Abstract
The immunodominance of Mycobacterium tuberculosis proteins malate synthase (MS) and MPT51 has been demonstrated in case-control studies with patients from countries in which tuberculosis (TB) is endemic. The value of these antigens for the serodiagnosis of TB now is evaluated in a cross-sectional study of pulmonary TB suspects in the United States diagnosed to have TB, HIV-associated TB, or other respiratory diseases (ORD). Serum antibody reactivity to recombinant purified MS and MPT51 was determined by enzyme-linked immunosorbent assays (ELISAs) of samples from TB suspects and well-characterized control groups. TB suspects were diagnosed with TB (n = 87; 49% sputum microscopy negative, 20% HIV+) or ORD (n = 63; 58% HIV+). Antibody reactivity to MS and MPT51 was significantly higher in U.S. HIV+/TB samples than in HIV−/TB samples (P < 0.001), and it was significantly higher in both TB groups than in control groups with latent TB infection (P < 0.001). Antibody reactivity to both antigens was higher in U.S. HIV+/TB samples than in HIV+/ORD samples (P = 0.052 for MS, P = 0.001 for MPT51) but not significantly different between HIV−/TB and HIV−/ORD. Among U.S. HIV+ TB suspects, a positive anti-MPT51 antibody response was strongly and significantly associated with TB (odds ratio, 11.0; 95% confidence interval, 2.3 to 51.2; P = 0.002). These findings have implications for the adjunctive use of TB serodiagnosis with these antigens in HIV+ subjects.
The detection and treatment of individuals who are at early stages of active pulmonary tuberculosis (TB) is critical for the successful control and elimination of TB (34, 38). Mycobacterium tuberculosis is a slow-growing pathogen, and it takes months to years for an infection (and, presumably, reactivation) to progress to clinical TB. In resource-limited countries, the microscopic examination of smears made directly from unprocessed sputum are used for diagnosis, resulting in the identification of only advanced TB patients with high bacillary burden. In contrast, in industrialized settings the combined use of the fluorescence microscopy of decontaminated and concentrated sputum, mycobacterial culture, and nucleic acid amplification technologies permits the identification of patients with much lower bacillary burden and, thus, in the early stages of TB. Still, only around 50% of TB cases are rapidly diagnosed by optimized microscopy (5, 18). While adjunctive amplification methods increase the yield of confirmed TB, albeit with added cost and delays, around 20% of TB cases remain without microbiologic confirmation (5, 18). Additional tests that can enhance the rapid identification of patients at early stages of TB are required to add to the armamentarium of TB diagnostic tests.
The amplification power of immune responses potentially can detect TB at a low antigen threshold and without requiring a specimen from the site of infection. Assays that detect TB infection by measuring the gamma interferon release of circulating lymphocytes in response to M. tuberculosis-specific antigens (IGRAs) cannot distinguish active from latent TB infection (LTBI) (reviewed in reference 15) and have limited utility in patients with advanced HIV infection (3, 32). In contrast, positive antibody (Ab) responses to several new antigens during active TB but not LTBI have been reported. However, most studies have focused on patients with advanced TB, and the utility of these responses appears to be limited in patients with low mycobacterial burden (reviewed in references 29 and 30).
Two immunodominant M. tuberculosis proteins, the 81-kDa malate synthase (MS; Rv 1837c, GlcB) and the 27-kDa MPT51 (Rv3803c), are reported to elicit Ab responses during early and advanced stages of TB in both HIV− and HIV+ patients (1, 2, 10, 16, 24, 27, 37). This is important because HIV+ TB patients appear to develop Ab responses to a smaller repertoire of M. tuberculosis antigens than that of HIV− TB patients (24, 25). In previous case-control studies with HIV− and HIV+ patients, pulmonary and extrapulmonary TB patients from settings in which TB is endemic demonstrated the presence of anti-MS and/or anti-MPT51 Abs in about 80% of the TB patients but not in tuberculin skin test (TST)-negative and -positive volunteers (27, 37). Similar studies with U.S. patients demonstrated that while anti-MS and/or anti-MPT51 Abs were present in only ∼40% of the HIV− patients at early stages of TB, ∼80% of the U.S. HIV+ TB patients were Ab positive (1). Nevertheless, combining serology with sputum microscopy improved the detection of TB in both groups compared to that of microscopy alone, and it led to the identification of 90% of HIV+ TB patients, compared to 60% by microscopy alone (1). These findings are of high clinical relevance, since the rapid identification and treatment of early TB is crucial for HIV+ patients, in whom the dual infection leads to the acceleration of both diseases (20, 35).
While antigen discovery, selection, and validation initially relies on case-control studies comparing known TB cases to healthy controls, such comparisons result in the overestimation of accuracy (14, 23), and the real value of any antigen needs to be ascertained by cross-sectional studies in clinical settings where the TB suspects include patients with a variety of respiratory diseases. The goals of the current investigations were to (i) identify the range of Ab reactivities to MS and MPT51 in a cross-sectional study of U.S. TB suspects, (ii) compare Ab reactivities between U.S. HIV− and HIV+ TB patients and to asymptomatic U.S. non-TB as well as endemic TB controls; and (iii) compare Ab reactivities of HIV− and HIV+ TB suspects diagnosed to have respiratory diseases other than TB (ORD) to those of HIV− and HIV+ TB patients.
MATERIALS AND METHODS
TB suspects.
Consecutive subjects with a high clinical suspicion for TB were enrolled in a cross-sectional study from September 2006 to October 2008 from four public hospitals in New York City, NY: Bellevue Hospital Center, located in Manhattan, and Jacobi Medical Center, North Central Bronx Hospital, and Montefiore Medical Center, all three of which are located in the Bronx. Inclusion criteria were an age of >21 years and the receipt of sputum smears for acid-fast bacilli (AFB) and mycobacterial cultures, mostly by induced sputum production. Subjects on anti-tuberculous treatment (ATT) for >2 weeks or those with a history of ATT for active TB within the year prior to enrollment were excluded.
Control groups.
There were four control groups: (i) U.S. HIV− healthy volunteers categorized by tuberculin skin test (TST) and IGRA (QuantiFERON-TB Gold blood test [QFT]; Celestis, Australia) results; (ii) asymptomatic U.S. HIV+ persons categorized by TST results; (iii) asymptomatic HIV− individuals from settings in which TB is endemic, mostly India, categorized by TST result; and (iv) HIV− and HIV+ pulmonary TB patients from India.
Subjects were bled at the time of enrollment, and sera were stored at −80°C until tested. Informed consent was obtained from all subjects. Approval for human subject research for U.S. subjects was obtained from the Institutional Review Boards of the New York University School of Medicine and the Albert Einstein College of Medicine, and for Indian patients from the Lala Ram Sarup Institute for TB, New Delhi, and the Post Graduate Institute for Medical Education and Research, Chandigarh.
Measurements, diagnoses, and definitions.
Demographic and clinical information was obtained from interviews and medical records. A TST was considered positive if it was known or newly reported to be positive per CDC guidelines (4). The gold standard for TB diagnosis was a respiratory specimen culture positive for M. tuberculosis or, if culture negative, clinical and radiologic responses to ATT. Subjects were categorized as ORD if they had a respiratory culture negative for M. tuberculosis and an ORD diagnosis per a physician's discharge note. The diagnosis of nontuberculous mycobacterial lung disease (NTM) was based on diagnostic criteria according to the American Thoracic Society (36).
Protein preparation.
Recombinant bacterial expression plasmids pMRLB8 and pMRLB38, encoding C-terminal hexahistidine fusions of MS (Rv1837c) and MPT51 (Rv3803c), were obtained from the Tuberculosis Research Materials and Vaccine Testing Contract at Colorado State University. Recombinant proteins were expressed and purified essentially as described previously for pMLRB8-borne Rv1837c, with the exception that the final Ni affinity chromatographic steps were implemented on an AktaFPLC system (GE Healthcare) equipped with 1 ml HisTrapHP (GE Healthcare) columns (27).
ELISAs.
The ELISAs were performed as reported previously (27). Briefly, wells of 96-well microtiter plates (Immulon 2HB; Dynax) were coated with MS or MPT51 at 4 μg/ml (50 μl/well). Sera diluted at 1:50 (for MS) or 1:25 (for MPT51) were added to the antigen-coated wells, and the bound Abs were detected with a mixture of protein A-alkaline phosphatase (1:2,000; Sigma) and anti-human IgA-alkaline phosphatase (1:1,000; Sigma) followed by p-nitrophenyl phosphate substrate (60 min at 37°C). The optical densities (OD) were measured at 405 nm. In each assay, the mean ODs of sera from the same 20 TST-negative healthy volunteers (negative controls) plus 3 standard deviations (SD) were used to determine the cutoff for positive Ab responses and the change in OD after the subtraction of the cutoff value (ΔOD). Two wells on each plate were processed as described above, except for the addition of serum. Sera from two patients with advanced TB known to be positive for anti-MS and anti-MPT51 Abs were included as positive controls. All sera were tested three times, and sera testing positive 2/3 or 3/3 times were considered positive.
Statistical analysis.
Statistical analysis was performed using STATA software, version 9.2 (StataCorp, College Station, TX). A two-tailed α of <0.05 was considered to be statistically significant. The t test was used when variables were normally distributed, and when skewed (e.g., all OD and ΔOD values) the Mann-Whitney U test was used for two-group comparison or the Kruskall-Wallis test for multiple-group comparison. Categorical variables were compared with the chi-square test without correction for continuity. The Spearman rank test was used for correlations between ΔOD values for MS and MPT51. Associations between a positive Ab response to either protein and the diagnosis of pulmonary TB were tested in logistic regression models.
RESULTS
TB suspects.
Of the 180 TB suspects enrolled, 150 were included in the studies after the exclusion of 30 subjects who did not receive a confirmed diagnosis. Suspects were categorized as pulmonary TB (n = 87) or ORD (n = 63) (Table 1). Presenting symptoms in the two groups were mostly similar, although symptom duration was longer in the TB group. Table 2 lists the most common diagnoses of subjects with ORD categorized by HIV status.
TABLE 1.
Characteristics of U.S. TB suspectsa
| Characteristic | TB (n = 87) | ORD (n = 63) | P |
|---|---|---|---|
| Age, mean yr (±SD) | 40 (±12) | 51 (±13) | <0.001b |
| Male (%) | 61 (70) | 46 (74) | 0.546c |
| Foreign born (%) | 67 (77) | 18 (29) | <0.001c |
| HIV+ (%) | 13/64 (20) | 30/52 (58) | <0.001c |
| CD4+ if HIV+, median no. (range) | 137 (17-483) | 71 (4-919) | 0.149d |
| TST+ (%) | 38/46 (83) | 15/39 (38) | <0.001c |
| AFB+ (%) | 44 (51) | 3 (5) | <0.001c |
| M. tuberculosis culture+ (%) | 78 (90) | 0 | NA |
| Cavitary lesions (%) | 28 (32) | 6 (10) | 0.001c |
| Nodular opacities (%) | 50 (57) | 8 (13) | <0.001c |
| Cough (%) | 56/73 (77) | 43/54 (80) | 0.695c |
| Fever (%) | 42/74 (57) | 35/54 (65) | 0.358c |
| Night sweats (%) | 39/73 (53) | 18/54 (33) | 0.024c |
| Weight loss (%) | 32/71 (45) | 21/54 (39) | 0.488c |
| Symptom duration, median wk (range) | 4 (0-52) | 1 (0-36) | <0.001d |
TB, tuberculosis; ORD, respiratory diseases other than TB; AFB, sputum smears for acid-fast bacilli, which were considered positive if at least one of the initial three smears was positive.
t test.
Chi-square test.
Mann-Whitney U test.
TABLE 2.
Diagnoses of subjects with ORD (n = 63)
| HIV status | No. (%) diagnosed witha: |
||||
|---|---|---|---|---|---|
| CAP (n = 26) | PCP (n = 7) | NTM (n = 6) | Bronchitis (n = 6) | Other (n = 18) | |
| HIV− | 9 (35) | 0 | 2 (33) | 3 (50) | 8 (44) |
| HIV+ | 11 (42) | 7 (100) | 3 (50) | 1 (17) | 8 (44) |
| HIV unknown | 6 (23) | 0 | 1 (17) | 2 (33) | 2 (11) |
CAP, community-acquired pneumonia; PCP, Pneumocystis jiroveci pneumonia; NTM, nontuberculous mycobacterial lung disease; other, other respiratory diseases, such as lung cancer, sarcoidosis, lung abscess, empyema, etc.
Control groups.
Asymptomatic U.S. control groups were categorized by TST result and HIV status, and if they were HIV−/TST+ then they were categorized further by QFT result (Table 3). Asymptomatic HIV− individuals from countries in which TB is endemic were TST− (n = 8) or TST+ (n = 12). Indian HIV−/TB (n = 20) and HIV+/TB patients (n = 17) were pulmonary TB patients with positive smears on unprocessed sputum samples. All Indian subjects had received BCG vaccination during their early childhood years.
TABLE 3.
Characteristics of asymptomatic US control groups categorized by TST and HIV statusa
| Characteristic | HIV−/TST− (n = 20) | HIV−/TST+ (n = 22) | HIV+/TST− (n = 20) | HIV+/TST+ (n = 11) |
|---|---|---|---|---|
| Age, mean yr (±SD) | 38 (±8) | 43 (±11) | 50 (±10) | 40 (±9) |
| No. (%) male | 5 (25) | 12 (55) | 9 (45) | 6 (55) |
| No. (%) foreign born | 6 (30) | 19 (86) | 3 (15) | 4 (36) |
| No. (%) BCG vaccinated | 6 (30) | 22 (100) | 3 (15) | 4 (36) |
| No. (%) with prior INH prophylaxis | 0 | 5 (23) | 0 | 7 (64) |
| No. (%) QFT+ | NA | 10 (46) | NA | NA |
| No. (%) CXR WNL | NA | 22 (100) | 20 (100) | 10 (91) |
| No. (%) on ART | NA | NA | 19 (95) | 11 (100) |
| No. CD4+, median cells/mm3 (range) | NA | NA | 632 (269-1,157) | 592 (298-1,541) |
| VL, median copies/ml (range) | NA | NA | <50 (<50-1,646) | 92 (<50-8,120) |
INH, isoniazid; QFT, QuantiFERON-TB Gold test; CXR WNL, chest X-ray findings within normal limits; ART, antiretroviral treatment; VL, HIV viral load; NA, not applicable.
Anti-MS and anti-MPT51 Abs in asymptomatic control groups.
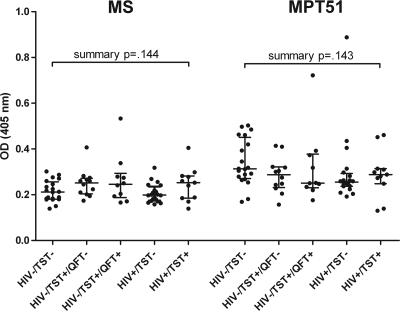
There was no statistically significant difference in the median ODs for either protein between the U.S. control groups regardless of HIV status or TST or QFT results (Fig. 1). Moreover, there was no significant difference in ODs for either protein between asymptomatic U.S. controls and asymptomatic individuals from settings in which TB is endemic regardless of TST result (data not shown). The highest OD values for MS and MPT51 in the HIV−/TST+/QFT+ control group were from two different individuals, neither of whom developed any signs of active TB during a 3-year follow-up period.
FIG. 1.
Antibody reactivities to MS and MPT51 in asymptomatic U.S. control groups categorized by TST result and HIV status. Bars show median ODs with interquartile ranges. The Kruskal-Wallis test was used for the group comparison of median antibody reactivities.
Anti-MS and anti-MPT51 Abs in TB patients.
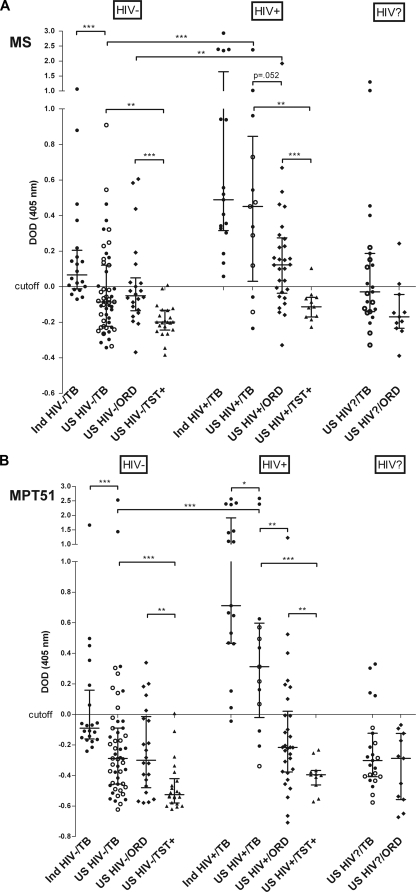
The median Ab reactivity to both proteins was significantly higher in the U.S. HIV−/TB subjects than in HIV−/TST+ subjects but was significantly lower than that in the Indian HIV−/TB patients (P < 0.001) (Fig. 2A and B). Only 15/51 (29%) and 9/51 (18%) U.S. HIV−/TB patients had Abs to MS and MPT51, respectively, while among the Indian HIV−/TB patients, 15/20 (75%) had anti-MS and 6/20 (30%) had anti-MPT51 Abs. The median Ab reactivity to both proteins also was significantly higher in the U.S. HIV+/TB subjects than in HIV+/TST+ subjects (P < 0.001), and it was significantly higher than that of U.S. HIV−/TB subjects (P < 0.001). However, the median Ab reactivity for U.S. HIV+/TB patients was only slightly lower than that of Indian HIV+/TB (not significant for MS; P = 0.045 for MPT51). Moreover, in contrast to the U.S. HIV−/TB patients, where few patients were Ab positive, 10/13 (77%) U.S. HIV+/TB patients had anti-MS and anti-MPT51 Abs. Anti-MS Abs were detected in 20/20 and anti-MPT51 Abs in 19/20 (95%) Indian HIV+/TB patients. Twenty-six suspects diagnosed to have TB refused HIV testing; 10/23 (43%) had anti-MS and 4/23 (17%) had anti-MPT51 Abs. Similarly to the U.S. TB patients, Ab reactivities to both proteins were significantly higher in Indian HIV+/TB than Indian HIV−/TB patients.
FIG. 2.
Antibody reactivities to MS (A) and MPT51 (B) in subjects with TB, other respiratory diseases (ORD), and controls, categorized by HIV status. Cutoff values were derived from the mean OD of HIV−/TST− healthy volunteers plus 3 standard deviations; ΔOD, change in optical density after the subtraction of the cutoff value; •, sputum AFB smear positive; ○, sputum AFB smear negative; Ind, Indian; bars show median ΔODs with interquartile ranges. The Mann-Whitney U test was used for two-group comparisons of median antibody reactivities. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Anti-MS and anti-MPT51 Abs in U.S. patients with ORD.
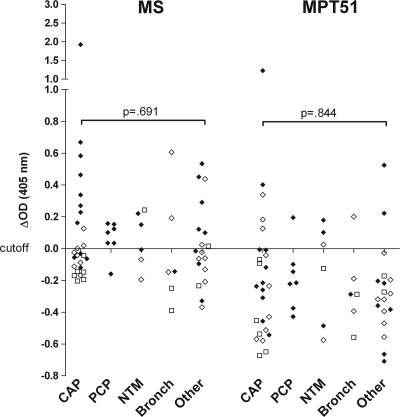
The median Ab reactivity to both proteins was significantly higher for the HIV−/ORD group than the HIV−/TST+ group (P < 0.001 for MS; P < 0.003 for MPT51) and was not significantly different from that for the U.S. HIV−/TB patients (Fig. 2A and B). Of the HIV−/ORD patients, 7/22 (32%) had anti-MS and 5/22 (23%) had anti-MPT51 Abs. Ab reactivity was significantly higher in the HIV+/ORD group than the HIV+/TST+ group (P < 0.001 for MS; P < 0.007 for MPT51). In contrast to HIV− TB suspects, Ab reactivity in the HIV+/ORD patients was much lower than that in the HIV+/TB patients (P = 0.052 for MS; P < 0.001 for MPT51). Of the HIV+/ORD patients, 20/30 (67%) had anti-MS and 7/30 (23%) anti-MPT51 Abs. Two of the 11 ORD patients (18%) with unknown HIV status had anti-MS Abs, and none were positive for anti-MPT51 Abs. There were no significant differences in the Ab reactivities to both proteins between the different types of ORD (Fig. 3). Of note, the HIV+/ORD subject with the highest ΔOD (>1.0 for MS and MPT51 Abs) had bilateral lung nodules and hilar adenopathy consistent with active TB but was diagnosed with pneumonia due to clinical improvement on treatment with antibacterial antibiotics.
FIG. 3.
Antibody reactivities to MS and MPT51 by type of ORD. Cutoff values were derived from the mean OD of HIV−/TST− healthy volunteers plus 3 standard deviations; ΔOD, change in optical density after the subtraction of the cutoff value; ⧫, HIV+; ⋄, HIV−; □, HIV status unknown; CAP, community-acquired pneumonia; PCP, Pneumocystis jiroveci pneumonia; NTM, nontuberculous mycobacterial lung disease; bronch, bronchitis; other, respiratory diseases other than TB, such as lung cancer, sarcoidosis, lung abscess, empyema, immune reconstitution syndrome, etc.
Association between positive Ab reactivity and pulmonary TB in U.S. TB suspects.
In logistic regression models stratified by HIV status, a positive Ab response to MPT51 was strongly and significantly associated with the diagnosis of TB among HIV+ TB suspects (odds ratio [OR], 11.0; 95% confidence intervals [CI], 2.3 to 51.2; P = 0.002), while no significant association was found for MS or for either of the proteins among HIV−/TB suspects.
Ab reactivity to MS and MPT in U.S. TB patients according to clinical presentation.
No significant differences in Ab reactivity were found between smear-positive and smear-negative TB samples either among HIV− or among HIV+ U.S. patients, and no significant differences were found when HIV−/TB patients were further categorized by the presence of cavitary lesions, which mostly were small and seen only on computed tomography (CT). Furthermore, no significant associations were found among U.S. TB patients between a positive Ab response to MS or MPT51 and symptom duration.
Correlation between Ab reactivity to MS and MPT51.
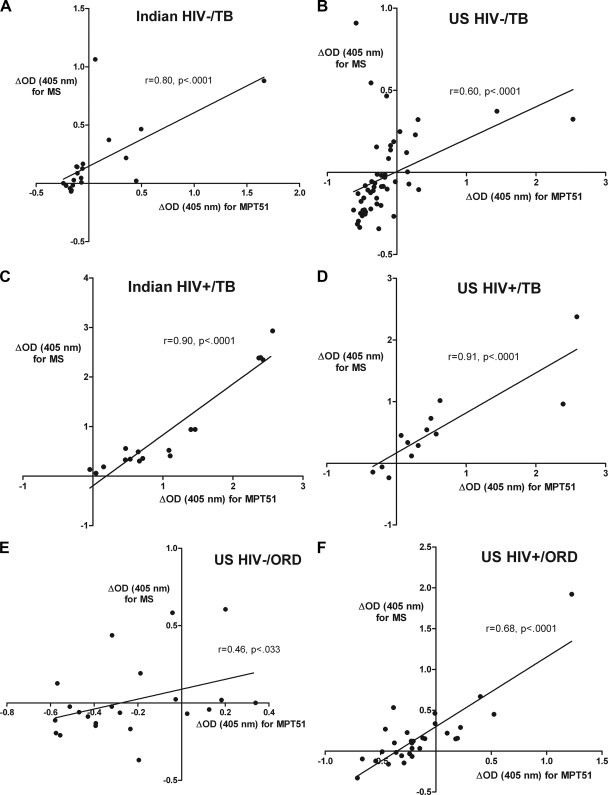
Stratified by HIV status, Ab reactivity to the two proteins correlated very strongly in both U.S. and Indian HIV−/TB as well as HIV+/TB patients (P < 0.001 in all correlations) (Fig. 4A to D), indicating that Abs to the two antigens are detected simultaneously in patients with active TB. Interestingly, while the correlation remained strong in the HIV+/ORD patients (P < 0.001), it was much weaker in the HIV−/ORD patients (P < 0.033) (Fig. 4E and F).
FIG. 4.
Correlation between antibody reactivity to MS and MPT51 in Indian HIV−/TB (A), U.S. HIV−/TB (B), Indian HIV+/TB (C), U.S. HIV+/TB (D), U.S. HIV−/ORD (E), and U.S. HIV+/ORD subjects (F). Spearman rank correlation was used to test for statistical significance.
DISCUSSION
The results of this first cross-sectional study of U.S. TB suspects provide important insights into the value of MS and MPT51 for TB diagnosis in industrialized settings, and the comparison to control groups from settings in which TB is endemic allows inferences for such settings. This is also the first study wherein Ab responses to both proteins have been tested in asymptomatic subjects categorized on the basis of their IGRA results, confirming the lack of anti-MS and anti-MPT51 Abs in subjects with positive TSTs due to BCG vaccination, exposure to atypical mycobacteria, or LTBI. Moreover, there was no difference of Ab responses to either antigen between asymptomatic U.S. controls and TST− and TST+ individuals from settings in which TB is endemic. As was observed in the prior U.S. case-control study, both HIV−/TB and HIV+/TB patients had significantly higher Ab reactivities than subjects with LTBI, and U.S. HIV+/TB demonstrated significantly higher reactivities irrespective of CD4+ T-cell counts, viral loads, or sputum smear results compared to those of HIV−/TB patients (1, 26, 37). The evaluation of Ab reactivities in TB suspects with ORD revealed higher values in U.S. HIV+/TB than U.S. HIV+/ORD patients, while no such differences were observed among U.S. HIV− TB suspects. Among U.S. HIV+/TB suspects, the strong and significant association of a positive anti-MPT51 Ab response with pulmonary TB is a finding of potential clinical relevance.
Ab responses to both antigens were higher in both U.S. and Indian HIV+/TB patients than in the respective HIV−/TB patients. Interestingly, the anti-MS responses in the U.S. and Indian HIV+/TB patients were similar, and the anti-MPT51 responses were only marginally greater in the Indian patients. The impaired granuloma formation and ineffective containment of M. tuberculosis in HIV+/TB patients is known to result in high in vivo bacterial burden even when the patients are bacteriologically negative on sputum examination. Coupled to the polyclonal B-cell stimulation that accompanies HIV infection, the higher bacterial burden likely results in the stronger stimulation of the humoral responses in HIV+/TB patients compared to that of the HIV−/TB patients (13). That the bacterial burden influences Ab titers is supported by the significantly higher Ab reactivities to both proteins in advanced Indian HIV−/TB compared to early U.S. HIV−/TB patients. Note that the sensitivity of Ab detection with either antigen in U.S. HIV+/TB patients in the current cross-sectional study is similar to the combined sensitivity with both antigens in our earlier case-control study, 77 and 80%, respectively (1). The confirmation of the high sensitivity in the U.S., where TB can be diagnosed early due to the use of multiple technologies, and the high Ab responses in Indian HIV+/TB patients together emphasize the value of these antigens in the rapid diagnosis of TB in countries where TB is rampant in HIV+ patients. Furthermore, the previously demonstrated diagnostic adjunct effect of serology with these antigens to sputum microscopy in U.S. HIV+/TB patients (1), together with the strong association of a positive anti-MPT51 Ab response with active TB among U.S. HIV+/TB suspects in this study, indicate that serology with these antigens is a valuable tool as an adjunct test to rapidly identify TB in HIV+ patients, even in industrialized settings where no serodiagnostic test has ever been found to contribute to TB diagnosis.
The lower Ab responses to both proteins in U.S. patients compared to that in Indian HIV−/TB patients are in accordance with our earlier case-control studies. Similar differences in reactivity to all other proteins that were tested in TB patients from industrialized and endemic settings have been observed (reviewed in reference 29). Considering that despite sputum induction and optimized microscopy ∼50% of our patients were smear negative and ∼10% were even culture negative, the low Ab reactivities likely are due to the detection of very early TB. Compared to countries in which TB is endemic, where most patients have symptoms for months before they access even the lowest levels of health care, the median symptom duration in our patients was ∼4 weeks. While the vast majority of the TB patients in endemic settings have extensive pulmonary infiltration and cavitation by the time they are diagnosed, less than a third of our U.S. TB patients had cavitary lesions, and most of them were so small that they were seen only on chest CT. Studies with many infectious organisms have demonstrated significant correlations between pathogen burden and host responses (17, 33), thus it is plausible that at such early stages of TB the titers of Abs are low and likely are trapped in immune complexes, making the detection of free Abs difficult. This is further supported by the observation that in our population the presence of neither smear positivity nor cavitary lesions had an impact on the level of Ab reactivity, while both of these clinical parameters were shown to have an impact on the Ab reactivities in Indian TB patients (37). While the inclusion of serology with these two proteins is unlikely to improve the diagnosis of HIV− TB in industrialized countries, its value in settings in which TB is endemic, even with low HIV prevalence, likely will be much higher, although cross-sectional studies to confirm this remain to be done.
The presence of anti-MS and anti-MPT51 Abs in several TB suspects diagnosed with ORD could be ascribed to poor specificity with these antigens or the presence of incipient TB. All subjects diagnosed to have ORD had a high clinical suspicion for TB, either due to their risk factors or clinical features and/or radiographs characteristic of TB. The hypotheses that a number of these patients, particularly those who were HIV+, have incipient TB is supported by the following observations: (i) HIV infection is a known risk factor for progression from LTBI to active TB, and Ab reactivities to MS and MPT51 had a similar strong and significant correlation in HIV+/ORD and both HIV−/TB and HIV+/TB patients; (ii) around 45% of HIV+ patients from a country in which TB is endemic with high risk for progression to active TB had positive Ab responses to MS and/or MPT51 (37); (iii) active TB was estimated to be present up to 1.3 years prior to diagnosis in HIV+ and up to 4.2 years in HIV− African gold miners (6); (iv) Ab reactivities against both MS and MPT51 were shown to be increased by months to years in HIV+ subjects from the U.S. and India before developing clinically detectable active TB (26); and (v) Abs to other M. tuberculosis-specific antigens (ESAT-6, CFP-10, and PPE55) have been reported to emerge 24 to 30 months before the clinical diagnosis of active TB (7, 9, 26, 28).
It is possible that the Ab responses in some of the ORD patients, particularly in HIV− patients, are due to the lack of specificity of MS and MPT51 for the serodiagnosis of TB, since neither is M. tuberculosis specific and genes that show >80% homology are present in other mycobacteria, including M. avium, M. kansassi, and M. intracellulare (12, 39). However, we do not consider this to be the likely explanation for most ORD patients with positive Ab responses, because (i) in prior studies, anti-MS Abs were not detected in patients with lung infections due to atypical mycobacteria, pneumonia, or lung cancer (8, 10); (ii) in our studies, subjects with ORD due to NTM did not have higher Ab responses than subjects with ORD due to other etiologies; (iii) consistently with the high specificity reported in other studies (1, 21, 27), most of our asymptomatic subjects lacked positive Ab responses to both proteins regardless of HIV status, history of BCG vaccine, or LTBI; and (iv) consistently with our findings, Ab reactivities to M. tuberculosis-specific proteins (TB9.7, ESAT6, and CFP10) were higher in Gambian TB suspects diagnosed with ORD than in BCG-vaccinated healthy controls from Denmark (22).
Unfortunately, the long-term monitoring of our ORD patients was not feasible, making it impossible to determine the prognostic value of the high Ab responses in some of these patients. Although response to antibiotics plays a major role in the clinical decision of ruling out TB, studies from settings in which TB is endemic have shown that between 8 and 44% of smear-negative TB patients who ultimately had positive mycobacterial cultures showed symptomatic responses to a trial of antibiotics (19). Thus, although all ORD patients had negative mycobacterial cultures, it is possible that despite clinical response to antibiotics, some of them had underlying incipient or culture-negative TB. Of note, the patient with the highest ΔOD values in the HIV+/ORD group had bilateral lung nodules and hilar adenopathy that was consistent with active TB but was diagnosed with community-acquired pneumonia due to clinical improvement on treatment with antibiotics. Long-term monitoring would have further allowed serial assessments of Ab reactivities in ORD patients over time to determine whether increases wane or persist. Progression from LTBI to active TB is likely a dynamic process between resolution and progression in the early stages of the disease, depending on the regulatory mechanisms activated (11, 31).
In summary, these results suggest that among U.S. HIV+/TB suspects, Ab responses to MPT51 can help differentiate active TB from ORD, which likely is generalizable to HIV+ patients in settings in which TB is endemic. The prognostic potential of anti-MS and anti-MPT51 Ab responses for identifying incipient TB or a risk for progression to TB should be assessed in longitudinal studies.
Acknowledgments
This work was supported in part by grants from various institutes and centers of the National Institutes of Health (NIH), mostly from the National Institute of Allergy and Infectious Diseases (NIAID) (AI-067665 to J.M.A.; T32 AI-07501 in support of P.W.B.; AI-07289 to S.C.A.; AI-033774, AI-052733, HL-059842, and AI-033146 to A.C.; and AI-056257 to S.L.) and the Center for AIDS Research (AI-51519), as well as the Institute for Clinical and Translational Research (UL1 RR-025750, KL2 RR-025749, and TL1 RR-025748) at the Albert Einstein College of Medicine and Montefiore Medical Center, the Tuberculosis Research Materials and Vaccine Testing Contract at Colorado State University (AI-40091), the General Clinical Research Center at the New York University School of Medicine (M01 RR-00096), and the Fogarty Institute Center (TW01409). Non-NIH support sources included the Research Enhancement Award Program and the Merit Review Award (S.L.) funded by the Department of Veterans Affairs and the Macromolecular Therapeutic Development Facility at the Albert Einstein College of Medicine.
We thank H. Cohen for his statistical advice and A. Wanchu and D. Behera for their help with enrolling TB patients at their hospital in India.
We have no conflicts of interest.
Footnotes
Published ahead of print on 13 January 2010.
REFERENCES
- 1.Achkar, J. M., Y. Dong, R. S. Holzman, J. Belisle, I. S. Kourbeti, T. Sherpa, R. Condos, W. N. Rom, and S. Laal. 2006. Mycobacterium tuberculosis malate synthase- and MPT51-based serodiagnostic assay as an adjunct to rapid identification of pulmonary tuberculosis. Clin. Vaccine Immunol. 13:1291-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethunaickan, R., A. R. Baulard, C. Locht, and A. Raja. 2007. Antibody response in pulmonary tuberculosis against recombinant 27kDa (MPT51, Rv3803c) protein of Mycobacterium tuberculosis. Scand. J. Infect. Dis. 39:867-874. [DOI] [PubMed] [Google Scholar]
- 3.Brock, I., M. Ruhwald, B. Lundgren, H. Westh, L. R. Mathiesen, and P. Ravn. 2006. Latent tuberculosis in HIV positive, diagnosed by the M-tuberculosis specific interferon-gamma test. Resp. Res. 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1990. Screening for tuberculosis and tuberculous infection in high-risk populations. Recommendations of the Advisory Committee for Elimination of Tuberculosis. MMWR Recomm. Rep. 39:1-7. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2007. Reported tuberculosis in the United States 2006. Centers for Disease Control and Prevention, U. S. Department of Health and Human Services, Atlanta, GA.
- 6.Corbett, E. L., S. Charalambous, V. M. Moloi, K. Fielding, A. D. Grant, C. Dye, K. M. De Cock, R. J. Hayes, B. G. Williams, and G. J. Churchyard. 2004. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am. J. Resp. Crit. Care Med. 170:673-679. [DOI] [PubMed] [Google Scholar]
- 7.Gennaro, M. L., M. Affouf, G. V. Kanaujia, P. N. Brusasca, B. Mangura, and L. Reichman. 2007. Antibody markers of incident tuberculosis among HIV−infected adults in the U.S.A.: a historical prospective study. Int. J. Tuber. Lung Dis. 11:624-631. [PubMed] [Google Scholar]
- 8.Hendrickson, R. C., J. F. Douglass, L. D. Reynolds, P. D. McNeill, D. Carter, S. G. Reed, and R. L. Houghton. 2000. Mass spectrometric identification of mtb81, a novel serological marker for tuberculosis. J. Clin. Microbiol. 38:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoff, S. T., M. Abebe, P. Ravn, N. Range, W. Malenganisho, D. S. Rodriques, E. G. Kallas, C. Soborg, T. Mark Doherty, P. Andersen, and K. Weldingh. 2007. Evaluation of Mycobacterium tuberculosis-specific antibody responses in populations with different levels of exposure from Tanzania, Ethiopia, Brazil, and Denmark. Clin. Infect. Dis. 45:575-582. [DOI] [PubMed] [Google Scholar]
- 10.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain, R., N. Talat, F. Shahid, and G. Dawood. 2007. Longitudinal tracking of cytokines after acute exposure to tuberculosis: association of distinct cytokine patterns with protection and disease development. Clin. Vaccine Immunol. 14:1578-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinhikar, A. G., D. Vargas, H. Li, S. B. Mahaffey, L. Hinds, J. T. Belisle, and S. Laal. 2006. Mycobacterium tuberculosis malate synthase is a laminin-binding adhesin. Mol. Microbiol. 60:999-1013. [DOI] [PubMed] [Google Scholar]
- 13.Lane, H. C., H. Masur, L. C. Edgar, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 14.Lijmer, J. G., B. W. Mol, S. Heisterkamp, G. J. Bonsel, M. H. Prins, J. H. van der Meulen, and P. M. Bossuyt. 1999. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 282:1061-1066. [DOI] [PubMed] [Google Scholar]
- 15.Menzies, D., M. Pai, and G. Comstock. 2007. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann. Intern. Med. 146:340-354. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee, S., N. Daifalla, Y. Zhang, J. Douglass, L. Brooks, T. Vedvick, R. Houghton, S. G. Reed, and A. Campos-Neto. 2004. Potential serological use of a recombinant protein that is a replica of a Mycobacterium tuberculosis protein found in the urine of infected mice. Clin. Diagn. Lab. Immunol. 11:280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutapi, F., T. Mduluza, N. Gomez-Escobar, W. F. Gregory, C. Fernandez, N. Midzi, and R. M. Maizels. 2006. Immuno-epidemiology of human Schistosoma haematobium infection: preferential IgG3 antibody responsiveness to a recombinant antigen dependent on age and parasite burden. BMC Infect. Dis. 6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.New York City Department of Health and Mental Hygiene. 2008. 2006 Annual TB Summary. New York City Department of Health and Mental Hygiene, Bureau of Tuberculosis Control, NY.
- 19.O'Brien, R. J., and E. A. Talbot. 2003. The utility of an antibiotic trial for diagnosis of AFB-negative tuberculosis. Int. J. Tuber. Lung Dis. 7:198. [PubMed] [Google Scholar]
- 20.Perkins, M. D., and J. Cunningham. 2007. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J. Infect. Dis. 196(Suppl. 1):S15-S27. [DOI] [PubMed] [Google Scholar]
- 21.Ramalingam, B., K. R. Uma Devi, and A. Raja. 2003. Isotype-specific anti-38 and 27 kDa (mpt 51) response in pulmonary tuberculosis with human immunodeficiency virus coinfection. Scand. J. Infect. Dis. 35:234-239. [DOI] [PubMed] [Google Scholar]
- 22.Rosenkrands, I., C. Aagaard, K. Weldingh, I. Brock, M. H. Dziegiel, M. Singh, S. Hoff, P. Ravn, and P. Andersen. 2008. Identification of Rv0222 from RD4 as a novel serodiagnostic target for tuberculosis. Tuberculosis (Edinburg) 88:335-343. [DOI] [PubMed] [Google Scholar]
- 23.Rutjes, A. W., J. B. Reitsma, M. Di Nisio, N. Smidt, J. C. van Rijn, and P. M. Bossuyt. 2006. Evidence of bias and variation in diagnostic accuracy studies. CMAJ 174:469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samanich, K., J. T. Belisle, and S. Laal. 2001. Homogeneity of antibody responses in tuberculosis patients. Infect. Immun. 69:4600-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartain, M. J., R. A. Slayden, K. K. Singh, S. Laal, and J. T. Belisle. 2006. Disease state differentiation and identification of tuberculosis biomarkers via native antigen array profiling. Mol. Cell Proteomics 5:2102-2113. [DOI] [PubMed] [Google Scholar]
- 26.Singh, K. K., Y. Dong, J. T. Belisle, J. Harder, V. K. Arora, and S. Laal. 2005. Antigens of Mycobacterium tuberculosis recognized by antibodies during incipient, subclinical tuberculosis. Clin. Diagn. Lab. Immunol. 12:354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh, K. K., Y. Dong, L. Hinds, M. A. Keen, J. T. Belisle, S. Zolla-Pazner, J. M. Achkar, A. J. Nadas, V. K. Arora, and S. Laal. 2003. Combined use of serum and urinary antibody for diagnosis of tuberculosis. J. Infect. Dis. 188:371-377. [DOI] [PubMed] [Google Scholar]
- 28.Singh, K. K., Y. Dong, S. A. Patibandla, D. N. McMurray, V. K. Arora, and S. Laal. 2005. Immunogenicity of the Mycobacterium tuberculosis PPE55 (Rv3347c) protein during incipient and clinical tuberculosis. Infect. Immun. 73:5004-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steingart, K. R., N. Dendukuri, M. Henry, I. Schiller, P. Nahid, P. C. Hopewell, A. Ramsay, M. Pai, and S. Laal. 2009. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16:260-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steingart, K. R., M. Henry, S. Laal, P. C. Hopewell, A. Ramsay, D. Menzies, J. Cunningham, K. Weldingh, and M. Pai. 2007. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 4:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talat, N., F. Shahid, G. Dawood, and R. Hussain. 2009. Dynamic changes in biomarker profiles associated with clinical and subclinical tuberculosis in a high transmission setting: a four-year follow-up study. Scandinavian J. Immunol. 69:537-546. [DOI] [PubMed] [Google Scholar]
- 32.Talati, N. J., U. Seybold, B. Humphrey, A. Aina, J. Tapia, P. Weinfurter, R. Albalak, and H. M. Blumberg. 2009. Poor concordance between interferon-gamma release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect. Dis. 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka, S., and T. Suzuki. 1990. Anti-Treponema pallidum IgM, IgA, and IgG subclass antibody responses after treatment in patients with syphilis at various stages: 1. Assessments by enzyme-linked immunosorbent assay. Genitourin Med. 66:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor, Z., C. M. Nolan, and H. M. Blumberg. 2005. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm. Rep. 54:1-81. [PubMed] [Google Scholar]
- 35.Toossi, Z. 2003. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J. Infect. Dis. 188:1146-1155. [DOI] [PubMed] [Google Scholar]
- 36.Wallace, R. J., Jr., J. Glassroth, D. Griffith, K. N. Olivier, J. L. Cook, and F. Gordin. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed] [Google Scholar]
- 37.Wanchu, A., Y. Dong, S. Sethi, V. P. Myneedu, A. Nadas, Z. Liu, J. Belisle, and S. Laal. 2008. Biomarkers for clinical and incipient tuberculosis: performance in a TB-endemic country. PLoS One 3:e2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. 2006, posting date. The Stop TB strategy. World Health Organization, Geneva, Switzerland.
- 39.Wilson, R. A., W. N. Maughan, L. Kremer, G. S. Besra, and K. Futterer. 2004. The structure of Mycobacterium tuberculosis MPT51 (FbpC1) defines a new family of non-catalytic alpha/beta hydrolases. J. Mol. Biol. 335:519-530. [DOI] [PubMed] [Google Scholar]