in DR animals may contribute to their life-span extension by improving stress-coping capability.
The results from this and previous studies show that NPFR1 enables foraging larvae to become resistant to stressors in thermal, gustatory and mechanical forms (Wu et al., 2005a; Wu et al., 2005b). These findings suggest that the fly NPY-like system is a central organizer of diverse aspects of motivational food response. Consistent with this notion, NPY has been strongly implicated in the suppression of stress and anxiety in rodent models (Thorsell and Heilig, 2002; Heilig, 2004; Karl and Herzog, 2007). It remains to be determined how NPFR1 neurons, modulated positively by NPF and negatively by DILPs, promote resistance to such a diverse array of stressors. One possibility is that different subsets of NPFR1 neurons may be associated with distinct sensory neuronal circuits.
Under the cold conditions, a majority of larvae display two opposite responses to food within the 20-min test period. They showed either sustained feeding activity or no food intake, as evidenced by a large amount of green food or a complete lack of it in the gut (data not shown). Therefore, the data from this assay appear to provide an indirect quantification of the binary decision of eat or flight by individual foraging larvae. Conceivably, this simple behavioral paradigm may be useful for screening novel genes essential for balancing risk and reward by foragers.
Acknowledgments
We thank B. Parrott for discussions, the Bloomington Drosophila Stock Center and Exelixis, Inc for flies, and National Institutes of Health (grant DK-58348 to P.S) for support.
Abbreviations
- D. melanogaster
Drosophila melanogaster
- NPF
Neruopeptide F
- NPY
Neuropeptide Y
- UAS
Upstream Activation Sequence
- NPFR1, npfr1
Neuropeptide F Receptor-1
- cDNA, npfr1cDNA
Complimentary DNA
- dsRNA, npfr1dsRNA
Double-stranded RNA
- dILPs
Drosophila insulin-like peptides
- dInR, dInR
Drosophila insulin-like receptor
- dInRACT
Constitutively active
- dInRDN
Dominant-negative
- AEL
After egg laying
- DR
Dietary Restriction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bateson M. Recent advances in our understanding of risk-sensitive foraging preferences. Proc Nutr Soc. 2002;61:509–516. doi: 10.1079/pns2002181. [DOI] [PubMed] [Google Scholar]
- Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Johnson KG. Analysis of a conflict between palatability and cold exposure in rats. Physiol Behav. 1983;31:249–253. doi: 10.1016/0031-9384(83)90128-2. [DOI] [PubMed] [Google Scholar]
- Charnov EL. Optimal foraging, the marginal value theorem. Theor Popul Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptide. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Karl T, Herzog H. Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides. 2007;2:326–333. doi: 10.1016/j.peptides.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Hafen E. Characterization of a functional neuropeptide F receptor from Drosophila melanogaster. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Heilig M. Diverse functions of neuropeptide Y revealed using genetically modified animals. Neuropeptides. 2002;36:182–193. doi: 10.1054/npep.2002.0897. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci USA. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci USA. 2005a;102:13289–13294. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat Neurosci. 2005b;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]


