Abstract
Between October 2005 and May 2006, a total of 727 badgers found dead in Wales were reported, and 550 were delivered to the Regional Laboratories of the Veterinary Laboratories Agency (VLA). Of the 459 carcasses suitable for examination, 55 were deemed to be infected with Mycobacterium bovis on the basis of culture, spoligotyping, and variable-number tandem repeat typing. Acid-fast bacteria were observed histologically in a further six badgers, but these bacteria were not confirmed as M. bovis by culture. A rapid serological test (BrockTB Stat-Pak) performed on thoracic blood showed a sensitivity of 35% and a specificity of 99%. Presence of M. bovis infection was 45 times more likely to be confirmed postmortem by culture in BrockTB Stat-Pak-reactive animals than in seronegative ones. Using visible carcass lesions as a marker of bovine tuberculosis (bTB) infection had a similar sensitivity (38%) but was significantly less specific (84%) than serology. The overall accuracy of the antibody detection was 93% (346 correct results from 374 tests), whereas the accuracy of regarding visible lesions as a marker for bTB infection was 78% (354 correct from 453 carcasses examined). Culture remains the gold standard method for detecting M. bovis infection in badgers. However, where resources are limited and/or an instant result is preferred, the BrockTB Stat-Pak could be used in field surveillance efforts to identify animals which should be examined further by only submitting test-negative animals to more detailed postmortem examination and culture.
Mycobacterium bovis infection is the cause of bovine tuberculosis (bTB) in a wide range of mammal species, including domestic livestock and captive and free-ranging wildlife. Bovine TB remains an important zoonotic disease with significant impacts on the economy in many countries (6, 22, 23). Eurasian badgers (Meles meles) are a wildlife maintenance host of bTB in Great Britain and Ireland (5, 15) and are implicated in the maintenance and onward transmission of M. bovis infection to cattle (10, 19).
Surveillance of wildlife vectors of disease for prevalence estimates of infection may be valuable in disease control strategies and for the assessment of risk of transmission to livestock. Diagnosis of bTB in live badgers has been demonstrated using assays of both serological (4, 20) and cell-mediated (8, 9) immunity. While isolation of M. bovis from clinical samples is definitive, it is too insensitive for badgers, as infected animals yield positive samples infrequently and intermittently (3). A rapid serological test (BrockTB Stat-Pak; Chembio Diagnostic Systems, Inc.) has recently been developed for the diagnosis of bTB in multiple wildlife species (20). The test has modest sensitivity (46 to 55%) for antibody detection in live, infected badgers, but it has the advantages of being simple, rapid, inexpensive, and suitable for field application. Its utility as an animal-side test for badgers, however, is limited by the difficulties associated with obtaining a blood sample from a nonanesthetized animal.
Where carcasses are recovered and submitted for mycobacterial culture, the sensitivity of diagnosis depends on the effort taken for careful examination and on the number of tissue samples submitted for culture testing and histopathology (7), as well as on the condition of the carcass. In many cases, the cost involved may prove prohibitive. Reliance on the presence of visible lesions as indicative of bTB is fraught with difficulties, as infected animals may present with no visible lesions or lesions may be the result of other infections while having the appearance of bTB (reviewed in reference 13). The purpose of this study was to determine whether the BrockTB Stat-Pak test could detect M. bovis antibody in blood collected from the carcasses of dead badgers as an alternative means of diagnosis and decision making. Animals were obtained as part of a separate government-funded study to determine the prevalence of bTB in badgers found dead in Wales (http://new.wales.gov.uk/depc/publications/environmentandcountryside/animalhealthandwelfare/diseasesurveillancecontrol/bovinetb/2567889/publicationindex/2326585/badgerfounddeadreport?lang=en). Our results reveal that the BrockTB Stat-Pak test used on thoracic blood samples was very specific (99%) but less sensitive (35%) than found previously for live badgers (2, 14). However, bTB was 45 times more likely to be confirmed in BrockTB Stat-Pak-positive animals than in BrockTB Stat-Pak-negative ones, whereas using visible carcass lesions as a marker of infection was less reliable.
MATERIALS AND METHODS
Examination of found-dead badgers.
The public was asked to notify the State Veterinary Service (now Animal Health) when they saw a dead badger. Badger carcasses were collected and transported to local Veterinary Laboratories Agency (VLA) regional laboratories, usually within 24 h of collection, by animal health officers of the State Veterinary Service. Badgers were deemed unsuitable for postmortem examination if the carcass was not intact, if the carcass was distended with gas, if there was severe myiasis, or if the carcass was flattened. Carcasses were refrigerated at VLA between 2 and 8°C and examined as soon as possible after receipt and always within 72 h. Postmortem examination was conducted using a standard protocol as previously described (17). Relevant to this study, data were also collected on animal sex, weight, and tooth wear, the latter two measures being used to approximate the ages of the badgers. Cubs were considered to have tooth wear between 0 and 25% (16) and to weigh less than 8 kg (based on unpublished analyses of other badgers). The severity of lesions in each tissue examined was scored subjectively from 0 to 4, with 0 indicating no visible lesions. For confirmation of bTB, an attempt was made to collect a standard sample of one-half of each of the retropharyngeal and bronchial lymph nodes and half of the mediastinal lymph nodes if available (half of the hepatic lymph node was also collected after 15 March 2006) plus any other lesions suggestive of bTB into 15 ml of 1% cetylpyridinium chloride (CPC). Any lesions suggestive of bTB available after this sampling were preserved in 10% buffered formaldehyde solution. Bite wounds were divided between a separate container of CPC and 10% buffered formaldehyde solution. The standard sample and any bite wounds were cultured for mycobacteria, usually the next day, using the methods of the standard protocol (17) with the exception that 12 slopes, rather than 6 slopes, of modified Middlebrook 7H11 agar were incubated for 12 weeks rather than 6 weeks. Whenever M. bovis was isolated, its molecular type (spoligotype and variable-number tandem repeat [VNTR] type) was determined using published methods (11, 18). In 134 badgers that had visible carcass lesions or bite wounds and where sufficient tissue was available after sampling for culture, preserved samples of visible carcass lesions or bite wounds were stained by the Ziehl-Neelsen method and examined microscopically for acid-fast bacilli (AFB). For the purposes of this study, only animals in which M. bovis was isolated by culture were classified as bTB positive.
BrockTB Stat-Pak testing.
A simple and rapid antibody detection assay was developed by Chembio Diagnostic Systems, Inc., using colored latex-based lateral flow technology and a cocktail of selected M. bovis antigens, including ESAT-6, CFP10, and MPB83 (14). Where possible, a blood sample was collected for the lateral flow immunoassay, either by using a syringe and 14-gauge needle from the thorax via the chest wall before the carcass was opened or, if this was not possible, during the necropsy. The blood sample was collected from the heart or free from within the thorax. An assessment of the condition of the blood was made on a scale of 1 to 4, with 1 being fresh with little hemolysis and 4 being severely hemolysed. Blood samples were tested for the presence of specific antibody as previously described (21), immediately after collection. Results were read at 20 min after adding sample buffer. Any visible band in the test area of the BrockTB Stat-Pak, in addition to the control line, was considered an antibody-positive result, whereas no band in the test area in addition to the visible control line was considered a negative result.
Data analyses.
Diagnostic performance was evaluated against bTB positivity (determined by culture and/or histology). The test sensitivity, specificity, and positive predictive value (PPV) were calculated using GraphPad InStat (version 3.06 for Windows; GraphPad Software, San Diego, CA) and are reported with 95% confidence interval (CI). Tests of significance between proportions (Fisher's exact test) or between medians (Mann-Whitney test) and the calculation of odds ratio were performed using the same software.
RESULTS AND DISCUSSION
Confirmation of bTB infection and its prevalence.
Between 26 October 2005 and 31 May 2006, a total of 727 badgers found dead in Wales were reported, and 550 were delivered to the Regional Laboratories of the VLA. Of the 459 carcasses that were sampled, M. bovis was isolated from 55 badgers, for which spoligotype and VNTR type were determined. Stained tissue samples from 117 culture-negative badgers revealed a further 6 culture-negative badgers considered likely to be infected with M. bovis on the basis of AFB being present. However, given that these could not be confirmed by culture they were discounted from the calculations of sensitivity and specificity, etc. Mycobacterium avium was isolated from two badgers. No acid-fast organisms were seen histologically, and no M. bovis was isolated from either of these animals.
Further details of the geographical distribution and prevalence of bTB in Welsh badgers is the subject of another paper (submitted for publication). Only four distinct molecular types of bTB were seen in the 55 badgers: spoligotype 17 (with the unique VNTR type 7555*33.1), spoligotype 22 (with VNTR type 7524*33.1), and two VNTR types of spoligotype 9. One of these shares its VNTR type with spoligotype 22, and one had the unique VNTR type 7555*32.1.
BrockTB Stat-Pak test and/or lesions as diagnostic criteria.
A lateral flow immunoassay based on the detection of serum antibodies to M. bovis antigens (BrockTB Stat-Pak test; Chembio Diagnostic Systems, Inc.) (14) was evaluated for the first time using unseparated thoracic blood samples obtained from animals found dead. The test was originally designed for use with serum obtained from live animals, where it was demonstrated to have a sensitivity of 51% (95% CI, 46 to 55%) and a specificity of 93% (95% CI, 91 to 95%) following validation on 1,532 badgers against culture postmortem (20). Recently, the test has been shown to also work with unclotted (freshly obtained) whole blood or plasma or even diaphragm extract from white-tailed deer experimentally inoculated with M. bovis (20). In this study, 379 badgers of the total 459 were examined by using the BrockTB Stat-Pak (37 samples from infected badgers, 337 samples from noninfected badgers, and 5 of the 6 badgers for which only histological evidence of infection was obtained). In some cases it was not possible to obtain blood from the carcass, and testing by BrockTB Stat-Pak was not carried out at the end of the study due to test kits being temporarily unavailable.
The ratio of males to females submitted to postmortem examination was 3:2 and was no different for the subset tested with the Stat-Pak (P = 0.57, Fisher's exact test). For all badgers examined, the median weight was 9.6 kg, the median body length was 68 cm, and the median tooth wear was 0.5. The subset of animals tested with the Stat-Pak was representative of the whole in each respect (P > 0.5, Mann-Whitney Test). Based on the weight and tooth wear data to approximate the age of the badgers, there were 164 cubs (36%) and 295 adults (64%) examined. These proportions were the same for the subset tested by Stat-Pak (P = 0.79, Fisher's exact test).
Blood could be obtained from 226 animals (60%) through the chest wall before the carcass was opened. In a further 152 cases (40%) this was not possible. Reasons for this were either that the needle became blocked by a blood clot or thoracic viscera that had become macerated through trauma or that there was only a small amount of blood in the heart/thorax. In these cases the sample was taken during the necropsy. Details of the procedure used were not recorded in one case. Blood quality was significantly poorer (P < 0.0001, Mann-Whitney test) if it had to be obtained during necropsy. The median score was 3 in the latter case, versus 2 for blood obtained before the carcass was opened. The sensitivity and specificity of the BrockTB Stat-Pak were 32% and 99% for blood obtained before the carcasses was opened and 44% and 99% for blood obtained at necropsy. However, the sensitivity and specificity of the BrockTB Stat-Pak were not significantly affected by the condition of the blood or by the method used to obtain the sample (Fisher's exact test) (data not shown). Overall, the sensitivity for bTB detection was 35% (95% CI, 20 to 53%) with a specificity of 99% (95% CI, 97 to 100%) (Table 1). The lower sensitivity of the test when performed postmortem rather than on live animals can be accounted for by degradation of immunoglobulins after death. This was demonstrated to be the case in studies in foxes (24) and white-tailed deer (1). In the fox study, blood samples were readily obtained from intact carcasses stored for up to 11 days at 10°C, and sample degradation was less if it was obtained postmortem from the thoracic cavity rather than a blood vessel. These observations suggest that samples obtained from the thoracic cavity postmortem are particularly suitable for this application.
TABLE 1.
Association between infection in badgers and result of blood test or presence of lesions
| Test (no. tested) | Infecteda badgers (55 examined) |
Uninfected badgers (398 examined) |
Relative riskb | P value | ||||
|---|---|---|---|---|---|---|---|---|
| No. negative | No. positive | Sensitivity (%) | No. positive | No. negative | Specificity (%) | |||
| Brock TB Stat-Pak (374) | 24 | 13 | 35 | 4 | 333 | 99 | 11 | <0.0001 |
| Presence of any lesion (453) | 34 | 21 | 38 | 65 | 333 | 84 | 3 | <0.003 |
As determined by positive culture confirmed by spoligotyping/VNTR (n = 55) only. Six badgers with AFB present histologically but from which no positive culture was obtained were discounted from the analysis as having ambiguous disease status.
Proportion of test-positive animals that were infected divided by the proportion of test-negative animals that were infected.
The median score for the condition of the blood from all samples was 2 (range, 1 to 4, indicating best to worst conditions). As we have previously found the hemolysis of badger serum to significantly reduce the sensitivity of the BrockTB Stat-Pak but not influence its specificity (2), we tested for any significant difference between the median score of blood from which a correct positive BrockTB Stat-Pak result was obtained and the score from those samples giving a false-negative result (Fig. 1). The median blood score for the true positive samples was 3, and the score was 2 for the false-negative samples. This difference was not statistically significant (P = 0.49, Mann-Whitney test).
FIG. 1.
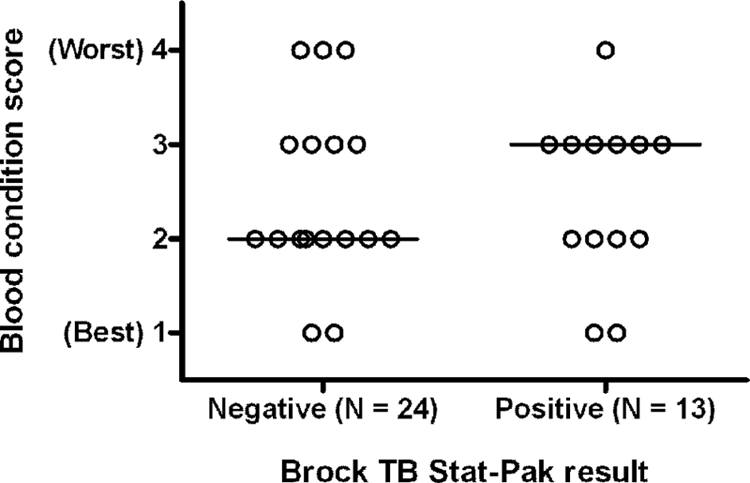
The influence of blood condition, scored from 1 (fresh with little hemolysis) to 4 (severely hemolysed), on the Brock TB Stat-Pak result for badgers confirmed as infected by culture only.
In each badger, between 5 and 18 sites were examined for lesions (mean, 15 sites). In some badgers it was not possible to examine all sites due to traumatic damage to viscera or the inability to locate all lymph nodes. Using carcass lesions as a marker of infection was significantly less accurate (78%; 95% CI, 74 to 82%) than the BrockTB Stat-Pak test (93%; 95% CI, 89 to 95%; P < 0.0001, Fisher's exact test), reflecting the fact that other infections can produce visible lesions reminiscent of bTB (12, 13). An example of this was evident in the current study. One badger had an enlarged/lesioned popliteal lymph node from which M. avium was subsequently isolated. This badger was negative in the Stat-Pak test. Taking the sum of the lesion scores in each tissue as a relatively crude indicator of the severity of infection among M. bovis culture-positive badgers, there was evidence that Stat-Pak positivity was associated with more severe bTB, as reported previously (2). The median score for the 13 Stat-Pak-positive badgers was 2 (range, 0 to 9), in contrast to a score of 0 (range, 0 to 11) for the 24 Stat-Pak-negative badgers. This difference was significant (P < 0.05, Mann-Whitney test).
The estimates of sensitivity and specificity were relative, having been measured against culture on a limited range of tissues. Gross examination of more tissues and obtainment of more cultures from each badger would result in bTB being detected in more carcasses (7). However, although culture may miss M. bovis infection in some cases, it would be difficult to replace this gold standard method with either the BrockTB Stat-Pak test or postmortem examination alone.
Despite the relatively low sensitivity, the close association of the BrockTB Stat-Pak test result with bTB status was highly significant (P < 0.0001, Fisher's exact test), and the proportion of seropositive animals that were infected divided by the proportion of test-negative animals that were infected (relative risk) was 11, compared with 3 for the application of visible lesions (Table 1). Calculation of the odds ratio for the BrockTB Stat-Pak test was 45 (95% CI, 14 to 149), which means bTB positivity was 45 times more likely to be confirmed in antibody-reactive animals than in the seronegative population. This is in contrast to an odds ratio of 3 (95% CI, 2 to 6) for the presence of visible lesions indicating bTB infection.
The PPV of the BrockTB Stat-Pak test was 76% (95% CI, 50 to 93%) and represented the percentage of badgers with a positive test that were confirmed by culture to have bTB at the prevalence of bTB represented by the 374 test samples (37/374; 10%). The bTB prevalence calculated from all animals submitted to culture was 12% (55/459), so the subset used for BrockTB Stat-Pak testing was representative of the whole. The broad confidence intervals for the estimated test sensitivity and PPV reflect the fact that only 17 seropositive results were obtained from a total of 379 badgers tested. Based on these results, over three-quarters of BrockTB Stat-Pak test-reactive badgers needn't have been submitted to detailed postmortem examination and culture in order to confirm their bTB status. However, the PPV is dependent on both the properties of the test and the population tested, because the lower the actual disease prevalence, the lower the predictive value of the diagnostic test. Also, failure to attempt culture from a badger would by definition mean a loss of molecular typing data, which may be undesirable.
The ability of the BrockTB Stat-Pak test to detect bTB in badgers and wild deer (20) using samples that we were able to obtain from dead animals suggests that this may be a useful approach generally applicable to other wildlife species. Given its high specificity, low cost, ease of operation, and rapidity, the BrockTB Stat-Pak test could be used to reduce the number of badger carcasses submitted for examination, especially in situations where resources are limited, a prompt management decision is needed, and/or investigation by the gold standard method of culture is not possible. Accordingly, a positive BrockTB Stat-Pak result would be accepted as a true positive, while only animals negative by the test would be submitted to more detailed postmortem examination and culture.
Acknowledgments
This study was funded by the Welsh Assembly Government (projects OG0017 and OG0130).
The VLA acknowledges the contribution of the State Veterinary Service (now Animal Health) and those who reported and transported dead badgers for examination, as well as the veterinary investigation officers at the Aberystwyth, Carmarthen, and Shrewsbury VLA regional laboratories who carried out the postmortem examinations, the Laboratory Testing Division staff at the laboratories above and at VLA Truro and Bury who carried out the serological testing, culture, and histology, and those at VLA Weybridge who carried out the molecular typing and data handling (CERA3). We thank Richard Delahay of the Food and Environment Research Agency for his advice on aging badgers.
Footnotes
Published ahead of print on 30 December 2009.
REFERENCES
- 1.Anderson, T., A. DeJardin, D. K. Howe, J. P. Dubey, and M. L. Michalski. 2007. Neospora caninum antibodies detected in Midwestern white-tailed deer (Odocoileus virginianus) by Western blot and ELISA. Vet. Parasitol. 145:152-155. [DOI] [PubMed] [Google Scholar]
- 2.Chambers, M. A., T. Crawshaw, S. Waterhouse, R. Delahay, R. G. Hewinson, and K. P. Lyashchenko. 2008. Validation of the BrockTB Stat-Pak assay for detection of tuberculosis in Eurasian badgers (Meles meles) and influence of disease severity on diagnostic accuracy. J. Clin. Microbiol. 46:1498-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers, M. A., W. A. Pressling, C. L. Cheeseman, R. S. Clifton-Hadley, and R. G. Hewinson. 2002. Value of existing serological tests for identifying badgers that shed Mycobacterium bovis. Vet. Microbiol. 86:183-189. [DOI] [PubMed] [Google Scholar]
- 4.Clifton-Hadley, R. S., A. R. Sayers, and M. P. Stock. 1995. Evaluation of an ELISA for Mycobacterium bovis infection in badgers (Meles meles). Vet. Rec. 137:555-558. [DOI] [PubMed] [Google Scholar]
- 5.Clifton-Hadley, R. S., J. W. Wilesmith, and F. A. Stuart. 1993. Mycobacterium bovis in the European badger (Meles meles): epidemiological findings in tuberculous badgers from a naturally infected population. Epidemiol. Infect. 111:9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corner, L. A. 2006. The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: how to assess the risk. Vet. Microbiol. 112:303-312. [DOI] [PubMed] [Google Scholar]
- 7.Crawshaw, T. R., I. B. Griffiths, and R. S. Clifton-Hadley. 2008. Comparison of a standard and a detailed postmortem protocol for detecting Mycobacterium bovis in badgers. Vet. Rec. 163:473-477. [DOI] [PubMed] [Google Scholar]
- 8.Dalley, D., M. A. Chambers, P. Cockle, W. Pressling, D. Gavier-Widen, and R. G. Hewinson. 1999. A lymphocyte transformation assay for the detection of Mycobacterium bovis infection in the Eurasian badger (Meles meles). Vet. Immunol. Immunopathol. 70:85-94. [DOI] [PubMed] [Google Scholar]
- 9.Dalley, D., D. Dave, S. Lesellier, S. Palmer, T. Crawshaw, R. G. Hewinson, and M. Chambers. 2008. Development and evaluation of a gamma-interferon assay for tuberculosis in badgers (Meles meles). Tuberculosis (Edinburgh) 88:235-243. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly, C. A., G. Wei, W. T. Johnston, D. R. Cox, R. Woodroffe, F. J. Bourne, C. L. Cheeseman, R. S. Clifton-Hadley, G. Gettinby, P. Gilks, H. E. Jenkins, A. M. Le Fevre, J. P. McInerney, and W. I. Morrison. 2007. Impacts of widespread badger culling on cattle tuberculosis: concluding analyses from a large-scale field trial. Int. J. Infect. Dis. 11:300-308. [DOI] [PubMed] [Google Scholar]
- 11.Frothingham, R., and W. A. Meeker-O'Connell. 1998. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 144:1189-1196. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher, J., R. Monies, M. Gavier-Widen, and B. Rule. 1998. Role of infected, non-diseased badgers in the pathogenesis of tuberculosis in the badger. Vet. Rec. 142:710-714. [DOI] [PubMed] [Google Scholar]
- 13.Gavier-Widen, D., M. M. Cooke, J. Gallagher, M. A. Chambers, and C. Gortazar. 2009. A review of infection of wildlife hosts with Mycobacterium bovis and the diagnostic difficulties of the “no visible lesion” presentation. N. Z. Vet. J. 57:122-131. [DOI] [PubMed] [Google Scholar]
- 14.Greenwald, R., J. Esfandiari, S. Lesellier, R. Houghton, J. Pollock, C. Aagaard, P. Andersen, R. G. Hewinson, M. Chambers, and K. Lyashchenko. 2003. Improved serodetection of Mycobacterium bovis infection in badgers (Meles meles) using multiantigen test formats. Diagn. Microbiol. Infect. Dis. 46:197-203. [DOI] [PubMed] [Google Scholar]
- 15.Griffin, J. M., D. H. Williams, G. E. Kelly, T. A. Clegg, I. O'Boyle, J. D. Collins, and S. J. More. 2005. The impact of badger removal on the control of tuberculosis in cattle herds in Ireland. Prev. Vet. Med. 67:237-266. [DOI] [PubMed] [Google Scholar]
- 16.Hancox, M. 1988. Field age determination in the European badger. Rev. Ecol. 23:399-404. [Google Scholar]
- 17.Jenkins, H. E., W. I. Morrison, D. R. Cox, C. A. Donnelly, W. T. Johnston, F. J. Bourne, R. S. Clifton-Hadley, G. Gettinby, J. P. McInerney, G. H. Watkins, and R. Woodroffe. 2008. The prevalence, distribution and severity of detectable pathological lesions in badgers naturally infected with Mycobacterium bovis. Epidemiol. Infect. 136:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly, G. E., J. Condon, S. J. More, L. Dolan, I. Higgins, and J. Eves. 2008. A long-term observational study of the impact of badger removal on herd restrictions due to bovine TB in the Irish midlands during 1989-2004. Epidemiol. Infect. 136:1362-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, M. A. Chambers, J. Vicente, C. Gortazar, N. Santos, M. Correia-Neves, B. M. Buddle, R. Jackson, D. J. O'Brien, S. Schmitt, M. V. Palmer, R. J. Delahay, and W. R. Waters. 2008. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet. Microbiol. 132:283-292. [DOI] [PubMed] [Google Scholar]
- 21.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, J. H. Olsen, R. Ball, G. Dumonceaux, F. Dunker, C. Buckley, M. Richard, S. Murray, J. B. Payeur, P. Andersen, J. M. Pollock, S. Mikota, M. Miller, D. Sofranko, and W. R. Waters. 2006. Tuberculosis in elephants: antibody responses to defined antigens of Mycobacterium tuberculosis, potential for early diagnosis, and monitoring of treatment. Clin. Vaccine Immunol. 13:722-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel, A. L., R. G. Bengis, D. F. Keet, M. Hofmeyr, L. M. Klerk, P. C. Cross, A. E. Jolles, D. Cooper, I. J. Whyte, P. Buss, and J. Godfroid. 2006. Wildlife tuberculosis in South African conservation areas: implications and challenges. Vet. Microbiol. 112:91-100. [DOI] [PubMed] [Google Scholar]
- 23.Thoen, C. O., P. A. Lobue, D. A. Enarson, J. B. Kaneene, and I. N. de Kantor. 2009. Tuberculosis: a re-emerging disease in animals and humans. Vet. Ital. 45:135-181. [PubMed] [Google Scholar]
- 24.Tryland, M., K. Handeland, A. M. Bratberg, I. T. Solbakk, and A. Oksanen. 2006. Persistence of antibodies in blood and body fluids in decaying fox carcasses, as exemplified by antibodies against Microsporum canis. Acta Vet. Scand. 48:10. [DOI] [PMC free article] [PubMed] [Google Scholar]


