Abstract
Protective immunity against dengue virus (DENV) is best reflected by the presence of neutralizing antibodies. The conventional plaque reduction neutralizing test (PRNT) is performed using Fcγ receptor (FcγR)-negative cells. Because FcγR plays a key role in antibody-dependent enhancement, we examined neutralizing antibody titers of mouse monoclonal antibodies and human serum samples in PRNTs using FcγRIIA-negative and FcγRIIA-expressing BHK cells. There was a discrepancy in dengue virus neutralizing antibody titers between PRNTs using FcγRIIA-negative versus FcγRIIA-expressing BHK cells. Neutralizing antibody titers to DENV-1 and DENV-2 tested with monoclonal antibodies, and with most of the human serum samples, were higher in assays using BHK cells than those using FcγRIIA-expressing BHK cells. The results suggest that neutralizing antibody titers determined using FcγRIIA-expressing cells may better reflect the protective capacity of anti-DENV antibodies, as the major target cells of DENV infection are FcγR-positive cells.
Dengue virus (DENV), a member of the family Flaviviridae, represents a major health problem in tropical and subtropical regions of the world. There are four serotypes, dengue virus types 1 to 4 (DENV-1 to DENV-4). DENV causes a wide range of symptoms, from mild febrile illness known as dengue fever (DF) to severe life-threatening illness, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). Infection with one serotype induces life-long protection against homologous serotypes, but protection against other serotypes is short-lived. In secondary infection, cross-reactive, nonneutralizing antibodies bind to DENV. DENV-antibody complexes are taken up more efficiently by Fcγ receptor (FcγR)-expressing cells, and higher levels of viremia develop (5, 7, 10, 12, 15, 16). This phenomenon, known as antibody-dependent enhancement (ADE), is considered to be a risk factor for DHF and DSS.
Protective immunity against DENV is best reflected by the presence of neutralizing antibody. High neutralizing antibody levels induced by primary infection are considered central in offering life-long protective immunity against the homologous serotypes. Thus, a vaccine against DENV infection is expected to induce high levels of neutralizing antibodies against all four serotypes. The plaque reduction neutralizing test (PRNT) is a widely accepted approach to measure the neutralizing activities of antibodies (14). PRNTs, which employ Vero, LLC-MK2, or BHK-21 cells (11, 14) are, however, limited to measuring neutralizing activities of viral infectivity in the absence of FcγR (1). It is possible that neutralizing antibody titers of anti-DENV antibodies induced by natural infection or by vaccines may differ when assayed in the presence of enhancing activity. The neutralizing antibody titers determined using FcγR-expressing BHK-21 cells may better reflect protective immunity, because the principal target cells of DENV are FcγR-expressing cells, such as monocytes (6). In the present study, we sought to determine if neutralizing antibody titers were at the same or different levels when BHK-21 cells and cell lines expressing FcγR were used as the assay cells.
MATERIALS AND METHODS
Cell lines.
BHK-21 cells, a hamster kidney cell line, and Vero cells (Vero 9013; Japan Health Science Research Resources Bank), which are an African green monkey kidney-derived epithelial cell line, were used. Two stable BHK-21 cell lines were established previously that express FcγRIIA (BHK-FcγRIIA/2 and BHK-FcγRIIA/4) and were used in this study (9). BHK-21 and Vero cells were cultured in Eagle's minimum essential medium (EMEM; Sigma, St. Louis, MO) supplemented with heat-inactivated 10% fetal calf serum (FCS; Sigma) without antibiotics at 37°C in 5% CO2. BHK-FcγRIIA/2 and BHK-FcγRIIA/4 cell lines were cultured in EMEM (Sigma) supplemented with heat-inactivated 10% FCS (Sigma) and 0.5 mg/ml neomycin (G418; PAA Laboratories GmbH, Austria) at 37°C in 5% CO2.
Virus.
DENV-1 strain 01-44-1HuNIID (GenBank accession number AB111070), DENV-2 strain D2/Hu/OPD030NIID/2005 (GenBank accession number AB219135), DENV-3 strain CH53962, and DENV-4 strain TVP-360 were used (see Table 1, below) (4, 9, 14). DENV-1 (01-44-1HuNIID) and DENV-2 (D2/Hu/OPD030NIID/2005) were isolated from imported dengue patients and established as laboratory strains for plaque reduction neutralizing assay at the Department of Virology I, National Institute of Infectious Diseases, Tokyo, Japan (4, 9). DENV-3 (CH53962 strain) and DENV-4 (TVP-360 strain) are World Health Organization laboratory strains and were supplied by the Walter Reed Army Institute of Research, Washington, DC (9, 14).
TABLE 1.
Neutralizing titers of anti-DENV monoclonal antibodies determined in assays using BHK-21, BHK-FcγRIIA/2, and BHK-FcγRIIA/4 cells
| Monoclonal antibody | Challenge virusa | Neutralizing titer (PRNT50)b in: |
||
|---|---|---|---|---|
| BHK-21 | BHK- FcγRIIA/2 | BHK- FcγRIIA/4 | ||
| 4G2 | DENV-1 | 320 | <10 | <10 |
| DENV-2 | 160 | <10 | <10 | |
| DENV-3 | 80 | 10 | 10 | |
| DENV-4 | 40 | <10 | <10 | |
| 3H5 | DENV-1 | <10 | <10 | <10 |
| DENV-2 | 40 | <10 | <10 | |
| DENV-3 | <10 | <10 | <10 | |
| DENV-4 | <10 | <10 | <10 | |
DENV-1 01-44-1HuNIID strain, DENV-2 D2/Hu/OPD030NIID/2005 strain, DENV-3 CH53962 strain, and DENV-4 TVP-360 strain were used as the challenge virus.
The PRNT50 was determined as described in Materials and Methods.
Monoclonal antibodies.
Flavivirus-cross-reactive mouse monoclonal IgG2a antibody (MAb HB-112 D1-4G2-4-15) and DENV-2 serotype-specific mouse monoclonal IgG1 antibody (MAb HB-46 3H5-1) (3) used in the assays were purchased from the American Type Culture Collection (Manassas, VA).
Serum specimens.
The serum samples were collected for laboratory diagnostic purposes from dengue patients from 2004 to 2009. Dengue virus infection was confirmed by positive type-specific real-time reverse transcriptase PCR (RT-PCR) and anti-DENV antibody based on IgG enzyme-linked immunosorbent assay (ELISA) and IgM ELISA in our laboratory (4). Serum specimens 1 to 18 were heat inactivated at 56°C for 30 min and used in the experiments. Serum samples 1 and 3 and samples 2 and 4 were paired serum samples obtained from two cases of primary DENV infections. Serum samples designated as early samples (1, 2, 7, 8, 9, and 10) were collected 1 to 3 days after the onset of the disease. Serum samples designated as late-phase samples (3, 4, 5, 6, 11, 12, 13, and 14) were obtained 6 to 14 days after the onset of the disease. The virus was isolated from primary cases 1 and 2 and secondary cases 7, 8, 9, and 10 previously (see Tables 2 and 3, below). Serum samples 15 to 18 were obtained from non-DF patients.
TABLE 2.
Neutralizing antibody titers of human serum samples against DENV-1 as determined using BHK, BHK-FcγRIIA/2, and BHK-FcγRIIA/4 cells
| Sample source | Disease phase | Serum ID no. | DENV typea | Neutralizing titer (PRNT50) to DENV-1 in: |
||
|---|---|---|---|---|---|---|
| BHK-21 | BHK- FcγRIIA/2b | BHK- FcγRIIA/4c | ||||
| Primary DENV infection | Earlyd | 1f | 1 | <5 | <5 | <5 |
| 2g | 2 | <5 | <5 | <5 | ||
| Latee | 3f | 1 | 160 | 160 | 160 | |
| 4g | 2 | <20j | <20j | <20j | ||
| 5h | 3 | 10 | <5 | <5 | ||
| 6h | 4 | 320 | 10 | 10 | ||
| Secondary DENV infection | Earlyd | 7h | 1i | 10 | <5 | <5 |
| 8h | 1i | <5 | <5 | <5 | ||
| 9h | 2i | 10 | <5 | <5 | ||
| 10h | 3i | 320 | 160 | 160 | ||
| Latee | 11h | 1i | 320 | 40 | 40 | |
| 12h | 2i | 320 | 40 | 40 | ||
| 13h | 3i | 160 | <5 | <5 | ||
| 14h | 4i | 640 | 10 | 10 | ||
| Non-DENV patient | DENV IgG negative | 15 | <5 | <5 | <5 | |
| 16 | <5 | <5 | <5 | |||
| 17 | <5 | <5 | <5 | |||
| 18 | <5 | <5 | <5 | |||
DENV types that infected the patients. The types of dengue virus were determined by type-specific real-time RT-PCR.
FcγRIIA-expressing BHK cell line 2.
FcγRIIA-expressing BHK cell line 4.
Days 1 to 3 after onset of the disease.
Days 6 to 14 after onset of the disease.
Samples 1 and 3 were obtained from the same patient, infected with DENV-1.
Samples 2 and 4 were obtained from the same patient, infected with DENV-2.
Serum samples 5 to 14 were obtained from patients 5 to 14, respectively.
Dengue virus types of primary infection of patient numbers 7 to 14 were not determined. Dengue virus types in secondary infections are included in the table.
Serum was serially diluted 2-fold, starting from 1:20.
TABLE 3.
Neutralizing titers of human serum samples against DENV-2 as determined using BHK-21, BHK-FcγRIIA/2, and BHK-FcγRIIA/4 cells
| Sample source | Disease phase | Serum ID no. | DENV typea | Neutralizing titer (PRNT50) to DENV-1 in: |
||
|---|---|---|---|---|---|---|
| BHK-21 | BHK- FcγRIIA/2b | BHK- FcγRIIA/4c | ||||
| Primary DENV infection | Early phased | 1f | 1 | <5 | <5 | <5 |
| 2g | 2 | <5 | <5 | <5 | ||
| Late phasee | 3f | 1 | 160 | 10 | 10 | |
| 4g | 2 | 40 | 10 | 10 | ||
| 5h | 3 | 20 | <5 | <5 | ||
| 6h | 4 | 40 | <5 | <5 | ||
| Secondary DENV infection | Early phased | 7h | 1i | 5 | <5 | <5 |
| 8h | 1i | 10 | <5 | <5 | ||
| 9h | 2i | 40 | <5 | <5 | ||
| 10h | 3i | 20 | <5 | <5 | ||
| Late phasee | 11h | 1i | <5 | <5 | <5 | |
| 12h | 2i | 80 | 5 | 5 | ||
| 13h | 3i | 40 | <5 | <5 | ||
| 14h | 4i | 320 | <5 | <5 | ||
| Non-DENV patient | DENV- IgG negative | 15 | <5 | <5 | <5 | |
| 16 | <5 | <5 | <5 | |||
| 17 | <5 | <5 | <5 | |||
| 18 | <5 | <5 | <5 | |||
DENV types that infected the patients. The types of dengue virus were determined by type-specific real-time RT-PCR.
FcγRIIA-expressing BHK cell line 2.
FcγRIIA-expressing BHK cell line 4.
Days 1 to 3 after onset of the disease.
Days 6 to 14 after onset of the disease.
Samples 1 and 3 were obtained from the same patient, infected with DENV-1.
Samples 2 and 4 were obtained from the same patient, infected with DENV-2.
Serum samples 5 to 14 were obtained from patients 5 to 14, respectively.
Dengue virus types of primary infection in patients 7 to 14 were not determined. Dengue virus types in secondary infection are included in the table.
Plaque reduction neutralizing assays.
Mouse monoclonal antibody 4G2 (HB-112 D1-4G2-4-15; 1.3 mg/ml; ATCC) and mouse monoclonal antibody 3H5 (HB-46 3H5-1; 2.5 mg/ml; ATCC) were serially diluted 2-fold from 1:10 to 1:5,120 with EMEM supplemented with 10% FCS. Human serum samples were serially diluted 2-fold from 1:5 to 1:2,560 with EMEM supplemented with 10% FCS. The virus-antibody mixture was prepared by mixing 25 μl of DENV-1 or DENV-2 at a titer of 2.5 × 103 PFU/ml with 25 μl of serially diluted antibody or serum sample. Control virus samples were prepared by mixing 25 μl of DENV-1 or DENV-2 at titers of 2.5 × 103 PFU/ml with 25 μl of EMEM supplemented with 10% FCS. The virus-antibody mixture was incubated at 37°C for 1 h. Fifty microliters of virus-antibody mixture was inoculated onto BHK-21 monolayers in 12-well plates. The plates were incubated for 1 h at 37°C in 5% CO2. After virus absorption, the cells were washed with 1 ml of EMEM and overlaid with maintenance medium containing 1% methylcellulose (Wako Pure Chemical Industry, Osaka, Japan). The plates were incubated at 37°C in 5% CO2 for 5 days. After 5 days of incubation, the cells were fixed with 3.7% (vol/vol) formaldehyde for 1 h at room temperature and washed with water. The cells were then stained with methylene blue solution for 1 h at room temperature and washed with water. Plaques were counted with the naked eye. The neutralization titer was defined as the highest serum dilution that reduced the number of plaques by 50% (PRNT50) (17). In cases of no plaque reduction or an increase in the number of plaques, the percentage plaque reduction was expressed as 0%.
RESULTS
Neutralizing titers of mouse monoclonal antibodies 4G2 and 3H5 determined by assays using parent BHK-21 and FcγRIIA cell lines.
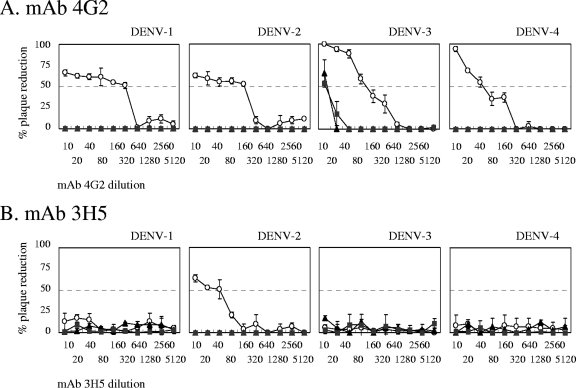
Neutralizing titers of MAbs 4G2 and 3H5 were examined against DENV-1, DENV-2, DENV-3, and DENV-4, using BHK-21 cells and two stable BHK-21 cell lines expressing FcγRIIA, BHK-FcγRIIA/2 and BHK-FcγRIIA/4 (Fig. 1 and 2). The MAb 4G2 demonstrated neutralizing titers of 1:320 to 1:40 to all four serotypes of DENV when parent BHK-21 cells were used. However, when BHK-FcγRIIA/2 and BHK-FcγRIIA/4 cells were employed, no neutralizing activity was detected for three of the four serotypes, and neutralizing titers as low as 10 were detected for DENV-3 (Table 1).
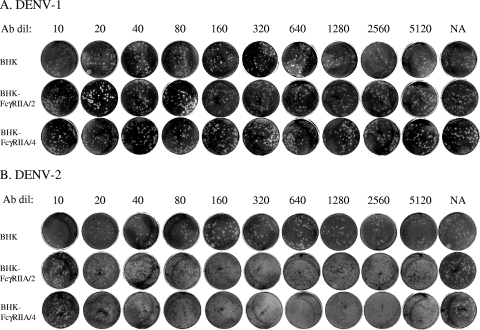
FIG. 1.
Plaque reduction neutralizing assays using BHK-21 cells and BHK-FcγRIIA cells. DENV-1 (A) and DENV-2 (B) were reacted with serially diluted mouse monoclonal 3H5 antibody in PRNT assays using BHK-21 and BHK-FcγRIIA cell lines in 12-well plates.
FIG. 2.
Patterns of plaque reduction against DENV in neutralization assays. (A) 4G2 MAb; (B) 3H5 MAb. ○, untransfected BHK-21 cells; ▴, BHK-FcγRIIA/2 cells; ▪, BHK-FcγRIIA/4 cells. Each curve is the mean of duplicate experiments.
The DENV-2 serotype-specific MAb 3H5 demonstrated a neutralizing titer of 1:40 in DENV-2 only when BHK-21 cells were used. Neutralizing activity to DENV-2 was not detected when FcγRIIA/2 and FcγRIIA/4 were used as assay cells (Table 1). The results indicate that there is a discrepancy in the neutralizing activities of the two MAbs between assays using Fcγ-negative BHK-21 cells and those using FcγRIIA-positive cells.
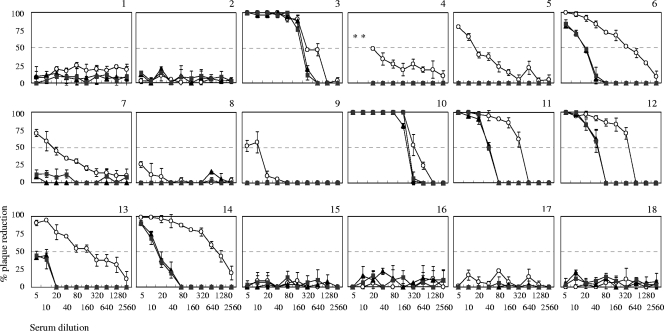
Neutralizing antibody titers to DENV-1 of human serum samples determined by assays using BHK-21 and BHK-FcγRIIA cells.
Eighteen human serum samples were tested for their neutralizing titers to DENV-1 by using BHK-21, BHK-FcγRIIA/2, and BHK-FcγRIIA/4 cell lines (Fig. 3). Serum samples 6, 11, 12, 13, and 14 demonstrated neutralizing antibody titers of 1:160 to 1:640 when Fcγ-negative BHK-21 cells were used as assay cells. However, they demonstrated neutralizing antibody titers of <1:5 to 1:40 when FcγR-positive cells were used (Table 2). Interestingly, serum samples 3 and 10 demonstrated similar levels of neutralizing antibody titers in assays using FcγR-negative and FcγR-positive BHK-21 cells. DENV antibody-negative samples (15 to 18) did not show any neutralizing activity in BHK-21, BHK-FcγRIIA/2, and BHK-FcγRIIA/4 cells. The results indicate that neutralizing antibody titers of human serum samples from dengue patients were different between assays using FcγR-negative and FcγR-positive BHK-21 cells.
FIG. 3.
Patterns of plaque reduction against DENV-1 in neutralization assays with human serum samples. DENV-1 was reacted with human serum samples 2-fold serially diluted from 1:5 to 1:2,560. Graphs are presented according to serum sample number. The characterization of samples is delineated in Tables 2 and 3. ○, untransfected BHK-21 cells; ▴, BHK-FcγRIIA/2 cells; ▪, BHK-FcγRIIA/4 cells. Each curve is the mean of duplicate experiments.
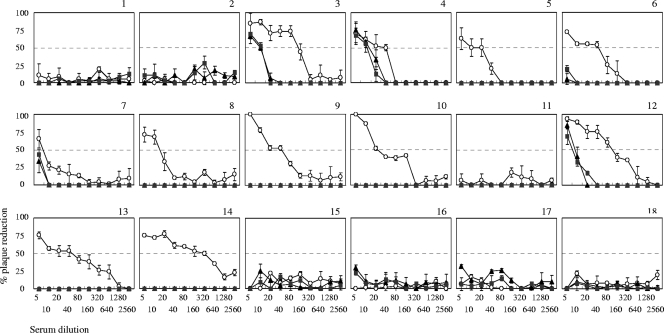
Neutralizing antibody titers to DENV-2 of human serum samples determined by assays using BHK-21 and BHK-FcγRIIA cells.
Eighteen human serum samples were also tested for their neutralizing titers to DENV-2 by using BHK-21, BHK-FcγRIIA/2, and BHK-FcγRIIA/4 cells (Fig. 4). Serum samples 5, 6, 9, 10, 13, and 14 demonstrated neutralizing titers of 1:20 to 1:320 when BHK-21 cells were used. However, they did not demonstrate detectable levels of neutralizing activity when BHK-FcγRIIA cells were used (Table 3). Serum samples 4 and 12 demonstrated neutralizing titers of 40 and 80, respectively, in BHK-21 cells, and 10 and 5, respectively, in BHK-FcγRIIA cells. These results were consistent with those shown in Tables 1 and 2 and indicated that neutralizing titers were higher when determined in assays using FcγR-negative cells than when using BHK-FcγRIIA cells.
FIG. 4.
Patterns of plaque reduction against DENV-2 in neutralization assays with human serum samples. DENV-2 was reacted with human serum samples 2-fold serially diluted from 1:5 to 1:2,560. Graphs are presented according to serum sample number. The characterization of samples is delineated in Tables 2 and 3. ○, untransfected BHK-21 cells; ▴, BHK-FcγRIIA/2 cells; ▪, BHK-FcγRIIA/4 cells. Each curve is the mean of duplicate determinants.
DISCUSSION
The PRNT is a widely accepted method of measuring the neutralizing capacities of antibodies against DENV. Conventional PRNTs employ Vero, LLC-MK2, or BHK-21 cells, which do not express FcγR (1, 11, 14). Thus, assays using these cell lines measure the effects of viral infectivity in the absence of FcγR, and activity measurements are excluded. In the present study, we compared DENV-neutralizing titers between stable BHK-21 cell lines expressing FcγRIIA, BHK-FcγRIIA/2 and BHK-FcγRIIA/4, and parent FcγR-negative BHK-21 cells to examine the influence of FcγR on DENV neutralization. The assay using BHK-FcγRIIA/2 and BHK-FcγRIIA/4 cell lines in this study was developed to examine neutralizing antibody titers of anti-DENV antibody present in serum samples obtained from DENV patients at various stages of the disease. The assay proved useful in studies of the role of antibodies in ADE of DENV infection when using human serum samples. The abilities to enhance the response to DENV by using the flavivirus group-reactive monoclonal mouse antibody 4G2 and BHK-FcγRIIA/2 and BHK-FcγRIIA/4 cells were similar (9).
In this study, the monoclonal antibody 4G2 neutralized all four DENV serotypes in BHK-21 cells. In contrast, when BHK-FcγRIIA cells were used, 4G2 did not neutralize three of the four serotypes and neutralized DENV-3 at antibody dilutions as low as 1:10. The 3H5 antibody neutralized only DENV-2, and the neutralizing activity was not detected using BHK-FcγRIIA cells. The absence of neutralization using BHK-FcγRIIA cells suggests that the presence of ADE lowers the neutralizing activity of the monoclonal antibody. Human serum samples from dengue patients demonstrated similar results. The neutralizing antibody titers of most of the tested samples were higher when determined using FcγR-negative BHK-21 cells than when determined with the FcγR-expressing BHK-21 cell lines BHK-FcγRIIA/2 and BHK-FcγRIIA/4.
Antibodies have two competing effects on DENV growth in the presence of FcγR: neutralization and infection enhancement (10). In the presence of FcγR, the infection enhancement effect may hamper neutralization (9). DENV immune complexes that formed with heterologous antibodies were less susceptible to neutralization in the presence of FcγRIIA-expressing BHK-21 cells, which is consistent with earlier findings by other investigators who used FcγRIIA-expressing CV-1 cells (13, 16). In contrast, DENV-1 immune complexes formed with DENV-1 antibodies, or DENV-2 immune complexes formed with DENV-2 antibodies (homologous DENV immune complexes) were susceptible to neutralization in both BHK-FcγRIIA cells and parent BHK-21 cells. Primary infection with one DENV serotype usually induces long-term protective immunity against homologous serotypes (2). Neutralization of heterologous DENV in assays using FcγR-expressing cells thus strongly reflects the effect of ADE activity. Some serum samples from primary infection also demonstrated higher neutralizing antibody titers when determined using BHK-21 cells than when determined using BHK-FcγRIIA cells. It is possible that some ADE activity exists in the neutralizing assay, even against homologous serotypes.
In the present study, a conventional PRNT was employed, and the serum samples used in this study were obtained from primary or secondary DENV-1 to -4 patients at both early and late phases of the disease; thus, our findings offer insights into individual patient's immunological responses during various stages of the disease (Table 2 and 3). The PRNTs using BHK-FcγRIIA cells satisfy a criterion for an acceptable alternative to conventional neutralization assays: the assay detects the sum of neutralizing and enhancing activities as neutralizing titers in the presence of FcγRIIA. At the same time, the simplicity and ease of performance using the cell lines in the present study meet or exceed those of previous studies (8, 13, 14, 16). The results suggest that PRNTs using BHK-FcγRIIA cells could be a feasible alternative for the detection of neutralizing titers of DENV. In addition, the assay holds potential in assessing the protective capacity against heterologous DENV challenge using in vivo animal models. However, subsequent studies are needed to determine whether PRNTs using FcγRIIA-expressing BHK-21 cells can better demonstrate the correlation between the PRNT titer using human serum samples and the protective capacity against DENV in vivo.
Acknowledgments
We thank Jeffrey V. Ravetch, Rockfeller University, NY, for generously providing the FcγRIIA cDNA. We also thank Susheela Tridandapani, Ohio State University College of Medicine, Columbus, for assistance in obtaining the FcγRIIA cDNA for this work.
This study was supported by grants KH 53333 and KHC 3332 from Research on Publicly Essential Drug and Medical Devices from the Japan Health Sciences Foundation and grants (H20-shinkou-ippan-13 and H20-shinkou-ippan-15) from Research on Emerging and Re-emerging Infectious Diseases by the Ministry of Health, Labor and Welfare, Japan.
Footnotes
Published ahead of print on 30 December 2009.
REFERENCES
- 1.Daëron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203-234. [DOI] [PubMed] [Google Scholar]
- 2.Endy, T. P., A. Nisalak, S. Chunsuttiwat, D. W. Vaughn, S. Green, F. A. Ennis, A. L. Rothman, and D. H. Libraty. 2004. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J. Infect. Dis. 189:990-1000. [DOI] [PubMed] [Google Scholar]
- 3.Henchal, E. A., M. K. Gentry, J. M. McCown, and W. E. Brandt. 1982. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am. J. Trop. Med. Hyg. 31:830-836. [DOI] [PubMed] [Google Scholar]
- 4.Ito, M., K. Yamada, T. Takasaki, B. Pandey, R. Nerome, S. Tajima, K. Morita, and I. Kurane. 2007. Phylogenetic analysis of dengue viruses isolated from imported dengue patients: possible aid for determining the countries where infections occurred. J. Trav. Med. 14:233-244. [DOI] [PubMed] [Google Scholar]
- 5.Kontny, U., I. Kurane, and F. A. Ennis. 1988. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J. Virol. 62:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kou, Z., M. Quinn, H. Chen, W. W. I. S. Rodrigo, R. C. Rose, J. J. Schlesinger, and X. Jin. 2008. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 80:134-146. [DOI] [PubMed] [Google Scholar]
- 7.Littaua, R., I. Kurane, and F. A. Ennis. 1990. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 144:3183-3186. [PubMed] [Google Scholar]
- 8.Martin, N. C., J. Pardo, M. Simmons, J. A. Tjaden, S. Widjaja, M. A. Marovich, W. Sun, K. R. Porter, and T. H. Burgess. 2006. An immunocytometric assay based on dengue infection via DC-SIGN permits rapid measurement of anti-dengue neutralizing antibodies. J. Virol. Methods 134:74-85. [DOI] [PubMed] [Google Scholar]
- 9.Moi, M. L., C. K. Lim, A. Kotaki, T. Takasaki, and I. Kurane. 23 September 2009, posting date. Development of an antibody-dependent enhancement assay for dengue virus using stable BHK-21 cell lines expressing FcγRIIA. J. Virol. Methods. [Epub ahead of print.] doi: 10.1016/j/viromet.2009.09.018. [DOI] [PubMed]
- 10.Moi, M. L., C. K. Lim, T. Takasaki, and I. Kurane. 23 September 2009, posting date. Involvement of the Fcγ receptor IIA cytoplasmic domain in antibody dependent enhancement of dengue virus infection. J. Gen. Virol. [Epub ahead of print.] doi: 10.1099/vir.0.014829-0. [DOI] [PubMed]
- 11.Morens, D. M., S. B. Halstead, P. M. Repik, R. Putvatana, and N. Raybourne. 1985. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J. Clin. Microbiol. 22:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigo, W. W., X. Jin, S. D. Blackley, R. C. Rose, and J. J. Schlesinger. 2006. Differential enhancement of dengue virus immune complex infectivity mediated by signaling-competent and signaling-incompetent human FcγRIA (CD64) or FcγRIIA (CD32). J. Virol. 80:10128-10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigo, W. W., D. C. Alcena, Z. Kou, T. J. Kochel, K. R. Porter, G. Comach, R. C. Rose, X. Jin, and J. J. Schlesinger. 2009. Difference between the abilities of human Fcγ receptor-expressing CV-1 cells to neutralize American and Asian genotypes of dengue virus 2. Clin. Vaccine Immunol. 16:285-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roehrig, J. 2007. Guidelines for plaque reduction neutralization testing of human antibodies to dengue viruses. World Health Organization, Geneva, Switzerland. [DOI] [PubMed]
- 15.Schlesinger, J. J., and S. E. Chapman. 1999. Influence of the human high-affinity IgG receptor FcγRI (CD64) on residual infectivity of neutralized dengue virus. Virology 260:84-88. [DOI] [PubMed] [Google Scholar]
- 16.Shanaka, W. W., I. Rodrigo, D. C. Alcena, Z. Kou, R. C. Rose, X. Jin, and J. J. Schlesinger. 2009. An automated dengue virus microneutralization plaque assay performed in human Fcγ receptor-expressing CV-1 cells. Am. J. Trop. Med. Hyg. 80:61-65. [PubMed] [Google Scholar]
- 17.Takasaki, T., S. Yabe, R. Nerome, M. Ito, K. I. Yamada, and I. Kurane. 2003. Partial protective effect of inactivated Japanese encephalitis vaccine on lethal West Nile virus infection in mice. Vaccine 21:4514-4518. [DOI] [PubMed] [Google Scholar]