Abstract
The most important animal reservoirs of Schistosoma japonicum in China are bovines. Diagnosis and control of bovine schistosomiasis is critical for reducing the prevalence of the disease. We screened defined diagnostic antigens that have the potential to increase the sensitivity and specificity of serological assays and to distinguish between active and prior infections. Five recombinant proteins with the potential to be diagnostic antigens were compared to the native soluble egg antigen preparation by enzyme-linked immunosorbent assay (ELISA). We evaluated the potentials of the recombinant proteins for discriminating active from prior infections, as well as the therapeutic efficacy of the established ELISA technique.
Schistosomiasis has a major public health impact in countries where it is endemic. It affects about 200 million people worldwide, causing more than 200,000 deaths per year (1). In China, approximately 0.51 million people are currently infected, and more than 65 million people are considered to be at risk of being infected with Schistosoma japonicum (10). Both the prevalence and the severity of the disease have decreased significantly after decades of successful national schistosomiasis control programs, but the disease is far from eradicated (14, 17, 20, 22). Schistosomiasis is particularly difficult to control because it is a zoonosis. The pathogen, Schistosoma japonicum, infects not only humans, but also more than 40 different wild and domestic animals (8). In China, the most important animal reservoirs are bovine, including cattle, and particularly water buffalo (3, 8, 16, 22). Thus, the control of bovine schistosomiasis is critical for reducing disease prevalence in China.
Schistosomiasis diagnosis is essential for epidemiology surveys and for evaluating the efficacy of control programs. Currently, definitive diagnosis of schistosomiasis still relies on the detection of viable ova in feces or histological samples (4, 9). However, direct parasitological diagnosis techniques are labor-intensive and time-consuming and have become relatively insensitive because of widespread chemotherapy with praziquantel, which lowers the worm burden in the host.
Immunodiagnostic techniques are rapid, sensitive, convenient, and easily applied, making them superior to parasitology techniques. Since the early 1980s, immunodiagnosis has been used to estimate infection rates and has been integrated into the Chinese control program with the goal of improving the diagnostic record in epidemiological surveys and identifying individuals to target for treatment. Nonetheless, cross-reaction is frequently a problem in immunodiagnostic assays, largely because of the use of crude antigens that are either intact material from the parasite or a soluble extract of the parasite or eggs, both of which contain many antigens that might be shared with unrelated pathogens (13, 19). In addition, antibody-based serological assays do not discriminate between active and prior infections and cannot be used to evaluate therapeutic efficacy, because eggs can persist in the liver and gut and continue to stimulate the immune response, even after cure (21). Native soluble egg antigen (SEA), which leads to the cross-reactivity problem described above, is commonly used as the antigen for diagnosing schistosomiasis in domestic animals. For this reason, a defined diagnostic antigen that might increase the sensitivity and specificity of a serological assay and potentially distinguish active from prior infections is needed. In this study, we evaluated the sensitivities of five recombinant proteins from S. japonicum for use as diagnostic antigens for detecting bovine and rabbit schistosomiasis. The proteins were compared to the SEA by enzyme-linked immunosorbent assay (ELISA) and investigated for use in potential applications that discriminate between active and prior infections and for evaluating therapeutic efficacy using the established ELISA technique.
MATERIALS AND METHODS
Parasites and animals. (i) Parasites.
The freshly shed cercariae of S. japonicum (Chinese strain) were obtained by exposing infected snails to light. The numbers and viability of cercariae were determined prior to infection using a light microscope. Adult worms were collected by perfusion of New Zealand rabbits 6 weeks postinfection. Schistosome eggs were separated from the liver tissues of infected rabbits as described previously by Doenhoff et al. (6).
(ii) Animals.
Male New Zealand rabbits (6 weeks old; 2.0 to 2.5 kg) were from the Shanghai Experimental Animal Center, Chinese Academy of Sciences.
Antigen preparation.
Specific primer sets containing suitable restriction enzyme sites (Table 1) were designed to amplify and fuse a desired segment against the cDNA sequence of each protein or peptide. PCRs were performed using the cDNA of Schistosoma japonicum as templates. PCR products were purified from agarose gel electrophoresis and fused properly with the 26-kDa glutathione-S-transferase (GST) of expression vector pGEX-4T-2 (Pharmacia) and sequenced to confirm its identity. Recombinant proteins with molecular weights as specified in Table 2 were expressed in Escherichia coli BL21(DE3). Induction was done with 1 mmol/liter isopropyl-d-galactopyranoside (IPTG) for 6 h at 37°C with agitation (200 rpm). Bacterial cells were centrifuged at 4,000 × g for 10 min. The pellet was resuspended in 10 ml phosphate-buffered saline (PBS), pH 7.2, containing 1% Triton X-100 and frozen and thawed three times. The lysate was submitted to 10 cycles of 30 s of sonication on ice to release the target protein. Fusion proteins were purified by glutathione-agarose affinity chromatography in a single step (GST Bind fractogel; Novagen). The purities and concentrations of purified recombinant proteins were determined by one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and UV spectroscopy. Preparation of SEA was as described by Doenhoff et al. (6).
TABLE 1.
Primer information for 6 recombinant antigens
| Recombinant antigen | Primer | Sequencea | Enzyme site |
|---|---|---|---|
| rSjGcp-Sj23-Sj28 | SjGCP upper primer | 5′-AATAGGATCCATGCGAATTGGATATG-3′ | BamHI |
| SjGCP lower primer | 5′-GCGCTCTAGAATAATAATCAACATCTTCAG-3′ | XhaI | |
| Sj23 upper primer | 5′-AATATCTAGAATGACTGGTGCTCTGGA-3′ | XbaI | |
| Sj23 lower primer | 5′-CGCGGAATTCGTTGCGTTTTAAG-3′ | EcoRI | |
| Sj28 upper primer | 5′-CGGCGAATTCAAGCCACCAGAAGAA-3′ | EcoRI | |
| Sj28 lower primer | 5′-ATTAAAGCTTCTATCCGACAGTCGGATTA-3′ | HindIII | |
| rSjGcp-Sj23 | SjGCP upper primer | 5′-AATAGGATCCATGCGAATTGGATATG-3′ | BamHI |
| SjGCP lower primer | 5′-GCGCTCTAGAATAATAATCAACATCTTCAG-3′ | XhaI | |
| Sj23 upper primer | 5′-AATATCTAGAATGACTGGTGCTCTGGA-3′ | XbaI | |
| Sj23 lower primer | 5′-CGCGAAGCTTTCAGTTGCGTTTTAAG-3′ | HindIII | |
| rSj23-LHD | Upper primer | 5′-AATAGAATTCATGACTGGTGCTCTGGA-3′ | EcoRI |
| Lower primer | 5′-CGCGGAATTCGTTGCGTTTTAAG-3′ | EcoRI | |
| rSjTPX1 | Upper primer | 5′-GGGATCCATGGTACTGATTCCA-3′ | BamHI |
| Lower primer | 5′-GCTCGAGTCAGTGATTCACTT-3′ | XholI | |
| rSjEF1 | Upper primer | 5′-GGGATCCATGCCTTCTGACAA-3′ | BamHI |
| Lower primer | 5′-GCTCGAGTTACTTCTTCTTCGC-3′ | XholI |
The sequences of enzyme sites are underlined.
TABLE 2.
Schistosoma japonicum recombinant antigens
| Recombinant antigen | Molecular mass (kDa) | Peptide (aa)a | Stage expressed | Reference |
|---|---|---|---|---|
| rSjGcp-Sj23-Sj28 | 43.4 | AAN39279 (539-590) | Somula and male adult | 2 |
| AAA29920 (120-183) | All stages | 5 | ||
| AAA29892 (115-153) | All stages | 18 | ||
| rSjGcp-Sj23 | 39.1 | AAN39279 (539-590) | Somula and male adult | 2 |
| AAA29920 (120-183) | All stages | 5 | ||
| rSj23-LHD | 23.0 | AAA29920 (120-183) | All stages | 5 |
| rSjTPX1 | 71 | BAD01572 (1-184) | Somula and male adult | 12 |
| rSjEF1 | 140 | AAQ16109 (1-465) | Somula and male adult | Unpublished |
aa, amino acids.
Serum samples.
Rabbits were infected with 300 cercariae of S. japonicum and treated with praziquantel (80 mg/kg of body weight; Nanjing Pharmaceutical Factory, China) at 6 weeks after infection. Sera were collected before infection and at 2, 4, 6, 8, 10, and 30 weeks post-cercaria challenge. Sera from 189 schistosomiasis-positive cattle were obtained from the local veterinary office of Yunnan Province, and infection was confirmed by the parasitological diagnosis method (9). As controls, 92 healthy cattle serum samples were obtained from Henan Province, where schistosomiasis is not endemic. In addition, serum samples from 12 Fasciola gigantica-infected bovines and 12 Trypanosoma evansi-infected rabbits were obtained. All sera were collected and stored at −80°C in our laboratory.
ELISA.
Polystyrene microtiter plates (Costar) were coated overnight at 4°C with 100 μl per well of 10 μg/ml recombinant antigen or SEA diluted with carbonate buffer (pH 9.6). Serum at a dilution of 1:100 was added to the wells and incubated for 1 h at 37°C. Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) or horseradish peroxidase-conjugated rabbit anti-bovine immunoglobulin G (Sigma, St. Louis, MO) was added at a dilution of 1:15,000. The wells were washed three times with 200 μl of PBS-Tween 20 per well. After incubation for 1 h at 37°C, a substrate solution of orthophenylene diamine plus H2O2 was added, and the reaction was stopped after 10 min with 2N H2SO4. The plates were read at 490 nm with an ELISA reader (Bio-Rad). This test was performed on all of the sera described above.
Statistics.
The cutoff optical density (OD) value was established from the mean plus 3 standard deviations of the tested sera.
RESULTS
ELISA for S. japonicum with bovine sera.
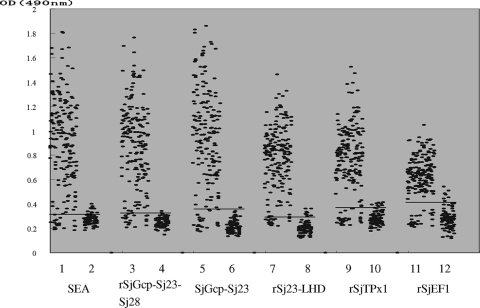
The ELISA results of 281 analyzed serum samples are presented in Fig. 1. Samples with confirmed schistosomiasis showed higher mean OD values, while samples of healthy sera from areas where schistosomiasis is not endemic showed lower mean OD values (Table 3). A receiver operating characteristic curve established from these results defined sensitivities and specificities at different cutoff OD values. pGEX-SjGcp-Sj23 had the highest positive predictive value at 91% and the best negative predictive value at 98% (Table 3). None of the sera from the controls with F. hepatica or T. evansi infections presented ODs higher than the cutoff values (data not shown).
FIG. 1.
Values of OD at 490 nm determined by the 6 different antigens in ELISA with sera from bovines included in this study. The sera from 189 schistosomiasis-positive bovines diagnosed with schistosomiasis by the detection of eggs and 92 healthy bovines from Henan Province were tested by SEA and 5 recombinant protein antigens, rSjGcp-Sj23-Sj28, rSjGcp-Sj23, rSj23-LHD, rSjTPx1, and rSjEF1. The horizontal lines represent the OD cutoff values.
TABLE 3.
ELISA analysis data
| Antigen | Cutoff value (OD at 490 nm) | Mean OD ± SD for group: |
Sensitivity (%) | Specificity (%) | Correlation with SEA (r) | |
|---|---|---|---|---|---|---|
| With confirmed schistosomiasis | From area without endemicity | |||||
| SEA | 0.325 | 0.8876 ± 0.379 | 0.271 ± 0.041 | 89.9 | 91.2 | |
| rSjGcp-Sj23-Sj28 | 0.355 | 0.8566 ± 0.352 | 0.249 ± 0.038 | 89.9 | 93.4 | 0.9262 |
| rSjGcp-Sj23 | 0.3326 | 0.9258 ± 0.417 | 0.214 ± 0.049 | 91.0 | 97.8 | 0.9363 |
| rSj23-LHD | 0.309 | 0.7704 ± 0.265 | 0.205 ± 0.053 | 89.9 | 91.2 | 0.6411 |
| rSjTPX1 | 0.382 | 0.7642 ± 0.283 | 0.286 ± 0.054 | 84.0 | 89.0 | 0.642 |
| rSjEF1 | 0.416 | 0.6135 ± 0.184 | 0.286 ± 0.086 | 83.5 | 89.0 | 0.4885 |
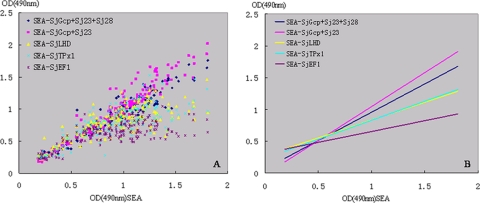
For the sera from confirmed schistosomiasis cases, we also determined the correlation between the ELISA OD values for SEA and for the recombinant antigens (Fig. 2), with the highest correlation seen between the SEA and the recombinant antigen SjGcp-Sj23 (r = 0.9363) (Table 3). For the healthy group, the values for SEA ELISA and recombinant antigen ELISA showed weak correlation (data not shown).
FIG. 2.
Correlations (A) and correlation trend lines (B) among ELISA OD values of sera analyzed with Schistosoma japonicum SEA and five Schistosoma japonicum recombinant antigens using 189 sera from schistosomiasis-positive bovines.
ELISA to detect infected and treated samples.
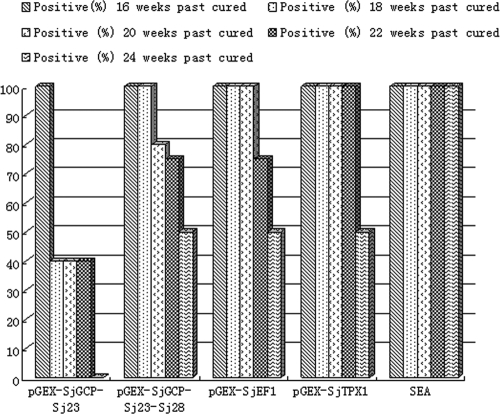
Rabbits were infected with 300 cercariae of S. japonicum and treated with praziquantel (80 mg/kg of body weight) at 6 weeks after infection and bled every 2 weeks after infection. Schistosomiasis was tested for at different time points by ELISA with either the five recombinant antigens or SEA as the diagnostic antigen. The results are presented as OD profiles in Fig. 2. Specific IgG antibodies against the six antigens were detected at 4 weeks after infection, with decreasing intensities in the order rSjGcp-Sj23 > rSjGcp-Sj23-Sj28 > SEA > rSjTPX1 > rSj23-LHD > rSjEF1. The ELISA results, measured as OD, were reduced to untreated levels at 4 weeks after treatment, with the OD value shown by rSjGcp-Sj23 clearly reduced (Fig. 3). At 24 weeks after treatment (30 weeks after infection), all live rabbits were schistosomiasis negative by tests with antigen rSjGcp-Sj23 but were positive by tests with SEA (Fig. 4).
FIG. 3.
Antibody profiles as OD values using six antigens. Error bars represent standard deviations.
FIG. 4.
Percentages of rabbits determined as positive after treatment, as tested with six antigens.
DISCUSSION
Although several immunodiagnostic techniques have been applied in the Chinese schistosomiasis control program, the available immunodiagnostic assays must be improved before they can offer a valid alternative to parasitological diagnosis. So far, no immunodiagnostic assays have provided a “gold standard” for determining prevalence, identifying infected individuals for selective population chemotherapy, or assessing the effectiveness of intervention, including chemotherapy follow-up. However, the available antigen-based serological assays that detect schistosome-specific circulating molecules are also not sufficiently sensitive for chronic cases, especially those with a low intensity of infection (7, 15). For these reasons, we must find ways to overcome the obstacles to developing immunodiagnostic techniques.
Pure or single-molecule antigenic reagents may improve the specificity of immunodiagnostic tests. Since the 1980s, several purified antigens have been fractionated from schistosome eggs or worms by biochemical or immunochemical methods and tested as diagnostics, including MSA1, isolated by affinity chromatography from SEA; TCA-S-A, a glycoprotein biochemically purified from adult worms; and a 31/32-kDa diagnostic antigen isolated from adult worms by starch electrophoresis (9). The application of these purified antigenic preparations did not appear to have an advantage over the crude antigens in terms of sensitivity, but some improved the diagnostic specificity of the existing methods.
Another issue is the production of sufficient quantities of defined antigens for large-scale testing. The development of genetic-engineering techniques has allowed the production of defined recombinant antigens (e.g., surface protein and stage-differentially expressed protein) in quantities sufficient for diagnostics. Recombinant DNA technology can also be used to construct multiepitope antigens that can be used to improve assay sensitivity and specificity. Several fusion proteins, such as LHD-Sm23, a large hydrophobic domain of the 23-kDa membrane protein of S. mansoni (5); 26-kDa GST; and 31/32-kDa antigen, have been used as diagnostic antigens for detecting human and domestic-animal schistosomiasis (11).
In this study, we compared schistosomiasis diagnosis with SEA to diagnosis with five fusion proteins, LHD-Sj23, a promising candidate diagnostic antigen; SjTPx1 and SjEF1, which are highly expressed schistosomulum and male adult worm proteins (12); and two multiepitope antigens, SjGcp-Sj23 and SjGcp-Sj23-Sj28 (SjGcps are highly expressed male adult worm proteins) (2). Using SEA, we detected 89.9% of the cases of confirmed schistosomiasis, giving a specificity of 91.2% for the 281 sera analyzed. For the multiepitope recombinant antigen pGEX-SjGcp-Sj23, we detected 91.0% of the confirmed schistosomiasis cases, for a specificity of 97.8%. Only two false positives occurred using rpGEX-SjGcp-Sj23.
We also tested rabbit sera by ELISA before and after treatment with praziquantel. The results suggested the multiepitope antigen rSjGCP-Sj23 was the best diagnostic antigen of the proteins tested. In summary, we conclude that the recombinant antigen rSjGCP-Sj23 compares positively to SEA and other recombinant antigens for use in the immunodiagnosis of schistosomiasis in cattle and that it is a good candidate diagnostic antigen for distinguishing active from prior infections.
Acknowledgments
This work was supported by the Chinese National Basic Research Program (no. 2007CB513108), the Chinese National Natural Science Foundation (no. 30671581 and no. 30800819), the Shanghai Pujing Program (no. 09PJ14118), and the Basic Scientific Research Operation Cost of State-Leveled Public Welfare Scientific Research Courtyard (no. 2006JB03).
Footnotes
Published ahead of print on 6 January 2010.
REFERENCES
- 1.Bergquist, N. R. 2002. Schistosomiasis: from risk assessment to control. Trends Parasitol. 18:309-314. [DOI] [PubMed] [Google Scholar]
- 2.Bostic, J. R., and M. Strand. 1996. Molecular cloning of a Schistosoma mansoni protein expressed in the gynecophoral canal of male worms. Mol. Biochem. Parasitol. 79:79-89. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, M., and F. Zheng. 1999. Schistosomiasis control in China. Parasitol. Int. 48:11-19. [DOI] [PubMed] [Google Scholar]
- 4.Corachan, M. 2002. Schistosomiasis and international travel. Clin. Infect. Dis. 35:446-450. [DOI] [PubMed] [Google Scholar]
- 5.Davern, K. M., M. D. Wright, V. R. Herrmann, and G. F. Mitchell. 1991. Further characterisation of the Schistosoma japonicum protein Sj23, a target antigen of an immunodiagnostic monoclonal antibody. Mol. Biochem. Parasitol. 48:67-75. [DOI] [PubMed] [Google Scholar]
- 6.Doenhoff, M. J., A. E. Butterworth, R. J. Hayes, R. F. Sturrock, J. H. Ouma, D. Koech, M. Prentice, and J. Bain. 1993. Seroepidemiology and serodiagnosis of schistosomiasis in Kenya using crude and purified egg antigens of Schistosomia mansoni in ELISA. Trans. R. Soc. Trop. Med. Hyg. 87:42-48. [DOI] [PubMed] [Google Scholar]
- 7.Doenhoff, M. J., J. G. Wheeler, K. Tricker, J. V. Hamilton, R. F. Sturrock, A. E. Butterworth, J. H. Ouma, G. G. Mbugua, C. Kariuki, and D. Koech. 2003. The detection of antibodies against Schistosoma mansoni soluble egg antigens (SEA) and CEF6 in ELISA, before and after chemotherapy. Ann. Trop. Med. Parasitol. 97:697-709. [DOI] [PubMed] [Google Scholar]
- 8.Gray, D. J., G. M. Williams, Y. Li, H. Chen, S. J. Forsyth, R. S. Li, A. G. Barnett, J. Guo, A. G. Ross, Z. Feng, and D. P. McManus. 2009. A cluster-randomised intervention trail against Schistosoma japonicum in the People's Republic of China: bovine and human transmission. PloS. ONE 4:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton, J. V., M. Klinkert, and M. J. Doenhoff. 1998. Diagnosis of schistosomiasis: antibody detection, with notes on parasitological and antigen detection methods. Parasitol. 117(Suppl.):S41-S57. [DOI] [PubMed] [Google Scholar]
- 10.Hao, Y., X. Wu, H. Zheng, L. Wang, J. Guo, and G. Xia. 2008. Schistosomiasis situation in People's Repulic of China in 2007. Chin. J. Schistosomiasis Control 20:401-404. (In Chinese.) [Google Scholar]
- 11.Klinkert, M. Q., K. Bommert, D. Moser, R. Felleisen, G. Link, O. Doumbo, and E. Beck. 1991. Immunological analysis of cloned Schistosoma mansoni antigens Sm31 and Sm32 with sera of schistosomiasis patients. Trop. Med. Parasitol. 42:319-324. [PubMed] [Google Scholar]
- 12.Kumagai, T., Y. Osada, and T. Kanazawa. 2006. 2-Cys peroxiredoxins from Schistosoma japonicum: the expression profile and localization in the life cycle. Mol. Biochem. Parasitol. 149:135-143. [DOI] [PubMed] [Google Scholar]
- 13.Maddison, S. E., S. B. Slemenda, V. C. Tsang, and R. A. Pollard. 1985. Serodiagnosis of Schistosoma mansoni with microsomal adult worm antigen in an enzyme-linked immunosorbent assay using a standard curve developed with a reference serum pool. Am. J. Trop. Med. Hyg. 34:484-494. [DOI] [PubMed] [Google Scholar]
- 14.Mao, S. B. 1986. Recent progress in the control of schistosomiasis in China. Clin. Med. J. 99:439-443. [PubMed] [Google Scholar]
- 15.Mott, K. E., H. Dixon, C. E. Carter, E. Garcia, A. Ishii, H. Matsuda, G. Mitchell, M. Owhashi, H. Tanaka, and V. C. Tsang. 1987. Collaborative study on antigens for immunodiagnosis of schistosomiasis japonicum infection. Bull. World Health Organ. 65:233-244. [PMC free article] [PubMed] [Google Scholar]
- 16.Ross, A. G., A. C. Sleigh, Y. Li, G. M. Davis, G. M. Williams, Z. Jiang, Z. Feng, and D. P. McManus. 2001. Schistosomiasis in the People's Republic of China: prospects and challenges for the 21st century. Clin. Microbiol. Rev. 14:270-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaoji, Z., and L. Dandan. 2002. The potential risk and control strategy in low endemic area of schistosomiasis in China. Acta Trop. 82:289-293. [DOI] [PubMed] [Google Scholar]
- 18.Trottein, F., C. Godin, R. J. Pierce, B. Sellin, M. G. Taylor, I. Gorillot, M. S. Silva, J. P. Lecocq, and A. Capron. 1992. Inter-species variation of schistosome 28-kDa glutathione S-transferases. Mol. Biochem. Parasitol. 54:63-72. [DOI] [PubMed] [Google Scholar]
- 19.Tsang, V. C., K. Hancock, S. E. Maddison, A. L. Beatty, and D. M. Moss. 1984. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA). J. Immunol. 132:2607-2613. [PubMed] [Google Scholar]
- 20.Utzinger, J., X. N. Zhou, M. G. Chen, and R. Bergquist. 2005. Conquering schistosomiasis in China: the long march. Acta Trop. 96:69-96. [DOI] [PubMed] [Google Scholar]
- 21.Van Dam, G. J., J. H. Wichers, T. M. Ferreira, D. Ghati, A. van Amerongen, and A. M. Deelder. 2004. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J. Clin. Microbiol. 42:5458-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou, X. N., L. Y. Wang, M. G. Chen, X. H. Wu, Q. W. Jiang, X. Y. Chen, J. Zheng, and J. Utzinger. 2005. The public health significance and control of schistosomiasis in China—then and now. Acta Trop. 96:97-105. [DOI] [PubMed] [Google Scholar]