Abstract
There currently are commercial fowlpox virus (FPV)-vectored vaccines for use in chickens, including TROVAC-AIV H5, which expresses the hemagglutinin (HA) antigen of an avian influenza virus and can confer immunity against avian influenza in chickens. Despite the use of recombinant FPV (rFPV) for vaccine delivery, very little is known about the immune responses generated by these viruses in chickens. The present study was designed to investigate host responses to rFPV in vivo and in vitro. In cultured cells infected with TROVAC-AIV H5, there was an early increase in the expression of type I interferons (IFN), Toll-like receptors 3 and 7 (TLR3 and TLR7, respectively), TRIF, and MyD88, which was followed by a decrease in the expression of these genes at later time points. There also was an increase in the expression of interleukin-1β (IL-1β), IL-8, and beta-defensin genes at early time points postinfection. In chickens immunized with TROVAC-AIV H5, there was higher expression of IFN-γ and IL-10 at day 5 postvaccination in spleen of vaccinated birds than in that of control birds. We further investigated the ability of the vaccine to induce immune responses against the HA antigen and discovered that there was a cell-mediated response elicited in vaccinated chickens against this antigen. The findings of this study demonstrate that FPV-vectored vaccines can elicit a repertoire of responses marked by the early expression of TLRs, type I interferons, and proinflammatory cytokines, as well as cytokines associated with adaptive immune responses. This study provides a platform for designing future generations of rFPV-vectored vaccines.
Fowlpox virus (FPV) belongs to the genus Avipoxvirus and causes a disease of economic importance in poultry. FPV infection occurs through insect bites, skin abrasions, or the respiratory system, and the disease caused by the virus is presented in two forms: cutaneous and/or diphtheric (5, 48). Attenuated FPVs have been employed as vaccines for chickens or as candidate vectors for the delivery of recombinant proteins in both mammalian and avian species (38, 53). FPV-based vectors have been demonstrated to efficiently elicit both antibody- and T-cell-mediated immune responses against the antigen that they express (23, 34, 43). In mammals, applications of FPV-vectored vaccines include infectious diseases and cancer. Immunization with a FPV vaccine expressing the glycoprotein antigen of rabies virus resulted in conferring protection in mice, dogs, and cats (53). More recently, the safety and efficacy of FPV vaccines against malaria and human immunodeficiency virus (HIV) have been assessed in experimental animal models as well as in human trials (9). In mice, an FPV-vectored vaccine expressing an epitope of the V3 loop of several HIV strains was shown to elicit T helper 1 (Th1) and CD8+ T-cell responses to the cognate HIV antigen (57). The delivery of gag proteins of HIV or simian immunodeficiency virus (SIV) by FPV in rabbits resulted in the induction of antibody- and cell-mediated responses to these proteins (44). Moreover, the administration of FPV expressing the E2 protein of bovine viral diarrhea virus (BVDV) to mice led to the induction of gamma interferon (IFN-γ) in spleen mononuclear cells of vaccinated mice following the incubation of their cells with BVDV (34). In the case of malaria, the use of FPV-vectored vaccines in human trials has shown promising results. For instance, Webster and colleagues (59) reported that a prime-boost vaccination regimen, which included FPV expressing the preerythrocytic-stage malaria antigen thrombospondin-related adhesion protein (TRAP) for priming and a vaccinia Ankara virus expressing TRAP for boosting, could confer protection in humans against challenge with Plasmodium falciparum. FPV also has been used in clinical trials of several anticancer vaccines. These FPV vectors express tumor-associated antigens, such as carcinoembryonic antigen, prostate-specific antigen, and NY-ESO-1, which is a malignancy/testis antigen (9, 22). In the case of NY-ESO-1, the delivery of this antigen by FPV resulted in the generation of antibody and T-cell responses against various epitopes of this antigen in the vaccinated patients. Moreover, vaccination appeared to have favorably affected the course of disease in the vaccinated patients (22).
In domestic animal species, FPV also has been used as a delivery mechanism for various vaccines, and these vaccines have been shown to induce T-cell responses. Shen and colleagues (47) demonstrated that the coexpression of GP5/GP3 proteins of porcine reproductive and respiratory syndrome virus by recombinant FPV (rFPV) led to enhanced T-cell responses, marked by increased IFN-γ concentrations in sera of immunized pigs. However, there also are some reports that, at least in mammals, FPV may not be able to elicit T-cell responses to same extent as other viruses (12). This may be associated with the suboptimal induction of type I interferons by FPV, leading to the perturbed development and expansion of effector CD8+ T cells (12).
Several experimental and commercial vaccines for chickens have employed FPV for the delivery of viral antigens. These vaccines are against Marek's disease virus, infectious bursal disease, Newcastle disease virus (NDV), and avian influenza virus (AIV) (18, 36, 50, 55), of which FPV-vectored vaccines against NDV and AIV are commercially available. Several rFPV vaccines containing the hemagglutinin (HA) gene of AIV have been studied in the past (8, 56). Recombinant FPV expressing HA from a highly pathogenic avian influenza (HPAI) strain, A/turkey/Ireland/1378/83 (H5N8), induced immune responses to and protection against AIV in chickens (52, 60). In addition, a similar construct (TROVAC-AIV H5 vaccine) induced antibodies to HA in cats (26). Swayne et al. (50) showed that the same vaccine prevented the morbidity and mortality of chickens, which were challenged with any of the eight different strains of HPAI virus. However, there also are some caveats associated with the use of FPV-vectored vaccines. For example, the presence of maternal antibodies against FPV could interfere with the vaccination of young birds. Furthermore, similarly to many other viral vectors, secondary immunization with the same vector may not be effective due to the elicitation of a memory immune response following primary immunization. Nevertheless, the efficiency of FPV as a vector for the delivery of chicken vaccines has been underscored by several studies. However, our understanding of avian host immune responses to FPV is very limited. There is only one report of induction cell-mediated responses by FPV in immunized chickens (49). Therefore, the present study was designed to elucidate immune responses to a recombinant FPV in chickens. We reasoned that gaining a better understanding of these responses should lead to the development of better FPV vectors that have a superior capacity of inducing appropriate responses, both quantitatively and qualitatively, against the insert that they deliver. In the present study, we have profiled host responses to FPV at the molecular and cellular levels.
MATERIALS AND METHODS
Chickens and housing.
Specific-pathogen-free (SPF), 1-day-old chicks were obtained from the Animal Disease Research Institute, Canadian Food Inspection Agency (Ottawa, Ontario, Canada). The birds were maintained in floor pens on clean wood shavings at the Ontario Ministry of Agriculture and Food Isolation Unit (University of Guelph, Ontario, Canada). The research complied with University of Guelph Animal Care Committee guidelines.
Recombinant vaccine.
The TROVAC-AIV H5 vaccine was kindly provided by Merial Select Inc. (Gainesville, GA). This vaccine is a recombinant fowlpox virus vector that expresses AIV HA antigen derived from the A/turkey/Ireland/1378/83 (H5N8) isolate. The vaccine was stored at 4°C as recommended by the manufacturer.
Experimental design.
Chicken embryo fibroblasts (CEFs) were infected with TROVAC-AIV H5 and harvested at several time points postinfection for RNA extraction and cDNA synthesis before the quantitative real-time PCR analysis of cDNA for several immune system genes.
For in vivo experiments, 14-day-old chickens were divided into two groups: 34 were vaccinated subcutaneously with one dose of TROVAC-AIV H5 as recommended by the manufacturer, and 34 control chickens received the vaccine diluent only. Six birds from each group were euthanized on days 1, 5, 7, 14, and 21 postinoculation, spleen tissues were aseptically removed, and 100 mg was stored in RNAlater (Qiagen Inc., Mississauga, Ontario, Canada) at −80°C for RNA extraction. The rest of the spleen tissue was kept in Hank's balanced salt solution (HBSS) on ice following removal from chickens and used immediately for fluorescence-activated cell sorter (FACS) analysis. To determine cell-mediated responses, four vaccinated and four control chickens were euthanized on day 21 postvaccination. Spleen tissues were aseptically removed, and spleen mononuclear cells were prepared to determine their proliferative response to the HA antigen.
Single-cell suspension of spleen and separation of mononuclear cells.
Spleens were kept in HBSS buffer on ice following removal from chickens. After being rinsed with HBSS, the tissue was minced with a scalpel and crushed with the flat end of a syringe in sterile petri dishes. Larger pieces were allowed to settle out of the cell suspensions, which subsequently were filtered through 40-μm BD cell strainers (BD Biosciences, Bedford, MA). Cell numbers and viability were assessed using a hemacytometer and the trypan blue exclusion method.
To isolate mononuclear cells, 1.5 × 108 spleen cells in a 4-ml volume were layered onto 4 ml Histopaque 1077 (Sigma-Aldrich Canada Ltd., Oakville, ON, Canada) and centrifuged at 400 × g for 20 min to allow the separation of mononuclear cells. Mononuclear cells were removed from the interface and washed three times in cell culture medium, RPMI 1640 medium containing 10% fetal bovine serum, 50 μg/ml gentamicin, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen Canada Inc., Burlington, Ontario, Canada). Cells were resuspended in either FACS buffer (1% bovine serum albumin in phosphate-buffered saline [PBS]) or cell culture medium.
Cloning and expression of hemagglutinin.
The entire coding sequence of the HA protein expressed by the vaccine was amplified by PCR using the following primers: forward primer, 5′-ATCGGATCCGAGGAAATAGTGCTTCTTTTTGC-3′; reverse primer, 5′-ATCCTCGAGCAATGCAAATTCTGCACTGCAATG-3′. The full length of HA was inserted into the BamHI-HindIII cloning site of the expression vector pET-28a (Novagen, Madison, WI) to express the His-tagged HA protein in Escherichia coli strain BL21. Expression was induced by treatment with isopropyl-β-d-thiogalactopyranoside (IPTG), and the expression product was purified using a His-tagged protein purification kit (QIAexpressionist; Qiagen Inc., Mississauga, Canada) according to the manufacturer's manual.
In vitro spleen cell stimulation and proliferative response.
Splenocytes were prepared from vaccinated and unvaccinated birds. Cells were suspended in cell culture medium to a density of 1 × 106 cells/ml and plated at 100 μl per well in 96-well round-bottom plates. The HA antigen, which was homologous to the one produced by TROVAC-AIV H5, was expressed in vitro in E. coli and added to splenocytes at a final concentration of 3.2 ng per ml of cell culture medium. This concentration was chosen based on a pilot experiment using different concentrations of HA. As a positive control for the proliferation assay, spleen mononuclear cells also were treated with concanavalin A (ConA) (Sigma, Oakville, Ontario, Canada) at 20 μg/ml. Untreated cells served as a negative control. Following the addition of treatment, cells were incubated at 41°C with 5% CO2 in a humidified incubator for 24, 48, or 72 h. To measure proliferative responses, 1 μCi of methyl-3H-thymidine (25 Ci/mmol) (Amersham, Buckinghamshire, United Kingdom) was added to each well 18 h before the end of each time point. Plates were kept frozen at −80°C until processed. Cells were harvested onto glass fiber filters, and thymidine incorporation into newly synthesized DNA was measured using the TopCount NXT Microplate Scintillation Counter (Packard/PerkinElmer, Waltham, MA). Results are presented as mean counts per minute (cpm) ± standard errors (SE).
In vitro spleen cell stimulation and cytokine expression.
Spleen mononuclear cells were suspended at a density of 1 × 107 cells/ml in RPMI 1640 medium (Invitrogen, Grand Island, NY) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. One ml of splenocyte suspension was added into each well of a 24-well flat-bottomed plate. The cells were treated in duplicate by three different treatments, including (i) HA, (ii) ConA as a positive control, and (iii) medium only as negative control. The cells were incubated at 41°C in an incubator with 5% CO2 and collected at 2, 8, 16 h poststimulation for the extraction of RNA.
CEFs.
Primary CEFs were prepared from 9- to 11-day-old SPF embryonated chicken eggs. The embryos were dissected using aseptic procedures and then minced and trypsinized with 0.025% of trypsin in PBS at 37°C. After centrifugation, the cells were counted, and 106 cells were plated onto 60-mm dishes supplemented with Dulbecco's minimal essential medium containing 5% fetal bovine serum and incubated at 37°C in the presence of 5% CO2. Twenty-four hours after being seeded, the cells were either mock infected or infected with the TROVAC-AIV H5 vaccine at a multiplicity of infection (MOI) of 0.5. Infected and uninfected cells were washed twice with PBS at different time points postinfection (p.i.) and harvested for RNA extraction.
RNA extraction and reverse transcription-PCR.
Total RNA was extracted from spleens and CEFs as described previously (3) using TRIzol reagent (Invitrogen Canada, Inc., Burlington, Ontario, Canada). RNA then was treated with DNase using the DNA-free system (Ambion, Austin, TX) to remove any remaining DNA contamination. The RNA concentration was measured by spectrophotometry (GeneQuant; Biochrom Limited, Cambridge, England).
RNA was reverse transcribed to cDNA by following the instructions of the manufacturer (Superscript First-Strand cDNA Synthesis; Invitrogen Corporation, Carlsbad, CA). The oligo(dT)12-18 primer from this kit was used. The cDNA was stored at −20°C until it was used for real-time PCR.
Standard curves and real-time PCR.
The expression of different immune system genes was quantified by real-time PCR using standard curves. The preparation of the constructs, designing of primers, and creation of standard curves for the genes used in this study have been previously described: β-actin, IFN-γ, and IL-18 (3); IL-10 (2); IL-1β, chicken IL-8 (chIL-8), IFN-α, and IFN-β (1); IL-1β, Toll-like receptor 3 (TLR3), and TLR7 (M. F. Abdul-Careem, unpublished data); myeloid differentiation primary response gene 88 (MyD88) (10); and avian beta defensin 4 (AvBD4) and AvBD6 (7). The sequence of the TIR domain-containing adapter inducing IFN-β (TRIF) gene was obtained from GenBank (accession number EF025853). Primers specific for TRIF (forward, 5′-GCTGACCAAGAACTTCCTGTGC-3′; reverse, 5′-AGAGTTCTCATCCAAGGCCACC-3′) were designed using the PrimerDesign program. Real-time PCR was carried out on the cDNA samples described above. β-Actin was amplified as a reference gene. The PCRs were made up in 20-μl total volumes: 3 μl of PCR-grade water, 0.25 μM each primer, 5 μl of cDNA template (1:10 dilution), and 10 μl of 2× SYBR green reaction mix (containing MgCl2 and FastStart Taq DNA polymerase; Lightcycler 480 SYBR green I master mix; Roche Diagnostics GmbH, Mannheim, Germany). All primers used in this study were synthesized by Sigma-Aldrich Canada, Ltd., Oakville, Ontario, Canada. The samples were amplified in 384-well plates using a Lightcycler 480 instrument (Roche Diagnostics GmbH, Mannheim, Germany). Every real-time PCR assay was run with a negative control (PCR-grade water replacing cDNA template) and a related standard sample as a calibrator.
Data analysis.
For the real-time PCR data, efficiency was calculated as E = 10−1/slope of standard curve. With the cytokine of interest as the target and β-actin as the reference, the relative gene expression ratio was determined as follows:
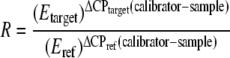 |
where Etarget and Eref are the efficiencies of the target cytokine and β-actin, respectively, and ΔCP is the difference of crossing points between calibrator and samples (40). The relative expression ratios, R, were used to determine differences in cytokine expression among different groups, excluding outliers.
The expression of normalized target genes in TROVAC-AIV H5-infected CEF cells, vaccinated birds, or HA-stimulated cells was compared to that of control uninfected CEF cells, sham-immunized birds, or unstimulated cells, respectively, using the relative expression software tool (REST-MCS) (41). In REST-MCS, the difference between the means of crossing points of tested groups and control groups are presented as the fold change. The difference between treated and control groups was tested for significance by Student's t test (P ≤ 0.05).
For cell proliferation data, the mean cpm was compared among all groups at each time point using analysis of variance (ANOVA). Differences were considered significant when P ≤ 0.05, while comparisons with 0.05 > P ≤ 0.1 were considered to show a trend toward statistical significance.
RESULTS
Profiling the expression of cytokine, Toll-like receptor, and defensin genes in CEF cells infected with TROVAC-AIV H5.
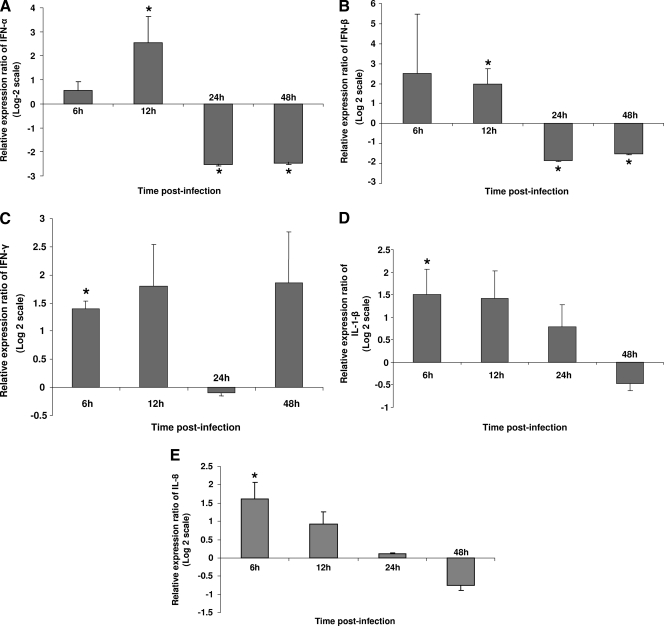
To investigate the effects of FPV on innate responses of chicken cells in vitro, CEF cells were infected with TROVAC-AIV H5, and the expression of a panel of immunologically important genes was evaluated at several time points postinfection. These genes were selected based on their potential relevance for innate responses against FPV. Furthermore, consideration was given to the ability of CEF cells to express these genes based on the available literature (11, 21, 25, 63), and those genes that are expressed exclusively by cells of lymphoid or myeloid origin were excluded from our analysis. CEF cells were used in this experiment, because FPV can infect chicken fibroblasts. Among the genes that were studied here, IFN-α, IFN-β, and IFN-γ have antiviral effects, whereas IL-1β and IL-8 are known to play a proinflammatory role in the chicken. Figure 1 illustrates the expression of IFN-α, IFN-β, IFN-γ, IL-1β, and chIL-8 in mock- and TROVAC-AIV H5-infected CEF cells (Fig. 1A to E, respectively). The levels of expression of both IFN-α and IFN-β in TROVAC-AIV H5-infected CEF cells was significantly higher than those of mock-infected CEF cells at 12 h postinfection (h p.i); however, the expression of both cytokines was significantly lower than that of uninfected CEF cells at 24 and 48 h p.i. (P < 0.05). The expression of IFN-γ in TROVAC-AIV H5-treated cells was significantly higher than that of mock-infected cells only at 6 h p.i. (P ≤ 0.05). Also, the expression of both IL-1β and chIL-8 in TROVAC-AIV H5-treated CEF cells was significantly higher than that of mock-infected cells at 6 h p.i. only (P ≤ 0.05). Overall, these results demonstrate that at early time points, FPV is able to induce antiviral and proinflammatory cytokines. However, at later time points, the expression of some of these genes may be significantly dampened.
FIG. 1.
Relative expression ratio of cytokines following infection of CEF cells with TROVAC-AIV H5 compared to that of control cells. The relative expression of IFN-α (A), IFN-β (B), IFN-γ (C), IL-1 β (D), and chIL-8 (E) in CEF cells was measured following mock infection or infection with TROVAC-AIV H5 at different time points postinfection in four independent experiments. Target and reference gene expression in the cells was quantified by real-time reverse transcription-PCR, and the expression ratio was calculated as the means of gene expression in samples of TROVAC-AIV H5-treated mice compared to that of the controls. An asterisk indicates significant difference (P < 0.05). Error bars represent standard errors from the means.
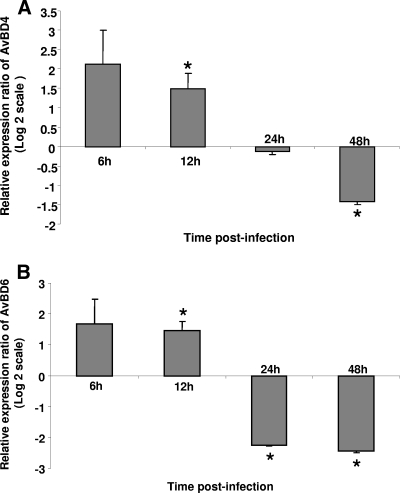
Beta-defensins are known to be involved in antiviral activities, and they also can act as chemoattractants for cells of the immune system (13). AvBD4 and AvBD6 were selected because we have previously shown their expression in the chicken immune system (7). The expression of AvBD4 and AvBD6 in TROVAC-AIV H5-treated CEF cells is illustrated in Fig. 2A and B, respectively. The expression of AvBD4 was significantly higher in TROVAC-AIV H5-treated CEF cells than in control cells at 6 and 12 h p.i. (P ≤ 0.05). The levels of transcripts of AvBD4 were significantly lower (P ≤ 0.05) in TROVAC-AIV H5-treated cells than in controls at 48 h p.i (Fig. 2A). The AvBD6 expression was increased significantly in TROVAC-AIV H5-infected CEF cells compared to that of uninfected cells at 12 h p.i. (Fig. 2B). However, the expression of AvBD6 was significantly (P ≤ 0.05) lower in TROVAC-AIV H5-treated cells than in control cells at 24 and 48 h p.i. (Fig. 2B). These findings underline the relevance of defensins in the host response to FPV.
FIG. 2.
Relative expression ratio of antimicrobial peptides in CEF cells after TROVAC-AIV H5 infection compared to that of uninfected cells. Relative expression of AvBD4 and AvBD6 in CEF cells was measured after infection with TROVAC-AIV H5 in four independent experiments. The expression of AvBD4 (A) and AvBD6 (B) in TROVAC-AIV H5-treated CEF cells is illustrated. The levels of target and reference gene expression in the cells were quantified by real-time RT-PCR and are presented as expression ratios of target genes to reference genes. An asterisk indicates significant difference (P < 0.05). Error bars represent standard errors from the means.
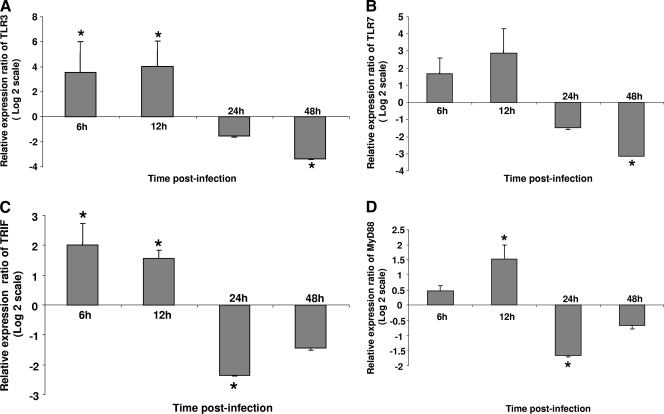
TLRs and their adaptor molecules were examined in this study. Although FPV is a DNA virus, the ortholog of TLR9, which recognizes the unmethylated DNA of pathogens, has not been identified in the chicken. Among the other TLRs that play a role in antiviral immunity, only orthologs of TLR3 and TLR7 have been identified in chickens (30). Therefore, these two TLRs were selected for further analysis. We also examined the expression of TRIF, the adaptor molecule for TLR3, and MyD88, the adaptor molecule for TLR7. The expression of TLR3 in TROVAC-AIV H5-treated cells was significantly (P ≤ 0.05) higher than that in control cells at 6 and 12 h p.i. However, its expression was significantly lower than that of control cells at 48 h. p.i. (Fig. 3A). A similar pattern was observed for TLR7, where the expression of this gene was significantly lower at 48 h p.i. in infected than in mock-infected cells (P ≤ 0.05) (Fig. 3B). The expression of TRIF in TROVAC-AIV H5-treated cells was significantly (P ≤ 0.05) higher than that in mock-infected cells at 6 and 12 h p.i.; however, there was a significant downregulation in the expression of this gene in TROVAC-AIV H5-treated cells compared to that in mock-infected control cells (Fig. 3C). The expression of MyD88 in TROVAC-AIV H5-treated cells was significantly (P ≤ 0.05) higher than that in control cells at 12 h p.i.; however, the expression of this gene was significantly lower in TROVAC-AIV H5-treated cells than in control cells at 24 h p.i. (Fig. 3D). These results clearly show that TLR pathways are triggered after FPV infection and that, similarly to type I interferons, the expression of TLRs may be downregulated at late time points postinfection.
FIG. 3.
Relative expression ratio of TLRs and TLR adaptors in infected and mock-treated CEF cells. The expression of TLR3 (A), TLR7 (B), TRIF (C), and MyD88 (D) in the mock-treated and TROVAC-AIV H5-infected cells in four biological replicates are illustrated. Target and reference gene expression in the cells were quantified by real-time RT-PCR and are presented as the expression ratio of target genes to reference genes. An asterisk indicates significant difference (P < 0.05). Error bars represent standard errors from the means.
Effects of vaccination with TROVAC-AIV H5 on the expression of cytokines in spleen of chickens.
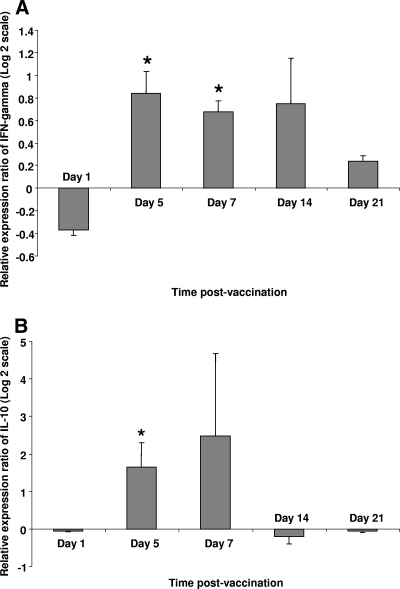
The expression of IFN-γ and IL-10 was examined in vaccinated and control birds at indicated time points postvaccination. These cytokines serve as indicators of the activation of Th1/CD8+ T cells and regulatory T cells, respectively. The expression of IFN-γ was significantly higher in spleens of vaccinated chickens than in controls on days 5 and 7 postvaccination (Fig. 4A). The expression of IL-10 also was detected in both vaccinated and control groups at all time points throughout the trial; however, IL-10 expression was significantly higher in vaccinated chickens than in controls on day 5 postvaccination (Fig. 4B).
FIG. 4.
Relative expression ratio of cytokines in spleen of vaccinated chickens compared to that of control birds. The expression of IFN-γ (A) and IL-10 (B) was compared between chickens vaccinated with TROVAC (n = 6) and control chickens that were treated with vaccine diluent only (n = 6). Target and reference gene expression in spleen cells was quantified by real-time RT-PCR, and expression ratios were calculated as the means of gene expression in samples of vaccinated group compared to that of controls. An asterisk indicates significant difference (P < 0.05). Error bars represent standard errors from the means.
In vitro responses of splenocytes derived from vaccinated birds.
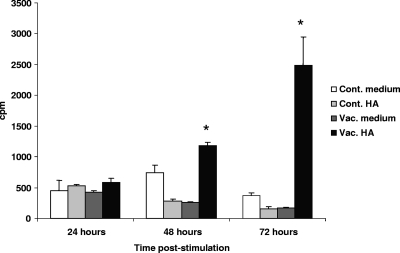
To confirm that chickens immunized with the vaccine can mount an antigen-specific cell-mediated response, splenocytes from vaccinated birds were treated with the HA protein expressed by TROVAC-AIV H5, and the proliferation response of cells was measured at different time points poststimulation. Stimulation with HA significantly elicited proliferative responses in splenocytes of vaccinated birds compared to responses of controls at 48 and 72 h poststimulation (Fig. 5). The stimulation of cells from either vaccinated or control birds with ConA resulted in significant proliferation (data not shown), whereas the stimulation of cells from control chickens with HA did not induce proliferation. Taken together, these results indicate that the HA protein expressed by TROVAC-AIV H5 can elicit a cell-mediated response in vaccinated chickens.
FIG. 5.
In vitro proliferative responses of splenocytes to recombinant HA antigen. Chickens were vaccinated with TROVAC-AIV H5 or received vaccine diluent as a control. Splenocytes from four vaccinated chickens were treated with HA or medium and are shown as Vac. HA and Vac. medium, respectively. Also, splenocytes from four unvaccinated chickens (as controls) were treated with HA and medium and are shown as Cont. HA and Cont. medium. The differences in proliferative responses among the groups at each time point were tested and considered significant at P ≤ 0.05 (*).
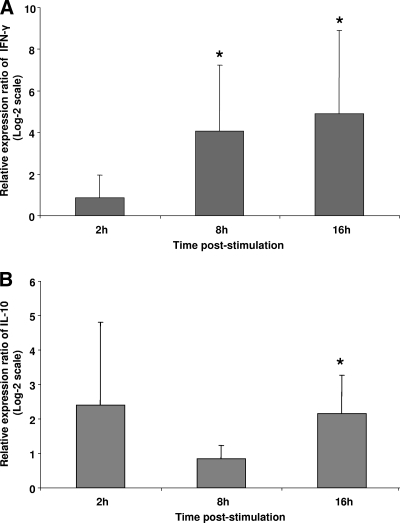
To further examine antigen-specific cytokine responses elicited by TROVAC-AIV H5, spleen mononuclear cells from vaccinated or control chickens were cultured and treated with HA. The fold change in IFN-γ and IL-10 levels for cells derived from vaccinated chickens compared to those of control chickens at 2, 8, and 16 h poststimulation are shown in Fig. 6A and B. The treatment of cells with HA resulted in a significantly higher expression of IFN-γ in cells of vaccinated birds at 8 and 16 h poststimulation compared to that of controls (Fig. 6A). In the case of IL-10, the higher expression of this cytokine in cells derived from vaccinated birds was significant only at 16 h poststimulation compared to that of control cells (Fig. 6B). These findings again underscore the ability of HA to induce a cell-mediated response in chickens.
FIG. 6.
Relative expression ratio of cytokines in spleen cells of vaccinated chickens following stimulation with HA antigen compared to that of control cells. The expression levels of IFN-γ (A) and IL-10 (B) were compared between the HA-stimulated group and medium-treated controls in four biological replicates. Target and reference gene expression levels in spleen cells were quantified by real-time RT-PCR, and the expression ratio was calculated as the means of gene expression in samples of HA-treated group compared to that of medium-treated controls. An asterisk indicates significant difference (P < 0.05). Error bars represent standard errors from the means.
DISCUSSION
The use of FPV as a vaccine delivery vector has been extensively explored experimentally in mammalian and avian species. This vector also has been used in a commercial poultry vaccine against avian influenza virus. However, responses mounted by the chicken host to this vector have not been fully studied. Here, we report innate and cytokine responses mounted by cultured cells infected with recombinant FPV. In addition, cellular and cytokine responses to FPV in vivo in chickens immunized with TROVAC-AIV H5 are presented.
To study innate responses to FPV mounted by chicken cells, we examined the expression of type I and II interferons, IL-1β, and IL-8, as well as other genes that usually are associated with innate responses, including defensins, TLRs, and TLR adaptors, in CEF cells infected with TROVAC AIV H5. In general, a common and striking feature of these expression data was the enhanced expression of almost all of the genes at early time points, 6 and 12 h p.i., followed by a decline in their expression at later time points, namely, 24 and 48 h poststimulation. The upregulation of these genes indicates the ability of FPV to elicit proinflammatory responses leading to the recruitment and activation of adaptive immune system cells. IL-1β and IL-8 both are proinflammatory cytokines whose function has been studied in the chicken (61, 62). The expression of both cytokines is enhanced following infection with certain viruses, such as reovirus and infectious bursal disease virus (27, 28, 61).
Type I interferons are involved in antiviral responses and also play a part in the induction of adaptive immune responses. Plasmocytoid DCs (pDCs) as well as other host cells, such as fibroblasts, are responsible for the production of these cytokines (54). Interestingly, it has been reported that rFPV can induce only suboptimal levels of type I interferon expression by pDCs, which presumably leads to inefficient CD4+ T-cell help and transient cytotoxic T-cell activity (12). Despite this finding, we observed a significant increase in the expression of type I interferons at 12 h postinfection. The discrepancy between our observations and those previously reported may be related to the cells used in the present study, i.e., fibroblasts as opposed to pDCs. Also, the ability of FPV to replicate in avian cells should be taken into account.
Here, we report for the first time the induction of TLRs and their adaptor molecules after FPV infection in chicken cells. We found that TLR3 and TLR7 as well as TRIF and MyD88, which act as adaptor molecules for TLR3 and TLR7, respectively, are upregulated early after the infection of cells with rFPV. TLR3 and TLR7 are the only TLRs known to date to be involved in antiviral responses in chickens. TLR3 is involved in the recognition of double-stranded RNA (dsRNA). In addition to the recognition of RNA viruses, TLR3 also has been shown to be involved in the recognition of some DNA viruses, such as cytomegalovirus and herpes simplex virus type 2 (HSV-2) (15, 51). Importantly, DNA viruses such as adenovirus, HSV, and vaccinia virus may produce dsRNA during their replication cycles, hence activating the TLR3 pathway (58). The engagement of TLR3 may lead to triggering the expression of proinflammatory cytokines and chemokines, such as IL-1β and IL-8 (16, 31, 32). Interestingly, TLR3 appears to have a detrimental effect in the vaccinia infection model, as the deletion of this gene results in the lower morbidity and mortality caused by vaccinia virus in mice (20). TLR7 also may be involved in protection against DNA viruses, because TLR7−/− mice are susceptible to infection with murine cytomegalovirus (64). However, the involvement of TLR7 in the host response to FPV has not been explored previously. Overall, the results described above support the notion that both TLR3 and TLR7 play a part in early host response to FPV. It is noteworthy that the ortholog of TLR9, which is the receptor for CpG DNA and that may be involved in recognition of DNA viruses, has not been identified in the chicken genome. Therefore, it is possible that TLR3 and TLR7 act as substitutes for TLR9 in the chicken. Although the results described above demonstrate an increase only in the abundance of gene transcripts and not necessarily an increase in the function or cellular translocation of the protein products of these genes, they underscore the involvement of TLRs in the process of host response to rFPV.
In the studies presented here, there was an increase in the expression of beta-defensins (AvBD4 and AvBD6). In addition to their antimicrobial activities, beta-defensins have other functions. For example, they act as a chemotactic factor or are involved in the maturation, differentiation, and activation of various cells of the immune system (46). Beta-defensins have antiviral effects against some viruses, such as adenovirus, HIV, and influenza virus, while their effects against vaccinia virus remain somewhat contradictory (19, 29). We have demonstrated previously that the expression of AvBD4 and AvBD6 are induced following infection with Salmonella enterica serovar Typhimurium (7). To our knowledge, this is the first report of the induction of beta-defensins to a virus infection in chickens. Although the efficacy of AvBD4 and AvBD6 in neutralizing rFPV was not studied here, it is tempting to speculate that these antimicrobial peptides can neutralize rFPV or be involved in the process of the elicitation of immune responses.
Despite the upregulation of many of the genes studied here, at early time points after the infection of CEF cells with FPV, we noticed a decline in the expression of some of the genes (IFN-α and IFN-β, AvBD4 and AvBD6, TLR3 and TLR7, TRIF, and MyD88) at late time points postinfection. In general, poxviruses express an arsenal of proteins with immune evasive properties (45). Among these, a protein has been identified with sequence homology to type I interferon receptors that is involved in binding to IFN-α and IFN-β, hence neutralizing their activity (45). In the case of TLR molecules, several vaccinia virus proteins have been identified that target and interfere with signaling through TLRs. For instance, A52R inhibits the activation of NF-κB after TLR engagement (17). A52R also appears to bind to two of the TLR signaling proteins, IRAK2 and TRAF6, hence interfering with the process of signaling (17). Another protein of vaccinia virus, A46R, interferes with the process of signaling through TRIF and MyD88 (17). It is not known whether FPV also encodes the same proteins and can interfere with TLR signaling or IFN activity in the same fashion, but based on our results, it appears that this is a possible scenario.
Vaccinia virus and some of the other poxviruses encode proteins that can bind and neutralize IL-1β (17, 45). Furthermore, proteins with IFN-γ binding capacity have been identified in a wide range of poxviruses, including FPV (42, 45). However, in the study presented here, there was no significant decline in the abundance of IL-1β and IFN-γ transcripts over time. This may be due partly to the possibility that although cytokine activity is affected at the protein level, the expression of these cytokines may not be affected. Therefore, further investigations are needed to decipher the potential immune evasive mechanisms of FPV. This information will be important for the design of future FPV vectors, because immune evasive mechanisms of the vector may affect the immunogenicity of the vector itself, as well as its ability to elicit a response against the insert that it expresses.
An in vivo study also was conducted to further examine host responses to FPV. Cellular responses to FPV were characterized by a slight, albeit statistically significant, increase in the percentage of CD4+ cells and a decrease in the percentage of CD8+ cells among spleen cells at 1 day p.i. Although due to experimental limitations we could not examine the antigen specificity of CD4+/CD8+ cells in our system, our findings are somewhat different from those of Diener and colleagues (12). These authors showed that in the mouse model, recombinant FPV expressing ovalbumin (OVA) could elicit a strong, albeit transient, cytotoxic T-cell response. This was, however, accompanied by poor CD4+ T-cell help (12). In our study, we did not directly investigate the effector functions of FPV-specific T cells, thus it is possible that despite the observed decrease in the percentage of CD8+ cells, the absolute number of these cells had not changed, or even if the numbers had changed, the effector activity on a per-cell basis might actually have increased. The other issue that needs some consideration to explain the difference between the study reported here and that reported by Diener and colleagues (12) is the fact that recombinant FPV does not replicate in the mammalian host, whereas it can replicate in the avian host (39, 53). This ability of the virus might impact the elicitation of immune responses in avian versus mammalian species. Nevertheless, we observed a significant increase in IFN-γ expression on days 5 and 7 postimmunization, as well as IL-10 expression on day 5 postimmunization. Furthermore, when antigen-specific responses to HA, which is expressed by the rFPV used in this study, were examined, there was a significant activation of cells in response to HA as well as an increase in the expression of both IFN-γ and IL-10. The coexpression of IL-10 and IFN-γ may be counterintuitive, because IL-10 is considered a regulatory cytokine whose production usually suppresses the expression of Th1 cytokine, such as IFN-γ. However, there are reports that Th1 cells also may promiscuously express regulatory cytokines, including IL-10, as a part of the self-regulation process (37). In addition, exposure to certain cytokines, such as IL-12, may trigger CD4+ and CD8+ T-cell subsets to secrete both IFN-γ and IL-10 (14). Aside from T cells, dendritic cells (DCs) also may act as a source of cytokines. Indeed, Agrawal and coworkers (6) have reported the production of IL-10 and IL-12 (another Th1 cytokine) by human DCs stimulated with three vaccinia virus proteins, including D8L, D13L, and H3L. The coexpression of IL-10 and IFN-γ also has been shown in domestic animal species in response to viral infections. In piglets infected with porcine reproductive and respiratory syndrome virus (PRRS), the simultaneous expression of IFN-γ and IL-10 genes was observed in peripheral blood mononuclear cells (24). Our group also has shown a concomitant increase in the expression of IL-10 and IFN-γ in chicken spleens following vaccination with herpesvirus of turkeys against Marek's disease (4). Therefore, the results of the study presented here raise the possibility that, similarly to some other infections, IL-10 has a dual role, both regulatory and stimulatory, in chickens infected or vaccinated with FPV (33, 35, 37). Another scenario is that FPV exploits IL-10 as a mechanism to evade the host immune system. However, these possibilities need further investigation.
In conclusion, we have demonstrated the elicitation of host responses both in vivo and in vitro after infection with rFPV. This information will become important for the design of the future generation of rFPV vectors for vaccine delivery. The future vectors should induce an appropriate response, both quantitatively and qualitatively. Moreover, these vectors should be devoid of proteins with immune-evasive properties so that a protective immune response can be elicited.
Acknowledgments
Funding was provided by the Poultry Industry Council, Canadian Poultry Research Council, Saskatchewan Chicken Industry, Ontario Ministry of Agriculture, Food and Rural Affairs, and Natural Sciences and Engineering Research Council of Canada-Agriculture and Agri-Food Canada Partnerships Program.
Footnotes
Published ahead of print on 13 January 2010.
REFERENCES
- 1.Abdul-Careem, M. F., B. D. Hunter, L. F. Lee, J. H. Fairbrother, H. R. Haghighi, L. Read, P. Parvizi, M. Heidari, and S. Sharif. 2008. Host responses in the bursa of Fabricius of chickens infected with virulent Marek's disease virus. Virology 379:256-265. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Careem, M. F., B. D. Hunter, P. Parvizi, H. R. Haghighi, N. Thanthrige-Don, and S. Sharif. 2007. Cytokine gene expression patterns associated with immunization against Marek's disease in chickens. Vaccine 25:424-432. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Careem, M. F., B. D. Hunter, A. J. Sarson, A. Mayameei, H. Zhou, and S. Sharif. 2006. Marek's disease virus-induced transient paralysis is associated with cytokine gene expression in the nervous system. Viral Immunol. 19:167-176. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Careem, M. F., D. B. Hunter, M. D. Lambourne, L. R. Read, P. Parvizi, and S. Sharif. 2008. Expression of cytokine genes following pre- and post-hatch immunization of chickens with herpesvirus of turkeys. Vaccine 26:2369-2377. [DOI] [PubMed] [Google Scholar]
- 5.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2000. The genome of fowlpox virus. J. Virol. 74:3815-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal, S., S. Gupta, and A. Agrawal. 2009. Vaccinia virus proteins activate human dendritic cells to induce T-cell responses in vitro. Vaccine 27:88-92. [DOI] [PubMed] [Google Scholar]
- 7.Akbari, M. R., H. R. Haghighi, J. R. Chambers, J. Brisbin, L. R. Read, and S. Sharif. 2008. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clin. Vaccine Immunol. 15:1689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beard, C. W., W. M. Schnitzlein, and D. N. Tripathy. 1991. Protection of chickens against highly pathogenic avian influenza virus (H5N2) by recombinant fowlpox viruses. Avian Dis. 35:356-359. [PubMed] [Google Scholar]
- 9.Beukema, E. L., M. P. Brown, and J. D. Hayball. 2006. The potential role of fowlpox virus in rational vaccine design. Expert Rev. Vaccines 5:565-577. [DOI] [PubMed] [Google Scholar]
- 10.Brisbin, J. T., H. Zhou, J. Gong, P. Sabour, M. R. Akbari, H. R. Haghighi, H. Yu, A. Clarke, A. J. Sarson, and S. Sharif. 2008. Gene expression profiling of chicken lymphoid cells after treatment with Lactobacillus acidophilus cellular components. Dev. Comp. Immunol. 32:563-574. [DOI] [PubMed] [Google Scholar]
- 11.Dar, A., S. Munir, S. Vishwanathan, A. Manuja, P. Griebel, S. Tikoo, H. Townsend, A. Potter, V. Kapur, and L. A. Babiuk. 2005. Transcriptional analysis of avian embryonic tissues following infection with avian infectious bronchitis virus. Virus Res. 110:41-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diener, K. R., E. L. Lousberg, E. L. Beukema, A. Yu, P. M. Howley, M. P. Brown, and J. D. Hayball. 2008. Recombinant fowlpox virus elicits transient cytotoxic T-cell responses due to suboptimal innate recognition and recruitment of T-cell help. Vaccine 26:3566-3573. [DOI] [PubMed] [Google Scholar]
- 13.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 14.Gerosa, F., C. Paganin, D. Peritt, F. Paiola, M. T. Scupoli, M. Aste-Amezaga, I. Frank, and G. Trinchieri. 1996. Interleukin-12 primes human CD4 and CD8 T-cell clones for high production of both interferon-gamma and interleukin-10. J. Exp. Med. 183:2559-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill, N., P. M. Deacon, B. Lichty, K. L. Mossman, and A. A. Ashkar. 2006. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J. Virol. 80:9943-9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillot, L., R. Le Goffic, S. Bloch, N. Escriou, S. Akira, M. Chignard, and M. Si-Tahar. 2005. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571-5580. [DOI] [PubMed] [Google Scholar]
- 17.Haga, I. R., and A. G. Bowie. 2005. Evasion of innate immunity by vaccinia virus. Parasitology 130(Suppl.):S11-S25. [DOI] [PubMed] [Google Scholar]
- 18.Haygreen, E. A., P. Kaiser, S. C. Burgess, and T. F. Davison. 2006. In ovo DNA immunisation followed by a recombinant fowlpox boost is fully protective to challenge with virulent IBDV. Vaccine 24:4951-4961. [DOI] [PubMed] [Google Scholar]
- 19.Howell, M. D., J. E. Streib, and D. Y. Leung. 2007. Antiviral activity of human beta-defensin 3 against vaccinia virus. J. Allergy Clin. Immunol. 119:1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchens, M., K. E. Luker, P. Sottile, J. Sonstein, N. W. Lukacs, G. Nunez, J. L. Curtis, and G. D. Luker. 2008. TLR3 increases disease morbidity and mortality from vaccinia infection. J. Immunol. 180:483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal, M., V. J. Philbin, and A. L. Smith. 2005. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 104:117-127. [DOI] [PubMed] [Google Scholar]
- 22.Jäger, E., J. Karbach, S. Gnjatic, A. Neumann, A. Bender, D. Valmori, M. Ayyoub, E. Ritter, G. Ritter, D. Jager, D. Panicali, E. Hoffman, L. Pan, H. Oettgen, L. J. Old, and A. Knuth. 2006. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc. Natl. Acad. Sci. USA 103:14453-14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, W., N. Jin, S. Cui, Z. Li, L. Zhang, H. Zhang, H. Wang, and W. Han. 2005. Construction and characterization of recombinant fowlpox virus coexpressing HIV-1(CN) gp120 and IL-2. J. Virol. Methods 130:95-101. [DOI] [PubMed] [Google Scholar]
- 24.Johnsen, C. K., A. Botner, S. Kamstrup, P. Lind, and J. Nielsen. 2002. Cytokine mRNA profiles in bronchoalveolar cells of piglets experimentally infected in utero with porcine reproductive and respiratory syndrome virus: association of sustained expression of IFN-gamma and IL-10 after viral clearance. Viral Immunol. 15:549-556. [DOI] [PubMed] [Google Scholar]
- 25.Karaca, G., J. Anobile, D. Downs, J. Burnside, and C. J. Schmidt. 2004. Herpesvirus of turkeys: microarray analysis of host gene responses to infection. Virology 318:102-111. [DOI] [PubMed] [Google Scholar]
- 26.Karaca, K., D. E. Swayne, D. Grosenbaugh, M. Bublot, A. Robles, E. Spackman, and R. Nordgren. 2005. Immunogenicity of fowlpox virus expressing the avian influenza virus H5 gene (TROVAC AIV-H5) in cats. Clin. Diagn. Lab. Immunol. 12:1340-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khatri, M., J. M. Palmquist, R. M. Cha, and J. M. Sharma. 2005. Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res. 113:44-50. [DOI] [PubMed] [Google Scholar]
- 28.Khatri, M., and J. M. Sharma. 2006. Infectious bursal disease virus infection induces macrophage activation via p38 MAPK and NF-kappaB pathways. Virus Res. 118:70-77. [DOI] [PubMed] [Google Scholar]
- 29.Klotman, M. E., and T. L. Chang. 2006. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 6:447-456. [DOI] [PubMed] [Google Scholar]
- 30.Kogut, M. H., M. Iqbal, H. He, V. Philbin, P. Kaiser, and A. Smith. 2005. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 29:791-807. [DOI] [PubMed] [Google Scholar]
- 31.Krasowska-Zoladek, A., M. Banaszewska, M. Kraszpulski, and G. W. Konat. 2007. Kinetics of inflammatory response of astrocytes induced by TLR 3 and TLR4 ligation. J. Neurosci. Res. 85:205-212. [DOI] [PubMed] [Google Scholar]
- 32.Maelfait, J., E. Vercammen, S. Janssens, P. Schotte, M. Haegman, S. Magez, and R. Beyaert. 2008. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J. Exp. Med. 205:1967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mege, J. L., S. Meghari, A. Honstettre, C. Capo, and D. Raoult. 2006. The two faces of interleukin 10 in human infectious diseases. Lancet Infect. Dis. 6:557-569. [DOI] [PubMed] [Google Scholar]
- 34.Mehdy Elahi, S., J. Bergeron, E. Nagy, B. G. Talbot, S. Harpin, S. H. Shen, and Y. Elazhary. 1999. Induction of humoral and cellular immune responses in mice by a recombinant fowlpox virus expressing the E2 protein of bovine viral diarrhea virus. FEMS Microbiol. Lett. 171:107-114. [DOI] [PubMed] [Google Scholar]
- 35.Mocellin, S., F. Marincola, C. R. Rossi, D. Nitti, and M. Lise. 2004. The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 15:61-76. [DOI] [PubMed] [Google Scholar]
- 36.Nazerian, K., L. F. Lee, N. Yanagida, and R. Ogawa. 1992. Protection against Marek's disease by a fowlpox virus recombinant expressing the glycoprotein B of Marek's disease virus. J. Virol. 66:1409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Garra, A., and P. Vieira. 2007. T(H)1 cells control themselves by producing interleukin-10. Nat. Rev. Immunol. 7:425-428. [DOI] [PubMed] [Google Scholar]
- 38.Paoletti, E. 1990. Poxvirus recombinant vaccines. Ann. N. Y. Acad. Sci. 590:309-325. [DOI] [PubMed] [Google Scholar]
- 39.Paoletti, E. 1996. Applications of pox virus vectors to vaccination: an update. Proc. Natl. Acad. Sci. USA 93:11349-11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puehler, F., H. Schwarz, B. Waidner, J. Kalinowski, B. Kaspers, S. Bereswill, and P. Staeheli. 2003. An interferon-gamma-binding protein of novel structure encoded by the fowlpox virus. J. Biol. Chem. 278:6905-6911. [DOI] [PubMed] [Google Scholar]
- 43.Radaelli, A., M. Gimelli, C. Cremonesi, C. Scarpini, and C. De Giuli Morghen. 1994. Humoral and cell-mediated immunity in rabbits immunized with live non-replicating avipox recombinants expressing the HIV-1SF2 env gene. Vaccine 12:1110-1117. [DOI] [PubMed] [Google Scholar]
- 44.Radaelli, A., C. Zanotto, G. Perletti, V. Elli, E. Vicenzi, G. Poli, and C. De Giuli Morghen. 2003. Comparative analysis of immune responses and cytokine profiles elicited in rabbits by the combined use of recombinant fowlpox viruses, plasmids and virus-like particles in prime-boost vaccination protocols against SHIV. Vaccine 21:2052-2064. [DOI] [PubMed] [Google Scholar]
- 45.Seet, B. T., C. A. McCaughan, T. M. Handel, A. Mercer, C. Brunetti, G. McFadden, and S. B. Fleming. 2003. Analysis of an orf virus chemokine-binding protein: Shifting ligand specificities among a family of poxvirus viroceptors. Proc. Natl. Acad. Sci. USA 100:15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selsted, M. E., and A. J. Ouellette. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551-557. [DOI] [PubMed] [Google Scholar]
- 47.Shen, G., N. Jin, M. Ma, K. Jin, M. Zheng, T. Zhuang, H. Lu, G. Zhu, H. Jin, M. Jin, X. Huo, X. Qin, R. Yin, C. Li, H. Li, Y. Li, Z. Han, Y. Chen, and M. Jin. 2007. Immune responses of pigs inoculated with a recombinant fowlpox virus coexpressing GP5/GP3 of porcine reproductive and respiratory syndrome virus and swine IL-18. Vaccine 25:4193-4202. [DOI] [PubMed] [Google Scholar]
- 48.Singh, P., T. J. Kim, and D. N. Tripathy. 2000. Re-emerging fowlpox: evaluation of isolates from vaccinated flocks. Avian Pathol. 29:449-455. [DOI] [PubMed] [Google Scholar]
- 49.Singh, P., and D. N. Tripathy. 2003. Fowlpox virus infection causes a lymphoproliferative response in chickens. Viral Immunol. 16:223-227. [DOI] [PubMed] [Google Scholar]
- 50.Swayne, D. E., M. Garcia, J. R. Beck, N. Kinney, and D. L. Suarez. 2000. Protection against diverse highly pathogenic H5 avian influenza viruses in chickens immunized with a recombinant fowlpox vaccine containing an H5 avian influenza hemagglutinin gene insert. Vaccine 18:1088-1095. [DOI] [PubMed] [Google Scholar]
- 51.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor, J., R. Weinberg, Y. Kawaoka, R. G. Webster, and E. Paoletti. 1988. Protective immunity against avian influenza induced by a fowlpox virus recombinant. Vaccine 6:504-508. [DOI] [PubMed] [Google Scholar]
- 53.Taylor, J., R. Weinberg, B. Languet, P. Desmettre, and E. Paoletti. 1988. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine 6:497-503. [DOI] [PubMed] [Google Scholar]
- 54.Tovey, M. G., C. Lallemand, and G. Thyphronitis. 2008. Adjuvant activity of type I interferons. Biol. Chem. 389:541-545. [DOI] [PubMed] [Google Scholar]
- 55.Tripathy, D. N. 2004. The impact of vaccines and the future of genetically modified poxvirus vaccines for poultry. Anim. Health Res. Rev. 5:263-266. [DOI] [PubMed] [Google Scholar]
- 56.Tripathy, D. N., and W. M. Schnitzlein. 1991. Expression of avian influenza virus hemagglutinin by recombinant fowlpox virus. Avian Dis. 35:186-191. [PubMed] [Google Scholar]
- 57.Vázquez-Blomquist, D., S. Gonzalez, and C. A. Duarte. 2002. Effect of promoters on cellular immune response induced by recombinant fowlpox virus expressing multi-epitope polypeptides from HIV-1. Biotechnol. Appl. Biochem. 36:171-179. [DOI] [PubMed] [Google Scholar]
- 58.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webster, D. P., S. Dunachie, J. M. Vuola, T. Berthoud, S. Keating, S. M. Laidlaw, S. J. McConkey, I. Poulton, L. Andrews, R. F. Andersen, P. Bejon, G. Butcher, R. Sinden, M. A. Skinner, S. C. Gilbert, and A. V. Hill. 2005. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc. Natl. Acad. Sci. USA 102:4836-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webster, R. G., Y. Kawaoka, J. Taylor, R. Weinberg, and E. Paoletti. 1991. Efficacy of nucleoprotein and haemagglutinin antigens expressed in fowlpox virus as vaccine for influenza in chickens. Vaccine 9:303-308. [DOI] [PubMed] [Google Scholar]
- 61.Wu, Y. F., H. J. Liu, J. H. Shien, S. H. Chiou, and L. H. Lee. 2008. Characterization of interleukin-1beta mRNA expression in chicken macrophages in response to avian reovirus. J. Gen. Virol. 89:1059-1068. [DOI] [PubMed] [Google Scholar]
- 62.Wu, Y. F., J. H. Shien, H. H. Yin, S. H. Chiow, and L. H. Lee. 2008. Structural and functional homology among chicken, duck, goose, turkey and pigeon interleukin-8 proteins. Vet. Immunol. Immunopathol. 125:205-215. [DOI] [PubMed] [Google Scholar]
- 63.Xing, Z., and K. A. Schat. 2000. Expression of cytokine genes in Marek's disease virus-infected chickens and chicken embryo fibroblast cultures. Immunology 100:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zucchini, N., G. Bessou, S. Traub, S. H. Robbins, S. Uematsu, S. Akira, L. Alexopoulou, and M. Dalod. 2008. Cutting edge: overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J. Immunol. 180:5799-5803. [DOI] [PubMed] [Google Scholar]