Abstract
Papillomatous digital dermatitis (PDD) is a major infectious disease of the foot skin in dairy cattle. Treponema phagedenis-like spirochetes have been consistently detected in PDD lesions, and antibodies against these organisms have been demonstrated in affected cattle. However, little is known about the dominant antigens recognized by the immune system of affected cattle. Here, we investigated the IgG immune response to T. phagedenis-like isolates by Western blotting with different sera using whole-cell lysates and extracted glycolipid from 18 and 8 isolates, respectively, including those from different cattle on the same or different farms, isolates from different lesions affecting a single cow, and different isolates from the same lesion affecting a single cow. The reactivity of sera in Western blot assays revealed different banding patterns or showed no bands, suggesting that considerable antigenic variations, including glycolipid, may exist among the isolates, even in those from single individuals. With use of a total of 151 serum samples collected from three groups of cattle, i.e., PDD-positive cows on PDD-positive farms (group A), PDD-negative cows on PDD-positive farms (group B), and cows on PDD-free farms (group C), the levels of IgG antibodies against four T. phagedenis-like isolates were measured by enzyme-linked immunosorbent assay (ELISA). The optical density in groups A and B was significantly higher than that in group C, even though the value varied among the antigens used. Therefore, combinations of multiple Treponema species should be used for serological analysis and the development of a suitable vaccine because of antigenic variations.
Papillomatous digital dermatitis (PDD) is an important leading cause of severe epidemic lameness in dairy cows (18), with resulting economic losses due to decreases in milk production and reproductive performance and the costs of treatment (10, 15, 27).
Interestingly, among bacteria in the lesions, a large number of spirochetes have been consistently detected, and these have been identified as Treponema species which are closely related to Treponema phagedenis, which is an inhabitant of the human genital tract, and human oral treponemes, including T. denticola, T. vincentii, and T. medium (2, 7, 12). Their presence in both superficial lesions and deeper layers of the epidermis implies that they may be one of the most predominant populations in the lesions and play a role in the pathogenesis of PDD (12, 17, 19).
Humoral and cell-mediated immune responses to T. phagedenis-like spirochetes have been demonstrated in cattle with PDD (4, 5, 6, 29, 30, 31). It has been reported that the levels of antibodies against treponemes in PDD-positive cattle were significantly higher than those in PDD-negative cattle (4, 30, 31). However, little is known about the dominant antigens recognized by the immune system of affected individual cattle. Because no culture methods for isolating treponemes from PDD lesions have been established, there has been a paucity of antigenic and serological analyses using sufficient numbers of isolates.
Recently, for the first time in Japan, we successfully isolated 40 spirochete isolates from dairy cattle with PDD lesions by using a simple two-step culture technique, and these isolates were identified as Treponema phagedenis-like spirochetes on the basis of their biochemical traits and enzyme activities, which were identical to those of T. phagedenis ATCC 27087, and the results of sequencing of the 16S rRNA gene showing that all isolates had >99% identity to those of the T. phagedenis type strain and T. phagedenis-like spirochetes isolated from PDD lesions in the United States and Europe (33). Interestingly, considerable genetic diversity was detected among the isolates, not only from different cattle but also from the same individuals, using pulsed-field gel electrophoresis and PCR-based random amplified polymorphism DNA methods (33). Such genetic diversity may underlie the antigenic diversity of these spirochetes, and determination of the bacterial antigens recognized by infected cattle would be useful for the development of a suitable vaccine.
In the present study, we investigated the serological characteristics of individual cattle with PDD from which T. phagedenis-like spirochetes were isolated and searched for the main bacterial antigens recognized by affected cattle by using Western blot analysis. Furthermore, we evaluated the potential of the enzyme-linked immunosorbent assay (ELISA) for detecting differences in the IgG antibody response against T. phagedenis-like spirochetes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
To examine the antigens recognized by antibodies in sera of affected cattle, we selected 18 isolates isolated from 12 cattle on 7 farms among the 40 isolates of T. phagedenis-like spirochetes stored in our laboratory (33). As shown in Table 1, the isolates examined included isolates from different cattle on the same or different farms, isolates from different lesions affecting a single cow, and different isolates from the same lesion affecting a single cow. For ELISA, T. phagedenis-like spirochete isolates HT201, YG3903R, HD26-43, and IZ6-2 were used as antigens. Two type strains, i.e., Treponema phagedenis ATCC 27087 and Treponema denticola JCM 8225, were also included as controls. These strains were suspended in brucella broth (BBL Becton Dickinson, Sparks, MD) supplemented with 10% glycerol and stored at −80°C until use. Bacteria grown at 37°C for 2 weeks on agar plates containing an anaerobic medium named PDDTp, as described in a previous report (33), were used for further experiments.
TABLE 1.
Treponema phagedenis-like isolates used in this study
| Cow no.a | Isolate | Lesion sourceb | Region | Farm |
|---|---|---|---|---|
| 1 | HT201 | R-L | Kumamoto | H |
| 2 | YG3903R | R-R | Yamagata | C |
| 3 | YG5618 | R-R | Yamagata | C |
| 4 | HG42 | NA | Hyogo | F |
| 5 | IZ6-2 | NA | Kagoshima | K |
| 6 | IZ7-2 | R-R | Kagoshima | K |
| 7 | CH6 | R-R | Chiba | D |
| 8 | CH9 | R-L | Chiba | E |
| 9 | HD21-66 | R-L | Hokkaido | A |
| HD21-R7 | R-R | Hokkaido | A | |
| 10 | HD22-63 | R-L | Hokkaido | A |
| HD22-R16 | R-R | Hokkaido | A | |
| 11 | HD26-43 | R-R | Hokkaido | A |
| HD26-67 | R-R | Hokkaido | A | |
| HD26-R5 | R-R | Hokkaido | A | |
| 12 | HD27-4 | R-L | Hokkaido | B |
| HD27-24 | R-L | Hokkaido | B | |
| HD27-R6 | R-L | Hokkaido | B |
For cows 9 and 10, the multiple isolates came from different lesions on the same cow; for cows 11 and 12, the multiple isolates came from the same lesion on the same cow.
R-L, rear left foot; R-R, rear right foot; NA, not available.
Collection of sera from cattle.
A total of 151 serum samples were obtained from different locations in Japan between 2005 and 2008. Eighty-five of the samples were collected from cattle with PDD on 11 PDD-positive farms (group A) and 33 from cattle without PDD lesions on 3 PDD-positive farms (group B). The other 33 sera were collected from cattle on 2 PDD-free farms, i.e., farms with no history of PDD and no individual cattle with lameness or visible papillomatous or erosive digital skin lesions upon physical examination of lower limbs before serum collection (group C). Most of the PDD-positive cattle were in the chronic stage of infection at the time of serum collection. All sera examined were heat inactivated at 56°C for 30 min and stored at −20°C before testing.
Preparation of polyclonal antisera.
Polyclonal antisera against 3 T. phagedenis-like spirochetes, i.e., YG3903R, HT201, and HD26-43, and 2 control strains, T. phagedenis ATCC 27087 and T. denticola JCM 8225, were prepared in New Zealand White rabbits. Experimental protocols were approved by the institutional review board for animal experiments of the University of Miyazaki (approval no. 2007-24). In brief, equal volumes of bacterial suspension and Freund's incomplete adjuvant (Nacalai Tesque, Kyoto, Japan) were mixed thoroughly, and 2-month-old rabbits were inoculated intracutaneously with each suspension twice with a 2-week interval. After the second injection, the rabbits received 1 ml of bacterial suspension in phosphate-buffered saline (PBS) injected through an ear vein. One week after the third immunization, whole blood was collected from the immunized rabbits. Each serum sample was inactivated by incubation at 56°C for 30 min. These antisera were then used as positive controls for Western blotting and ELISA.
Glycolipid extraction from T. phagedenis-like spirochetes.
Glycolipid was extracted from the treponemes, including the T. phagedenis type strain ATCC 27087 and 8 T. phagedenis-like spirochetes isolated from different individual cows with PDD from different locations in Japan. Briefly, a bacterial suspension of 2-week-old growth cells was harvested in PBS, and the optical density (OD) was adjusted to 1.0 at 550 nm. Glycolipid was extracted using a lipopolysaccharide (LPS) extraction kit (Intron Biotechnology, Seongnam, South Korea) in accordance with the manufacturer's instructions.
SDS-PAGE.
Antigen samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (14). The electrophoresis materials were obtained from Japan Bio-Rad Laboratories (Tokyo, Japan). Discontinuous SDS-PAGE was done using 1.5-mm-thick slab gels with a 4% stacking gel and a 12.5% separating gel. Bacterial cells on PDDTp agar plates were harvested with a sterile cotton swab and suspended in PBS. Then, the cell suspensions were centrifuged at 3,000 × g at 20°C for 20 min and the pellet was washed three times in PBS. Finally, the pellet was resuspended in PBS and the optical density was adjusted to 0.5 at 550 nm. Each bacterial suspension was mixed with sample buffer containing SDS (4%), 2-mercaptoethanol (0.4%), bromophenol blue (0.2%), glycerol (35%), and Tris base (0.38%) at pH 6.8 and boiled at 100°C for 5 min. The sample was then placed on ice for 5 min and centrifuged at 13,000 rpm for 5 min. Ten microliters of the supernatant was used as the antigen, and the gel was visualized by being stained with Coomassie brilliant blue. The extracted glycolipid described above was mixed with a 1/5 volume of sample buffer and heated at 100°C for 2 min. Five microliters of each glycolipid sample was used for SDS-PAGE, and the gel was stained using a modified silver staining method described elsewhere (8). Electrophoresis was carried out with a constant voltage of 200 V for 45 min.
Western blotting.
Western blotting was performed to detect the bacterial antigens recognized by the affected cattle using whole-cell lysates or glycolipids. T. phagedenis type strain ATCC 27087 and 18 T. phagedenis-like spirochetes isolated from different individual cows with PDD, including 8 isolates isolated from different individuals on the same or different farms (cattle 1 to 8) and 10 isolates isolated from a single lesion or both feet of four individuals on farms A and B (cattle 9 to 12), were used in the analysis (Table 1). Eleven serum samples from PDD-positive cows from which T. phagedenis-like spirochetes had been isolated, 6 serum samples from PDD-negative cows on PDD-positive farms, and 5 serum samples from cows on two PDD-free farms were used. Bacterial cell lysates or glycolipids of Treponema species separated by SDS-PAGE were transblotted onto nitrocellulose sheets (NCS) as described previously (28). Unoccupied sites of NCS were blocked with 5% skim milk in PBS by incubation at 25°C for 2 h or at 4°C overnight. Then, the NCS was washed three times for 5 min with PBS supplemented with 0.05% Tween 20 (PBST). A washed NCS was incubated with serum from a cow (diluted 1:500) in PBST containing 5% skim milk at 37°C for 1 h with shaking. The NCS was washed three times with PBST and incubated with goat anti-bovine polyclonal IgG labeled with alkaline phosphate (Gene Tex, Inc., Irvine, CA) diluted 1:12,000 in PBST containing 5% skim milk at 37°C for 1 h with shaking. The NCS was washed three times, and the bound antibody was detected with a 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt/nitroblue tetrazolium chloride (BCIP/NBT) substrate system (Sigma-Aldrich, Tokyo, Japan). The reaction was stopped with distilled water when visible bands developed.
Preparation of soluble antigens for ELISA.
Antigens for ELISA were prepared by an acid extraction method (16) since cell surface antigens are suitable for the ELISA to eliminate the effect of cross-reactivity to common antigens. Briefly, bacterial cells grown on PDDTp agar plates were harvested in PBS by centrifugation at 7,000 × g at 4°C for 15 min. The pellet was washed three times with PBS. Then, 3 ml of sterile distilled water was added to make a bacterial suspension in a 50-ml conical flask, and 7 ml of 100 mM glycine-HCl buffer (pH 2.2) containing 150 mM NaCl was added. The flask was placed on a magnetic stirrer and stirred for 20 min at room temperature. The extracted fraction was separated by centrifugation at 13,000 × g at 4°C for 10 min. The extraction was neutralized by adding Tris base (Sigma-Aldrich, Japan), and the protein concentration was determined using a protein assay kit (Japan Bio-Rad) in accordance with the manufacturer's instructions and stored at −20°C until use for ELISA.
ELISA.
ELISA was performed on serum samples from cattle to detect antibodies against four Treponema phagedenis-like spirochete isolates—HT201, YG3903R, HD26-43, and IZ6-2—isolated from cows with PDD raised in four different locations (three different islands) in Japan and two control type strains—T. phagedenis ATCC 27087 and T. denticola JCM 8225. Fifty microliters of each bacterial antigen (5 μg/ml) was added to each of the wells of disposable polyvinyl chloride 96-well microplates (BD Falcon, Franklin Lakes, NJ) to coat the wells with the antigen, and each microplate was allowed to stand at 37°C for 1 h and then at 4°C overnight. The unbound antigens were then removed, and each well of the microplate was blocked by adding 200 μl of 1% skim milk in 10 mM PBS for 2 h at 37°C. The plates were washed three times with PBST. Sera from cattle, diluted 1:500 in PBST supplemented with 1% skim milk, were added to ELISA plate wells in triplicate and incubated at 37°C for 1 h. Positive (rabbit antiserum described above)- and negative (newborn calf serum)-control sera were used in every ELISA plate for each test. The plates were washed again, and then protein G labeled with horseradish peroxidase (Bio-Rad, Hercules, CA) diluted 1:2,500 in 1% skim milk in PBST was added as a conjugate, followed by incubation at 37°C for 1 h. Then, 50 μl of substrate solution (0.446 g of citric acid monohydrate, 0.73 g of sodium hydrogen phosphate, 40 mg of O-phenylenediamine, and 40 μl of 30% H2O2 in 100 ml of distilled water) was added to each well, followed by incubation at 37°C for 15 min. The reaction was stopped by adding 2.5 M sulfuric acid, and the optical density at 492 nm was measured with a multiwell ELISA plate reader (Benchmark Plus; Bio-Rad).
Statistical analysis.
Differences in antibody titers were compared statistically between pairs among the three groups, A, B, and C. Each datum was analyzed by the Mann-Whitney test using SPSS for Windows v.16.0 (SPSS Inc., Chicago, IL). For all analyses, differences at P < 0.05 were considered to be statistically significant.
RESULTS
SDS-PAGE profiles of T. phagedenis-like isolates.
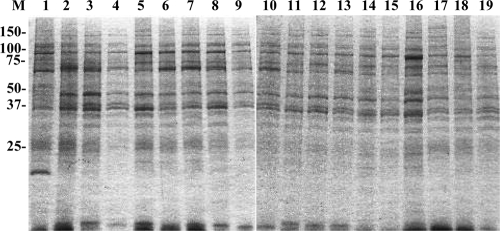
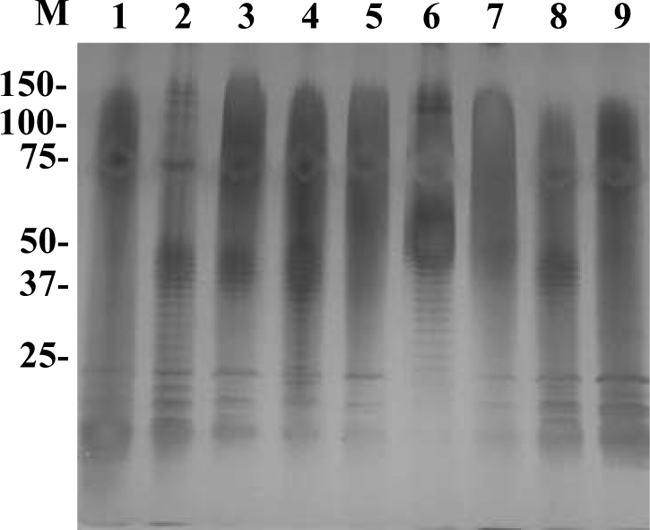
Coomassie brilliant blue staining showed that the banding patterns of whole-cell lysates were quite similar among all T. phagedenis-like spirochetes isolated from the PDD lesions examined, whereas the staining pattern of T. phagedenis type strain ATCC 27087 was partly different from that of the PDD isolates (Fig. 1).
FIG. 1.
SDS-PAGE profiles of whole-cell lysates of Treponema phagedenis-like spirochetes isolated from cattle with PDD. Lanes and isolates are as follow: M, prestained molecular mass markers shown in kilodaltons; 1, T. phagedenis type strain ATCC 27087; 2, HT201; 3, YG3903R; 4, YG5618; 5, HG42; 6, IZ6-2; 7, IZ7-2; 8, CH6; 9, CH9; 10, HD21-66; 11, HD21-R7; 12, HD22-63; 13, HD22-R16; 14, HD26-43; 15, HD26-67; 16, HD26-R4; 17, HD27-4; 18, HD27-24; 19, HD27-R6.
Western blot analysis.
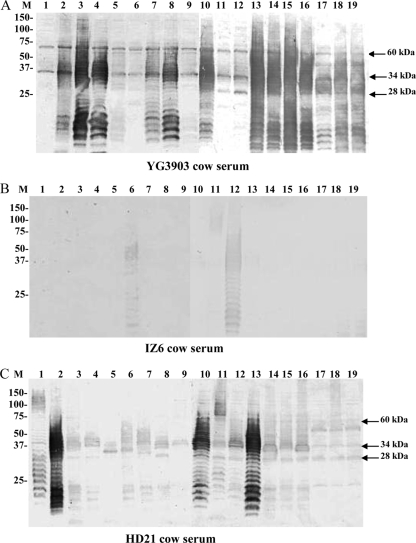
Western blot analysis demonstrated that the treponemes examined had considerable antigenic diversity in view of their different banding patterns (Fig. 2A), although the SDS-PAGE profiles of their whole-cell lysates were similar (Fig. 1). Furthermore, ladder-like bands were detected by some sera against several strains used as antigens, suggesting the presence of the glycolipid. However, some sera did not recognize such ladder-like antigen patterns. Although most of the serum samples reacted not only with isolates from the same animal but also with isolates isolated from different animals, serum from cow IZ6 reacted strongly with isolate IZ6-2 from the same animal and another isolate, HD22-63, but reacted weakly or partially with other isolates (Fig. 2B). When reactivities among multiple isolates from a single lesion or from different feet of the same animal were compared, different banding profiles were detected, as shown for the two isolates isolated from different feet of cow HD21 (Fig. 2C).
FIG. 2.
Western blotting profiles of sera from cow YG3903 (A), cow IZ6 (B), and cow HD21 (C) harboring PDD lesions reacted with bacterial whole-cell lysates of Treponema phagedenis-like organisms isolated from different cattle. Lanes and isolates are as follow: M, prestained molecular mass markers shown in kilodaltons; 1, T. phagedenis type strain ATCC 27087; 2, HT201; 3, YG3903R; 4, YG5618; 5, HG42; 6, IZ6-2; 7, IZ7-2; 8, CH6; 9, CH9; 10, HD21-66; 11, HD21-R7; 12, HD22-63; 13, HD22-R16; 14, HD26-43; 15, HD26-67; 16, HD26-R4; 17, HD27-4; 18, HD27-24; 19, HD27-R6.
We then examined whether individual cattle recognize a common antigen(s) against the T. phagedenis-like spirochetes tested. Most of the sera from cattle on PDD-positive farms, regardless of whether they harbored lesions, recognized a 60-kDa antigen as well as two other antigens with molecular masses of 34 kDa and 28 kDa (Table 2). Furthermore, the 60-kDa antigen was recognized by sera from both groups A and B, whereas the 34- and 28-kDa antigens were recognized by sera from group B rather than from group A. In contrast, sera taken from cattle on PDD-free farms (group C) showed no reactivity with the antigens of each of the strains used in this study (Table 2). None of the sera examined in this study reacted with a human isolate, T. denticola JCM 8225 (data not shown).
TABLE 2.
Antigens of T. phagedenis-like isolates recognized by sera of individual cows
| Cow serumb | No. of antigenic sites recognized by individual seraa |
||
|---|---|---|---|
| 60 kDa | 34 kDa | 28 kDa | |
| Group A | |||
| YG3903 | 18 | 18 | 6 |
| YG5618 | 18 | ||
| HG42 | 14 | 10 | |
| IZ6 | |||
| IZ7 | 18 | ||
| CH6 | 18 | ||
| CH9 | 8 | ||
| HD21 | 16 | 4 | 10 |
| HD22 | 18 | ||
| HD26 | 18 | 6 | 18 |
| HD27 | 12 | 5 | 14 |
| Group B | |||
| K1307 | 18 | 18 | |
| K1308 | 18 | 9 | 14 |
| K1374 | 18 | 18 | 18 |
| YG3904 | 18 | 10 | 12 |
| YG3927 | 18 | 10 | 9 |
| HD24(B) | 18 | 9 | 7 |
| Group C | |||
| U2416 | |||
| U9410 | |||
| U9412 | |||
| SUM2 | |||
| SUM5 | |||
Whole-cell lysate antigens of 18 T. phagedenis-like isolates were examined, and the numbers of isolates recognized by each individual serum sample were reported for each antigenic site.
Group A, PDD-positive cows on PDD-positive farms; group B, PDD-negative cows on PDD-positive farms; group C, cows on PDD-free farms.
Antibody reaction with glycolipid extracted from T. phagedenis-like spirochetes.
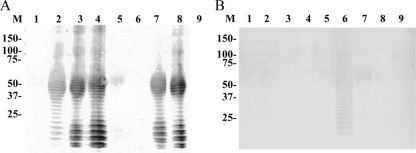
Silver staining of glycolipid extracted from the T. phagedenis ATCC 27087 type strain and 8 PDD isolates demonstrated ladder-like banding patterns indicating the presence of the glycolipid (Fig. 3). Western blot analysis using sera from two cows, YG3903 and IZ6, showed different reactivities against glycolipid from the 9 strains examined. The serum from cow YG3903 reacted with 5 of the 9 strains including homologous glycolipid (Fig. 4A). In contrast, the serum from cow IZ6 reacted strongly with the homologous glycolipid extracted from isolate IZ6-2 and weakly with glycolipid extracted from the two heterologous isolates, HT201 and IZ7-2 (Fig. 4B).
FIG. 3.
Silver-stained profiles of glycolipid of Treponema phagedenis-like organisms isolated from cattle with PDD. Lanes and isolates are as follows: M, prestained molecular mass marker shown in kilodaltons; 1, T. phagedenis type strain ATCC 27087; 2, HT201; 3, YG3903R; 4, YG5618; 5, HG42; 6, IZ6-2; 7, IZ7-2; 8, CH6; 9, CH9.
FIG. 4.
Western blot reaction profiles of IgG antibody in sera from two individuals, cow YG3903 (A) and cow IZ6 (B), harboring PDD lesions, against glycolipid of Treponema phagedenis-like organisms isolated from different cattle with PDD. Lanes and isolates are as follows: M, prestained molecular mass markers shown in kilodaltons; 1, T. phagedenis type strain ATCC 27087; 2, HT201; 3, YG3903R; 4, YG5618; 5, HG42; 6, IZ6-2; 7, IZ7-2; 8, CH6; 9, CH9.
Antibody response in ELISA.
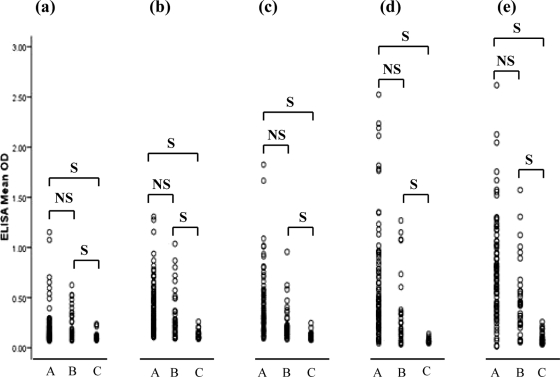
The level of IgG antibody against the T. phagedenis type strain and four T. phagedenis-like isolates (HT201, YG3903R, HD26-43, and IZ6-2) was measured by ELISA. The serum samples were divided into three groups (groups A, B, and C) as described above. As shown in Fig. 5, the optical density in groups A and B was significantly higher than that in group C. However, the optical densities for each T. phagedenis-like isolate varied among the sera. The antibody response to the T. phagedenis type strain was also similar to that seen in PDD isolates from groups A and B and group C (Fig. 5). However, the titer of IgG antibody against T. denticola JCM 8225 was very low, regardless of whether PDD was present or absent (data not shown).
FIG. 5.
IgG antibody responses in sera of cows determined by ELISA, against T. phagedenis ATCC 27087 (a), T. phagedenis-like HT201 (b), T. phagedenis-like YG3903R (c), T. phagedenis-like HD26-43 (d), and T. phagedenis-like IZ6-2 (e). Group A, PDD-positive cows on PDD-positive farms (n = 85); group B, PDD-negative cows on PDD-positive farms (n = 33); group C, cows on PDD-free farms (n = 33) for each ELISA. Each circle represents optical density at a wavelength of 492 nm. IgG levels against T. phagedenis ATCC 27087 and four T. phagedenis-like spirochetes of groups A and B are significantly higher than that of group C, but there are no significant differences between groups A and B. S, significant (P < 0.05); NS, not significant.
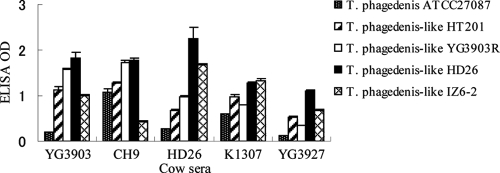
Finally, we compared the optical density values among sera from individuals against the same isolate. ELISA OD values of three sera from individual cows with PDD (YG3903, CH9, and HD26) and two sera from individuals without PDD (K1307 and YG3927) on PDD-positive farms were compared between each of the five ELISA antigens described above. As shown in Fig. 6, the optical densities of the serum varied among the antigens used in the ELISA.
FIG. 6.
Comparisons of ELISA mean ODs for five individual cow sera against T. phagedenis ATCC 27087 and four T. phagedenis-like spirochetes isolated from cows with PDD.
DISCUSSION
Although the precise etiology of PDD remains unclear, large numbers of spirochetes have been observed consistently among the bacteria detected in PDD lesions in many countries (1, 3, 13, 23). Our identification of candidate pathogens of PDD in dairy cattle using quantitative 16S rRNA clonal analysis confirmed that T. phagedenis-like isolates are among the most prevalent populations in PDD lesions but not in healthy foot skin (32). Therefore, understanding the immune response to these organisms in infected cattle is an important first step for designing effective vaccines.
In previous studies, systemic antibodies against T. phagedenis-like spirochetes were detected (4, 5, 6, 29, 30, 31). However, recurrent infection has also been reported even in a large proportion of some treated cows, i.e., 7 of 27 Holstein dairy cows (33%) in southern California (22) and 26 of 63 dairy cows (41%) in Japan (21). Therefore, they may not have been protective. One possible reason may be the presence of antigenic variations among T. phagedenis-like spirochetes, which may allow them to escape detection by the host immune system. To examine this hypothesis, we investigated the humoral immune response to isolates from individual cattle using Western blotting and ELISA.
First, using Western blotting, we examined the IgG immune response to multiple T. phagedenis-like isolates employing whole-cell lysates. We used samples of serum from cattle that had yielded each of the isolates used as the antigen, since we expected that the immune response against isolates isolated from the host would be analyzed, and the cattle produced a sufficient level of antibodies in the serum upon recognition of the bacterial antigens. Although the sera from affected cows recognized homologous and heterologous cell lysates, different banding patterns were detected even though the SDS-PAGE profiles were similar (Fig. 1). In some cases, no band was detected using heterologous sera. Interestingly, different banding patterns were detected between two isolates (Fig. 2C) that had been isolated from the same individuals (cows HD21 and HD22). These findings implied that antigenic heterogeneity such as serotypes exists among isolates or that treponemal antigens may vary in the host during infection. However, the antibodies against the T. phagedenis-like isolates detected here may have included some cross-reactive antibodies produced in part by other coinfecting treponeme species or unknown bacteria, and these antibodies may have contributed to the production of different banding patterns. Moreover, differences in the clinical stages of individual cows and/or the period of infection may have affected the antibody responses to the infecting treponemes. To resolve these issues, development of an animal model of PDD will be required.
Western blotting using whole-cell lysates showed that most of the reaction profiles had repeated ladder-like bands resembling the O-polysaccharide side chain of Gram-negative lipopolysaccharide (LPS). However, the presence of LPS in Treponema was ruled out recently because of the lack of LPS synthesis genes (9, 20, 26). The presence of glycolipid in Treponema resembling bacterial LPS was postulated, (11), and the detailed structure of a glycolipid in Treponema was first described by Schultz et al. (25). Silver staining of glycolipid derived from Treponema maltophilum and Treponema brennaborense revealed the presence of several “repeating units” (24). To confirm the immune response to this glycolipid, we extracted it from 8 T. phagedenis-like spirochetes and confirmed its reactivity with sera from affected cattle, suggesting that this glycolipid is one of the antigens most widely recognized in infected cattle. Silver staining of all glycolipid samples demonstrated repeated ladder-like bands, with profiles that were similar among the strains tested (Fig. 3). Although sera from two affected cows (YG3903 and IZ6) reacted with the glycolipid and the banding profile corresponded to that of the silver staining, these sera recognized only some of the glycolipids extracted from the isolates examined, suggesting the presence of O-like serotypes, as seen in Gram-negative bacteria such as Salmonella enterica and Escherichia coli (Fig. 4A and B). Using Western blot analysis, Trott et al. demonstrated the presence of antigenic diversity among 6 treponeme isolates using proteinase K-treated whole-bacterial-cell lysates and pooled sera from cows with PDD lesions (29). Our analysis of the immune response in individual cattle from PDD-positive farms was consistent with their observations.
In a previous study by Demirkan et al., Western blotting of antigens of treponemes isolated in the United States probed with PDD-positive sera of dairy cattle from the United Kingdom demonstrated reactivity with 34-, 41-, and 55-kDa antigens, although some sera responded to glycolipid-like material (4). The authors suggested that the flagellum, with a molecular mass of 41 kDa, may be the common antigenic site in spirochetes (4). In the present study, however, a 60-kDa antigen was the one most widely recognized in cattle from PDD-positive farms. An immunoblotting study by Trott et al. detected an 80-kDa band by using proteinase K-treated antigen from T. phagedenis-like isolate 4A, which was intensely reactive with pooled sera from three Iowa dairy cows with erosive PDD lesions (29). These differences in the recognition of bacterial antigens may be caused by both bacterial and host-related factors. To explain these differences, it will be necessary to conduct a comparative study of antigens of T. phagedenis-like spirochetes isolated in different countries as well as differences in antigenic recognition among different cattle at the species and individual level at various stages of infection.
In the present study, sera from cows with and without lesions on PDD-positive farms (groups A and B) showed significant levels of IgG against all of the four treponemal isolates examined, whereas IgG levels were very low in sera of cows on PDD-free farms (group C) in ELISA (Fig. 5). These results are consistent with previous serological investigations of antibody responses in dairy cattle to T. phagedenis-like isolates (4, 5, 31).
Vink et al. (30) suggested that application of serology is attractive because the outcome is objectively quantified on a continuous scale and taking serum samples is less time-consuming than performing foot inspection. They also pointed out that serology is potentially a better indicator of biological infection, enabling a more realistic and detailed description of the epidemiology of PDD (30). However, we found that the optical density of the serum varied among the antigens used for the ELISA in this study (Fig. 5). As expected, ELISA values for a single serum varied among the isolates used as the antigen, due to antigenic heterogeneity (Fig. 6). Elliott and Alt also reported that naturally exposed field cattle and test cattle that were primarily and secondarily inoculated with a mixed inoculum containing four live PDD treponemes in an equal ratio showed a humoral response to these PDD isolates but the reactivity varied among the spirochetes subjected to ELISA (6). Vink et al. also implemented antigen-specific ELISAs using 20 Treponema isolates cultured from PDD lesion biopsy samples and demonstrated that there were three apparent serogroups among the isolates tested (30). ELISA using only a single strain may detect PDD-positive farms. However, it cannot detect antibodies in individual cattle against T. phagedenis-like isolates, and evaluation of the presence of PDD in cattle may be misinterpreted. Therefore, pooled multiple treponemal species including different serotypes should be used in ELISA screening for accurate and useful serological analysis of anti-T. phagedenis-like antibody.
To prevent PDD in cattle, development of a vaccine with potential to protect against PDD infection is desirable. The present study was able to reveal some facts about the presence of variations in treponemal antigens and humoral immune responses. A combination of these multiple treponemal antigens may be more effective as a vaccine than the use of a single one. However, Trott et al. reported that the immune response in cattle appeared to be of short duration and diminished rapidly, suggesting that reinfection may occur in convalescent animals, although cattle with active stages of infection produce significant cellular and humoral antibody responses to T. phagedenis-like spirochetes (29). It is currently difficult to conclude whether treponemal antigen is useful to sustain protective immunity in cattle. Further detailed studies of immune responses to Treponema spp. in PDD lesions and the serotypes of heat-labile and heat-stable antigens will be required for the production of an effective vaccine. Furthermore, antigenic variations in T. phagedenis-like spirochetes in the host during infection will also need to be clarified.
Acknowledgments
This work was supported by the Japan Farriers Association; KAKENHI (Grant-in-Aid for Scientific Research) on Priority Areas “Applied Genomics” from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and Project for Zoonoses Education and Research, University of Miyazaki.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Blowey, R. W., and M. W. Sharp. 1988. Digital dermatitis in dairy cattle. Vet. Rec. 122:505-508. [DOI] [PubMed] [Google Scholar]
- 2.Choi, B. K., H. Nattermann, S. Grund, W. Haider, and U. B. Göbel. 1997. Spirochetes from digital dermatitis lesions in cattle are closely related to treponemes associated with human periodontitis. Int. J. Syst. Bacteriol. 47:175-181. [DOI] [PubMed] [Google Scholar]
- 3.Collighan, R. J., and M. J. Woodward. 1997. Spirochaetes and other bacterial species associated with bovine digital dermatitis. FEMS Microbiol. Lett. 156:37-41. [DOI] [PubMed] [Google Scholar]
- 4.Demirkan, I., R. L. Walker, R. D. Murray, R. W. Blowey, and S. D. Carter. 1999. Serological evidence of spirochaetal infections associated with digital dermatitis in dairy cattle. Vet. J. 157:69-77. [DOI] [PubMed] [Google Scholar]
- 5.Dhawi, A., C. A. Hart, I. Demirkan, I. H. Davies, and S. D. Carter. 2005. Bovine digital dermatitis and severe virulent ovine foot rot: a common spirochaetal pathogenesis. Vet. J. 169:232-241. [DOI] [PubMed] [Google Scholar]
- 6.Elliott, M. K., and D. P. Alt. 2009. Bovine immune response to papillomatous digital dermatitis (PDD)-associated spirochetes is skewed in isolate reactivity and subclass elicitation. Vet. Immunol. Immunopathol. 130:256-261. [DOI] [PubMed] [Google Scholar]
- 7.Evans, N. J., J. M. Brown, I. Demirkan, R. D. Murray, W. D. Vink, R. W. Blowey, C. A. Hart, and S. D. Carter. 2008. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 131:141-150. [DOI] [PubMed] [Google Scholar]
- 8.Fomsgaard, A., M. A. Freudenberg, and C. Galanos. 1990. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J. Clin. Microbiol. 28:2627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, C. Fujii, S. Garland, B. Hatch, K. Horst, K. Roberts, M. Sandusky, J. Weidman, H. O. Smith, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez, J., J. K. Shearer, and D. W. Webb. 2001. Effect of lameness on the calving-to-conception interval in dairy cows. J. Am. Vet. Med. Assoc. 218:1611-1614. [DOI] [PubMed] [Google Scholar]
- 11.Jackson, S. W., and P. N. Zey. 1973. Ultrastructure of lipopolysaccharide isolated from Treponema pallidum. J. Bacteriol. 114:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klitgaard, K., M. Boye, N. Capion, and T. K. Jensen. 2008. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 46:3012-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koniarová, I., A. Orsag, and V. Ledeckŷ. 1993. The role anaerobes in dermatitis digitalis et interdigitalis in cattle. Vet. Med. (Prague) 38:589-596. [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Losinger, W. C. 2006. Economic impacts of reduced milk production associated with papillomatous digital dermatitis in dairy cows in the USA. J. Dairy. Res. 73:244-256. [DOI] [PubMed] [Google Scholar]
- 16.McCoy, E. C., D. Doyle, K. Burda, L. B. Corbeil, and A. J. Winter. 1975. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of an antiphagocytic component. Infect. Immun. 11:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moter, A., G. Leist, R. Rudolph, K. Schrank, B. K. Choi, M. Wagner, and U. B. Göbel. 1998. Fluorescence in situ hybridization shows spatial distribution of as yet uncultured treponemes in biopsies from digital dermatitis lesions. Microbiology 144:2459-2467. [DOI] [PubMed] [Google Scholar]
- 18.Murray, R. D., D. Y. Downham, M. J. Clarkson, W. B. Faull, J. W. Hughes, F. J. Manson, J. B. Merritt, W. B. Russell, J. E. Sutherst, and W. R. Ward. 1996. Epidemiology of lameness in dairy cattle: description and analysis of foot lesions. Vet. Rec. 138:586-591. [DOI] [PubMed] [Google Scholar]
- 19.Nordhoff, M., A. Moter, K. Schrank, and L. H. Wieler. 2008. High prevalence of treponemes in bovine digital dermatitis—a molecular epidemiology. Vet. Microbiol. 131:293-300. [DOI] [PubMed] [Google Scholar]
- 20.Norris, S. J., and G. M. Weinstock. 2000. The genome sequence of Treponema pallidum, the syphilis spirochete: will clinicians benefit? Curr. Opin. Infect. Dis. 13:29-36. [DOI] [PubMed] [Google Scholar]
- 21.Ohtake, O., H. Noya, and T. Okuyama. 1999. Verrucous dermatitis of milk cow recently occurring in a frequent tendency. J. Livestock Med. 429:163-168. (In Japanese.) [Google Scholar]
- 22.Read, D. H., and R. L. Walker. 1998. Papillomatous digital dermatitis (footwarts) in California dairy cattle: clinical and gross pathologic findings. J. Vet. Diagn. Invest. 10:67-76. [DOI] [PubMed] [Google Scholar]
- 23.Read, D. H., R. L. Walker, A. E. Castro, J. P. Sundberg, and M. C. Thurmond. 1992. An invasive spirochaete associated with interdigital papillomatosis of dairy cattle. Vet. Rec. 130:59-60. [DOI] [PubMed] [Google Scholar]
- 24.Schröder, N. W., B. Opitz, N. Lamping, K. S. Michelsen, U. Zähringer, U. B. Göbel, and R. R. Schumann. 2000. Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J. Immunol. 65:2683-2693. [DOI] [PubMed] [Google Scholar]
- 25.Schultz, C. P., V. Wolf, R. Lange, E. Mertens, J. Wecke, D. Naumann, and U. Zahringer. 1998. Evidence for a new type of outer membrane lipid in oral spirochete Treponema denticola. Functioning permeation barrier without lipopolysaccharides. J. Biol. Chem. 273:15661-15666. [DOI] [PubMed] [Google Scholar]
- 26.Seshadri, R., G. S. Myers, H. Tettelin, J. A. Eisen, J. F. Heidelberg, R. J. Dodson, T. M. Davidsen, R. T. DeBoy, D. E. Fouts, D. H. Haft, J. Selengut, Q. Ren, L. M. Brinkac, R. Madupu, J. Kolonay, S. A. Durkin, S. C. Daugherty, J. Shetty, A. Shvartsbeyn, E. Gebregeorgis, K. Geer, G. Tsegaye, J. Malek, B. Ayodeji, S. Shatsman, M. P. McLeod, D. Smajs, J. K. Howell, S. Pal, A. Amin, P. Vashisth, T. Z. McNeill, Q. Xiang, E. Sodergren, E. Baca, G. M. Weinstock, S. J. Norris, C. M. Fraser, and I. T. Paulsen. 2004. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc. Natl. Acad. Sci. U. S. A. 101:5646-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shearer, J. K., and S. R. Van Amstel. 2000. Manual for the Master Hoof Care Technician Program, p.6-14. Department of Large Animal Clinical Sciences-College of Veterinary Medicine, University of Florida, Gainesville, FL.
- 28.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trott, D. J., M. R. Moeller, R. L. Zuerner, J. P. Goff, W. R. Waters, D. P. Alt, R. L. Walker, and M. J. Wannemuehler. 2003. Characterization of Treponema phagedenis-like spirochetes isolated from papillomatous digital dermatitis lesions in dairy cattle. J. Clin. Microbiol. 41:2522-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vink, W. D., G. Jones, W. O. Johnson, J. Brown, I. Demirkan, S. D. Carter, and N. P. French. 2009. Diagnostic assessment without cut-offs: application of serology for the modelling of bovine digital dermatitis infection. Prev. Vet. Med. 92:235-248. [DOI] [PubMed] [Google Scholar]
- 31.Walker, R. L., D. H. Read, K. J. Loretz, D. W. Hird, and S. L. Berry. 1997. Humoral response of dairy cattle to spirochetes isolated from papillomatous digital dermatitis lesions. Am. J. Vet. Res. 58:744-748. [PubMed] [Google Scholar]
- 32.Yano, T., K. K. Moe, K. Yamazaki, T. Ooka, T. Hayashi, and N. Misawa. 17 December 2009, posting date. Identification of candidate pathogens of papillomatous digital dermatitis in dairy cattle from quantitative 16S rRNA clonal analysis. Vet. Microbiol. doi: 10.1016/j.vetmic.2009.12.009. [DOI] [PubMed]
- 33.Yano, T., R. Yamagami, K. Misumi, C. Kubota, K. K. Moe, T. Hayashi, K. Yoshitani, O. Ohtake, and N. Misawa. 2009. Genetic heterogeneity among strains of Treponema phagedenis-like spirochetes isolated from dairy cattle with papillomatous digital dermatitis in Japan. J. Clin. Microbiol. 47:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]