Abstract
In July 2006, the seven-valent pneumococcal conjugate vaccine (PCV7) was introduced in Norway with a reduced (2 doses + 1 boost) dose schedule. Post-PCV7 shifts in pneumococcal reservoirs were assessed by two point prevalence studies of nasopharyngeal colonization among children in day care centers, before (2006) and after (2008) widespread use of PCV7. Nasopharyngeal swabs were obtained from 1,213 children, 611 in 2006 and 602 in 2008. A total of 1,102 pneumococcal isolates were recovered. Serotyping, multilocus sequence typing, and antimicrobial drug susceptibility testing were performed on all isolates. Although carriage of PCV7 serotypes decreased among both vaccinated and unvaccinated children, the overall prevalence of pneumococcal carriage remained high (80.4%) after vaccine introduction. The pneumococcal populations were diverse, and in the shift toward non-PCV7 serotypes, expansion of a limited number of established clonal complexes was observed. While non-antimicrobial-susceptible clones persisted among PCV7 serotypes, antimicrobial resistance did not increase among non-PCV7 serotypes. Direct and indirect protection of PCV7 against nasopharyngeal colonization was inferred from an overall decrease in carriage of PCV7 serotypes. No preference was found for nonsusceptible clones among the replacing non-PCV7 serotypes.
Streptococcus pneumoniae is a leading cause of otitis media, sinusitis, pneumonia, and meningitis worldwide (35). S. pneumoniae colonizes the nasopharynx and is considered a part of the normal flora in early childhood (1). Following the implementation of childhood vaccination with the seven-valent conjugated pneumococcal vaccine (PCV7), reports from several locations have described declines in carriage of the seven serotypes included in the vaccine, i.e., serotypes 4, 6B, 9V, 14, 18C, 19F and 23F (4, 6, 13, 19, 24). Due to reduced transmission of PCV7 serotypes, the incidence of invasive pneumococcal disease (IPD) declines also among the unvaccinated, which is an indirect effect of conjugate pneumococcal vaccination. However, the effect of PCV7 may in part be eroded over time as non-PCV7 serotypes emerge as a more frequent cause of IPD (11). In the United States, non-antimicrobial-susceptible clones seem to have an advantage among the emerging and expanding non-PCV7 serotypes, both in asymptomatic colonization and IPD (10, 20). This is primarily demonstrated by increasing incidence rates of drug-resistant clones of serotype 19A (23).
PCV7 was introduced into the Norwegian childhood vaccination program in a dose schedule of two doses and one boost (2 + 1 dose schedule) in July 2006 and has been offered free of charge to all children born in 2006 and since. A high level of effectiveness of the vaccination program among children was demonstrated quickly after vaccine introduction, and the effect included a decline in IPD caused by erythromycin-resistant S. pneumoniae (34).
As part of the Norwegian surveillance of PCV7 introduction, a cross-sectional study of nasopharyngeal carriage of Streptococcus pneumoniae among children attending day care centers (DCC) was performed in the early autumn of 2006. Data from this study, with exception of data regarding 38 vaccinated participants, have been reported previously (33). To assess the impact of the 2 + 1 dose schedule PCV7 vaccination program on carriage of S. pneumoniae, a follow-up was performed in 2008. Serotyping, antimicrobial susceptibility testing, and genotyping of the isolates from 2008 were performed, and the results were compared to those from analyses of the previous collection. Shifts in clonal compositions of the pneumococcal populations were analyzed and are reported here.
Limited outpatient use of antimicrobial agents is recommended in Norway, and the levels of nonsusceptibility to antimicrobials among S. pneumoniae isolates from both local infections and IPD are low (25). Hence, emphasis has been put on post-PCV7 changes in nonsusceptibility to antimicrobials and nonsusceptible clones in a setting with limited antimicrobial use and resistance.
MATERIALS AND METHODS
Study population.
Cross-sectional studies of pneumococcal carriage were performed in 29 DCCs in 2006 (33) and 27 DCCs in 2008 in suburban areas around Oslo, the capital of Norway. Parents/guardians were informed about the study by leaflets, letters, and information meetings, and written consent was obtained from all who agreed to participate.
By use of a questionnaire, information on household size, risk factors for carriage, pneumococcal vaccination, any respiratory tract infection (RTI), and antimicrobial use in the 3 months preceding sampling were obtained from the parents/guardians.
The study was approved by the Regional Committee for Medical Research Ethics, Southern Norway.
Sample collection.
Transnasal nasopharyngeal samples were collected at DCCs between 13 September and 7 November 2006 and between 3 September and 28 October 2008. Nasopharyngeal sampling was performed as previously described (33). The swab was then inserted into a tube containing 1.5 ml of serum broth from beef infusion, enriched with 5% horse serum, and 3.3% defibrinated horse blood (Statens Serum Institut, Copenhagen, Denmark). The swabs were transported to the laboratory and further processed within 3 to 4 h.
Bacterial identification and serotyping.
Processing of swabs was performed as previously described (33). Briefly, the swabs were plated onto a nonselective chocolate agar and a Columbia horse blood agar containing 5.0 μg/ml gentamicin and reinserted into the respective enrichment broth used for transport. The presence of pneumococci was detected by direct serotyping of all enrichment cultures by using a commercial kit for latex agglutination (Pneumotest-Latex kit; Statens Serum Institut, Copenhagen, Denmark). By this method, the presence of multiple serotypes in one sample can be easily detected (17). Growth on the selective blood agar was examined for alpha-hemolytic colonies with typical pneumococcal morphology to confirm the findings from the broths. If more serogroups/serotypes were identified in the enrichment culture, up to 16 colonies were passaged in the attempt to identify the different strains. The serotype was determined by the capsular reaction test (Quellung reaction) with specific antisera (Statens Serum Institut, Copenhagen, Denmark). In cases in which there was positive agglutination of the enrichment culture but no growth on the selective blood agar, a new droplet of the enrichment broth was plated on blood agar and incubated overnight for isolation of S. pneumoniae colonies. The recently described serotype 6C was identified directly in the 2008 sample collection by use of the Quellung reaction; strains identified as serotype 6A in the 2006 sample collection were reserotyped in order to differentiate serotype 6C (27). Strains that were identified as serotype 15B or 15C are reported together as serotype 15B/C due to frequent transition between the two serotypes (32).
Antimicrobial susceptibility testing.
For all identified isolates, antimicrobial susceptibility testing was performed by determination of the MIC by using the antimicrobial gradient strip diffusion method (AB Biodisk, Solna, Sweden). Isolates were tested for susceptibility to penicillin G, cefotaxime, ceftriaxone, erythromycin, clindamycin, tetracycline, trimethoprim-sulfamethoxazole, and chloramphenicol, using ATCC 49619 as a quality control strain and breakpoints from the Clinical and Laboratory Standards Institute (CLSI) (3). Nonsusceptibility to penicillin was reported according to the breakpoint for oral administration, i.e., MIC > 0.064 μg/ml.
Genotyping.
Genomic DNA was prepared as previously described (33). Multilocus sequence typing (MLST) was performed as described by Enright and Spratt (7), and novel alleles and sequence types (STs) were submitted to the curator of the MLST database (http://www.mlst.net). Clonal relationships in the strain collection were visualized using eBURST version 3 (http://eburst.mlst.net) (8). STs sharing 6 of the 7 MLST alleles were named single-locus variants (SLVs), and groups of STs connected by SLVs were assigned to clonal complexes (CCs). STs belonging to internationally spread and antibiotic-resistant clones described by the Pneumococcal Molecular Epidemiology Network (PMEN) were identified (http://www.sph.emory.edu/PMEN).
Statistical analyses.
Chi-square and Fisher's exact test analyses were performed in SPSS 14.0 for Windows and GraphPad Prism 5.01. P values of <0.05 were considered significant. The genetic diversity in the total population and within each DCC was calculated using Simpson's index of diversity (D) (31). D is a measure of the probability that two random and independent samples from a population will belong to the same group and is defined as 1 − λ, where λ is given by the equation λ = Σ[ni(ni − 1)]/N(N − 1), where ni is the number of isolates with the ith ST in the population and N is the number of isolates in the population. Confidence intervals (CI) were determined using the method described by Grundmann et al (9). The classification index (C), was calculated in order to compare the ST distributions in the two data sets. C is defined as 1 − Σ(ρi1ρi2/ρ), where ρi1 and ρi2 are the frequencies of the ith ST in populations 1 and 2, respectively, and ρ is the frequency of the ith ST in the combined data set (16). If the two populations have identical proportions of each ST, the index is 0; as the populations become more dissimilar, the index increases toward 1.
RESULTS
Study population.
Children were recruited from 29 DCCs in 2006 (33) and from 27 DCCs in the same area in 2008; 17 DCCs participated in both studies. The average numbers of children attending the DCCs were 53.1 in 2006 and 56.0 in 2008. The proportions of DCC attendees volunteering to participate were 611 (39.7%) of 1,540 children in 2006 and 602 (43.0%) of 1,399 children in 2008.
The median ages of the children participating were 45 months in 2006 and 42 months in 2008. The male/female ratios, distributions per age group, numbers of siblings <6 years of age, and proportions exposed to passive smoking in the household did not differ (Table 1).
TABLE 1.
Characteristics of the study participants and changes from 2006 to 2008
| Parameter | No. (%) of children in yr |
P valuec | |
|---|---|---|---|
| 2006 | 2008 | ||
| Age group (mo) | NS | ||
| <24 | 87 (14.2) | 115 (19.1) | |
| 24-35 | 123 (20.1) | 108 (17.9) | |
| 36-47 | 133 (21.8) | 124 (20.6) | |
| 48-50 | 162 (26.5) | 140 (23.3) | |
| ≥60 | 106 (17.3) | 115 (19.1) | |
| Overall | 611 (100) | 602 (100) | |
| Sex | NS | ||
| Male | 326 (53.4) | 316 (52.5) | |
| Female | 285 (46.6) | 286 (47.5) | |
| Antibiotic use in the past 3 mo | 0.011 | ||
| No | 557 (91.2) | 570 (94.7) | |
| Yes | 54 (8.8) | 32 (5.3) | |
| RTIa in the past 3 mo | 0.016 | ||
| No | 553 (90.5) | 567 (94.2) | |
| Yes | 58 (9.5) | 35 (5.8) | |
| PCV7b immunization (no. of doses) | <0.001 | ||
| 0 | 573 (93.8) | 354 (58.8) | |
| 1 | 17 (2.8) | 11 (1.8) | |
| ≥2 | 21 (3.4) | 237 (39.4) | |
| No. of siblings < 6 yrs old | |||
| 0 | 303 (49.6) | 279 (46.3) | NS |
| 1 | 294 (48.1) | 296 (49.2) | NS |
| ≥2 | 14 (2.3) | 27 (4.5) | NS |
| Passive smoking | NS | ||
| No | 501 (82.0) | 520 (86.4) | |
| Yes | 110 (18.0) | 82 (13.6) | |
| Nasopharyngeal carriage per age group (mo) | NS | ||
| <24 | 71 (81.6) | 96 (83.5) | |
| 24-35 | 107 (87.0) | 95 (88.0) | |
| 36-47 | 103 (77.4) | 97 (78.2) | |
| 48-50 | 120 (74.1) | 110 (78.6) | |
| ≥60 | 74 (69.8) | 85 (73.9) | |
| Overall | 475 (77.7) | 483 (80.2) | |
RTI, upper respiratory tract infection.
PCV7, seven-valent pneumococcal conjugate vaccine.
P values are based on chi-square and Fisher's exact tests. NS, nonsignificant.
The proportion of children vaccinated at least twice with PCV7 increased from 3.4% in 2006 to 39.4% in 2008 (P < 0.001) (Table 1). In 2008, 93.6% of children born in 2006 and later had received ≥2 immunizations with PCV7.
The proportion of children reported to have any RTI during the 3 months preceding participation decreased, as did the proportion of children who had used antimicrobial agents (Table 1).
Pneumococcal carriage.
Nasopharyngeal swabs were obtained from 1,213 children, 611 in the 2006 study and 602 in the 2008 study (Table 1). The overall pneumococcal carriage rates were similar (77.7% in 2006 and 80.2% in 2008). The prevalence peaked among 2-year-olds in both study years (87.0% in 2006 and 88.0% in 2008).
Multiple pneumococcal serotypes were identified from 132 (13.8%) of 959 positive samples, 60 (12.6%) samples in 2006 and 72 (14.9%) samples in 2008. Three distinct strains were recovered from 12 (9.1%) cocolonized children, 4 children in 2006 and 8 children in 2008.
Carriage of specific pneumococcal serotypes.
A total of 1,102 pneumococcal isolates were recovered in the two studies, 539 in 2006 and 563 in 2008. Carriage of PCV7 serotypes decreased in all age groups; the overall carriage of PCV7 serotypes decreased by 48.4% (P < 0.001). The decrease was nonsignificant for the 48- to 60-month and ≥60-month age groups but significant for the group of children ≥48 months of age (P = 0.048).
In 2008, PCV7 serotypes were carried by 30 (12.7%) of 237 study participants who had been immunized at least twice and by 85 (23.3%) of 365 participants who had received ≤1 vaccine doses (P = 0.001). Relative to the overall 37.0% carriage of PCV7 serotypes in 2006, carriage of PCV7 serotypes decreased by 65.8% among vaccinated children (P < 0.001) and by 37.0% among children who had received one dose or no immunization (P < 0.001).
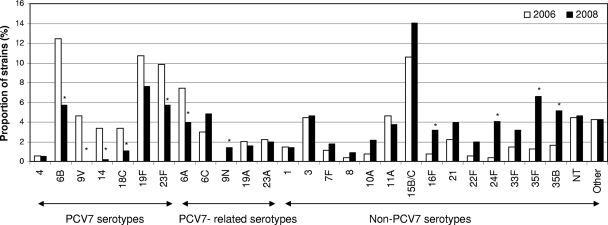
Carriage of five of the PCV7 serotypes (6B, 9V, 14, 18C, and 23F) significantly decreased, while carriage of serotypes 4 and 19F did not change significantly (Fig. 1 and Table 2). Decreased carriage was also observed for PCV7-related serotype 6A.
FIG. 1.
Distribution of pneumococcal serotypes, as proportions of all isolates, according to sampling period (539 isolates in 2006 and 563 isolates in 2008). Statistically significant differences in serotype-specific proportional carriage (P < 0.05; Fisher's exact tests) are indicated by asterisks.
TABLE 2.
Distribution of serotypes and genotypes for 1,102 strains of S. pneumoniae isolated from children attending DCCs in Norway in 2006 and 2008
| Serotype | No. of isolates in yr |
P valuea | ST | CC | No. of isolates with ST in yr |
P value | ||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2008 | 2006 | 2008 | |||||
| PCV7 serotypes | ||||||||
| 4 | 3 | 3 | NSb | 205 | Sc | 3 | 3 | NS |
| 6B | 67 | 32 | <0.0001 | 138 | CC138 | 18 | 1 | <0.0001 |
| 2547 | CC138 | 1 | 1 | NS | ||||
| 2556 | CC138 | 2 | NS | |||||
| 2720 | CC138 | 4 | NS | |||||
| 146 | S | 3 | NS | |||||
| 176 | CC176 | 35 | 16 | 0.004 | ||||
| 469 | CC176 | 1 | 9 | 0.0214 | ||||
| 490 | CC490 (6A) | 2 | NS | |||||
| 639 | S | 1 | NS | |||||
| 2779 | S | 5 | NS | |||||
| 9V | 25 | 0 | <0.0001 | 162 | S | 25 | <0.0001 | |
| 14 | 18 | 1 | <0.0001 | 9 | S | 11 | 0.0009 | |
| 124 | CC124 | 6 | 1 | NS | ||||
| 2551 | CC124 | 1 | NS | |||||
| 18C | 18 | 6 | 0.012 | 110 | CC110 | 4 | 1 | NS |
| 113 | CC110 | 5 | 5 | NS | ||||
| 2559 | CC110 | 1 | NS | |||||
| 496 | S | 8 | 0.0032 | |||||
| 19F | 58 | 43 | NS | 43 | S | 1 | NS | |
| 177 | CC177 | 11 | 22 | NS | ||||
| 179 | CC177 | 9 | 4 | NS | ||||
| 391 | CC177 | 3 | 1 | NS | ||||
| 2550 | CC177 | 1 | NS | |||||
| 2620 | CC177 | 1 | NS | |||||
| 2731 | CC177 | 1 | NS | |||||
| 236 | CC236 | 4 | NS | |||||
| 320 | CC236 | 2 | NS | |||||
| 420 | S | 1 | NS | |||||
| 423 | CC423 | 2 | NS | |||||
| 3016 | CC423 | 2 | NS | |||||
| 425 | S | 1 | NS | |||||
| 462 | S | 4 | 4 | NS | ||||
| 476 | S | 2 | NS | |||||
| 654 | S | 1 | NS | |||||
| 1173 | S | 3 | 3 | NS | ||||
| 2439 | S | 1 | NS | |||||
| 2581 | S | 8 | 0.0032 | |||||
| 2621 | S | 6 | 0.0135 | |||||
| 2631 | S | 2 | NS | |||||
| 4057 | S | 1 | NS | |||||
| 23F | 53 | 32 | 0.013 | 33 | CC439 | 2 | NS | |
| 439 | CC439 | 1 | NS | |||||
| 1011 | CC439 | 5 | NS | |||||
| 36 | S | 31 | 11 | 0.0014 | ||||
| 242 | S | 2 | NS | |||||
| 338 | S | 2 | NS | |||||
| 440 | CC440 | 9 | 18 | NS | ||||
| 2558 | S | 2 | 1 | NS | ||||
| 2624 | S | 1 | NS | |||||
| Non-PCV7 serotypes | ||||||||
| 6A | 40 | 22 | 0.013 | 65 | CC460 | 1 | NS | |
| 460 | CC460 | 5 | 13 | NS | ||||
| 2579 | CC460 | 1 | NS | |||||
| 138 | CC138 | 1 | NS | |||||
| 176 | CC167 | 1 | NS | |||||
| 490 | CC490 | 8 | 1 | 0.0186 | ||||
| 681 | CC490 | 2 | 1 | NS | ||||
| 2221 | CC490 | 5 | 4 | NS | ||||
| 1978 | S | 9 | 1 | 0.0099 | ||||
| 2068 | CC2068 | 1 | NS | |||||
| 2728 | CC2068 | 5 | NS | |||||
| 2557 | S | 1 | NS | |||||
| 2622 | S | 1 | NS | |||||
| 2623 | S | 1 | NS | |||||
| 6C | 16 | 27 | NS | 473 | CC473 | 1 | NS | |
| 639 | S | 1 | NS | |||||
| 1379 | CC1379 | 3 | 2 | NS | ||||
| 2629 | CC1379 | 1 | NS | |||||
| 1390 | S | 1 | NS | |||||
| 1692 | CC1692 | 9 | 0.0038 | |||||
| 1714 | CC1692 | 11 | 14 | NS | ||||
| 9N | 0 | 8 | 0.008 | 66 | S | 2 | NS | |
| 405 | S | 6 | 0.0312 | |||||
| 18A | 1 | 0 | NS | 1232 | S | 1 | NS | |
| 19A | 11 | 9 | NS | 199 | CC199 | 7 | 7 | NS |
| 645 | CC199 | 2 | NS | |||||
| 667 | CC199 | 2 | NS | |||||
| 2548 | S | 1 | NS | |||||
| 2632 | S | 1 | NS | |||||
| 23A | 12 | 11 | NS | 42 | CC439 | 3 | 2 | NS |
| 439 | CC439 | 4 | 9 | NS | ||||
| 2560 | CC439 | 5 | NS | |||||
| 1 | 8 | 8 | NS | 306 | S | 8 | 8 | NS |
| 3 | 24 | 26 | NS | 180 | CC180 | 22 | 24 | NS |
| 2630 | CC180 | 2 | NS | |||||
| 4054 | CC180 | 1 | NS | |||||
| 1377 | S | 1 | NS | |||||
| 7F | 6 | 10 | NS | 191 | CC191 | 6 | 1 | NS |
| 3795 | CC191 | 9 | 0.0038 | |||||
| 8 | 2 | 5 | NS | 53 | CC53 | 1 | 4 | NS |
| 2582 | CC53 | 1 | NS | |||||
| 3778 | CC53 | 1 | NS | |||||
| 10A | 4 | 12 | NS | 97 | CC460 | 2 | 1 | NS |
| 1551 | CC460 | 1 | 8 | 0.0387 | ||||
| 2068 | CC2068 | 1 | 2 | NS | ||||
| 4113 | S | 1 | NS | |||||
| 10B | 1 | 0 | NS | 2553 | CC473 | 1 | NS | |
| 11A | 25 | 21 | NS | 62 | CC62 | 20 | 19 | NS |
| 2625 | CC62 | 1 | NS | |||||
| 2649 | CC62 | 1 | NS | |||||
| 2549 | S | 3 | 2 | NS | ||||
| 15A | 0 | 2 | NS | 63 | S | 1 | NS | |
| 73 | S | 1 | NS | |||||
| 15B | 31 | 31 | NS | 199 | CC199 | 23 | 13 | NS |
| 200 | CC199 | 1 | NS | |||||
| 2729 | CC199 | 1 | NS | |||||
| 3976 | CC199 | 1 | NS | |||||
| 2344 | CC199 | 1 | NS | |||||
| 275 | S | 1 | NS | |||||
| 1262 | S | 5 | 15 | 0.0405 | ||||
| 2577 | CC1054 | 1 | NS | |||||
| 15C | 26 | 48 | 0.016 | 199 | CC199 | 18 | 20 | NS |
| 200 | CC199 | 6 | 0.03 | |||||
| 4056 | CC199 | 2 | NS | |||||
| 1262 | S | 8 | 20 | 0.0346 | ||||
| 16F | 4 | 18 | 0.004 | 30 | CC30 | 4 | 9 | NS |
| 414 | CC30 | 1 | NS | |||||
| 3273 | CC30 | 2 | NS | |||||
| 3796 | CC30 | 6 | 0.03 | |||||
| 17F | 0 | 2 | NS | 392 | CC440 | 1 | NS | |
| 4112 | S | 1 | NS | |||||
| 20 | 0 | 1 | NS | 235 | S | 1 | NS | |
| 21 | 12 | 22 | NS | 193 | S | 1 | 3 | NS |
| 1877 | S | 11 | 17 | NS | ||||
| 4055 | S | 2 | NS | |||||
| 22F | 3 | 11 | NS | 433 | CC433 | 3 | 8 | NS |
| 819 | CC433 | 1 | NS | |||||
| 4110 | CC433 | 2 | NS | |||||
| 24F | 2 | 23 | <0.0001 | 72 | S | 2 | 23 | <0.0001 |
| 31 | 2 | 3 | NS | 444 | S | 1 | 1 | NS |
| 1601 | S | 1 | 1 | NS | ||||
| 1766 | S | 1 | NS | |||||
| 33F | 8 | 18 | NS | 100 | S | 5 | 11 | NS |
| 673 | S | 3 | 4 | NS | ||||
| 4115 | S | 3 | NS | |||||
| 33A | 0 | 1 | NS | 4115 | S | 1 | NS | |
| 34 | 6 | 1 | NS | 1046 | S | 6 | 0.0135 | |
| 3922 | S | 1 | NS | |||||
| 35F | 7 | 37 | <0.0001 | 446 | CC446 | 7 | 27 | 0.0008 |
| 1635 | CC446 | 9 | 0.0038 | |||||
| 4111 | CC446 | 1 | NS | |||||
| 35B | 9 | 29 | 0.002 | 198 | S | 4 | 5 | NS |
| 452 | CC452 | 4 | 23 | 0.0003 | ||||
| 2628 | CC452 | 1 | NS | |||||
| 4114 | CC452 | 1 | NS | |||||
| 37 | 4 | 1 | NS | 447 | S | 4 | 1 | NS |
| 38 | 9 | 13 | NS | 393 | CC393 | 9 | 11 | NS |
| 4058 | CC393 | 2 | NS | |||||
| NTd | 24 | 26 | NS | 124 | CC124 | 1 | NS | |
| 448 | S | 2 | 2 | NS | ||||
| 550 | CC423 | 1 | NS | |||||
| 941 | S | 12 | 0.0002 | |||||
| 1054 | CC1054 | 7 | 21 | 0.012 | ||||
| 3108 | CC1054 | 1 | NS | |||||
| 449 | CC1054 | 1 | 2 | NS | ||||
Fisher's exact test.
NS, nonsignificant (P ≥ 0.05).
S, singleton, i.e., ≤5 of 7 alleles in common with other isolated sequence types.
NT, nontypeable.
Carriage of 21 distinct non-PCV7 serotypes and nontypeable strains increased, the increase being statistically significant for serotypes 9N, 16F, 24F, 35F, and 35B (Fig. 1 and Table 2). A significant increase was observed for serotype 15C, while the overall increase of serotype 15B/C proved nonsignificant. The prevalence of serotype 6C increased nonsignificantly, while the prevalence of serotype 19A decreased slightly from 2006 to 2008.
In the two collections of strains, serotypes 19F, 3, and 15C and nontypeable pneumococci were isolated more frequently from cocolonized children than from children carrying a single strain, as already reported for the 2006 samples (33). In 2008, this association was observed for further four serotypes, i.e., serotypes 23F, 8, 21, and 33F (P < 0.05 for all; Fisher's exact test).
Genotype distribution and population snapshot.
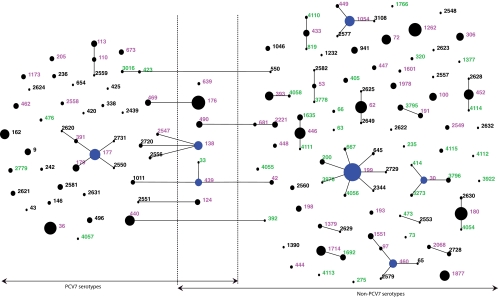
In the 2006 strain collection, 104 distinct STs had been identified, 33 (31.7%) being new in the MLST database at the time of analysis. In 2008, 93 STs were identified, including 17 (18.3%) new STs. Overall, 143 STs were identified in the two studies, 53 of which were present in both data sets (Table 2). The pneumococcal populations sampled in 2006 and 2008 are described in a population snapshot showing which STs were identified in either one or both collections (Fig. 2). Of the 33 new STs identified in 2006, only two were also isolated in 2008. The two sample collections were genetically different (classification index = 0.366). However, the genotypic diversity measured by Simpson's index of diversity (D) remained unchanged (Table 3).
FIG. 2.
Population snapshot based on eBURST analysis of 1,102 pneumococcal isolates, 539 and 563 isolates recovered in 2006 and 2008, respectively. Each sequence type is represented by a numbered circle, the size of which corresponds to the number of isolates. STs linked by a line differ in one of seven alleles; the STs that are linked by lines constitute a clonal complex. STs considered founders of clonal complexes are indicated by blue circles. STs whose numbers are shown in red were identified in both data sets, those shown in black were identified in 2006 only, and those shown in green were identified in 2008 only. As indicated by lines, STs are clustered according to associated serotype, with PCV7 serotypes to the left, non-PCV7 serotypes to the right, and STs identified with both PCV7 and non-PCV7 serotypes in the middle.
TABLE 3.
Genotypic diversity among strains of S. pneumoniae isolates from children attending DCCs in Norway
| Serotypes and study yr | No. of isolates | No. of STs | D (95% CI)a |
|---|---|---|---|
| Total | |||
| 2006 | 539 | 104 | 0.978 (0.975-0.981) |
| 2008 | 563 | 93 | 0.978 (0.975-0.980) |
| PCV7 serotypes | |||
| 2006 | 242 | 43 | 0.937 (0.924-0.950) |
| 2008 | 117 | 23 | 0.906 (0.882-0.930) |
| Non-PCV7 serotypes | |||
| 2006 | 297 | 63 | 0.969 (0.963-0.978) |
| 2008 | 446 | 73 | 0.971 (0.968-0.975) |
D, Simpson's index of diversity; 95% CI, 95% confidence interval.
Clonal changes among PCV7 serotypes.
The number of distinct STs identified among PCV7 serotypes decreased from 43 in 2006 to 23 in 2008; 32 STs either disappeared or decreased in frequency, while 3 STs expanded and 7 STs emerged. The genotypic diversity among PCV7 serotypes decreased, but there were slightly overlapping 95% CIs (Table 3).
Within serotype 6B, reductions of CC138 and CC176 accounted for the overall decrease, while ST 469 significantly increased. The elimination of ST 162 accounted for the disappearance of serotype 9V. Similarly, within serotype 14, ST 9 disappeared, and CC124 was significantly reduced. Carriage of serotype 19F did not change significantly; the dominating CC within serotype 19F, CC177, persisted.
Clonal changes among non-PCV7 serotypes.
Among non-PCV7 serotypes, the number of distinct STs increased from 63 in 2006 to 73 in 2008; 28 STs disappeared, 7 of which belonged to PCV7-related serotype 6A. The increase in non-PCV7 serotypes was caused both by emergence of 37 STs and by expansion of 20 existing STs. The emerging STs accounted for 88 (19.7%) of 446 non-PCV7 strains in 2008, while 358 (80.3%) of the non-PCV7 strains belonged to clones which were also isolated in 2006. A nonsignificant increase in genotypic diversity was observed (Table 3).
Although a diverse set of STs expanded among the non-PCV7 serotypes, expansion of a limited number of distinct CCs was associated with the significant increases in serotype-specific carriage. All isolates of serotypes 16F, 24F, and 35F belonged to one clone or CC, namely, CC30, ST 72, and CC446, respectively. Within serotype 35B, 24 (82.8%) of 29 isolates sampled in 2008 belonged to CC452. Expansion of serotype 9N was dominated by ST 405 (6 of 8 isolates in 2008).
Seven isolates expressing serotype 15B/C capsules were genotyped as ST 200, an SLV of ST 199, previously associated only with serotype 14. This accounted for the only possible serotype switch observed in the study. One single isolate belonging to ST 124 recovered in 2008 was nontypeable; ST 124 was associated with serotype 14 in 2006.
All strains of the recently described serotype 6C belonged to clones that had previously been entered into the MLST database as serotype 6A, i.e., no new STs expressing serotype 6C were observed following the introduction of PCV7.
Antimicrobial susceptibility.
Overall, the proportion of isolates with nonsusceptibility to penicillin G increased nonsignificantly from 1.7% in 2006 to 2.1% in 2008; one penicillin G-resistant isolate (MIC ≥ 2 μg/ml) was detected (Table 4) . Nonsusceptibility to erythromycin decreased significantly, while a small and nonsignificant decrease was observed for non-clindamycin-susceptible isolates (Table 4). The proportion of strains with resistance to trimethoprim-sulfamethoxazole decreased from 4.6% to 1.9%. The prevalence of multidrug-resistant pneumococci (MDRSP), defined as strains with nonsusceptibility to ≥2 β-lactams (i.e., penicillin G, cefotaxime, or ceftriaxone), erythromycin, clindamycin, tetracycline, or trimethoprim-sulfamethoxazole, decreased from 4.5% to 1.4%.
TABLE 4.
Antimicrobial susceptibility of S. pneumoniae isolates from children attending DCCs in Norway
| Antimicrobial agent | MIC breakpoint (μg/ml) | No. (%) of resistant isolates by serotypes and yr |
|||||
|---|---|---|---|---|---|---|---|
| All serotypes |
PCV7 serotypes |
Non-PCV7 serotypes |
|||||
| 2006 (n = 539) | 2008 (n = 563) | 2006 (n = 242) | 2008 (n = 117) | 2006 (n = 297) | 2008 (n = 446) | ||
| Penicillin G | 0.12-1.0 | 9 (1.7) | 11 (1.9) | 5 (2.1) | 7 (6.0)a | 4 (1.3) | 4 (0.9) |
| ≥2.0 | 1 (0.2) | 1 (0.8) | |||||
| Erythromycin | >0.25 | 32 (5.9) | 18 (3.2)a | 27 (11.2) | 15 (12.8) | 5 (1.7) | 3 (0.7) |
| Clindamycin | >0.25 | 12 (2.2) | 8 (1.4) | 10 (4.4) | 8 (6.7) | 2 (0.7) | 2 (0.4) |
| Tetracycline | >2.0 | 32 (5.9) | 38 (6.7) | 29 (12.0) | 32 (27.4)a | 3 (1.0) | 6 (1.3) |
| SXTb | ≥4.0 | 25 (4.6) | 11 (1.9)a | 3 (1.2) | 3 (2.6) | 22 (7.4) | 8 (1.8)a |
| MDRSP | 24 (4.5) | 8 (1.4)a | 15 (6.2) | 6 (5.1) | 9 (3.0) | 2 (0.4)a | |
Change statistically significant (P < 0.05; Fisher's exact test).
The breakpoint for resistant strains is given. SXT, trimethoprim-sulfamethoxazole.
Among PCV7 serotype strains, the proportion of non-penicillin-susceptible pneumococci increased from 2.1% in 2006 to 6.8% in 2008, and nonsusceptibility to tetracycline increased from 12.0% to 27.4% (Table 4).
Four PMEN clones, or SLVs of PMEN clones, expressing PCV7 serotypes disappeared following PCV7 introduction, including ST 9 (England14-ST 9), ST 162 (Spain9V-ST 156 SLV), ST 338 (Colombia23F-ST 338), and ST 236 (Taiwan19F-ST 236). The disappearance of ST 162 alone accounted for the decrease of serotype 9V, while the disappearance of ST 9 largely accounted for the decrease of serotype 14 (Table 2).
On the other hand, two PMEN clones, ST 179 (a SLV of Portugal19F-ST 177) and ST 320 (a double-locus variant [DLV] of Taiwan19F-ST 236), expanded and emerged, respectively, from 2006 to 2008. The persisting CC177 (Portugal19F-ST 177) dominated among serotype 19F strains in 2008, accounting for an increased proportion of tetracycline-resistant strains among PCV7 serotypes. Overall, among 10 expanding STs within PCV7 serotypes, 4 STs were nonsusceptible to one or more antimicrobial agents, accounting for 38 (32.5%) of the 117 PCV7 strains in 2008.
The proportion of strains with nonsusceptibility to any antimicrobial agent among non-PCV7 serotypes did not increase, but a slight increase in the proportion with tetracycline resistance was observed (Table 4). The proportions of MDRSP and strains resistant to trimethoprim-sulfamethoxazole decreased among these serotypes from 2006 to 2008 (P values of 0.009 and <0.001, respectively). The MDRSP PMEN clone ST 63 (Sweden15A-ST63) emerged and was represented by a single isolate among non-PCV7 serotypes in 2008.
DISCUSSION
The prevalence of nasopharyngeal pneumococcal colonization among children in Norway is very high compared to prevalences reported from other European and North American settings (2, 4, 13, 15, 19, 28). Furthermore, the prevalence remained unchanged following the introduction of PCV7 (77.7% before and 80.2% after widespread vaccination). The decrease of PCV7 serotypes among carriers was completely replaced by an increase of non-PCV7 serotypes, similar to what has been reported from other locations (13, 28).
Sample collection was performed in DCCs, a setting well known to be associated with a high risk of nasopharyngeal carriage of S. pneumoniae (29). The impact of DCC attendance on transmission of pneumococci has been further demonstrated by modeling; with an increase in the fraction of children attending DCCs and with increasing time spent in DCCs, transmission of pneumococci increases among all children within a community (12). The proportion of children 1 to 5 years of age attending DCCs in Norway is 84.3%, and more than 95% of these children attend the DCC for at least 25 h per week. Thus, level of transmission of pneumococci among children in Norway is probably very high, both among DCC-attending children and children not attending DCCs, and the observed carriage rate is presumably representative. The geographic distributions of the included DCCs in the two studies were limited but similar. The pneumococci circulating in the DCCs might be less heterogenic than those circulating in the community. This might bias the results regarding ST distribution. However, DCC attendance is very high, and the sample size is large; thus, the results are believed to be representative of the overall community.
The follow-up was performed only 2 years after vaccine introduction. Vaccine coverage increased rapidly after vaccine introduction in the Norwegian vaccination program (34). Thus, as the study population consisted of 1- to 5-year-olds, both vaccinated and unvaccinated children were included; approximately 40% of children had received ≥2 immunizations with PCV7, with high vaccine coverage among study participants born in 2006 or later. The overall rate of carriage of PCV7 serotypes decreased by 48%; carriage decreased both among children who had received ≤1 vaccine dose and among children who had been immunized at least twice, although the latter group carried fewer PCV7 serotypes. Thus, both direct and indirect effects of the PCV7 vaccination program were observed among colonized children. This is consistent with post-PCV7 carriage studies performed in the United States and Canada (19, 24). However, these studies were performed in settings where a 3 + 1 PCV7 vaccination schedule is recommended, while the present study demonstrates a similar impact with a 2 + 1 dose schedule.
Significant reduction of carriage was observed for five of the PCV7 serotypes; serotypes 4 and 19F did not change significantly. Less inhibition of colonization with serotype 19F by PCV7 has also been observed in other studies (22, 24). The prevalence of serotype 19A remained unchanged. Importantly, the prevalence of serotype 6B, against which the antibody response corresponding to the 2 + 1 dose schedule has been shown to be somewhat lower than the antibody response after a 3 + 1 dose schedule (18), decreased significantly. Reduced carriage was also observed for related serotype 6A, while serotype 6C increased nonsignificantly. Interestingly, all serotype 6C strains belonged to STs that had previously been entered in the MLST database and associated with serotype 6A. These isolates might actually be serotype 6C; retyping of the isolates and an update of the database should be considered.
Serotype replacement might be caused by a shift in circulating strains. However, it might also, in part, result from a sampling artifact, i.e., sampling might fail to identify a non-vaccine-serotype strain from cocolonized carriers, but following vaccination, these serotypes might be more easily detected. This phenomenon has been called “unmasking” (21). A sensitive sampling method was employed in the present study, and cocolonization was observed in 12.6% of samples. Hence, the observed changes are believed to represent a true serotype replacement (26). Certain serotypes, including serotypes 3 and 19F, were associated with cocolonization. However, no recombinational events in cocolonization were indicated by MLST data.
The post-PCV7 change in serotype distribution was generated by a shift in clonal composition of the carried pneumococcal population, including expansion, emergence, reduction, and disappearance of clones. A limited number of clones or CCs within serotypes accounted for the shift in serotype prevalence, while genetic diversity was generated by a large number of STs sharing less than 6 of 7 alleles with other STs (singletons). The possibility of capsule switch was suspected for one clone: ST 200, previously associated with serotype 14, was serotyped as 15B/C. Within PCV7 serotypes, effective reductions, including elimination of internationally dispersed nonsusceptible clones, were observed. However, clonal persistence, expansion, and emergence were observed, in particular for nonsusceptible clones. Statistically significant increases of non-PCV7 serotypes were related to a limited number of corresponding STs and CCs. Importantly, nonsusceptibility to antimicrobials was not observed among these rapidly expanding clones.
Data on the use of antimicrobial agents reported by parents on questionnaires were comparable to usage data obtained from the Norwegian Prescription Database (www.norpd.no) and indicate that overall usage among children in Norway is low. From 2006 to 2008, a significant decrease in antibiotic use was reported. Although the overall use decreased, the numbers were too small for differentiation between use among vaccinated and unvaccinated children. Similarly, the proportion of children reported to have any RTI during the 3 months preceding participation decreased overall. As for the case with antibiotic usage, differences between vaccinated and unvaccinated children were not significant. These findings are consistent with results from a randomized controlled study in DCCs (5), while postlicensure surveillance has not unambiguously demonstrated such reductions (14, 24).
Following the introduction of PCV7, the proportions of erythromycin-resistant, trimethoprim-sulfamethoxazole-resistant, and multidrug-resistant pneumococci decreased. Prior to PCV7 introduction, the PMEN clone England14-ST 9 dominated among erythromycin-resistant pneumococci. The reduced proportion of strains with erythromycin resistance in the carried population was closely linked to the disappearance of this clone and corresponds well to the decline in IPD caused by erythromycin-resistant pneumococci among children after PCV7 introduction (34).
The proportions of nonsusceptibility to penicillin and tetracycline, however, remained stable overall and increased among PCV7 serotypes. These increases were related to the expansion and introduction of a few distinct nonsusceptible clones, in particular, clones with serotype 19F capsules. Persistence of non-penicillin-susceptible pneumococci, and in particular non-penicillin-susceptible pneumococcus serotype 19F, following vaccine introduction has also been described in other settings. The tetracycline-resistant serotype 19F clone Portugal19F-ST 177 was described as one of the most prevalent and persistent clones in a longitudinal DCC carriage study in Portugal (30). Thus, both ST and susceptibility phenotype, in addition to serotype, might influence selection and persistence.
Interestingly, emergence of serotype 19F MDRSP belonging to ST 320 was observed. This clone has been a major contributor to the post-PCV7 increase in nonsusceptible serotype 19A in the United States (23). Vaccine-induced mucosal immunity against serotype 19F being weaker than that against other PCV7 serotypes might explain this persistence. However, the persistence and expansion of nonsusceptible serotype 19F clones under a fairly constant, even likely lower, antibiotic selective pressure suggest an additional selective advantage among these clones.
An association between carriage prevalence and nonsusceptibility to penicillin among non-PCV7 serotypes has been suggested (14), indicating an effect of continued antibiotic selection. Increased carriage of serotype 19A and, in particular, emergence of non-penicillin-susceptible serotype 19A were evident in studies from the United States with 3 years of follow-up (13, 28). Hence, emergence of serotype 19A and nonsusceptibility among colonizing non-PCV7 serotypes in Norway might increase, in particular, among the most prevalent serotypes, e.g., serogroups 15 and 35, and this should be assessed by further surveillance of nasopharyngeal carriage.
In conclusion, we have demonstrated that a 2 + 1 dose vaccination program rapidly leads to reduced circulation of PCV7 serotypes among both vaccinated and unvaccinated children in Norwegian DCCs, a setting where pneumococcal carriage is highly prevalent and the transmission rate is presumably very high. The decreased serotypes, however, were completely replaced by non-PCV7 serotypes. Drug resistance either decreased or persisted among carried strains; it is noteworthy that the proportion of nonsusceptible strains did not increase among non-PCV7 serotypes. Expansion of established clones and introduction of new clones among non-PCV7 serotypes were observed in the carried pneumococcal population; the major serotype-specific increases were caused by expansion of a corresponding ST or CC. Among PCV7 serotypes, nonsusceptible clones persisted, in particular, clones belonging to serotype 19F, an important colonizing serotype that did not significantly decrease post-PCV7. Thus, a differential immunologic selective pressure might interact with an antimicrobial selective pressure and clone-specific advantages to create the shifts observed in the population with pneumococcal carriage.
Acknowledgments
This study was in part supported by grants 06_14 and 08_01 from the Norwegian Surveillance System for Antimicrobial Resistance (NORM).
Anne R. Alme, Torill Alvestad, Martha L. Bjørnstad, Anne M. Klem, and Gunnhild Rødal are thanked for excellent technical assistance.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Bogaert, D., R. de Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert, D., A. van Belkum, M. Sluijter, A. Luijendijk, R. de Groot, H. C. Rumke, H. A. Verbrugh, and P. W. Hermans. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871-1872. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Cohen, R., C. Levy, F. de La Rocque, N. Gelbert, A. Wollner, B. Fritzell, E. Bonnet, R. Tetelboum, and E. Varon. 2006. Impact of pneumococcal conjugate vaccine and of reduction of antibiotic use on nasopharyngeal carriage of nonsusceptible pneumococci in children with acute otitis media. Pediatr. Infect. Dis. J. 25:1001-1007. [DOI] [PubMed] [Google Scholar]
- 5.Dagan, R., M. Sikuler-Cohen, O. Zamir, J. Janco, N. Givon-Lavi, and D. Fraser. 2001. Effect of a conjugate pneumococcal vaccine on the occurrence of respiratory infections and antibiotic use in day-care center attendees. Pediatr. Infect. Dis. J. 20:951-958. [DOI] [PubMed] [Google Scholar]
- 6.Dunais, B., P. Bruno, H. Carsenti-Dellamonica, P. Touboul, P. Dellamonica, and C. Pradier. 2008. Trends in nasopharyngeal carriage of Streptococcus pneumoniae among children attending daycare centers in southeastern France from 1999 to 2006. Pediatr. Infect. Dis. J. 27:1033-1035. [DOI] [PubMed] [Google Scholar]
- 7.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 8.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanage, W. P., S. S. Huang, M. Lipsitch, C. J. Bishop, D. Godoy, S. I. Pelton, R. Goldstein, H. Huot, and J. A. Finkelstein. 2007. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J. Infect. Dis. 195:347-352. [DOI] [PubMed] [Google Scholar]
- 11.Hicks, L. A., L. H. Harrison, B. Flannery, J. L. Hadler, W. Schaffner, A. S. Craig, D. Jackson, A. Thomas, B. Beall, R. Lynfield, A. Reingold, M. Farley, and C. G. Whitney. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 196:1346-1354. [DOI] [PubMed] [Google Scholar]
- 12.Huang, S. S., J. A. Finkelstein, and M. Lipsitch. 2005. Modeling community- and individual-level effects of child-care center attendance on pneumococcal carriage. Clin. Infect. Dis. 40:1215-1222. [DOI] [PubMed] [Google Scholar]
- 13.Huang, S. S., R. Platt, S. L. Rifas-Shiman, S. I. Pelton, D. Goldmann, and J. A. Finkelstein. 2005. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116:e408-e413. [DOI] [PubMed] [Google Scholar]
- 14.Huang, S. S., V. L. Hinrichsen, A. E. Stevenson, S. L. Rifas-Shiman, K. Kleinman, S. I. Pelton, M. Lipsitch, W. P. Hanage, G. M. Lee, and J. A. Finkelstein. 2009. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 124:e1-e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain, M., A. Melegaro, R. G. Pebody, R. George, W. J. Edmunds, R. Talukdar, S. A. Martin, A. Efstratiou, and E. Miller. 2005. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol. Infect. 133:891-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolley, K. A., D. J. Wilson, P. Kriz, G. McVean, and M. C. Maiden. 2005. The influence of mutation, recombination, population history, and selection on patterns of genetic diversity in Neisseria meningitidis. Mol. Biol. Evol. 22:562-569. [DOI] [PubMed] [Google Scholar]
- 17.Kaltoft, M. S., U. B. Skov Sorensen, H. C. Slotved, and H. B. Konradsen. 2008. An easy method for detection of nasopharyngeal carriage of multiple Streptococcus pneumoniae serotypes. J. Microbiol. Methods 75:540-544. [DOI] [PubMed] [Google Scholar]
- 18.Käyhty, H., H. Ahman, K. Eriksson, M. Sorberg, and L. Nilsson. 2005. Immunogenicity and tolerability of a heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 12 months of age. Pediatr. Infect. Dis. J. 24:108-114. [DOI] [PubMed] [Google Scholar]
- 19.Kellner, J. D., D. Scheifele, O. G. Vanderkooi, J. Macdonald, D. L. Church, and G. J. Tyrrell. 2008. Effects of routine infant vaccination with the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization with Streptococcus pneumoniae in children in Calgary, Canada. Pediatr. Infect. Dis. J. 27:526-532. [DOI] [PubMed] [Google Scholar]
- 20.Kyaw, M. H., R. Lynfield, W. Schaffner, A. S. Craig, J. Hadler, A. Reingold, A. R. Thomas, L. H. Harrison, N. M. Bennett, M. M. Farley, R. R. Facklam, J. H. Jorgensen, J. Besser, E. R. Zell, A. Schuchat, C. G. Whitney, and Active Bacterial Core Surveillance of the Emerging Infections Program Network. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354:1455-1463. [DOI] [PubMed] [Google Scholar]
- 21.Lipsitch, M. 1999. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg. Infect. Dis. 5:336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipsitch, M., K. O'Neill, D. Cordy, B. Bugalter, K. Trzcinski, C. M. Thompson, R. Goldstein, S. Pelton, H. Huot, V. Bouchet, R. Reid, M. Santosham, and K. L. O'Brien. 2007. Strain characteristics of Streptococcus pneumoniae carriage and invasive disease isolates during a cluster-randomized clinical trial of the 7-valent pneumococcal conjugate vaccine. J. Infect. Dis. 196:1221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore, M. R., R. E. Gertz, Jr., R. L. Woodbury, G. A. Barkocy-Gallagher, W. Schaffner, C. Lexau, K. Gershman, A. Reingold, M. Farley, L. H. Harrison, J. L. Hadler, N. M. Bennett, A. R. Thomas, L. McGee, T. Pilishvili, A. B. Brueggemann, C. G. Whitney, J. H. Jorgensen, and B. Beall. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197:1016-1027. [DOI] [PubMed] [Google Scholar]
- 24.Moore, M. R., T. B. Hyde, T. W. Hennessy, D. J. Parks, A. L. Reasonover, M. Harker-Jones, J. Gove, D. L. Bruden, K. Rudolph, A. Parkinson, J. C. Butler, and A. Schuchat. 2004. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J. Infect. Dis. 190:2031-2038. [DOI] [PubMed] [Google Scholar]
- 25.NORM/NORM-VET. 2007. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. NORM/NORM-VET, Tromsø/Oslo, Norway.
- 26.O'Brien, K. L., E. V. Millar, E. R. Zell, M. Bronsdon, R. Weatherholtz, R. Reid, J. Becenti, S. Kvamme, C. G. Whitney, and M. Santosham. 2007. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J. Infect. Dis. 196:1211-1220. [DOI] [PubMed] [Google Scholar]
- 27.Park, I. H., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, S. Y., M. R. Moore, D. L. Bruden, T. B. Hyde, A. L. Reasonover, M. Harker-Jones, K. M. Rudolph, D. A. Hurlburt, D. J. Parks, A. J. Parkinson, A. Schuchat, and T. W. Hennessy. 2008. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr. Infect. Dis. J. 27:335-340. [DOI] [PubMed] [Google Scholar]
- 29.Regev-Yochay, G., M. Raz, R. Dagan, N. Porat, B. Shainberg, E. Pinco, N. Keller, and E. Rubinstein. 2004. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin. Infect. Dis. 38:632-639. [DOI] [PubMed] [Google Scholar]
- 30.Sá-Leão, R., S. Nunes, A. Brito-Avo, C. R. Alves, J. A. Carrico, J. Saldanha, J. S. Almeida, I. Santos-Sanches, and H. De Lencastre. 2008. High rates of transmission of and colonization by Streptococcus pneumoniae and Haemophilus influenzae within a day care center revealed in a longitudinal study. J. Clin. Microbiol. 46:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson, E. H. 1949. Measurement of diversity. Nature 163:688. [Google Scholar]
- 32.van Selm, S., L. M. van Cann, M. A. Kolkman, B. A. van der Zeijst, and J. P. van Putten. 2003. Genetic basis for the structural difference between Streptococcus pneumoniae serotype 15B and 15C capsular polysaccharides. Infect. Immun. 71:6192-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vestrheim, D. F., E. A. Hoiby, I. S. Aaberge, and D. A. Caugant. 2008. Phenotypic and genotypic characterization of Streptococcus pneumoniae strains colonizing children attending day-care centers in Norway. J. Clin. Microbiol. 46:2508-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vestrheim, D. F., O. Lovoll, I. S. Aaberge, D. A. Caugant, E. A. Hoiby, H. Bakke, and M. R. Bergsaker. 2008. Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine 26:3277-3281. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. 2007. Pneumococcal conjugate vaccine for childhood immunization. WHO position paper. Wkly. Epidemiol. Rec. 82:93-104. [PubMed] [Google Scholar]