Abstract
An understanding of the correlation of the specific antibody responses and the disease phase is essential in evaluating diagnostic values of immunological tests in human echinococcosis. In this study, 422 echinococcosis patients diagnosed by ultrasonography, including 246 with cystic echinococcosis (CE), 173 with alveolar echinococcosis (AE), and 3 with dual infection, were tested for specific IgG in sera against recombinant AgB (rAgB) and recombinant Em18 (rEm18) in an enzyme-linked immunosorbent assay. As a result, rAgB-specific antibody was detected in 77.6% of CE and 86.1% of AE patients, while rEm18-specific antibody was present in 28.9% of CE and 87.3% of AE patients. Additionally, all three patients with dual infection exhibited specific antibodies responding to rAgB and rEm18. Further analysis revealed that rAgB-specific antibody was elevated in a significantly greater proportion (87.3%) of CE patients with cysts at active or transitional stages (CE1, CE2, or CE3), compared to 54.8% of other patients with cysts at an early or an inactive stage (CL or CE4 or CE5). Furthermore, rAgB-specific antibody was detected in 95.6% of CE2 cases, which was statistically greater than that (73.7%) in CE1 patients. Although rEm18-specific antibody was elevated in 28.9% of CE patients, the positive reaction was much weaker in CE than in AE cases. Serum levels and concentrations of rEm18-specific antibody were further indicated to be strongly disease phase correlated in AE patients, with positive rates of 97.4% in cases with alveolar lesions containing central necrosis and 66.7% in patients with early alveolar lesions that measured ≤5 cm.
Humans acquire the infection of echinococcosis by accidental ingestion of eggs excreted with feces of carnivores harboring the adult worms of Echinococcus spp. The eggs hatch in the small intestine of humans, releasing the oncosphere, which migrates via the portal system into various organs and then develops into the metacestode stage. The larval parasite can establish itself in any part of the human body but most frequently does so in the liver (32). Diagnosis of human echinococcosis is primarily based on the pathognomonic features in images obtained using imaging techniques including ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). Of these techniques, B-ultrasound is much more widely applied, as CT and MRI are too expensive and largely inaccessible in most areas where echinococcosis is endemic. Criteria for classification of cystic echinococcosis (CE) and alveolar echinococcosis (AE) have been proposed based on stage-specific ultrasound images (20, 36). Briefly, on the basis of conformational features of cysts, CE lesions are differentiated into six types: CL, CE1, CE2, CE3, CE4, and CE5. The CL type refers to a cystic lesion of a parasite origin and without a clear rim, indicating the parasite is at a very early stage of development. The CE1 type describes a unilocular simple cyst with uniform anechoic content and, importantly, with a visible wall, while the CE2 type is characterized by multivesicular, multiseptated cysts in which daughter cysts may partially or completely fill the unilocular mother cyst. The presence of CE1 or CE2 cysts is indicative of an active stage of the disease. The CE3 type is distinguished by detachment of the cyst membrane and/or partial degeneration of cyst content, suggestive of a transitional parasite. A CE4 or CE5 type of cyst shows an involution, with a necrotic or inactive parasite, with the features of complete degeneration of cyst content for CE4 and a calcified cyst wall for CE5 (36). In contrast, AE lesions are characterized by a nonhomogenous hyperechoic tumor-like structure with a poorly defined verge and containing scattered calcifications and/or a central necrotic cavity (1), and they are further differentiated into three types and eight subtypes based on the features and sizes of lesions, including AE1, AE2, and AE3 (20). In detail, AE1 refers to alveolar lesions measuring ≤5 cm, normally without central necrosis detected, and the type is differentiated further as AE1s (single lesion) and AE1m (multiple lesion) subtypes and indicates an early stage of the disease. Alveolar lesions that measure >5 cm and ≤10 cm are classified as AE2 and include three subtypes, recorded as AE2s (single lesion), AE2m (multiple lesions), and AE2f (presence of central necrotic fluid, regardless of the number of lesions), suggestive of a developing parasite, while AE lesions that measure >10 cm in diameter are confirmed as AE3, indicative of an advanced stage of the disease; this type includes three subtypes, i.e., AE3s (single lesion), AE3m (multiple lesions), and AE3f (presence of central necrotic fluid).
Meanwhile, several antigens, such as antigen B (AgB) (15, 23, 24, 26) for cystic echinococcosis and for Echinococcus multilocularis Em2a (8), II/3 (34), II/3-10 (27), EM10 (5), EM4 (9), and Em18 (12, 30), have been confirmed to be of potential use in serodiagnosis of human echinococcosis. However, relatively little information about the correlation between the specific antibody levels in humans and disease pathology or stage is available (29).
In this study, serum levels and concentrations of specific IgG antibodies in human CE and AE patients at different stages were determined by enzyme-linked immunosorbent assay (ELISA) using recombinant antigen B (rAgB) and recombinant Em18 (rEm18) as antigens.
MATERIALS AND METHODS
Serum samples.
A total of 422 serum samples were collected from 422 individuals with confirmative ultrasound images of echinococcal lesions during 2001 to 2008 in Tibetan communities of northwest Sichuan (23). We also performed all ultrasound examinations. Of these 422 individuals, 246 were diagnosed as CE, 173 as AE, and 3 as dual infection with both CE and AE. According to the criteria for classification of ultrasound images of cystic echinococcosis (36), 5 of the 246 CE cases were determined to have CL cysts of a parasitic origin (CL cysts of nonparasite origin were excluded in this study), 57 had CE1-type cysts, 68 had cysts belonging to the CE2 type, 39 had CE3 cysts, and 68 had CE4 or CE5 cysts. Two or more cystic lesions belonging to different types were concurrently observed in nine additional cases. Of 173 AE cases, 21 were classified as AE1, 54 as AE2 (without necrotic cavity), 20 as AE3 (without necrosis), and an additional 78 were grouped as AEf, including AE2f and AE3f. Serum samples were stored at −20°C until tested.
rAgB and rEm18 ELISAs.
The rAgB and rEm18 antigens were prepared as described previously (26, 30). Each serum sample was analyzed in an ELISA for specific IgG antibody responses to rAgB and rEm18 as reported previously (26, 30), with a minor alteration. In the assays, a 100-μl volume was applied throughout unless otherwise stated and phosphate-buffered saline (PBS) containing 0.05% Tween 20 was employed as the washing buffer (PBST), while casein buffer (1% casein in 20 mM Tris-HCl [pH 7.6] containing 150 mM NaCl) was used as diluting solution of serum and conjugate and also as blocking solution. PBS was employed to dilute antigens. Briefly, 96-well microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with diluted antigen at a protein concentration of 0.5 μg/ml for rAgB and 1.0 μg/ml for rEm18 and incubated at 4°C overnight. After wells were rinsed three times with PBST, 300 μl of blocking solution was added to each well. Plates were incubated at 37°C for 1 h and washed five times. Serum samples diluted at 1:100 were added in duplicate wells and incubated at 37°C for 1 h. After washing five times, plates were incubated with rec-protein G-peroxidase conjugate (Invitrogen, Camarillo, CA) at a 1:4,000 dilution at 37°C for 1 h. Plates were washed five times and incubated with substrate solution [0.4 mM 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) in 0.1 M citric acid buffer and 0.2 M Na2HPO4] at room temperature for 30 min. The color reaction was stopped by application of 1% SDS in each well. The optical density (OD) was determined at 405 nm with a microplate ELISA reader (model 450; Bio-Rad Laboratories, Hercules, CA).
The cutoff points were determined as the mean optical density of 30 serum samples obtained from healthy donors plus 3 standard deviations (SD).
Statistical analyses.
A chi-square test was used for comparing sensitivities among patients grouped on the basis of the type of echinococcal lesion, and the Kruskal-Wallis H rank sum test was applied to compare ELISA OD values for multiple groups of patients with lesions at different stages, whereas the Wilcoxon rank sum test was used to compare OD values between two groups of patients. P values equal to or less than 0.05 were considered indicative of statistical significance.
RESULTS
The cutoff values (mean OD plus 3 SD) derived from analysis of negative-control sera (n = 30) were 0.048 for rAgB and 0.076 for rEm18.
CE. (i) rAgB ELISA.
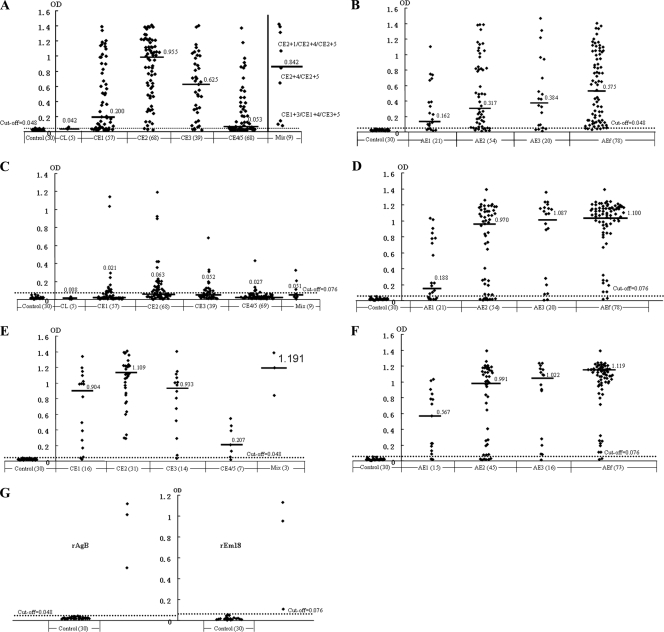
Of the 246 CE cases, a total of 77.6% (191) showed a positive IgG antibody response to rAgB, and the patients with positive reactions had a median OD of 0.640. However, patients with CL or CE4/CE5 cysts exhibited lower activities than those with CE1, CE2, or CE3 cysts. That is, 2 of 5 patients with CL cysts and 55.9% (38/68) of persons with CE4/CE5 cysts responded to rAgB, whereas specific antibody was detected in 73.7% (42/57) of CE1 cases, 95.6% (65/68) of CE2 cases, 89.7% (35/39) of CE3 cases, and in all 9 patients with mixed types of cysts (Table 1). Further analysis revealed that antibody activity against rAgB was significantly different between CE patients with cysts at the early CL or inactive CE4/CE5 stage (40/73; 54.8%) and patients with active or transitional cysts (CE1, CE2, or CE3; 151/173; 87.3%) (χ2 = 31.09; P = 0.000). Moreover, OD values in patients with active or transitional cysts were greater than those in patients with early or inactive cysts (P = 0.000) (Fig. 1A). Additionally, CE1 patients had a significantly lower positive rate (73.7%) than CE2 patients (95.6%; χ2 = 11.97; P = 0.005) (Table 1), and the difference in mean OD values was also significant (P = 0.000) (Fig. 1A).
TABLE 1.
Results of ELISAs with rAgB and rEm18 as antigens in 246 CE patients
| Cyst type(s) | No. of patients examined | No. (%) of patients with positive response to: |
|
|---|---|---|---|
| rAgB | rEm18 | ||
| CL | 5 | 2 (40.0) | 0 (0) |
| CE1 | 57 | 42 (73.7) | 16 (28.1) |
| CE2 | 68 | 65 (95.6) | 31 (45.6) |
| CE3 | 39 | 35 (89.7) | 14 (35.9) |
| CE4/CE5a | 68 | 38 (55.9) | 7 (10.3) |
| Mixed | 9 | 9 (100.0) | 3 (33.3) |
| Total | 246 | 191 (77.6) | 71 (28.9) |
The patients with CE4 or CE5 cysts (indicative of inactive parasites) were grouped together.
FIG. 1.
Results of rAgB and rEm18 ELISAs for CE and AE patients with echinococcal lesions at different stages. (A) rAgB ELISA in 246 CE cases; (B) rAgB ELISA in 173 AE cases; (C) rEm18 ELISA in 246 CE cases; (D) rEm18 ELISA in 173 AE cases; (E) rAgB ELISA in 71 CE cases with a positive response to rEm18; (F) rEm18 ELISA in 149 AE cases with a positive response to rAgB; (G) rAgB and rEM18 ELISAs for 3 cases with dual infections of both CE and AE. The dashed lines indicate the cutoff values, and black bars refer to OD medians. Controls were healthy persons. The numbers in parentheses indicate numbers of tested cases or persons.
(ii) rEm18 ELISA.
Sera from CE patients were also tested using rEm18 as antigen. Of the 246 CE cases, 28.9% (71) showed a positive response to rEm18, and all the cases with seropositivity had an OD median of 0.135. Antibody levels against rEm18 varied among patients with different types of cysts. That is, all 5 patients with CL cysts showed a negative response, while specific antibody was detected in 45.6% (31/68) of CE2 patients, 35.9% (14/39) of CE3 cases, 3 of 9 CE cases with mixed types of cysts, 28.1% (16/57) of CE1 patients, and 10.3% (7/68) of CE4/CE5 cases (Table 1). However, reactions with rEm18 in CE patients were generally weak, with respective OD medians for each group (in parentheses), as follows: CL (0.008), CE1 (0.021), CE2 (0.063), mixed (0.051), CE3 (0.052), and CE4/5 (0.027) (Fig. 1C). The difference was significant (P = 0.000).
Of the 71 CE cases with a positive response to rEm18, all except 4 also exhibited specific antibody to rAgB at a rather high level (median, 0.994) (Fig. 1E). The four negative sera consisted of CE1 (two), CE3 (one), and CE4/CE5 (one).
AE. (i) rAgB ELISA.
In 173 AE patients, 149 (86.1%) contained protein G binding antibodies (IgG) that recognized rAgB from Echinococcus granulosus, with an OD median of 0.489 for the positive cases. Serum levels and concentrations of specific antibody were shown to be elevated in patients with late-stage disease (AE2, AE3, or AEf) (Fig. 1B). That is, 93.6% (73/78) of AEf patients exhibited a positive antibody response, while positive responses were observed in 83.3% (45/54) for AE2, 80.0% (16/20) for AE3, and 71.4% (15/21) for AE1 patients (Table 2). Further analysis revealed that the differences in the positive rates were significant (χ2 = 8.41; P = 0.0382).
TABLE 2.
Results of ELISAs with rAgB and rEm18 as antigens in 173 AE patients
| Cyst type | No. of patients examined | No. (%) of patients with positive response to: |
|
|---|---|---|---|
| rAgB | rEm18 | ||
| AE1 | 21 | 15 (71.4) | 14 (66.7) |
| AE2 | 54 | 45 (83.3) | 43 (79.6) |
| AE3 | 20 | 16 (80.0) | 18 (90.0) |
| AEf | 78 | 71 (91.0) | 76 (97.4) |
| Total | 173 | 147 (85.0) | 151 (87.3) |
(ii) rEm18 ELISA.
Of the same 173 AE cases, 87.3% (151) exhibited a specific antibody response to rEm18, with an OD median of 1.068 for the positive cases. Antibody levels and concentrations were observed to be greatly elevated with advanced disease (Fig. 1D). That is, 14 (66.7%) of 21 patients with AE1-type lesions showed positive reactions, while 79.6% (43/54) of AE2 and 90.0% (18/20) of AE3 cases exhibited specific antibody, and the positive rate reached 97.4% (76/78) in patients with AEf-type lesions (Table 2). The positive rates proved to be significantly different (χ2 = 18.27; P < 0.0005). In addition, the differences in OD medians between those patients at different stages (AE1, AE2, AE3, or AEf) were also significant (χ2 = 32.265; P = 0.000).
Of the 149 AE patients with a positive response to rAgB, 136 (91.3%) exhibited specific antibody to rEm18 (Fig. 1F). In other words, AE patients with a positive response to rEm18 were more likely to react with rAgB (136/151; 90.1%) than AE patients with a negative response to rEm18 (13/22; 59.1%) (χ2 = 15.33; P < 0.0001).
As expected, a much greater proportion of AE patients (87.3%) exhibited rEm18-specific antibodies than CE patients (28.9%; χ2 = 138.83; P = 0.000). Similarly, rEm18 ELISA OD values of AE patients (OD median, 1.029) were significantly higher than those of CE patients (OD median, 0.036; P = 0.000). In contrast, antibody activities with rAgB in CE patients (77.6%) or AE patients (86.1%) were different (χ2 = 4.77; P = 0.0290), but the difference in OD medians was not significant (P = 0.473).
Cases with dual infection.
All three cases with dual CE/AE infection showed positive responses to both rAgB and rEm18 (Fig. 1G).
DISCUSSION
rAgB and rEm18 have recently been produced and have proved to be highly useful for serodiagnosis of human echinococcosis, with a high sensitivity and 100% specificity (26, 30, 37). Our current study focused on testing the sensitivity, and the results indicated that rAgB detected Echinococcus genus-specific antibodies, because both CE and AE patients were seropositive at similar levels, whereas rEm18 antigen exhibited higher E. multilocularis species specificity, with 87.3% of AE cases classified as seropositive and, by contrast, only weak reactions observed in 28.9% of CE patients. In addition, specific IgG antibody levels and concentrations measured against rAgB and rEm18 proved to be strongly correlated with disease stage in CE and AE cases, respectively.
Both human CE and AE are highly endemic in northwest Sichuan Province, China (21, 22), where a large number of echinococcosis cases at different stages were detected in the field through mass screening programs by portable ultrasound scan, which permitted us to analyze the correlation of specific antibody response and disease stage. Considering the natural history of cystic echinococcosis, cysts are classified into six types: CL (refers to cysts of a parasitic origin) and CE1, CE2, CE3, CE4, and CE5, indicating the different pathological/growth activities of the parasite in human hosts (36). Diagnosis of CE is currently primarily based on the imaging features of the cysts, but specific serology is also important as a complementary diagnostic tool. As one of the most important immunogenic antigens, E. granulosus native AgB detects about 80% to 90% of CE cases (15, 19, 24, 25), while rAgB has shown a similar diagnostic value, with positive reactions in about 70% to 90% of CE cases (26, 28, 33). Our current study revealed that rAgB had a similar positive rate (77.6%) in CE patients, and rAgB-specific antibody levels and concentrations in CE patients were strongly associated with the cyst type; i.e., when the parasite was at a very early (CL) or inactive (CE4 or CE5) stage, the specific IgG antibodies were present at a significantly lower concentration in a small proportion of patients, with a seropositive rate of 54.8% and an OD median of 0.050, compared to 87.3% seropositive and an OD median of 0.648 when the parasite was in an active (CE1 or CE2) or transitional (CE3) stage of development. Similar observations were made previously for ultrasound-confirmed CE cases detected in community studies (2, 3) and for hospitalized patients (28). Interestingly, patients with CE1 cysts in our study showed a markedly lower seropositivity (73.7%) and lower OD (median, 0.2) with rAgB than patients with CE2 cysts (95.6% seropositive and 0.995 OD median); this was probably caused by different structural features of the cysts, which can lead to the release of fewer antigens, including antigen B in the blood circulation, in CE1 cases than in CE2 cases. However, a similar seropositivity with native AgB in CE1 and CE2 cases was reported by Ortona et al. (28); this discrepancy might arise from differences in the time of serum sampling (before or after surgical or chemotherapeutic intervention), but it may be because CE2 and CE3 were revised in the original Gharbi classification (7, 35) before the WHO recommendation to change these criteria were published (36). In our study, 86.1% of AE sera were also recognized by rAgB, which was exceptionally higher than that where AE is exclusively endemic (approximately 40.0%), as reported previously (15, 19, 26). One possibility is that these AE cases might be coinfected with CE in other organs, such as the lung. However, a more likely probability is that rAgB applied in our study refers to rAg8/1 from E. granulosus protoscoleces (rEgAgB8/1), which is 92.6% homologous at the amino acid level to AgB8/1 from E. multilocularis metacestodes (EmAgB8/1) (26). These two antigens have been shown to have very similar immunoreactive regions which are thought to stimulate human hosts to produce similar IgG antibodies in CE and AE patients, respectively (26). Therefore, rAgB, although from E. granulosus, can bind IgG antibodies in both CE and AE sera to a similar level.
Several E. multilocularis antigens, such as EM10 (5), II/3 (34), II/3-10 (27), and EM4 (10), have proved to have potential for use in differential serodiagnosis of AE from CE. Em18 from E. multilocularis protoscoleces was confirmed to be a fragment of EM10 (30) and demonstrated its usefulness for highly sensitive and specific diagnosis of AE (12, 13, 14, 17, 18). In the current study, 87.3% of AE patients exhibited a specific antibody response to rEm18, which was identical to results of a previous report (30) but was lower than the results from other studies in which rEm18 detected almost 100% of AE cases (16, 37). This discrepancy is probably caused by the differences of the disease stages, as our study indicated the rEm18-specific antibody levels and concentrations in AE patients were strongly correlated with the stage of alveolar lesions. When the disease became aggravated, with the lesion changing from AE1 to AE2, AE3, or AEf, antibody activities against rEm18 were significantly elevated, from 66.7% to 97.4%, and concurrently serum concentrations of specific antibody also evidently increased with the OD value, changing from 0.188 to 1.100. This observation indicates that Em18 can be highly useful for assessing the parasite activities in human AE patients following interventional measures. Similar results have recently been published in which rEm18 serology was shown to be reliable for monitoring the progression of AE (11, 31). A much greater proportion (28.9%) of CE cases were observed to exhibit specific antibody against rEm18 than those (3% to 13%) described in previous studies (16, 18, 30, 37), in which positive patients were found to have complicated or multiple cysts. In the current study, CE patients with all types of cysts except for CL were observed to respond to rEm18; of these, cases with CE2 (45.6%) were most likely to have specific antibody compared to the other groups (ranging from 10.3% to 35.9%). Nevertheless, all the seropositive reactions in CE patients were much weaker than in AE patients (OD medians, 0.135 versus 1.068). Similar results were obtained with antigens EM10 or II/3, described in previous studies (4, 6), in which AE patients were found to have raised specific antibody to EM10 more frequently than CE patients, despite there being a protein with a high level of homology to EM10 expressed by E. granulosus metacestodes. The significant differences in activities with rEm18 or EM10 in AE and CE patients may be partially attributable to different pathological features of metacestodes of E. multilocularis and E. granulosus. The E. granulosus metacestode grows in a cyst with a double wall by endogenous budding. Conversely, the E. multilocularis metacestode grows via exogenous budding, and it therefore has intimate contact with host tissues (32).
Acknowledgments
The study was supported by grant number RO1 TW001565 from the Fogarty International Center of NIH to P.S.C.; by the International Joint Research Project of the Japan Society for the Promotion of Science (JSPS 17256002 and 21256003), the JSPS—Asia/Africa Scientific Platform Fund (2006-2011), and the Infection Matrix Fund from the Ministry of Education, Japan, to A.I.; and by the Invitation Fund by Japan China Medical Association to M.N. This study was also supported in part by the Sichuan Provincial Department of Health, China, a Ph.D. split-site studentship (to T.L.) between the University of Salford, United Kingdom, and SIPD/Sichuan CDC, China.
Footnotes
Published ahead of print on 30 December 2009.
REFERENCES
- 1.Bresson-Hadni, S., E. Delabrousse, O. Blagosklonov, B. Bartholomot, S. Koch, J. P. Miguet, G. A. Mantion, and D. A. Vuitton. 2006. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol. Int. 55(Suppl.):S267-S272. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, H., E. Paolillo, R. Bonifacino, B. Botta, L. Parada, P. Cabrera, K. Snowden, R. Gasser, R. Tessier, L. Dibarboure, H. Wen, J. C. Allan, H. Soto de Alfaro, M. T. Rogan, and P. S. Craig. 1998. Human cystic echinococcosis in a Uruguayan community: a sonographic, serologic, and epidemiologic study. Am. J. Trop. Med. Hyg. 59:620-627. [DOI] [PubMed] [Google Scholar]
- 3.Daeki, A. O., P. S. Craig, and M. K. Shambesh. 2000. IgG-subclass antibody responses and the natural history of hepatic cystic echinococcosis in asymptomatic patients. Ann. Trop. Med. Parasitol. 94:319-328. [DOI] [PubMed] [Google Scholar]
- 4.Felleisen, R., and B. Gottstein. 1994. Comparative analysis of full-length antigen II/3 from Echinococcus multilocularis and E. granulosus. Parasitology 109:223-232. [DOI] [PubMed] [Google Scholar]
- 5.Frosch, P. M., M. Frosch, T. Pfister, V. Schaad, and D. Bitter-Suermann. 1991. Cloning and characterization of an immunodominant major surface antigen of Echinococcus multilocularis. Mol. Biochem. Parasitol. 48:121-130. [DOI] [PubMed] [Google Scholar]
- 6.Frosch, P. M., F. Muhlschlegel, L. Sygulla, M. Hartmann, and M. Frosch. 1994. Identification of a cDNA clone from the larval stage of Echinococcus granulosus with homologies to the E. multilocularis antigen EM10-expressing cDNA clone. Parasitol. Res. 80:703-705. [DOI] [PubMed] [Google Scholar]
- 7.Gharbi, H. A., B. Hassine, M. W. Braunner, and K. Dupuch. 1981. Ultrasound examination of hydatid liver. Radiology 139:459-463. [DOI] [PubMed] [Google Scholar]
- 8.Gottstein, B. 1985. Purification and characterization of a specific antigen from Echinococcus multilocularis. Parasite Immunol. 7:201-212. [DOI] [PubMed] [Google Scholar]
- 9.Hemmings, L., and D. P. McManus. 1989. The isolation, by differential antibody screening, of Echinococcus multilocularis antigen gene clones with potential for immunodiagnosis. Mol. Biochem. Parasitol. 33:171-182. [DOI] [PubMed] [Google Scholar]
- 10.Hemmings, L., and D. P. McManus. 1991. The diagnostic value and molecular characterization of an Echinococcus multilocularis antigen gene clone. Mol. Biochem. Parasitol. 44:56-62. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa, Y., Y. Sako, S. Itoh, T. Ohtake, Y. Kohgo, T. Matuno, Y. Ohsaki, N. Miyokawa, M. Nakao, K. Nakaya, and A. Ito. 2009. Serological monitoring of progression of alveolar echinococcosis with multi-organ involvement using recombinant Em18. J. Clin. Microbiol. 47:3191-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito, A., M. Nakao, H. Kutsumi, M. W. Lightowlers, M. Itoh, and S. Sato. 1993. Serodiagnosis of alveolar hydatid disease by Western blotting. Trans. R. Soc. Trop. Med. Hyg. 87:170-172. [DOI] [PubMed] [Google Scholar]
- 13.Ito, A., P. M. Schantz, and J. F. Wilson. 1995. Em18, a new serodiagnostic marker for differentiation of active and inactive cases of alveolar hydatid disease. Am. J. Trop. Med. Hyg. 52:41-44. [DOI] [PubMed] [Google Scholar]
- 14.Ito, A., L. Ma, M. Itoh, S. Y. Cho, Y. Kong, S. Y. Kang, T. Horii, X. L. Pang, M. Okamoto, T. Yamashita, M. W. Lightowlers, X. G. Wang, and Y. H. Liu. 1997. Immunodiagnosis of alveolar echinococcosis by enzyme-linked immunosorbent assay using a partially purified Em18/16 enriched fraction. Clin. Diagn. Lab. Immunol. 4:57-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito, A., L. Ma, P. M. Schantz, B. Gottstein, Y. H. Liu, J. J. Chai, S. K. Abdelhafez, N. Altintas, D. D. Joshi, M. W. Lightowlers, and Z. S. Pawlowski. 1999. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and Echinococcus multilocularis protoscolex (Em18). Am. J. Trop. Med. Hyg. 60:188-192. [DOI] [PubMed] [Google Scholar]
- 16.Ito, A., N. Xiao, M. Liance, M. O. Sato, Y. Sako, W. Mamuti, Y. Ishikawa, M. Nakao, H. Yamasaki, K. Nakaya, K. Bardonnet, S. Bresson-Hadni, and D. A. Vuitton. 2002. Evaluation of an enzyme-linked immunosorbent assay (ELISA) with affinity-purified Em18 and an ELISA with recombinant Em18 for differential diagnosis of alveolar echinococcosis: results of a blind test. J. Clin. Microbiol. 40:4161-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito, A., M. Nakao, and Y. Sako. 2007. Echinococcosis: serological detection of patients and molecular identification of parasites. Future Microbiol. 2:439-449. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, L., H. Wen, and A. Ito. 2001. Immunodiagnostic differentiation of alveolar and cystic echinococcosis using ELISA test with 18-kDa antigen extracted from Echinococcus protoscoleces. Trans. R. Soc. Trop. Med. Hyg. 95:285-288. [DOI] [PubMed] [Google Scholar]
- 19.Leggatt, G. R., W. Yang, and D. P. McManus. 1992. Serological evaluation of the 12 kDa subunit of antigen B in Echinococcus granulosus cyst fluid by immunoblot analysis. Trans. R. Soc. Trop. Med. Hyg. 86:1-4. [DOI] [PubMed] [Google Scholar]
- 20.Li, T., J. Qiu, P. S. Craig, A. Ito, W. Yang, D. A. Vuitton, N. Xiao, X. Chen, W. Yu, and P. M. Schantz. 2004. Review of 311 cases of alveolar echinococcosis and criteria for the classification of hepatic ultrasound images. Southeast Asian J. Trop. Med. Public Health 35(Suppl.):1-5. [Google Scholar]
- 21.Li, T., J. Qiu, W. Yang, P. S. Craig, X. Chen, N. Xiao, A. Ito, P. Giraudoux, W. Mamuti, W. Yu, and P. M. Schantz. 2005. Echinococcosis in Tibetan populations, western Sichuan Province, China. Emerg. Infect. Dis. 11:1866-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, T., X. Chen, Z. Ren, J. Qiu, D. Qiu, N. Xiao, A. Ito, H. Wang, P. Giraudoux, Y. Sako, M. Nakao, and P. S. Craig. Widespread co-endemicity of human cystic and alveolar echinococcosis on the eastern Tibetan Plateau, China. Acta Trop., in press. [DOI] [PMC free article] [PubMed]
- 23.Lightowlers, M. W., D. Liu, A. Haralambous, and M. D. Rickard. 1989. Subunit composition and specificity of major cyst fluid antigens of Echinococcus granulosus. Mol. Biochem. Parasitol. 37:171-182. [DOI] [PubMed] [Google Scholar]
- 24.Maddison, S. E., S. B. Slemenda, P. M. Schantz, J. A. Fried, M. Wilson, and V. C. W. Tsang. 1989. A specific diagnostic antigen of Echinococcus granulosus with an apparent molecular weight of 8 kDa. Am. J. Trop. Med. Hyg. 40:377-383. [DOI] [PubMed] [Google Scholar]
- 25.Mamuti, W., H. Yamasaki, Y. Sako, K. Nakaya, M. Nakao, M. W. Lightowlers, and A. Ito. 2002. Usefulness of hydatid cyst fluid of Echinococcus granulosus developed in mice with secondary infection for serodiagnosis of cystic echinococcosis in humans. Clin. Diagn. Lab. Immunol. 9:573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamuti, W., H. Yamasaki, Y. Sako, M. Nakao, N. Xiao, K. Nakaya, N. Sato, D. A. Vuitton, R. Piarroux, M. W. Lightowlers, P. S. Craig, and A. Ito. 2004. Molecular cloning, expression, and serological evaluation of an 8-kilodalton subunit of antigen B from Echinococcus multilocularis. J. Clin. Microbiol. 42:1082-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller, N., B. Gottstein, M. Vogel, K. Flury, and T. Seebeck. 1989. Application of a recombinant Echinococcus multilocularis antigen in an enzyme-linked immunosorbent assay for immunodiagnosis of human alveolar echinococcosis. Mol. Biochem. Parasitol. 36:151-159. [DOI] [PubMed] [Google Scholar]
- 28.Ortona, E., R. Rigano, P. Margutti, S. Notargiacomo, S. Ioppolo, S. Vaccari, S. Barca, B. Buttari, E. Profumo, A. Teggi, and A. Siracusano. 2000. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol. 22:553-559. [DOI] [PubMed] [Google Scholar]
- 29.Rogan, M. T., and P. S. Craig. 2001. Immunological approaches for transmission and epidemiological studies in cestode zoonoses: the role of serology in human infection, p. 135-145. In P. S. Craig (ed.), Tapeworm zoonoses, an emergent and global problem. NATO Science Series. IOS Press, Amsterdam, The Netherlands.
- 30.Sako, Y., M. Nakao, K. Nakaya, H. Yamasaki, B. Gottstein, M. W. Lightowers, P. M. Schantz, and A. Ito. 2002. Alveolar echinococcosis: characterization of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J. Clin. Microbiol. 40:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tappe, D., M. Frosch, Y. Sako, S. Itoh, B. Grüner, S. Reuter, M. Nakao, A. Ito, and P. Kern. 2009. Close relationship between clinical regression and specific serology in the follow-up of patients with alveolar echinococcosis in different clinical stages. Am. J. Trop. Med. Hyg. 80:792-797. [PubMed] [Google Scholar]
- 32.Thompson, R. C. A. 1995. Biology and systematics of Echinococcus, p. 1-50. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 33.Virginio, V. G., A. Hernandez, M. B. Rott, K. M. Monteiro, A. F. Zandonai, A. Nieto, A. Zaha, and H. B. Ferreira. 2003. A set of recombinant antigens from Echinococcus granulosus with potential for use in the immunodiagnosis of human cystic hydatid disease. Clin. Exp. Immunol. 132:309-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel, M., B. Gottstein, N. Muller, and T. Seebeck. 1988. Production of a recombinant antigen of Echinococcus multilocularis with high immunodiagnostic sensitivity and specificity. Mol. Biochem. Parasitol. 31:117-125. [DOI] [PubMed] [Google Scholar]
- 35.Wang, Y., X. Zhang, B. Bartholomot, B. Liu, J. Luo, T. Li, X. Wen, H. Zheng, H. Zhou, H. Wen, N. Davaadori, L. Gambolt, T. Mukhar, K. al-Qaoud, S. Abdel-Hafez, P. Giraudoux, D. A. Vuitton, A. Fraser, M. T. Rogan, and P. S. Craig. 2003. Classification, follow-up and recurrence of hepatic cystic echinococcosis using ultrasound images. Trans. R. Soc. Trop. Med. Hyg. 97:203-211. [DOI] [PubMed] [Google Scholar]
- 36.WHO Informal Working Group. 2003. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 85:253-261. [DOI] [PubMed] [Google Scholar]
- 37.Xiao, N., W. Mamuti, H. Yamasaki, Y. Sako, M. Nakao, K. Nakaya, B. Gottstein, P. M. Schantz, M. W. Lightowlers, P. S. Craig, and A. Ito. 2003. Evaluation of use of recombinant Em18 and affinity-purified Em18 for serological differentiation of alveolar echinococcosis from cystic echinococcosis and other parasitic infections. J. Clin. Microbiol. 41:3351-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]