Abstract
Rat maternal behavior is a complex social behavior. Most antipsychotic drugs disrupt active maternal responses (e.g., pup retrieval, pup licking and nest building). Our previous work shows that typical antipsychotic haloperidol disrupts maternal behavior by blocking dopamine D2 receptors, whereas atypical clozapine works by blocking 5-HT2A/2C receptors. The present study used c-Fos immunohistochemistry technique, together with pharmacological tools and behavioral observations, and delineated the neuroanatomical bases of the disruptive effects of haloperidol and clozapine. Postpartum female rats were treated with haloperidol (0.2 mg/kg, sc) or clozapine (10.0 mg/kg, sc), with or without pretreatment of quinpirole (a selective dopamine D2/D3 agonist, 1.0 mg/kg, sc) or 2,5-dimethoxy-4-iodo-amphetamine (DOI, a selective 5-HT2A/2C agonist, 2.5 mg/kg, sc). They were then sacrificed 2 h later after a maternal behavior test was conducted. Brain regions that have been previously implicated in the regulation of rat maternal behavior and/or in the antipsychotic action were examined. Behaviorally, both haloperidol and clozapine disrupted pup retrieval, pup licking and nest building. Pretreatment of quinpirole, but not DOI, reversed the haloperidol-induced disruptions. In contrast, pretreatment of DOI, but not quinpirole, reversed the clozapine-induced deficits. Neuroanatomically, the nucleus accumbens (both the shell and core), dorsolateral striatum and lateral septum showed increased c-Fos expression to the treatment of haloperidol. In contrast, the nucleus accumbens shell showed increased expression of c-Fos to the treatment of clozapine. More importantly, pretreatment of quinpirole and DOI produced opposite response profiles in the brain regions where haloperidol and clozapine had an effect. Based on these findings, we concluded that haloperidol disrupts active maternal behavior primarily by blocking dopamine D2 receptors in a neural circuitry involving the nucleus accumbens, dorsolateral striatum and lateral septum. In contrast, clozapine appears to disrupt maternal behavior mainly by blocking serotonin 5-HT2A/2C receptors in the nucleus accumbens shell.
Keywords: quinpirole, DOI, c-Fos, nucleus accumbens, antipsychotic drugs, rat maternal behavior
Animal models are valuable tools for the study of mechanisms of action of antipsychotic drugs (APDs) (Lieberman et al., 2008). In recent years, we have used rat maternal behavior to investigate the behavioral and neurochemical mechanisms of action of APDs. We chose this model because it is a complex social behavior system that cuts across mammalian species and shares many direct features with human mothering behaviors (Fleming and Corter, 1988; Rosenblatt, 1989). Thus, it reflects the complex and multi-dimensional actions of APDs better than other simple models.
Behaviorally, we and others have shown that clinically comparable doses of haloperidol (HAL), clozapine (CLZ), risperidone, quetiapine, amisulpride and aripiprazole (∼50%-80% dopamine D2 occupancy) disrupt active components of maternal behavior, such as pup retrieval, pup licking and nest building (Li et al., 2004; Stern and Keer, 1999; Zhao and Li, 2009a). We found that pup separation, which putatively increases maternal motivation, is able to reverse some of the maternal behavior deficits caused by HAL and CLZ (Zhao and Li, 2009a), suggesting that suppression of maternal motivation is one important behavioral mechanism underlying the disruptive effects of APDs. Neurochemically, we recently reported an interesting double dissociation of the receptor mechanisms between HAL- and CLZ-induced maternal behavior deficits (Zhao and Li, 2009b). We found that pretreatment of quinpirole (a selective dopamine D2/D3 agonist), but not 2,5-dimethoxy-4-iodo-amphetamine (DOI, a selective 5-HT2A/2C agonist), dose-dependently reversed the HAL-induced disruptions. In contrast, pretreatment of DOI, but not quinpirole, dose-dependently reversed the CLZ-induced deficits. This study suggests that HAL disrupts maternal behavior primarily by blocking dopamine D2 receptors, whereas CLZ works primarily by blocking 5-HT2A/2C receptors.
The present study represented our efforts to investigate the neuroanatomical basis of action of antipsychotics using the rat maternal behavior model. The traditional approach in this field is to examine brain regions that show drug-induced changes using biomarkers such as c-Fos (a protein product of immediate-early gene c-fos) (Mo et al., 2005; Natesan et al., 2006; Robertson and Fibiger, 1992; Robertson et al., 1994). Although it is straightforward, this approach has two problems. The first is that it fails to connect a drug's behavioral effects with its neuronal effects. In a typical study, animals are injected with an antipsychotic drug in their home cages, and sacrificed 2 h later for brain analysis. Animal behavior and behavioral effects of the drug treatment are generally ignored. Thus, it is impossible to determine whether the identified brain regions that show changes of c-Fos expression are the ones that mediate the behavioral effects of the drug. This issue is further complicated by the fact that animal behavior itself can also induce brain changes as indexed by c-Fos expression. For example, maternal behavior can stimulate c-Fos expression in the medial preoptic area (MPOA), the ventral bed nucleus of the stria terminalis (vBST) and the nucleus accumbens (Fleming et al., 1994; Lonstein and De Vries, 2000; Lonstein et al., 1998,2000; Numan and Insel, 2003; Numan and Numan, 1994,1995; Numan et al., 1998). The second problem is that it does not take the neurochemical mechanisms of different APDs into consideration. As mentioned above, although both HAL and CLZ disrupt active maternal responses, they do so via blocking dopamine D2 and 5-HT2A/2C receptors respectively. It is quite possible that HAL and CLZ may induce similar changes in c-Fos expression in the same brain regions through distinct receptor mechanisms. Simply relying on the drug-induced c-Fos expression does not guarantee a correct identification of receptor-mediated neuroanatomical basis of a drug action.
In the present study, we used the c-Fos immunohistochemistry technique, together with pharmacological tools and behavioral observations, to delineate the neural circuitries upon which HAL and CLZ act to achieve their disruptive effects on rat maternal behavior. Rats were treated with HAL (0.2 mg/kg, sc) or CLZ (10.0 mg/kg, sc), with or without pretreatment of QUI (1.0 mg/kg, sc) or DOI (2.5 mg/kg, sc). They were then sacrificed 2 h later after maternal behavior was tested. We considered a brain region to be part of the neural circuitry of HAL or CLZ only if it meets the following three criteria: (1) it shows sensitive c-Fos response to the treatment of HAL or CLZ; (2) it shows sensitive c-Fos response to the reversal effect of pretreatment of QUI on HAL or DOI on CLZ; and (3) it does not show or shows little c-Fos response to the pretreatment of DOI on HAL or QUI on CLZ. This approach ensured that the brain regions identified are behaviorally and neurochemically relevant to the specific action of HAL and CLZ.
Experimental Procedures
Subjects and housing
Naive pregnant female Sprague-Dawley rats (between gestational days 13-15 upon arrival to the animal facility) obtained from Charles River Inc. (Portage, MI) were housed individually in 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cages under a 12-h light/dark cycle (lights on at 6:30 AM) and temperature-controlled (22±1°C) condition, and were given free access to standard laboratory rat chow and water. All behavioral tests were performed in the light phase between 9:00 AM and 4:00 PM. All animal manipulations were reviewed and approved by the University of Nebraska Institutional Animal Care and Use Committee, and were carried out in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The number of animals used was minimized to the level that would provide sufficient statistical power, and every effort was made to minimize the suffering of the animals.
Drugs and choices of doses
Haloperidol (HAL, 5.0 mg/ml ampoules, Sicor Pharmaceuticals, Inc, Irvine, CA) was diluted with sterile water. Clozapine (CLZ, a gift from NIMH drug supply program) was dissolved in 1.0% glacial acetic acid in distilled water. Quinpirole (QUI) and DOI (RBI-Sigma, Natick, MA) were dissolved in 0.9% saline. HAL (0.2 mg/kg), CLZ (10.0 mg/kg), QUI (1.0 mg/kg), DOI (2.5 mg/kg) and vehicle (water or saline) were all administered subcutaneously in a volume of 1.0 ml/kg body weight. Choices of drug doses for HAL and CLZ were based on our previous studies showing that at the chosen doses, HAL and CLZ reliably disrupt active components of maternal behavior (Li et al., 2004). The doses of QUI and DOI were chosen based on our recent study showing that QUI 1.0 mg/kg completely reversed the HAL-induced disruptions of pup retrieval, pup licking and nest building; and DOI 2.5 mg/kg completely reversed the CLZ-induced disruptions of pup retrieval and licking (Zhao and Li, 2009b). We employed the same drug injection regimen and behavioral observation procedure as detailed in our recent study (Zhao and Li, 2009b), so that we could not only replicate our previous study but also use the behavioral effects as a guide to identify the relevant neural substrates underlying action of HAL and CLZ (as indexed by c-Fos expression).
Basic experimental procedure
Starting 2 or 3 days prior to the first possible expected parturition date, the subjects were monitored every morning for signs of parturition. Once the dam was found with pups in the morning (that day was designated as Day 1 postpartum), the mother was transferred into a clean cage with wood shavings for bedding. Two shredded paper towels were also provided as additional nesting material. The litter was culled to 8 pups (4 males and 4 females with the most visible milk bands). Maternal behavior tests were conducted on one day between Day 6 and 8 postpartum.
Maternal behavior test
The basic procedure was identical to that described by Zhao and Li (2009b). A total of 54 postpartum rats were randomly divided into the following nine groups (n=6/group) using a full factorial design (3 pretreatment conditions × 3 antipsychotic conditions): VEH+VEH (sterile water or saline), VEH+HAL, QUI-1.0+HAL, DOI-2.5+HAL, QUI-1.0+VEH, VEH+CLZ, DOI-2.5+CLZ, QUI-1.0+CLZ, DOI-2.5+VEH. On the drug test day (one day on either Postpartum Day 6, 7, or 8), all subjects were tested twice, with the first maternal behavior test starting at 30 min prior to the drug (e.g., HAL, CLZ, QUI or DOI) or vehicle injection (i.e., baseline) and the second test occurring at 120 min after drug or vehicle injection. QUI, DOI or vehicle was injected subcutaneously twice with the first injection at 10 min before and the second at 50 min after the HAL, CLZ or vehicle injection, as was done in Zhao and Li (2009b).
In each test (8 min), using a laptop computer equipped with an event recording program (JWatcher, http://www.jwatcher.ucla.edu/), we recorded the frequency or duration of various components of maternal behavior. Each test started by removing the 8 pups from the dam and destroying the nest. Ten seconds later, the pups were returned to the corner of the cage diagonal to the nest site or dam sleeping corner. When the subject picked up a pup in her mouth and carried it back to the nest site, it was referred to as a successful pup retrieval. The total number of pups retrieved was recorded. The occurrence of other behaviors was also recorded, including pup nursing behavior (a rat positioning herself over the pups with legs splayed to accommodate the pups, including hover, high and low crouching over postures), pup licking (a female rat placing its tongue on the anogenital area and the rest of a pup's body), nest building (a rat picking up nesting material in her mouth and transporting it back to the nest site or pushing the material with her forepaws toward the nest site). At the conclusion of the test, any unretrieved pups were returned to the nest site.
c-Fos immunohistochemistry
Immediately after the second maternal behavior test, all rats were anesthetized with a lethal dose of sodium pentobarbital (100.0 mg/kg) and then perfused transcardially with ice-cold 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB) containing 0.2% picric acid at pH 7.4. Brains were rapidly removed from the skulls and post-fixed in the same fixative overnight at 4°C. They were then transferred to 30% sucrose in 0.1 M PB until settled. Coronal sections at a thickness of 40 μm were cut on a freezing microtome and collected in 0.02 M phosphate-buffered saline (PBS) containing 0.1% sodium azide until processing. Procedures for c-Fos immunohistochemistry followed the protocol by Zhao et al. (2007) with slight modifications. Sections were pre-blocked for 1 h with 10% normal goat serum (NGS) and 0.3% Triton X-100 in 0.02 M PBS, incubated for 30 min with 1.5% hydrogen peroxide and 50% methanol to inhibit endogenous peroxidase. They were washed with wash buffer (0.02 M PBS containing 0.05% NGS and 0.3% Triton X-100) and then incubated with a rabbit polyclonal anti-c-Fos (Ab-5, PC38) antibody raised against residues 4-17 of human c-Fos (1:20000 dilution, Calbiochem, CA, USA) in PBS containing 0.3% Triton X-100, 1% NGS and 1% blocking reagent for 48 h at 4°C. After the primary immunoreaction, sections were rinsed with wash buffer and incubated with a biotinylated goat anti-rabbit secondary antibody (1:200 dilution, Vector Laboratories, Burlingame, CA, USA) in PBS containing 1% NGS for 2 h at RT. They were rinsed with PBS and processed with avidin-biotin horseradish peroxidase complex (1:200 dilution, Vectastain Elite ABC Kit, Vector Laboratories). The immunoreaction was visualized with peroxidase substrate (DAB Substrate Kit for Peroxidase, Vector Laboratories). After staining, sections were mounted on gelatin-coated slides, air-dried, dehydrated in a graded series of alcohols, cleared in xylene and coverslipped with permount. As a control, the primary antibody was substituted with normal rabbit serum. No corresponding nucleus or cytoplasm was immunostained in the control.
Fos-immunoreactive (Fos-I) cell counting
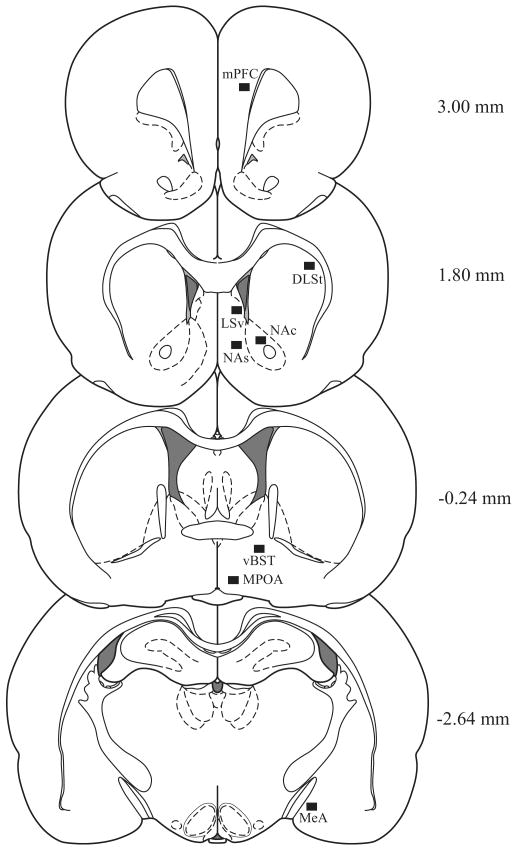
Microscopic images were captured with a digital camera (INFINITY lite, Canada) furnished with an Olympus CX41RF microscope (Japan) using ×10 objective lens. The number of Fos-I cells characterized by clearly labeled nuclei was counted unilaterally in three serial sections which were anatomically well-matched across the treatment groups. The brain regions analyzed included the neural sites that were either implicated in the action of antipsychotic drugs [e.g., the medial prefrontal cortex (mPFC), nucleus accumbence shell (NAs), nucleus accumbence core (NAc), dorsolateral striatum (DLSt), ventral part of lateral septal nucleus (LSv)], and/or in the regulation of maternal behavior [e.g., medial preoptic area (MPOA), ventral bed nucleus of the stria terminalis (vBST), medial amygdaloid nucleus (MeA) and nucleus accumbens shell and core] (Li and Fleming, 2003a,2003b; Numan et al., 2005)]. The levels of brain slices were: Bregma 3.00 mm for mPFC, 1.80 mm for NAs, NAc, DLSt and LSv, -0.24 for MPOA and vBST, -2.64 mm for MeA according to Paxinos and Watson (2007) (Fig. 1). With the help of ImageJ software (developed at the US National Institutes of Health), cell counts were made within a 680 × 510 μm2 unit area of each interest region by an experimenter blind to the treatment condition. The images were thresholded and then analyzed. In a given area from distinct treatments, the images were thresholded to the same value by means of eliminating background and noise staining to ensure that the Fos-I cells were selected. The number of Fos-I nuclei of a given brain region from unilateral sites per rat was averaged. The values from six rats of each treatment group were averaged to obtain the final mean ± SEM.
Fig. 1.
Schematic representation of the forebrain regions (black boxed areas) in which the c-Fos immunoreactive cells was counted. Distance from bregma in the rostrocaudal planes is indicated. Drawings were modified from Paxinos and Watson (2007).
Statistical analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Maternal behavior and c-Fos data were presented as mean ± SEM and analyzed using two-way analysis of variance (ANOVA) with pretreatment (3 levels: VEH, QUI-1.0, DOI-2.5) × antipsychotic treatment (3 levels: VEH, HAL, CLZ) as between-subject factors. When the overall significant effects were detected, multiple group differences were assessed using simple main effect tests (one-way ANOVA) followed by Fisher's protected least significant difference (PLSD) test for pairwise comparisons of means. A conventional two-tailed level of significance at the 0.05 level was required.
Results
Overall effects of drug pretreatment and antipsychotic treatment on maternal behavior
There was no significant effect of drug pretreatment and antipsychotic treatment on the baseline maternal behavior. For the maternal behavior data obtained at 2 h post-antipsychotic injection, two-way ANOVAs revealed a significant interaction between the pretreatment and antipsychotic treatment on pup retrieval [F (4, 45) = 20.05, p < 0.001], pup licking [F (4, 45) = 5.06, p = 0.002], nest building [F (4, 45) = 26.01, p < 0.001], and pup nursing [F (4, 45) = 10.56, p < 0.001], suggesting that the disruptive effects of antipsychotic treatment on maternal behavior were modulated by QUI and DOI pretreatment.
HAL or CLZ treatment disrupted various components of maternal behavior
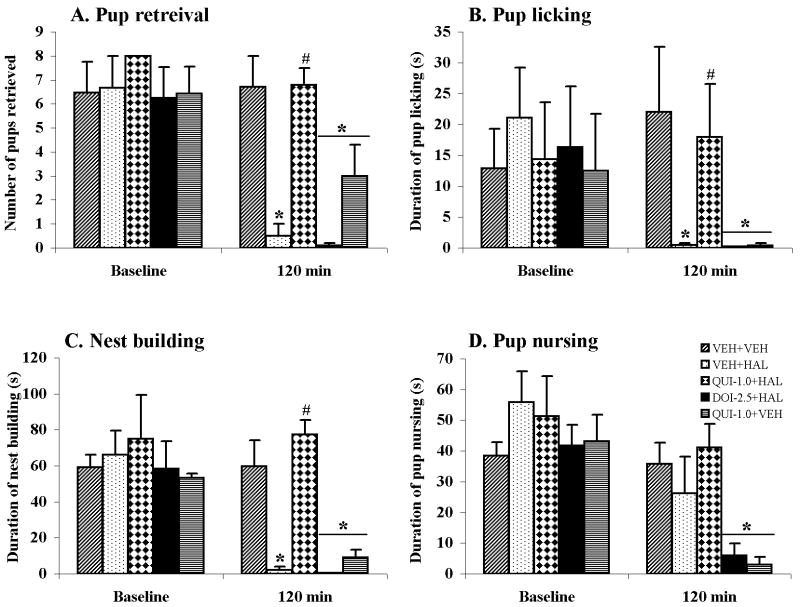
Consistent with our previous findings (Li et al., 2004; Zhao and Li, 2009a,2009b), a single injection of HAL or CLZ disrupted various components of maternal behavior. As can be seen in Figs. 2 and 3, at 2 h after antipsychotic administration, the HAL- or CLZ-treated rats retrieved fewer pups into the nest site, spent less time licking their pups and reconstructing the nest in comparison to the vehicle-treated ones (HAL: ps < 0.001 for pup retrieval and nest building, p = 0.027 for pup licking; CLZ: ps < 0.001 for pup retrieval and nest building, p = 0.024 for pup licking). HAL treatment had little effect on pup nursing, whereas CLZ significantly decreased pup nursing activity (HAL: p > 0.10; CLZ: p = 0.018).
Fig. 2.
Effects of pretreatment of quinpirole (QUI) or DOI on HAL-induced maternal behavior deficits in the postpartum female rats. Pup retrieval (A), pup licking (B), nest building (C) and pup nursing (D) were tested at baseline and at 120 min after injection of HAL or vehicle. HAL disrupted all active maternal responses, but leaving nursing behavior intact. Quinpirole, but not DOI, attenuated the HAL-induced disruptions of maternal behavior (A-C). Quinpirole itself also disrupted various components of maternal behavior (A-D). Each bar represents the mean + SEM of duplicate determinations from six rats. * P < 0.05 versus VEH+VEH control; # p < 0.05 versus VEH+HAL group.
Fig. 3.
Effects of pretreatment of quinpirole (QUI) or DOI on CLZ-induced maternal behavior deficits in the postpartum female rats. Pup retrieval (A), pup licking (B), nest building (C) and pup nursing (D) were tested at baseline and at 120 min after injection of CLZ or vehicle. CLZ disrupted various components of maternal behavior. DOI, but not quinpirole improved the CLZ-induced disruptions of pup retrieval and pup licking, but had less effect on nest building and pup nursing. In contrast, quinpirile failed to improve any maternal behavior deficit induced by CLZ (A-D). DOI itself also disrupted various components of maternal behavior (A-D). Each bar represents the mean + SEM of duplicate determinations from six rats. * P < 0.05 versus VEH+VEH control; # p < 0.05 versus VEH+CLZ group.
QUI or DOI treatment alone also disrupted various components of maternal behavior
In comparison to the VEH+VEH rats, the QUI- or DOI-treated ones retrieved fewer pups into the nest (QUI: p = 0.008; DOI: p < 0.001; Figs. 2A, 3A). They also spent less time on licking (QUI: p = 0.026; DOI: p = 0.024) and nursing pups (QUI: p = 0.002; DOI: p = 0.001), and on building the nest (both ps < 0.001) (Figs. 2B-D,3B-D), suggesting that QUI or DOI treatment itself has a disruptive effect on various components of maternal behavior.
Pretreatment of QUI attenuated HAL-induced disruptions of maternal behavior, but failed to improve CLZ-induced ones
Pretreatment of QUI significantly improved the HAL-induced maternal behavior deficits. In comparison to the VEH+HAL rats, the QUI+HAL ones retrieved more pups (p < 0.001), spent more time on pup licking (p = 0.049) and nest building (p < 0.001) (Fig. 2A-C). Furthermore, the reversal effect of QUI on these behaviors appeared to reach the normal level as there was no significant difference between the QUI+HAL group and VEH+VEH group. In contrast, QUI had little effect on CLZ-induced maternal behavior disruptions. There were no significant group differences between the QUI+CLZ group and the VEH+CLZ group (all ps > 0.10) (Fig. 3A-D).
Pretreatment of DOI attenuated CLZ-induced maternal behavior disruptions, but failed to improve HAL-induced ones
Pretreatment of DOI significantly alleviated the CLZ-induced maternal behavior deficits. In comparison to the VEH+CLZ rats, the DOI+CLZ ones retrieved more pups (p = 0.001) and spent more time licking their pups (p = 0.015) (Fig. 3A,B). There were also no significant group differences between the DOI+CLZ and VEH+VEH groups in these two behaviors (both ps > 0.10), suggesting that the reversal effect of DOI was complete. Pretreatment of DOI also tended to improve the CLZ-induced disruptions of nest building and pup nursing; however, this effect did not reach the significant level (nest building: p = 0.31; pup nursing: p = 0.28 vs VEH+CLZ group) (Fig. 3C,D). In contrast, pretreatment of DOI was ineffective in improving the HAL-induced maternal behavior deficits. There were no significant differences between the DOI+HAL group and the VEH+HAL group (all ps > 0.10; Fig. 2A-C).
Overall effects of drug pretreatment and antipsychotic treatment on c-Fos expression
Of the eight brain regions examined, six (mPFC, NAs, NAc, DLSt, LSv and vBST) showed a significant pretreatment effect [mPFC: F (2, 45) = 60.24, p < 0.001; NAs: F (2, 45) = 374.23, p < 0.001; NAc: F (2, 45) = 191.13, p < 0.001; DLSt: F (2, 45) = 774.41, p < 0.001; LSv: F (2, 45) = 8.74, p = 0.001; vBST: F (2, 45) = 10.59, p < 0.001], a significant antipsychotic treatment effect [mPFC: F (2, 45) = 10.98, p < 0.001; NAs: F (2, 45) = 195.74, p < 0.001; NAc: F (2, 45) = 538.23, p < 0.001; DLSt: F (2, 45) = 4506.09, p < 0.001; LSv: F (2, 45) = 35.75, p < 0.001; vBST: F (2, 45) = 5.69, p = 0.006], and a significant interaction between the two factors [mPFC: F (4, 45) = 19.16, p < 0.001; NAs: F (4, 45) = 107.70, p < 0.001; NAc: F (4, 45) = 60.22, p < 0.001; DLSt: F (4, 45) = 678.16, p < 0.001; LSv: F (4, 45) = 8.64, p < 0.001; vBST: F (4, 45) = 9.77, p < 0.001]. The MPOA showed only a significant pretreatment effect [F (2, 45) = 32.04, p < 0.001], but no significant antipsychotic treatment effect or interaction. The only brain region that did not show any significant effect was the MeA (see representative c-Fos staining images in Figs. 4 and 5, Table 1).
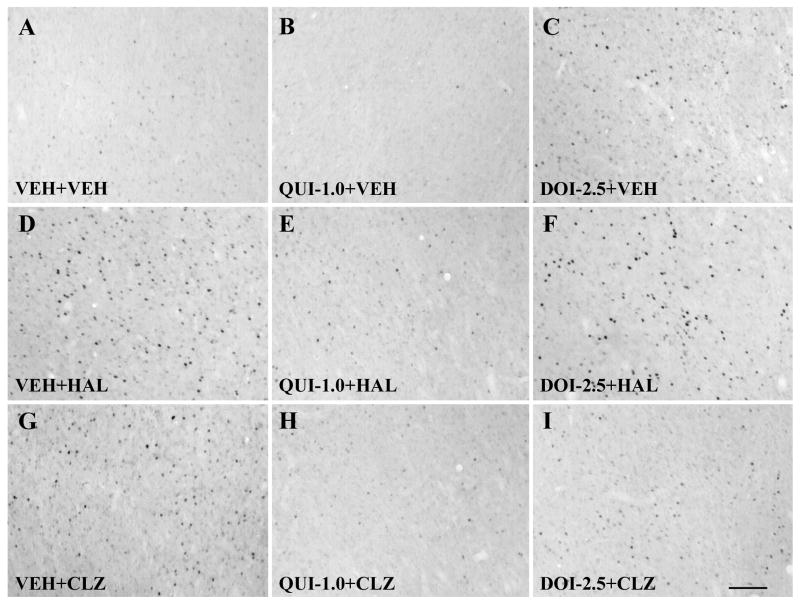
Fig. 4.
Photomicrographs of immunohistochemistry showing c-Fos expression in the nucleus accumbens shell. Note that in comparison to the vehicle treatment, quinpirole reduced the number of c-Fos immunoreactive cells (B), whereas DOI, HAL and CLZ significantly increased the c-Fos immunoreactive cells (C,D,G). Pretreatment with both quinpirole and DOI significantly reduced HAL-induced increase in c-Fos expression, although quinpirole had a more stronger effect than DOI (E,F). Similarly, both QUI and DOI significantly reduced CLZ-induced increase in c-Fos expression, while the effect of quinpirole was more prominent than DOI (H,I). Scale bar = 100 μm.
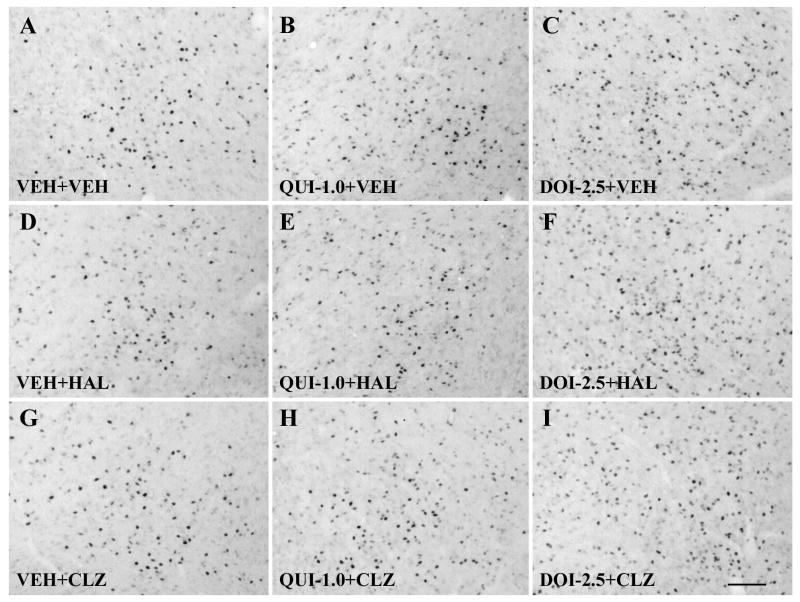
Fig. 5.
Photomicrographs of immunohistochemistry showing c-Fos expression in the medial preoptic area. Note that in comparison to the vehicle treatment, only DOI, but not quinpirole, HAL or CLZ, produced a significant increase in c-Fos expression in the MPOA (B,C,D,G). DOI pretreatment significantly increased c-Fos expression, while quinpirole had little effect in both HAL- and CLZ-treated rats (E,F,H,I). Scale bar = 100 μm.
Table 1.
Number of c-Fos immunoreactive cells in the MPOA, vBST and MeA of postpartum female rats
| Groups | N | MPOA | vBST | MeA |
|---|---|---|---|---|
| VEH+VEH | 96 ± 1.6 | 78 ± 1.9 | 76 ± 4.1 | |
| VEH+HAL | 6 | 103 ± 3.5 | 89 ± 5.3 | 78 ± 2.6 |
| QUI-1.0+HAL | 6 | 94 ± 4.1 | 71 ± 2.5b | 75 ± 2.6 |
| DOI-2.5+HAL | 6 | 134 ± 9.5a,b | 98 ± 5.0a | 78 ± 3.1 |
| QUI-1.0+VEH | 6 | 104 ± 2.7 | 100 ± 3.9a | 75 ± 2.7 |
| VEH+CLZ | 6 | 91 ± 2.0 | 99 ± 2.3a | 81 ± 5.8 |
| DOI-2.5+CLZ | 6 | 124 ± 8.0a,c | 97 ± 2.9a | 80 ± 2.5 |
| QUI-1.0+CLZ | 6 | 105 ± 2.7 | 92 ± 4.8a | 78 ± 3.5 |
| DOI-2.5+VEH | 6 | 123 ± 4.2a,c | 104 ± 2.7a | 72 ± 2.9 |
Data are presented as mean ± SEM.
p < 0.05 relative to the VEH+VEH group.
p < 0.05 relative to the VEH+HAL group.
p < 0.05 relative to the VEH+CLZ group.
HAL and CLZ treatment induced different patterns of c-Fos expression in the forebrain regions
Consistent with the previous reports on male rats (Binder et al., 2004; MacGibbon et al., 1994; Merchant and Dorsa, 1993; Nguyen et al., 1992; Robertson et al., 1994), HAL and CLZ induced different patterns of c-Fos expression in the forebrain in postpartum female rats. In comparison to the vehicle treatment, HAL significantly increased c-Fos expression in the NAs, NAc, DLSt and LSv (all ps<0.001; Figs. 4D,6B-D), but had little effect in the mPFC, MPOA, vBST and MeA (Figs. 5D,6A; Table 1). CLZ also increased c-Fos expression in the NAs and LSv (both ps<0.001; Figs. 4G,6B,D). In addition, it also significantly increased c-Fos expression in the mPFC (p<0.001, Fig. 6A), as well as in the vBST (p<0.001, Table 1), an area known to be critically involved in the regulation of maternal behavior, but failed to alter c-Fos expression in the NAc, DLSt, MPOA and MeA (Figs. 5G,6B,C; Table 1).
Fig. 6.
Effects of HAL, CLZ, QUI or DOI treatment alone, and the combined treatments of these drugs on HAL- or CLZ-induced c-Fos expression in the mPFC (A), NAs, NAc (B), DLSt (C) and LSv (D). Each bar represents the mean + SEM of duplicate determinations from six rats. * P < 0.05 versus VEH+VEH control; # p < 0.05 versus VEH+HAL group; £ P < 0.05 versus VEH+CLZ group.
QUI and DOI treatment alone also altered c-Fos expression in the forebrain regions
QUI alone significantly increased c-Fos expression in the mPFC and vBST (both ps < 0.001; Fig. 6A; Table 1), while it reduced c-Fos expression in the NAs and NAc (both ps<0.001; Figs. 4B,6B). QUI did not alter c-Fos expression in the DLSt, LSv, MPOA and MeA (Figs. 5B,6C,D; Table 1). DOI alone significantly increased c-Fos expression in the mPFC, NAs, NAc, MPOA and vBST (mPFC: p<0.001; NAs: p<0.001, NAc: p<0.001; MPOA: p = 0.001; vBST: p<0.001; Figs. 4C,5C,6A,B; Table 1), but had little effect in the DLST, LSv and MeA (Fig. 6C,D; Table 1).
The individual effects of HAL, CLZ, QUI and DOI treatment on the c-Fos expression in the eight brain regions are summarized in Table 2. We calculated the percent changes of drug treatment relative to the vehicle treatment. It appears that the nucleus accumbens, especially the shell region, is the common site on which all four drugs act. CLZ, QUI and DOI, but not HAL, also increased the c-Fos expression in the mPFC and vBST. In addition, only HAL increased the c-Fos expression in the DLSt, consistent with its EPS profile in the clinic.
Table 2.
Relative percent change of effects of drug treatments on c-Fos expression
| Treatment | mPFC | NAs | NAc | DLSt | LSv | MPOA | vBST | MeA |
|---|---|---|---|---|---|---|---|---|
| VEH+VEH | — | — | — | — | — | — | — | — |
| VEH+HAL | — | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑ | — | — | — |
| VEH+CLZ | ↑ | ↑↑ | — | — | ↑ | — | ↑ | — |
| QUI-1.0+VEH | ↑ | ↓ | ↓ | — | — | — | ↑ | — |
| DOI-2.5+VEH | ↑ | ↑↑ | ↑ | — | — | ↑ | ↑ | — |
—, no significant change; ↑or ↓, increase or decrease more than 20% but less than 100%; ↑↑, increase more than 100% but less than 200%; ↑↑↑, increase more than 200%. Percent change was calculated by subtracting the cell number in the vehicle control group (V) from the cell number in the treatment groups (T), dividing by cell number in the vehicle control group, and multiplying by 100 .
Pretreatment of QUI significantly reduced HAL-induced c-Fos increase in the NAs, NAc, DLSt and LSv, but also reduced CLZ-induced c-Fos increase in the NAs
In comparison to HAL treatment alone (i.e., VEH+HAL), pretreatment of QUI (i.e., QUI+HAL) significantly reduced the HAL-induced c-Fos increase in the NAs, NAc, DLSt and LSv (all ps<0.001; Figs. 4E,6B-D). In contrast, pretreatment of QUI only reduced CLZ-induced c-Fos increase in the NAs (p<0.001; Figs. 4H,6B), but had no effect on CLZ-induced c-Fos increase in the mPFC, LSv and vBST (Fig. 6A,D; Table 1).
Pretreatment of DOI significantly reduced CLZ-induced c-Fos increase in the NAs, but also reduced HAL-induced increase in the NAs, NAc and DLSt
In comparison to CLZ treatment alone (i.e. VEH+CLZ), pretreatment of DOI reduced CLZ-induced c-Fos increase in the NAs (Figs. 4I,6B, p<0.001). However, DOI did not alter CLZ-induced c-Fos increase in the mPFC, LSv and vBST (Fig. 6A,D; Table 1). The combined DOI and CLZ treatment produced an increased c-Fos expression in the MPOA, although CLZ itself did not alter c-Fos expression in this region (p<0.001; Fig. 5I; Table 1).
In comparison to HAL treatment alone (i.e. VEH+HAL), pretreatment of DOI (i.e. DOI+HAL) significantly reduced the number of HAL-induced c-Fos increase in the NAs, NAc and DLSt (all ps<0.001; Figs. 4F,6B,C). This effect was similar to that of the pretreatment effect of QUI. Although HAL treatment alone did not alter c-Fos expression in the mPFC and MPOA, the combined DOI and HAL treatment significantly increased c-Fos expression in these two regions (both ps<0.001; Figs. 5F,6A; Table 1), suggesting that this increasing effect was primarily due to DOI, consistent with the finding that DOI treatment alone increased c-Fos expression in both regions (see Figs. 5C,6A;Table 1).
The above analysis simply examined the direct effects of QUI and DOI pretreatment on HAL- and CLZ-induced c-Fos expression. It failed to take into consideration the intrinsic effects of QUI and DOI treatment alone on c-Fos expression. For example, because QUI or DOI itself had an opposite effect on c-Fos expression in the NAs (i.e., QUI reduced, while DOI increased c-Fos expression in the NAs), even though both drugs reduced HAL-induced c-Fos increase in the NAs, the nature of their impact would be different. To account for this intrinsic difference between QUI and DOI and to delineate a more accurate behavioral profile of QUI and DOI pretreatment on HAL and CLZ, we developed the following formulas and used the “group mean” (number of c-Fos immunoreactive cells) to calculate the overall pretreatment impact of QUI and DOI on HAL or CLZ for each brain region (using HAL as an example):
In this formula, the “numerator” calculates the reversal effect of pretreatment of QUI or DOI on the HAL-induced c-Fos expression (percent changes from the VEH+HAL level). The “denominator” calculates the intrinsic effect of pretreatment of QUI or DOI itself on the c-Fos expression (percent changes from the VEH+VEH level). For example, using this formula, we found that the pretreatment impact of QUI on HAL in the NAs was 0.89, whereas that of DOI was -0.42. The +/- sign captured the opposite directions of QUI and DOI pretreatment impact on HAL. This pattern of QUI and DOI pretreatment impact could not have been revealed by simply examining their direct reversal effects on HAL. The overall results are summarized in Fig. 7. As is evident, pretreatment of QUI and DOI produced opposite impacts on HAL- or CLZ-induced c-Fos expression in various brain regions (e,g., NAs, NAc, LSv, or DLSt). These dissociated pretreatment patterns were consistent with both our previous and present behavioral findings that only pretreatment of QUI (but not DOI) can reverse the HAL-induced disruptions, whereas only pretreatment of DOI (but not QUI) can reverse the CLZ-induced deficits. Together with the findings on the brain regions that showed increased c-Fos expression to HAL or CLZ treatment, it appears that the NAs, NAc, LSv and DLSt can be classified as the HAL neural system, whereas only the NAs should be considered as the CLZ neural system.
Fig. 7.
Pretreatment impact of quinpirole or DOI on c-Fos expression induced by HAL and CLZ. (A): Impact (as expressed in “ratio”) of quinpirole or DOI pretreatment on HAL-induced c-Fos expression; (B): Impact (as expressed in “ratio”) of quinpirole or DOI pretreatment on CLZ-induced c-Fos expression. Percent change of direct effect of quinpirole or DOI (M) was calculated by subtracting the number of c-Fos positive cells (group mean) in the VEH+HAL or VEH+CLZ group (T) from the cell number in the quinpirole or DOI pretreatment group (e.g., QUI-1.0+HAL/CLZ or DOI-2.5+HAL/CLZ groups, P), dividing by cell number in the VEH+HAL or VEH+CLZ group. Percent change of intrinsic effect of quinpirole or DOI (N) was calculated by subtracting the cell number in the VEH+VEH group (V) from the cell number in the VEH+QUI or VEH+DOI group (I), dividing by cell number in the VEH+VEH group. . Then pretreatment impact was obtained by M divided by N .
Discussion
The present study was built upon our recent work on the neurochemical basis of antipsychotic-induced disruptions of rat maternal behavior (Zhao and Li, 2009b). In that study, we administered QUI or DOI, together with HAL (0.2 mg/kg) or CLZ (10.0 mg/kg) to postpartum rats and examined which of these two agonists was able to reverse the disruptive effects induced by HAL or CLZ. We showed that pretreatment of QUI, but not DOI, dose-dependently improved the HAL-induced disruptions of pup approach, pup retrieval, pup licking and nest building, whereas pretreatment of DOI, but not QUI, dose-dependently improved the CLZ-induced disruptions of pup approach, pup retrieval and pup licking. The present study not only replicated these findings, but also showed that QUI or DOI treatment by itself also disrupted various components of maternal behavior. Rats treated with QUI or DOI retrieved fewer pups into the nest, spent less time on licking and nursing pups and on building nest. This finding is critically important because it suggests that the reversal effects of QUI on HAL and DOI on CLZ reflect the drug-drug interaction as opposed to the effect of a simple addition of individual drug, as both HAL and CLZ also disrupted active maternal responses when given alone. On the basis of these findings, we suggest that the HAL-induced maternal deficits are primarily mediated by its blockade of D2 dopamine receptors, whereas the CLZ-induced maternal deficits may be mediated by the blockade of 5-HT2A/2C receptors. We recently confirmed the same double dissociation receptor mechanisms between HAL and CLZ in a conditioned avoidance response task (unpublished observation), a model predictive of antipsychotic activity (Li et al., 2007), suggesting that the double dissociation of HAL and CLZ receptor mechanisms is closely related to their antipsychotic action, and is not task specific.
The most important finding of the present study is the identification of the distinct neural bases responsible for the maternal disruptive effects of HAL and CLZ. Our general strategy was to use c-Fos as a marker of neuronal activation to identify brain regions that show sensitive responses to the treatment of HAL or CLZ. Because we already know that pretreatment of QUI, but not DOI, reverses the HAL-induced maternal disruptions, whereas pretreatment of DOI, but not QUI, reverses the CLZ-induced ones (Zhao and Li, 2009b), to ensure that the identified systems are firmly grounded on this double dissociation receptor mechanisms and dissociated behavioral effects, we reasoned that the putative HAL neural system should also show sensitive responses to the reversal effects of QUI. In addition, it should show less or no c-Fos response to the pretreatment of DOI. Conversely, for the putative CLZ neural system, it should show sensitive response to CLZ, and sensitive response to the reversal effects of DOI but not QUI. We carefully selected two clusters of brain sites for c-Fos examination. One cluster includes brain regions that have been strongly implicated in the mediation of antipsychotic action, including the mPFC, NAs, NAc, DLSt and LSv (Binder et al., 2004; Guo et al., 1995; Mo et al., 2005; Robertson and Fibiger, 1992; Tremblay et al., 1998; Young et al., 1999). The second cluster includes brain regions that have been implicated in the mediation of rat maternal behavior, such as the NAs, MPOA, vBST and MeA (Bridges and Freemark, 1995; Li and Fleming, 2003a,2003b, Numan, 1988,2007; Numan and Sheehan, 1997; Numan and Stolzenberg, 2009; Numan et al., 2005). Of the eight brain regions examined, we found that only four (i.e. NAs, NAc, DLSt and LSv) met the criteria for the HAL neural system, and one (i.e. NAs) for the CLZ system. They all showed: (1) an increase in c-Fos expression in response to HAL or CLZ treatment; (2) a decrease in c-Fos expression in response to the reversal effects of pretreatment of QUI on HAL, or DOI on CLZ; and (3) opposing responses to the pretreatment of DOI on HAL or QUI on CLZ (see Fig. 7). According to these criteria, the brain regions such as mPFC, LSv and vBST are not considered be part of the CLZ system despite the fact that they all showed increased c-Fos expression to CLZ treatment and have been implicated in the mediation of maternal behavior (Afonso et al., 2007; Fleischer and Slotnick, 1978; Numan and Numan, 1994,1995,1996). This is because they failed to meet all three criteria. However, this is not to say that they are not important for the expression of maternal behavior. It only means that CLZ most likely does not act on the 5-HT2A/2C receptors in these regions to achieve its disruption.
Action of HAL and CLZ in the NAs may account for their suppressive effect on maternal motivation (Zhao and Li, 2009a). Both D2 and 5-HT2A/2C receptors are richly expressed in the nucleus accumbens, providing the neuroanatomical evidence for their action in this region (Clemett et al., 2000; Cornea-Hebert et al., 1999; Eberle-Wang et al., 1997; Mansour et al., 1990; Mengod et al., 1989,1990; Pompeiano et al., 1994). This finding is also consistent with a large number of studies indicating that NA, especially the shell region, plays a key role in the modulation of rat maternal behavior (Hansen, 1994; Hansen et al., 1991a,1991b; Keer and Stern, 1999; Lee et al., 2000; Li and Fleming, 2003a,2003b; Smith and Holland, 1975; Stolzenberg et al., 2007). Maternal interaction with pups produced increased release of dopamine into the NA (Hansen et al., 1993), and increased c-Fos expression in the NAs (Lonstein et al., 1998; Stack et al., 2002). The effect of HAL on the DLSt and NAc has been suggested to mediate its EPS liability (Deutch et al., 1992; Fibiger, 1994; Robertson and Fibiger, 1992; Robertson et al., 1994), Thus, this action of HAL may be related to its motor impairment effect, which may also contribute its disruptive effect on active maternal responses at this dose (0.2 mg/kg). The LSv is a brain region that has also been implicated in rat maternal behavior. Previous studies show that lesions of LSv disrupt rat maternal behavior, as evidenced by the lack of nest building and pup nursing due to the persistent disorganized pup retrieval (Fleischer and Slotnick, 1978; Numan, 1994). Together with the evidence showing that D2 receptors are expressed in the LSv (Mansour et al., 1990; Mengod et al., 1989), our finding supports that the D2 receptors in the LSv could also contribute to the HAL-induced disruption of maternal behavior.
We were surprised to learn that neither HAL nor CLZ alter c-Fos expression in the MPOA, vBNST and MeA, three critical brain regions that are directly involved in the regulation of rat maternal behavior (Kalinichev et al., 2000a; Numan, 1988,2007; Numan and Numan, 1996; Numan & Sheehan, 1997). The lack of effect by HAL is understandable on the basis of findings that the MPOA D2 receptors are not critical for the expression of maternal behavior. Dopamine D2 receptor antagonists, such as raclopride and eticlopride failed to disrupt maternal behavior, when infused directly into the MPOA (Miller and Lonstein, 2005; Numan et al., 2005). The lack of effect by CLZ may indicate that CLZ does not act on this hypothalamic region to affect maternal behavior. This conclusion is tentative because even the vehicle control rats exhibited increased c-Fos expression in the MPOA (Fleming et al., 1994; Kalinichev et al., 2000b; Lonstein et al., 1998; Numan and Insel, 2003; Stack and Numan, 2000; Stack et al., 2002), thus, the lack of strong CLZ treatment effects could have be masked by a ceiling effect. Our own unpublished observation suggests that the postpartum female rats did show increased c-Fos expression in the MPOA in comparison to the virgin females who were treated with HAL and CLZ but not exposed to rat pups. However, we think this ceiling effect explanation is not adequate because DOI treatment alone did increase c-Fos expression in the MPOA, suggesting that the level of c-Fos expression induced by HAL and CLZ had not reached the maximum level, and there is still room for HAL and CLZ to show an effect in this region.
We did notice that our control (VEH+VEH) animals had higher levels of c-Fos expression in the mPFC and NAs than those reported in other studies (Guo et al., 1995; Mo et al., 2005; Robertson and Fibiger, 1992; Robertson et al., 1994). Several factors may be contributable to such a relatively higher c-Fos expression. One factor is the effect of handling and injection procedure stress. It has been well demonstrated that stress itself stimulates Fos expression in various brain regions, such as the mPFC, LSv, vBST, MPOA and paraventricular nucleus of the hypothalamus (PVH) (De Medeiros et al., 2005; Monasterio et al., 2008; Senba and Ueyama, 1997). Our rats were behaviorally tested twice and injected three times before being sacrificed for c-Fos analysis. These manipulations may have produced higher levels of stress and c-Fos expression. Another possible factor is the differences in methodology of c-Fos analysis. We counted the Fos-positive cells in a 680 × 510 μm2 area of each region of a 40-μm section, while others counted Fos-positive cells in a less small area and/or a less thin section at 30 μm (Guo et al., 1995; Mo et al., 2005; Robertson and Fibiger, 1992; Robertson et al., 1994). Future research including more control groups (e.g. virgin females, nonhandled and noninjected, etc) is needed to sort out the different possibilities.
HAL-induced c-Fos expression is generally assumed to be attributed to its primary dopamine D2 receptor antagonism. The finding that QUI pretreatment reduced HAL-induced c-Fos increase in the NAs, NAc, DLSt and LSv is consistent with this hypothesis. The mechanisms underlying the CLZ-induced c-Fos increase have not been fully delineated. It has been suggested that multiple receptor systems, such as D3, D4, adenosine A2A, β-adrenergic receptors, may be involved (Guo et al., 1995; Ohashi et al., 2000; Pinna et al., 1999; Vahid-Ansari and Robertson, 1996). Our findings that both DOI and QUI reduced CLZ-induced c-Fos increase in the NAs are in agreement with this idea. Although CLZ blocks multiple receptors, not all its receptor actions contribute to its therapeutic effects. In the case of maternal behavior, it appears that antagonistic action on the 5-HT2A/2C receptors in the NAs is more important than others.
In addition to advancing our understanding of the neuroanatomical basis of action of HAL and CLZ, the present study also sheds new light on the neurochemical mechanisms involved in the regulation of normal expression of rat maternal behavior. Our results on the disruptive effects of QUI and DOI demonstrated that the dopaminergic neurotransmission through D2/D3 receptors and serotoninergic neurotransmission through 5-HT2A/2C receptors are involved in the regulation of maternal behavior. These findings are consistent with the view suggesting that dopamine transmission at a balanced level is necessary for the normal display of maternal behavior, and if this transmission is either too low or too high disruptions will occur (Numan and Insel, 2003; Stern and Protomastro, 2000). The present results further expand this view by showing that a balanced serotoninergic transmission is also critical for the normal expression of maternal behavior as both CLZ (which decreases 5-HT neurotransmission) and DOI (which increases 5-HT neurotransmission) disrupted maternal behaviors. Because DOI produced a broad increase in c-Fos expression in many forebrain regions that are implicated in rat maternal behavior, such as mPFC, NAs, NAc, MPOA and vBST, it suggests that serotoninergic transmissions in these regions are important for regulating maternal behavior.
Consistent with our recent work (Zhao and Li, 2009b), the present study provides pharmacological evidence supporting that 5-HT system is implicated in regulating rat maternal behavior based on the findings that systemic administration of DOI, a 5-HT2A/2C agonist, disrupts various components of rat maternal behavior (e.g., pup retrieval, pup licking, nest building and pup nursing). This finding is consistent with the growing literature. Brunner et al. (1999) reported that 5-HT1B receptor knockout mother mice did not display pup retrieval deficits. Other studies found that 5-HT1B and 5-HT2A/2C receptors are involved in maternal aggressive behavior (De Almeida et al., 2005, 2006a, 2006b; Olivier et al., 1995; Veiga et al., 2007). Recently, Lerch-Haner et al. (2008) reported that transgenic mouse dams with a specific disruption in serotonin transcription factor displayed profound maternal deficits (e.g., pup retrieval, pup nursing, nest building). Alenina et al. (2009) reported that the Tph2-deficient mouse dams lacking central serotonin exhibited disruptive maternal behavior as these dams neither retrieve their pups into the nest site nor nurturing them. Future work is required to address the mechanisms through which serotonin system participates in the regulation of maternal behavior.
We should point out several limitations with the current report. First, our search for the neural circuitries associated with the maternal behavioral effects of HAL and CLZ was largely a search within the brain regions associated either antipsychotic treatment or with the mediation of maternal behavior. There is a possibility that brain regions outside these circuitries (e.g. ventral tegmental area) may also be involved. Second, our work is limited by the sensitivity of c-Fos immunohistochemistry. It is quite possible that the QUI-HAL and DOI-CLZ interactions may produce brain changes that are unable to be detected by c-Fos expression. Finally, our work is correlational in nature. Future research using the microinjection technique to directly administer drugs into different brain regions is required to confirm our findings on the specific HAL and CLZ neural systems.
In conclusion, the present study used the c-Fos immunohistochemistry technique, together with pharmacological tools and behavioral observations and delineated the neuroanatomical bases of the disruptive effects of haloperidol and clozapine on rat maternal behavior. Our results show that the dopamine D2 receptor systems in the nucleus accumbens (both shell and core), dorsolateral striatum and lateral septum consist of the neural system that mediates the maternal disruptive effect of HAL. In contrast, the serotonin 5-HT2A/2C receptor system in the nucleus accumbens shell may be important for the maternal disruptive effect of CLZ.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (5R03MH080822-02). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. We are grateful to Dr. You Zhou and Mr. Wei He for their technical support for this work.
Abbreviations
- APDs
antipsychotic drugs
- CLZ
clozapine
- DLSt
dorsolateral striatum
- DOI
2,5-dimethoxy-4-iodo-amphetamine
- Fos-I
Fos-immunoreactivity
- HAL
haloperidol
- LSv
ventral part of lateral septal nucleus
- MeA
medial amygdaloid nucleus
- mPFC
medial prefrontal cortex
- MPOA
medial preoptic area
- NAc
nucleus accumbence core
- NAs
nucleus accumbence shell
- PBS
phosphate-buffered saline
- QUI
quinpirole
- vBST
ventral bed nucleus of the stria terminalis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121:515–526. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boyé P, Vilianovitch L, Sohr R, Tenner K, Hörtnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin receptor antagonist SR 142948A alters Fos expression and extrapyramidal side effect profile of typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2004;29:2200–2207. doi: 10.1038/sj.npp.1300546. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Freemark MS. Human placental lactogen infusions into the medial preoptic area stimulate maternal behavior in steroid-primed, nulliparous female rats. Horm Behav. 1995;29:216–226. doi: 10.1006/hbeh.1995.1016. [DOI] [PubMed] [Google Scholar]
- Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ. Maternal aggression in Wistar rats: effect of 5-HT2A/2C receptor agonist and antagonist microinjected into the dorsal periaqueductal gray matter and medial septum. Braz J Med Biol Res. 2005;38:597–602. doi: 10.1590/s0100-879x2005000400014. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ. The effect of 5-HT(2a/2c) receptor agonist microinjected into central amygdaloid nucleus and median preoptic area on maternal aggressive behavior in rats. Rev Bras Psiquiatr. 2006a;28:130–134. doi: 10.1590/s1516-44462006000200011. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA. 5-HT(1B) receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology (Berl) 2006b;185:441–450. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- De Medeiros MA, Carlos Reis L, Eugênio Mello L. Stress-induced c-Fos expression is differentially modulated by dexamethasone, diazepam and imipramine. Neuropsychopharmacology. 2005;30:1246–1256. doi: 10.1038/sj.npp.1300694. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Iadarola MJ. Regionally specific effects of atypical antipsychotic drugs on striatal Fos expression: the nucleus accumbens shell as a locus of antipsychotic action. Mol Cell Neurosci. 1992;3:332–341. doi: 10.1016/1044-7431(92)90030-6. [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF. Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol. 1997;384:233–247. [PubMed] [Google Scholar]
- Fibiger HC. Neuroanatomical targets of neuroleptic drugs as revealed by Fos immunochemistry. J Clin Psychiatry. 1994;55:33–36. [PubMed] [Google Scholar]
- Fleischer S, Slotnick BM. Disruption of maternal behavior in rats with lesions of the septal area. Physiol Behav. 1978;21:189–200. doi: 10.1016/0031-9384(78)90041-0. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C. Factors influencing maternal responsiveness in humans: usefulness of an animal model. Psychoneuroendocrinology. 1988;13:189–212. doi: 10.1016/0306-4530(88)90014-5. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav Neurosci. 1994;108:724–734. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- Guo N, Klitenick MA, Tham CS, Fibiger HC. Receptor mechanisms mediating clozapine-induced c-fos expression in the forebrain. Neuroscience. 1995;65:747–756. doi: 10.1016/0306-4522(94)00552-g. [DOI] [PubMed] [Google Scholar]
- Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991a;105:588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991b;39:71–77. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Morrell JI. The medial preoptic area, necessary for adult maternal behavior in rats, is only partially established as a component of the neural circuit that supports maternal behavior in juvenile rats. Behav Neurosci. 2000a;114:196–210. doi: 10.1037//0735-7044.114.1.196. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Nakabeppu Y, Morrell JI. Induction of c-fos-like and fosB-like immunoreactivity reveals forebrain neuronal populations involved differentially in pup-mediated maternal behavior in juvenile and adult rats. J Comp Neurol. 2000b;416:45–78. doi: 10.1002/(sici)1096-9861(20000103)416:1<45::aid-cne5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behav Neurosci. 2003a;117:426–445. doi: 10.1037/0735-7044.117.3.426. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS. The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behav Brain Res. 2003b;145:99–111. doi: 10.1016/s0166-4328(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Li M, Davidson P, Budin R, Kapur S, Fleming AS. Effects of typical and atypical antipsychotic drugs on maternal behavior in postpartum female rats. Schizophr Res. 2004;70:69–80. doi: 10.1016/j.schres.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Li M, Fletcher PJ, Kapur S. Time course of the antipsychotic effect and the underlying behavioral mechanisms. Neuropsychopharmacology. 2007;32:263–272. doi: 10.1038/sj.npp.1301110. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Maternal behaviour in lactating rats stimulates c-fos in glutamate decarboxylase-synthesizing neurons of the medial preoptic area, ventral bed nucleus of the stria terminalis, and ventrocaudal periaqueductal gray. Neuroscience. 2000;100:557–568. doi: 10.1016/s0306-4522(00)00287-6. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gréco B, De Vries GJ, Stern JM, Blaustein JD. Maternal behavior stimulates c-fos activity within estrogen receptor alpha-containing neurons in lactating rats. Neuroendocrinology. 2000;72:91–101. doi: 10.1159/000054576. [DOI] [PubMed] [Google Scholar]
- MacGibbon GA, Lawlor PA, Bravo R, Dragunow M. Clozapine and haloperidol produce a differential pattern of immediate early gene expression in rat caudate-putamen, nucleus accumbens, lateral septum and islands of Calleja. Brain Res Mol Brain Res. 1994;23:21–32. doi: 10.1016/0169-328x(94)90207-0. [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10:2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G, Martinez-Mir MI, Vilaró MT, Palacios JM. Localization of the mRNA for the dopamine D2 receptor in the rat brain by in situ hybridization histochemistry. Proc Natl Acad Sci U S A. 1989;86:8560–8564. doi: 10.1073/pnas.86.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G, Pompeiano M, Martínez-Mir MI, Palacios JM. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990;524:139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Dorsa DM. Differential induction of neurotensin and c-fos gene expression by typical versus atypical antipsychotics. Proc Natl Acad Sci U S A. 1993;90:3447–3451. doi: 10.1073/pnas.90.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine D1 and D2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav Neurosci. 2005;119:1072–1083. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Mo YQ, Jin XL, Chen YT, Jin GZ, Shi WX. Effects of l-stepholidine on forebrain Fos expression: comparison with clozapine and haloperidol. Neuropsychopharmacology. 2005;30:261–267. doi: 10.1038/sj.npp.1300628. [DOI] [PubMed] [Google Scholar]
- Monasterio N, Ramos E, Morales T. Changes in c-Fos and NOS expression in the PVH of lactating rats in response to excitotoxicity and stress. Ann N Y Acad Sci. 2008;1148:161–164. doi: 10.1196/annals.1410.046. [DOI] [PubMed] [Google Scholar]
- Natesan S, Svensson KA, Reckless GE, Nobrega JN, Barlow KB, Johansson AM, Kapur S. The dopamine stabilizers (S)-(-)-(3-methanesulfonyl-phenyl)-1-propyl-piperidine [(-)-OSU6162] and 4-(3-methanesulfonylphenyl)-1-propyl-piperidine (ACR16) show high in vivo D2 receptor occupancy, antipsychotic-like efficacy, and low potential for motor side effects in the rat. J Pharmacol Exp Ther. 2006;318:810–818. doi: 10.1124/jpet.106.102905. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci U S A. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Numan M. Maternal behavior. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 221–302. [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Expression of Fos-like immunoreactivity in the preoptic area of maternally behaving virgin and postpartum rats. Behav Neurosci. 1994;108:379–394. doi: 10.1037//0735-7044.108.2.379. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Importance of pup-related sensory inputs and maternal performance for the expression of Fos-like immunoreactivity in the preoptic area and ventral bed nucleus of the stria terminalis of postpartum rats. Behav Neurosci. 1995;109:135–149. doi: 10.1037//0735-7044.109.1.135. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann N Y Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Marzella SR, Palumbo A. Expression of c-fos, fos B, and egr-1 in the medial preoptic area and bed nucleus of the stria terminalis during maternal behavior in rats. Brain Res. 1998;792:348–352. doi: 10.1016/s0006-8993(98)00257-1. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Hamamura T, Lee Y, Fujiwara Y, Suzuki H, Kuroda S. Clozapine- and olanzapine-induced Fos expression in the rat medial prefrontal cortex is mediated by beta-adrenoceptors. Neuropsychopharmacology. 2000;23:162–169. doi: 10.1016/S0893-133X(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van Oorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry. 1995;28(Suppl 2):80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th. New York: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Pinna A, Wardas J, Cozzolino A, Morelli M. Involvement of adenosine A2A receptors in the induction of c-fos expression by clozapine and haloperidol. Neuropsychopharmacology. 1999;20:44–51. doi: 10.1016/S0893-133X(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Fibiger HC. Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience. 1992;46:315–328. doi: 10.1016/0306-4522(92)90054-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Matsumura H, Fibiger HC. Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther. 1994;271:1058–1066. [PubMed] [Google Scholar]
- Rosenblatt JS. The physiological and evolutionary background of maternal responsiveness. New Dir Child Dev. 1989;43:15–30. doi: 10.1002/cd.23219894304. [DOI] [PubMed] [Google Scholar]
- Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Smith MO, Holland RC. Effects of lesions of the nucleus accumbens on lactation and postpartum behavior. Physiol Psychol. 1975;3:331–336. [Google Scholar]
- Stack EC, Numan M. The temporal course of expression of c-Fos and Fos B within the medial preoptic area and other brain regions of postpartum female rats during prolonged mother--young interactions. Behav Neurosci. 2000;114:609–622. doi: 10.1037//0735-7044.114.3.609. [DOI] [PubMed] [Google Scholar]
- Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav Brain Res. 2002;131:17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- Stern JM, Keer SE. Maternal motivation of lactating rats is disrupted by low dosages of haloperidol. Behav Brain Res. 1999;99:231–239. doi: 10.1016/s0166-4328(98)00108-9. [DOI] [PubMed] [Google Scholar]
- Stern JM, Protomastro M. Effects of low dosages of apomorphine on maternal responsiveness in lactating rats. Pharmacol Biochem Behav. 2000;66:353–359. doi: 10.1016/s0091-3057(00)00180-5. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav Neurosci. 2007;121:907–919. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Tremblay PO, Gervais J, Rouillard C. Modification of haloperidol-induced pattern of c-fos expression by serotonin agonists. Eur J Neurosci. 1998;10:3546–3555. doi: 10.1046/j.1460-9568.1998.00372.x. [DOI] [PubMed] [Google Scholar]
- Vahid-Ansari F, Robertson GS. 7-OH-DPAT differentially reverses clozapine- and haloperidol-induced increases in Fos-like immunoreactivity in the rodent forebrain. Eur J Neurosci. 1996;8:2605–2611. doi: 10.1111/j.1460-9568.1996.tb01555.x. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, Almeida RM. Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Braz J Med Biol Res. 2007;40:825–830. doi: 10.1590/s0100-879x2006005000113. [DOI] [PubMed] [Google Scholar]
- Young CD, Bubser M, Meltzer HY, Deutch AY. Clozapine pretreatment modifies haloperidol-elicited forebrain Fos induction: a regionally-specific double dissociation. Psychopharmacology. 1999;144:255–263. doi: 10.1007/s002130051001. [DOI] [PubMed] [Google Scholar]
- Zhao C, Fujinaga R, Tanaka M, Yanai A, Nakahama K, Shinoda K. Region-specific expression and sex-steroidal regulation on aromatase and its mRNA in the male rat brain: immunohistochemical and in situ hybridization analyses. J Comp Neurol. 2007;500:557–573. doi: 10.1002/cne.21193. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li M. Sedation and disruption of maternal motivation underlie the disruptive effects of antipsychotic treatment on rat maternal behavior. Pharmacol Biochem Behav. 2009a;92:147–156. doi: 10.1016/j.pbb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Li M. The receptor mechanisms underlying the disruptive effects of haloperidol and clozapine on rat maternal behavior: A double dissociation between dopamine D2 and 5-HT2A/2C receptors. Pharmacol Biochem Behav. 2009b;93:433–442. doi: 10.1016/j.pbb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]