Abstract
The immunogenicities of conjugate pneumococcal vaccines have been demonstrated when they are administered at 2, 3, and 4 months of age. There is a paucity of data on the immunogenicity of this vaccine when it is administered concurrently with other vaccines in the primary immunization schedule of the United Kingdom. We immunized 55 term infants at 2, 3, and 4 months of age with the seven-valent pneumococcal conjugate vaccine (PCV7), the meningococcal group C conjugate (MCC) vaccine, and the diphtheria, tetanus, five-component acellular pertussis, inactivated polio, and Haemophilus influenzae type b (DTaP5/IPV/Hib-TT) vaccine. The immune responses to the H. influenzae type b (Hib), MCC, and tetanus vaccines were measured at 2, 5, and 12 months of age; and the immune responses to PCV7 were measured at 2 and 5 months and then either at 12 months or following a 4th dose of PCV7. There were increases in the geometric mean concentrations (GMCs) of all antigens postimmunization. Greater than or equal to 90% of the infants achieved putatively protective levels postimmunization for all vaccine antigens except pneumococcal serotype 6B and Hib. The GMCs of the PCV7 serotypes increased following a 4th dose, although one infant had not reached putative levels of protection against serotype 6B. In conclusion, when infants were vaccinated according to the schedule described above, they had lower postprimary immunization responses to Hib, meningococcus group C capsular polysaccharide, and pneumococcal serotype 6B than the responses demonstrated by use of the other schedules. Despite this finding, there was a good response following a 4th dose of PCV7.
The primary immunization schedule of the United Kingdom is continually evolving. While a vaccine may have been demonstrated to be immunogenic when it was administered according to one schedule, minor changes to that schedule can have an adverse impact on the response to the vaccine (2). In 2002, the chief medical officer of the United Kingdom recommended that infants considered to be at increased risk of invasive pneumococcal disease receive the seven-valent pneumococcal conjugate vaccine (PCV7) at 2, 3, and 4 months of age with their primary immunizations as well as a booster dose in the second year of life (8). In September 2004, the combined diphtheria, tetanus, five-component acellular pertussis, inactivated polio, and Haemophilus influenzae type b conjugate (DTaP5/IPV/Hib-TT) vaccine was introduced into the United Kingdom primary immunization schedule.
PCV7 has previously been demonstrated to have good immunogenicity when it is administered at 2, 4, and 6 months of age (3) and at 2, 3, and 4 months of age (7). There is a paucity of data examining the immunogenicity of PCV7 when it is administered concurrently with the DTaP5/IPV/Hib-TT vaccine and a meningococcal group C conjugate (MCC) vaccine. Additionally, at the time of this study, no other immunizations were boosted during the second year of life, and a questionnaire survey of neonatal units suggested that many at-risk infants were not receiving the recommended booster dose (14).
We therefore recruited healthy term infants to determine the immunogenicity of PCV7 when it was administered at 2, 3, and 4 months of age with the other vaccines in the primary immunization schedule of the United Kingdom in effect at that time. Additionally, we examined the effect of a booster dose in infants who responded poorly to the primary schedule and examined the antibody response at 12 months of age in infants who had had a good response to the primary immunization schedule.
MATERIALS AND METHODS
This study was approved by the Newcastle and North Tyneside, United Kingdom, local research ethics committees and the Medicines and Healthcare Research Authority.
Subjects.
Fifty-five healthy term infants were recruited within a few days of birth from the postnatal wards at the Royal Victoria Infirmary, Newcastle upon Tyne, between April and June 2005. We excluded infants who had received any neonatal intervention prior to recruitment.
Vaccines.
Following the provision of written informed parental consent, primary immunizations were given at home at 2, 3, and 4 months of age, in accordance with the routine United Kingdom immunization schedule in effect at that time. This comprised the combined DTaP/IPV/Hib vaccine (Pediacel; Aventis Pasteur) and an MCC conjugated to the mutant diphtheria toxin (CRM197) conjugate vaccine (Meningitec; Wyeth Vaccines), as well as PCV7 (Prevenar; Wyeth Vaccines). All vaccines were administered concomitantly in separate limbs. A single commercial batch of PCV7 was used. Prior to immunization, all infants were offered 0.5 to 2 ml of 25% sucrose solution orally as analgesia.
Booster dose.
Only infants who had not achieved putatively protective levels of antibodies to all vaccine serotypes following the primary course of PCV7 were offered a booster dose, as soon as the results from the primary immunizations were known.
Serology.
Venous blood samples were obtained at the time of the first immunization, at 4 weeks following the 3rd immunization (range, 21 to 51 days), and at either 12 months of age or 4 weeks after a booster dose of PCV7. The blood was allowed to clot at room temperature. The serum was then separated and stored at −80°C until analysis. Pneumococcal serotype-specific IgG antibody concentrations for all PCV7 serotypes and two control serotypes (serotypes 1 and 5) were analyzed by enzyme-linked immunosorbent assay (ELISA) at Newcastle University by a standardized protocol which included capsular polysaccharide and serotype 22F capsular polysaccharide adsorption (www.vaccine.uab.edu).
Sera were tested by the serum bactericidal assay (SBA) for functional antibody against group C Neisseria meningitidis by using baby rabbit complement, as described previously, at the Vaccine Evaluation Unit (VEU), Health Protection Agency (HPA), Manchester, United Kingdom (13).
SBA titers were expressed as the reciprocal serum dilution yielding 50% or greater killing after 60 min. Standardized ELISAs were also performed for the detection of IgG antibody responses to meningococcal group C capsular polysaccharide (VEU, HPA) (4, 6), tetanus, and Haemophilus influenzae type b (Hib) (Royal Victoria Infirmary) (15).
Statistical analysis.
For statistical analysis, all samples with titers below the lower limit of detection of an assay were assigned values of half the lower limit of detection. Antibody concentrations were then log transformed, and the geometric mean concentrations (GMCs) (or geometric mean titers [GMTs] for SBA) were calculated with 95% confidence intervals (CIs). The results were compared by paired t tests.
Putatively protective levels for all pneumococcal serotypes were defined as pneumococcal serotype-specific titers of ≥0.35 μg/ml, in line with WHO recommendations (19). The short-term correlates of protection were used for Hib (0.15 μg/ml) and SBA (1:8) (1, 4), and the accepted protective correlate of 0.1 μg/ml was used for tetanus. The proportion of infants achieving putatively protective levels to each antigen was also calculated, and the results were compared by the use of chi-square tests.
RESULTS
Study population.
Fifty-five infants were recruited, 1 infant emigrated during the study period, the 1st blood sample was not collected from 1 infant, and 1 infant had insufficient sera from the 2nd sample for complete analysis. Thirty-one infants received a 4th dose of PCV7, and postbooster serum was collected from 29 of them; serum was collected at 12 months of age from 23 infants who had not received a booster. The median age at the first immunization was 61.5 days (range, 56 to 71 days), the median age at the 3rd immunization was 118 days (range, 112 to 132 days), and the median age at the time that the 2nd blood sample was drawn was 147 days (range, 139 to 172 days). The median age at the time of receipt of the 4th PCV7 was 342 days (range, 325 to 361 days), and the median age at the time of collection of the postbooster samples was 376 days (range, 354 to 402 days). The median age at the time of collection of the 3rd blood sample from the infants who did not receive a booster dose was 373 days (range, 361 to 420 days).
Immunogenicity.
The pre- and postimmunization IgG GMCs or GMTs for all antigens tested are given in Table 1 . There was a statistically significant increase in the IgG levels from pre- to postimmunization for all vaccine antigens examined (P < 0.01), whereas the concentrations for the two pneumococcal control serotypes decreased over the same time period (P < 0.01).
TABLE 1.
Antibody responses to pneumococcus pre- and postimmunization and response to MCC, Hib, and tetanus vaccines preimmunization, postimmunization, and at 12 months
| Antigen and serotype or assay | IgG GMC (μg/ml) |
No. (%) of infants achieving putatively protective levelsa |
||||
|---|---|---|---|---|---|---|
| Preimmunization (n = 53) | Postimmunization (n = 53) | 12 mo (n = 52) | Preimmunization | Postimmunization | 12 mo | |
| Antipneumococcal | ||||||
| STb 4 | 0.04 (0.03, 0.05)c | 1.61 (1.26, 2.07) | 0 | 50 (96) | ||
| ST 6B | 0.08 (0.06, 0.10) | 0.29 (0.20, 0.42) | 6 (12) | 20 (38) | ||
| ST 9V | 0.07 (0.05, 0.11) | 1.07 (0.82, 1.39) | 9 (17) | 47 (90) | ||
| ST 14 | 0.33 (0.22, 0.49) | 3.80 (2.79, 5.19) | 23 (44) | 51 (98) | ||
| ST 18C | 0.26 (0.20, 3.04) | 1.39 (1.11, 1.75) | 23 (44) | 50 (96) | ||
| ST 19F | 0.45 (0.36, 0.56) | 2.64 (2.06, 3.39) | 33 (63) | 52 (100) | ||
| ST 23F | 0.12 (0.08, 0.16) | 1.46 (1.07, 2.00) | 11 (21) | 50 (96) | ||
| ST 1 | 0.03 (0.02, 0.03) | 0.02 (0.01, 0.02) | 1 (2) | 0 | ||
| ST 5 | 0.11 (0.08, 0.16) | 0.07 (0.06, 0.09) | 1 (2) | 1 (2) | ||
| Anti-meningoccocus group C | ||||||
| SBA | 2.87 (2.17, 3.78) | 376 (281, 504) | 9.09 (5.68, 14.53) | 6 (12) | 51 (98) | 26 (53) |
| ELISA | 0.31 (0.21, 0.47) | 11.72 (8.94, 15.34) | 0.89 (0.68, 1.13) | |||
| Anti-PRP | 0.18 (0.14, 0.24) | 0.77 (0.51, 1.16) | 0.23 (0.17, 0.31) | 27 (52) | 44 (85) | 32 (62) |
| Antitetanusd | 0.30 (0.22, 0.40) | 0.53 (0.44, 0.65) | 0.15 (0.13, 0.18) | 46 (89) | 52 (100) | 51 (100) |
Putatively protective levels were ≥0.35 μg/ml for all pneumococcal serotypes, an SBA titer of ≥1:8 for meningococcus group C, an anti-PRP titer of ≥0.15 μg/ml, and an antitetanus titer of ≥0.01 endotoxin units/ml.
ST, serotype.
Values in parentheses are 95% CIs.
Units are endotoxin units per milliliter.
The proportions of infants achieving putatively protective levels against each antigen pre- and post-primary immunization are given in Table 1. There was a statistically significant increase in the proportions of infants achieving these levels for all vaccine antigens examined from pre- to postimmunization (P < 0.01). For all vaccine antigens examined except pneumococcal serotype 6B and Hib, ≥90% of infants the achieved putatively protective levels postimmunization.
At 12 months of age, the GMCs of all antigens fell (Tables 1 and 2) (P < 0.03 for all antigens except pneumococcal serotype 6B, for which P was <0.06). The proportion of infants achieving putatively protective levels of antibody to an antigen had decreased significantly (P < 0.01) for all antigens except tetanus and pneumococcal serotypes 14 and 19F (Tables 1 and 2). At that time, only 22% of infants had putatively protective levels to all PCV7 serotypes.
TABLE 2.
Antibody responses to PCV7 postimmunization and at 12 months of age for 29 infants who did not receive a 4th dose of PCV7
| Serotype | Following primary immunizations |
At 12 mo |
||
|---|---|---|---|---|
| IgG GMC (μg/ml) | No. (%) with GMC ≥0.35 (μg/ml) | IgG GMC (μg/ml) | No. (%) with GMC ≥0.35 (μg/ml) | |
| 4 | 2.31 (1.72, 3.11)a | 22 (100) | 0.38 (0.25, 0.59)a | 13 (57) |
| 6B | 0.90 (0.54, 1.50) | 17 (77) | 0.50 (0.29, 0.86) | 7 (30) |
| 9V | 1.71 (1.26, 2.33) | 22 (100) | 0.46 (0.29, 0.75) | 11 (48) |
| 14 | 6.40 (4.27, 9.59) | 22 (100) | 2.09 (1.47, 2.96) | 23 (100) |
| 18C | 2.05 (1.43, 2.93) | 21 (96) | 0.60 (0.38, 0.94) | 16 (70) |
| 19F | 3.82 (2.88, 5.07) | 22 (100) | 1.59 (0.80, 3.18) | 20 (87) |
| 23F | 2.33 (1.55, 3.50) | 22 (100) | 0.36 (0.26, 0.51) | 11 (48) |
| 1 | 0.02 (0.01, 0.03) | 0 | 0.03 (0.02, 0.06) | 1 (4) |
| 5 | 0.09 (0.06, 0.15) | 1 (5) | 0.19 (0.12, 0.30) | 7 (30) |
Values in parentheses are 95% CIs.
Following a booster dose of PCV7, there was a significant increase in the GMCs for all vaccine serotypes (P < 0.01) (Table 3). All infants except one achieved putatively protective levels for all seven vaccine serotypes. The remaining infant had undetectable levels of antibodies to serotype 6B.
TABLE 3.
Antibody responses to PCV7 postimmunization and at 12 months of age for 23 infants who received a 4th dose of PCV7
| Serotype | IgG GMC (μg/ml) |
|
|---|---|---|
| Following primary immunizations | Following 4th dose of PCV7 | |
| 4 | 1.27 (0.88, 1.84)a | 3.84 (2.72, 5.42) |
| 6B | 0.13 (0.10, 0.17) | 5.95 (3.28, 10.78) |
| 9V | 0.81 (0.57, 1.16) | 3.60 (2.73, 4.75) |
| 14 | 2.62 (1.70, 4.04) | 10.54 (7.88, 14.10) |
| 18C | 1.08 (0.84, 1.37) | 1.99 (1.57, 2.51) |
| 19F | 2.09 (1.45, 2.99) | 4.51 (3.13, 6.51) |
| 23F | 1.01 (0.65, 1.57) | 4.79 (3.11, 7.37) |
| 1 | 0.02 (0.01, 0.02) | 0.05 (0.03, 0.11) |
| 5 | 0.07 (0.05, 0.10) | 0.29 (0.20, 0.43) |
Values in parentheses are 95% CIs.
DISCUSSION
Here we describe the first study to examine the immunogenicities of PCV7 and the Hib, tetanus, and MCC vaccines in term infants when the vaccines were administered according to the immunization schedule described. Although the response to all antigens tested increased from pre- to post-primary immunization, the majority of infants failed to achieve putatively protective levels to pneumococcal serotype 6B postimmunization. This is particularly important in the United Kingdom, as serotype 6B was responsible for approximately 7% of cases of invasive pneumococcal disease prior to the introduction of a universal program for pneumococcal vaccination (10). Additionally, only 85% of infants achieved minimally protective concentrations of ≥0.15 μg/ml to the Hib vaccine. The IgG GMCs for all antigens had decreased by 12 months of age, as had the proportion of infants achieving threshold levels for all antigens except tetanus. A 4th dose of PCV7 resulted in an increase in GMCs for all vaccine serotypes. However, 1 of the 29 infants still had undetectable levels of antibodies to serotype 6B.
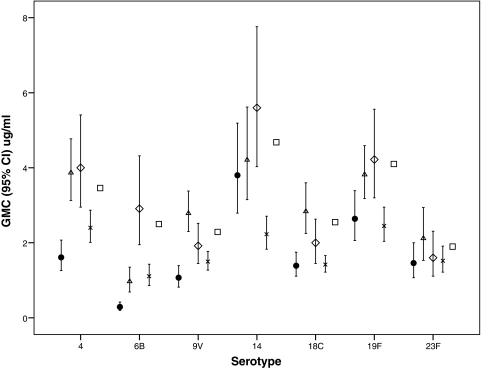
Our study differs from those reported previously in terms of the immunization schedule used, in the decision to give a booster dose only to those infants who responded poorly to the primary immunization schedule, and in the results. Four previous reports described that the PCV7 vaccine had good immunogenicity for all serotypes included in the PCV7 vaccine when it was administered to healthy term infants at 2, 3, and 4 months of age (7, 16-18). The postimmunization IgG GMCs for serotypes 14 and 23F from our study were similar to those presented in other reports. However, the GMC for serotype 6B in our study was markedly lower than that in any of the other studies (Fig. 1). The IgG GMCs for serotypes 4, 9V, 18C, and 19F were marginally lower than those described previously (7). When the proportions of infants achieving putatively protective levels to each serotype were compared, only the study by Ruggeberg et al. (17) used the minimum level of ≥0.35 μg/ml. In the study of Ruggeberg et al. and in our study, similar proportions of infants achieved these levels for serotypes 4, 14, 18C, 19F, and 23F; but in our study, only 38% of the infants achieved these levels for serotype 6B and 89% achieved these levels for serotype 9V, whereas the rates were 79% and 100%, respectively, in the study of Ruggeberg et al. (17). Ruggeberg et al. found that >90% of term infants had putatively protective levels against six or more serotypes, whereas in our study, only 76% of term infants achieved these levels. The other studies (7, 17, 18) examined the proportion of infants achieving titers of ≥0.15 μg/ml to each pneumococcal serotype. All of those studies found that >90% of infants achieved these levels of antibodies to each serotype, except for the arm in the study of Choo et al. (7) which received the combined PCV7-Hib vaccine, in which >85% of the infants achieved these levels of antibodies to each serotype (7, 16-18).
FIG. 1.
Comparison of the postimmunization serotype-specific anti-pneumococcal IgG GMCs from this study with those in other studies administering PCV7 with different vaccine combinations at 2, 3, and 4 months of age. Data are shown for a group that received PCV7 separately from the Hib vaccine. Symbols: •, this study; ▵, Ruggeberg et al. (17); ⋄, Reinert et al. (16); ×, Choo et al. (7); □, Schmitt et al. (18).
In our study, >90% of the infants achieved these levels of antibodies to each serotype except serotype 6B, with only 63% of the infants having levels of antibodies to serotype 6B of ≥0.15 μg/ml (data not shown).
In our study, the pneumococcal serotype-specific IgG GMCs at 12 months of age in infants who did not receive a 4th dose of PCV7 were comparable to those provided in other reports (7, 17, 18). However, the infants described previously had higher postimmunization IgG GMCs (Table 2) than the whole cohort in our study. The postimmunization IgG GMCs for this subgroup were comparable to those from the other studies. Although the booster PCV7 dose was given earlier in our study than in the only other study discussed here that gave a booster dose (18), the results of the two studies are comparable. This may indicate that even though the infants in our cohort had lower levels of antibodies to the serotypes included in PCV7, they may have induced immunological memory.
When the responses to the Hib, tetanus, and MCC vaccines found in our study are compared to those reported by Kitchin et al., who administered the same DTaP/IPV/Hib vaccine and a different MCC-CRM197 vaccine to infants in the United Kingdom at 2, 3, and 4 months of age but no pneumococcal vaccine (11), the antibody levels in our study were found to be markedly reduced (Table 4), although similar proportions of infants achieved threshold levels of antibodies to each antigen.
TABLE 4.
Comparison of postimmunization Hib, meningococcus group C, and tetanus responses from this study with those of a study administering the same DTaP5/IPV/Hib-TT and MCC vaccines but no pneumococcal vaccine
| Study | Anti-PRP IgG |
Anti-meningococcus group C IgG capsular polysaccharide (SBA) |
Antitetanus IgG |
|||
|---|---|---|---|---|---|---|
| GMC (μg/ml) | % with concn ≥0.15 μg/ml | GMT (μg/ml) | % with titer ≥1:8 | GMC (EU/mlc) | % with concn ≥0.01 EU/ml | |
| Kitchin et al. (11)a | 2.17 (1.34, 3.53)b | 87.8 | 2,165 (1,517, 3,089) | 100 | 1.30 (1.00, 1.68) | 100 |
| This study | 0.77 (0.51, 1.16) | 84.6 | 377 (281, 505) | 98.1 | 0.53 (0.44, 0.65) | 100 |
The results are for the group receiving MCC-CRM197 only.
Values in parentheses are 95% CIs.
EU/ml, endotoxin units per milliliter.
It seems unlikely that the difference in the results of our study and the results of the studies discussed above are due to differences in the study populations, as the majority of investigations were performed in the United Kingdom. A more likely explanation for the findings would be the combination of vaccines used in each study. In particular, only one of the studies administered an MCC vaccine concurrently with PCV7 (17). In addition, Choo et al. (7) and Ruggeberg et al. (17) both used whole-cell pertussis vaccines, and Choo et al. also administered a Hib-CRM197 conjugate vaccine. Few published studies examined the effects of different vaccine combinations on PCV7. Choo et al. (7) demonstrated that the use of PCV7 with the MCC-CRM197 vaccine in combination resulted in a diminished response compared to the responses obtained when the two vaccines were administered in separate limbs. Buttery et al. also demonstrated that the MCC vaccine portion of a combination nine-valent pneumococcal-meningococcal conjugate vaccine had decreased immunogenicity (5). Both of these reports highlight the importance of examining the effects of multiple conjugate vaccines, especially when they share a carrier protein. In our study, three conjugate vaccines were administered simultaneously, and two of them used the carrier protein CRM197. However, the study of Ruggeberg et al. also used three conjugate vaccines but found that term infants responded well to all pneumococcal serotypes (17). It is possible that some of these differences that have been observed could be attributed to interlaboratory and interassay variations.
It has previously been demonstrated that combining Hib conjugate vaccines with some acellular pertussis vaccines, as opposed to whole-cell pertussis vaccines, can result in the decreased immunogenicity of the Hib vaccine; this is also the case for separately administered inactivated polio vaccine (IPV) (2). Our study is the first to examine the effect of administering the combination DTaP5/IPV/Hib-TT vaccine currently used in the United Kingdom concurrently with PCV7. Kitchin et al. demonstrated a diminished response to Hib in infants who received the DTaP5/IPV/Hib-TT vaccine, with the infants who also received the MCC-CRM197 vaccine having the lowest Hib responses (11). Additionally, the anti-meningococcal group C SBA titer was diminished in infants who received the DTaP5/IPV/Hib-TT and MCC-TT vaccines as opposed to the diphtheria-tetanus-whole cell pertussis vaccine, but the SBA titers in those who received the MCC-CRM197 vaccine were not affected. The Hib and anti-meningococcal group C SBA titers for our study population were much lower than the those for comparable groups in the study of Kitchin et al. postimmunization (Table 4); this may have been due to the different MCC-CRM197 vaccines used. This may also indicate that there is a significant interaction between the DTaP5/IPV/Hib-TT vaccine, the MCC vaccine, and PCV7.
Our study demonstrates that PCV7, the MCC-CRM197 vaccine, and the DTaP5/IPV/Hib-TT vaccine have decreased immunogenicities when they are administered concomitantly at 2, 3, and 4 months of age. In September 2006, the primary immunization schedule of the United Kingdom underwent further changes. PCV7 was introduced for all infants, with doses administered at 2, 4 and 13 months of age. Additionally, the MCC vaccines are now administered at just 3 and 4 months of age and both the Hib and the MCC vaccines are boosted at 12 months of age. There are data to suggest a diminished response to serotypes 6B and 23F following only two doses of PCV7 during the primary immunization but adequate responses after the booster (12). In contrast, a study by Goldblatt et al. with a nine-valent conjugate pneumococcal vaccine showed equivalent responses in those infants receiving the vaccine on a reduced dose schedule and those receiving the vaccine at 2, 3, and 4 months of age (9). In view of the results of this study, the immunogenicity of the current United Kingdom immunization schedule should be carefully monitored and surveillance for invasive Hib, meningococcal group C, and pneumococcal disease should be continued.
Acknowledgments
This study was kindly funded by Wyeth Vaccines, The Children's Research Fund, and the Bubble Foundation.
Footnotes
Published ahead of print on 30 December 2009.
REFERENCES
- 1.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrington, J. E., A. J. Cant, J. N. Matthews, M. O'Keeffe, G. P. Spickett, and A. C. Fenton. 2006. Haemophilus influenzae type b immunization in infants in the United Kingdom: effects of diphtheria/tetanus/acellular pertussis/Hib combination vaccine, significant prematurity, and a fourth dose. Pediatrics 117:e717-e724. [DOI] [PubMed] [Google Scholar]
- 3.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, J. Aguilar, M. Bartlett, R. Bergen, M. Burman, S. Dorfman, W. Easter, A. Finkel, H. Froehlich, J. Glauber, A. Herz, D. Honeychurch, R. Kleinrock, I. Landaw, A. Lavetter, C. Le, S. McMurtry, P. Morozumi, P. Mullin, M. Rehbein, R. Rossin, G. Soe, I. Takahashi, G. Udkow, and R. Whitson. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, R., and E. B. Kaczmarksi. 2000. Meningococcal serology, p. 289-304. In A. J. Pollard and M. C. J. Maiden (ed.), Methods in molecular medicine. Human Press, Totowa, NJ.
- 5.Buttery, J. P., A. Riddell, J. McVernon, T. Chantler, L. Lane, J. Bowen-Morris, L. Diggle, R. Morris, A. Harnden, S. Lockhart, A. J. Pollard, K. Cartwright, and E. R. Moxon. 2005. Immunogenicity and safety of a combination pneumococcal-meningococcal vaccine in infants: a randomized controlled trial. JAMA 293:1751-1758. [DOI] [PubMed] [Google Scholar]
- 6.Carlone, G. M., C. E. Frasch, G. R. Siber, S. Quataert, L. L. Gheesling, S. H. Turner, B. D. Plikaytis, L. O. Helsel, W. E. DeWitt, W. F. Bibb, et al. 1992. Multicenter comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 30:154-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo, S., L. Seymour, R. Morris, S. Quataert, S. Lockhart, K. Cartwright, and A. Finn. 2000. Immunogenicity and reactogenicity of a pneumococcal conjugate vaccine administered combined with a Haemophilus influenzae type B conjugate vaccine in United Kingdom infants. Pediatr. Infect. Dis. J. 19:854-862. [DOI] [PubMed] [Google Scholar]
- 8.CMO. 2002, posting date. Pneumococcal vaccine for at-risk under 2 year olds. http://www.dh.gov.uk/en/publicationsandstatistics/Lettersandcirculars/Professionalletters/chiefpharmaceuticalofficerletters/DH_4004729.
- 9.Goldblatt, D., J. Southern, L. Ashton, P. Richmond, P. Burbidge, J. Tasevska, A. Crowley-Luke, N. Andrews, R. Morris, R. Borrow, K. Cartwright, and E. Miller. 2006. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 25:312-319. [DOI] [PubMed] [Google Scholar]
- 10.Health Protection Agency. 2007, posting date. Pneumococcal serotype distribution for samples referred for serotyping epidemiological years (June). Health Protection Agency, Manchester, United Kingdom. 2000/1-2005/6 www.hpa.org.uk/infections.
- 11.Kitchin, N. R., J. Southern, R. Morris, F. Hemme, S. Thomas, M. W. Watson, K. Cartwright, and E. Miller. 2007. Evaluation of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b vaccine given concurrently with meningococcal group C conjugate vaccine at 2, 3 and 4 months of age. Arch. Dis. Child. 92:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lockhart, S. P., J. G. Hackell, and B. Fritzell. 2006. Pneumococcal conjugate vaccines: emerging clinical information and its implications. Expert Rev. Vaccines 5:553-564. [DOI] [PubMed] [Google Scholar]
- 13.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. Peeters, S. Quataert, J. Y. Tai, and G. M. Carlone. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss, S. J., A. C. Fenton, and A. R. Gennery. 2005. Use of conjugate pneumococcal vaccine by United Kingdom neonatal intensive care units. Arch. Dis. Child. Fetal Neonatal Ed. 90:F187-F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps, D. C., J. West, R. Eby, M. Koster, D. V. Madore, and S. A. Quataert. 1990. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J. Immunol. Methods 135:121-128. [DOI] [PubMed] [Google Scholar]
- 16.Reinert, P., M. Guy, B. Girier, B. Szelechowski, B. Baudoin, P. Deberdt, A. Wollner, G. Kemeny, M. Amzallag, C. Moat, C. Szelechowski, H. Villain-Lemoine, C. A. Bouhanna, and F. Laudat. 2003. The safety and immunogenicity of an heptavalent pneumococcal polysaccharide conjugate vaccine (Prevenar) administered in association with a whole-cell pertussis-based pediatric combination vaccine (DTP-IPV/PRP-T) to French infants with a two-, three-, and four-month schedule. Arch. Pediatr. 10:1048-1055. (In French.) [DOI] [PubMed] [Google Scholar]
- 17.Ruggeberg, J. U., C. Collins, P. Clarke, N. Johnson, R. Sinha, N. Everest, J. Chang, E. Stanford, P. Balmer, R. Borrow, S. Martin, M. J. Robinson, E. R. Moxon, A. J. Pollard, and P. T. Heath. 2007. Immunogenicity and induction of immunological memory of the heptavalent pneumococcal conjugate vaccine in preterm UK infants. Vaccine 25:264-271. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt, H. J., J. Faber, I. Lorenz, A. B. Schmöle-Thoma, and N. Ahlers. 2003. The safety, reactogenicity and immunogenicity of a 7-valent pneumococcal conjugate vaccine (7VPnC) concurrently administered with a combination DTaP-IPV-Hib vaccine. Vaccine 21:3653-3662. [DOI] [PubMed] [Google Scholar]
- 19.WHO. 2005. Recommendations for the production and control of pneumoccocal conjugate vaccines. World Health Organ. Tech. Rep. Ser. 927:2007. [Google Scholar]