Abstract
Vi polysaccharide from Salmonella enterica serotype Typhi is used as one of the available vaccines to prevent typhoid fever. Measurement of Vi-specific serum antibodies after vaccination with Vi polysaccharide by enzyme-linked immunosorbent assay (ELISA) may be complicated due to poor binding of the Vi polysaccharide to ELISA plates resulting in poor reproducibility of measured antibody responses. We chemically conjugated Vi polysaccharide to fluorescent beads and performed studies to determine if a bead-based immunoassay provided a reproducible method to measure vaccine-induced anti-Vi serum IgG antibodies. Compared to ELISA, the Vi bead immunoassay had a lower background and therefore a greater signal-to-noise ratio. The Vi bead immunoassay was used to evaluate serum anti-Vi IgG in 996 subjects from the city of Kolkata, India, before and after vaccination. Due to the location being one where Salmonella serotype Typhi is endemic, approximately 45% of the subjects had protective levels of anti-Vi serum IgG (i.e., 1 μg/ml anti-Vi IgG) before vaccination, and nearly 98% of the subjects had protective levels of anti-Vi serum IgG after vaccination. Our results demonstrate that a bead-based immunoassay provides an effective, reproducible method to measure serum anti-Vi IgG responses before and after vaccination with the Vi polysaccharide vaccine.
Typhoid fever is caused by Salmonella enterica serotype Typhi (32). Humans are the only natural host and reservoir of S. enterica serotype Typhi (32, 41). Typhoid fever represents a spectrum of diseases ranging from an acute uncomplicated disease—including fever, headache, malaise, and disturbances of bowel function (constipation in adults and diarrhea in children)—to a more severe, complicated form of disease in 10 to 20% of infected patients that includes bleeding in the gastrointestinal tract, intestinal perforation (in 1 to 3% of hospital typhoid fever cases) and an altered mental state (32, 41). The case fatality rate is highly variable, depending on the medical treatment available and geographic location. For example, the average fatality rate is less than 1% overall but may range between 2% fatality in hospitalized patients in Pakistan and Vietnam and 50% fatality in hospitalized patients in some parts of Indonesia and Papua New Guinea (32, 41). Worldwide, typhoid fever remains a significant public health problem, with an estimated 17,000,000 cases of typhoid fever each year and up to 600,000 deaths (2, 10, 32, 41).
Typhoid vaccines currently available are composed of purified Vi polysaccharide or live attenuated S. enterica serotype Typhi (Ty21a) organisms (10, 39). The Vi polysaccharide vaccine induces protective serum antibody responses that reach a maximum at 28 days after a single intramuscular vaccination with 25 μg purified Vi polysaccharide (39), a capsular polysaccharide (Vi for virulence) that increases the virulence of S. enterica serotype Typhi (32). Protective antibody levels have been estimated to be 1 μg/ml anti-Vi IgG antibody in the serum (20). Protective efficacy of the Vi polysaccharide vaccine as determined by protection against disease is modest, with only 55 to 72% of subjects protected against disease through 3 years postvaccination (1, 20, 39). The live attenuated Ty21a vaccine is administered orally as three or four doses of enteric capsules (39). Due to its use as an oral, mucosally administered vaccine, the Ty21a vaccine induces protection against typhoid fever by induction of mucosal IgA and serum IgG antibodies specific for lipopolysaccharide antigens (39). The protective efficacy of the Ty21a vaccine at 3 years postvaccination was reported to range from 42 to 67% when using three doses of Ty21a enteric capsules (11, 39). Next-generation vaccines that utilize Vi conjugated to protein carriers that provide superior induction of anti-Vi antibodies are currently in development (14, 21, 25, 36).
Despite its ability to induce protective immune responses when used alone or conjugated to protein carriers, the use of Vi polysaccharide as a coating antigen in enzyme-linked immunosorbent assay (ELISA) to measure vaccine-induced anti-Vi antibody responses has been reported to be problematic. The use of polysaccharides (lipopolysaccharide [LPS], Haemophilus influenzae type b capsular polysaccharide, Vi polysaccharide) as coating antigens for immunoassays is plagued by problems such as a poor binding of polysaccharides to ELISA plates and inconsistent results (3, 15, 16, 26, 33). To increase binding of Vi antigen to ELISA plates and produce more-robust assays, others have biotinylated Vi and then added it to streptavidin-coated plates (12) or conjugated Vi to tyramine (22, 26). However, some reports indicate that Vi was used without any additional treatment as an ELISA coating antigen (7, 19, 21) although a Vi ELISA performed on plates was less sensitive than a radioimmunoassay procedure (19).
Immunoassays based on the use of fluorescent beads as the solid surface have recently been developed and compared to ELISA for the measurement of antigen-specific antibodies for polysaccharides from Streptococcus pneumoniae, Neisseria meningitidis, or Haemophilus influenzae type b (HiB) (5, 8, 23, 27, 34, 35). The fluorescent bead assays were comparable to ELISA and sometimes were noted as having enhanced dynamic ranges or increased sensitivity (5, 8, 27, 35). An additional benefit of fluorescent bead immunoassays is their ability to be multiplexed to permit the simultaneous measurement of antibodies specific for different antigens (8, 23, 27, 34, 35). This study was performed to evaluate a fluorescent bead immunoassay for its ability to measure vaccine-induced antibodies specific for Salmonella serotype Typhi Vi polysaccharide. The performance of the fluorescent bead assay was compared to that of ELISA.
MATERIALS AND METHODS
Vi polysaccharide.
The Vi polysaccharide antigen utilized in our initial screening ELISA to identify the samples to prepare the pooled standard curve was provided by Shousun C. Szu, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD (21, 25). Vi polysaccharide utilized for conjugation to fluorescent beads and used in ELISA performed in parallel with the bead assay was supplied by Fina Biosolutions (Rockville, MD).
Vaccine and immunization.
Vi polysaccharide was used as the vaccine. Subjects received a single intramuscular immunization containing 25 μg of purified Vi polysaccharide. Blood samples were collected before and 28 days postvaccination via venipuncture. Study participants provided informed consent, and the project had institutional review board (IRB) approval. Ethical approvals for this study were obtained from the Institutional Ethics Committees and the Health Ministry Screening Committee, Government of India, was well as the IRBs from Duke University and Research Triangle Institute. Approval for use of the Typherix vaccine was obtained from the Drug Controller General of India.
ELISA.
Day 0 and day 28 serum samples from study participants were screened by ELISA as described by others (21, 25) to identify high responders to make a pooled serum anti-Vi standard, using day 28 samples with high anti-Vi IgG responses. This anti-Vi standard was assigned an arbitrary anti-Vi IgG value of 200 ELISA units (EU) per ml. For comparison of the Vi bead assay to ELISA, we utilized an ELISA protocol similar to what we have previously reported (6, 28-30) with modifications as described below. Vi polysaccharide (1.0 μg/ml) was diluted in CBC coating buffer (pH 9.5; 15 mM Na2CO3 and 35 mM NaHCO3) before adding 15 μl to each well of a 384-well black Nunc Maxisorp plate (catalog no. 62409-062; Thermo Fisher Scientific, Rochester, NY) followed by incubation for 3 h at 37°C. After incubation, plates were washed four times with wash buffer (0.85% NaCl, 0.1% Brij 35, 0.1% Kathon) before the addition of 30 μl per well of blocking buffer (CBC buffer with 3% nonfat dry milk, 0.1% Kathon) followed by incubation at 4°C overnight. The Vi standard curve and serum samples were diluted in sample diluent buffer (phosphate-buffered saline [PBS], 0.1% Brij 35, 1% bovine serum albumin [BSA], 0.1% Kathon) before addition to the ELISA plates (15 μl per well) that had been washed four times with wash buffer followed by incubation at 37°C for 1 h. After incubation, plates were washed four times with wash buffer followed by the addition of 15 μl of detection antibody (goat anti-human IgG-AP; Southern Biotech Associates, Birmingham, AL) diluted 1:5,000 in sample diluent buffer and incubated at 37°C for 1 h. Plates were then washed four times with wash buffer followed by the addition of 15 μl per well of AttoPhos fluorescent substrate (catalog no. S1000; Promega, Madison, WI). After 60 min of room temperature incubation, plates were read by a fluorescent plate reader (excitation at 440 nm, emission at 570 nm). For comparison to the Vi bead assay, each ELISA plate included the anti-Vi serum standard as well as test samples tested at dilutions of 1:50 to 1:6,400. The values obtained for the serum standard were used to generate a standard curve that was used to calculate the anti-Vi IgG EU/ml in the test samples.
The serum/sample diluent for the ELISA is a buffered saline solution containing protein, detergent, and microbicide (PBS, 0.1% Brij 35, 1% BSA, 0.1% Kathon), and since we are using at least a 1:50 dilution, the maximum concentration of sample in the diluent is 2%. The sample diluent buffer is also used to dilute the detection antibodies. We therefore expect that matrix effects (40, 43) are minimal under the sample testing conditions (for either ELISA or the bead assay).
Conjugation of Vi polysaccharide to fluorescent beads.
Vi conjugation to fluorescent beads was performed by Solulink (San Diego, CA).
Derivatization of polysaccharide with 1,6-hexanediamine.
Vi polysaccharide was activated with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for conjugation to the fluorescent beads, using a protocol similar to that reported elsewhere (24). Polysaccharide was dissolved in molecular biology grade water at a concentration of 10 mg/ml. Periodic gentle vortexing over a period of several hours was required to dissolve the lyophilizate in its entirety. The resulting solution was notably viscous.
An aqueous solution of 0.2 M triethylamine was prepared, as was a solution of 0.5 M 1,6-hexanediamine in 0.75 M HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid). The pH of the hexanediamine in HEPES solution was slowly reduced to 7.50 via dropwise addition of 6 M hydrochloric acid under constant magnetic stirring. A 100 mg/ml solution of CDAP (1-cyano-4-dimethylaminopyridinium tetrafluoroborate) in dry acetonitrile was prepared immediately before use. Half a microgram (5 μl) CDAP solution was slowly added to 100 μl (1 mg) of polysaccharide solution with constant gentle vortexing. After 30 s, 5 μl of 0.2 M triethylamine was added with vortexing. A further 120 s elapsed, at which time 25 μl of 0.5 M 1,6-hexanediamine in 0.75 M HEPES (pH 7.50) was added while vortexing. The cyanate ester intermediate was allowed to react with the diamine overnight at 4°C. The next day, the solution was diluted with an equal volume of PBS (100 mM NaPO4, 150 mM NaCl; pH 7.40) and liberated of free diamine via two consecutive desaltings over appropriately sized Zeba columns (Thermo Scientific) equilibrated in PBS. The amino-derivatized polysaccharide solution tested mildly positive for 1° amines by ninhydrin assay.
Activation of Bio-Plex carboxyl beads.
One 1-ml vial of Bio-Plex beads (catalog no. 171-506028; 1.25 × 107 beads/ml; Bio-Rad, Hercules, CA) was vortexed for 30 s at medium-high speed to create monodisperse particles. This solution was moved to a 1.5-ml microcentrifuge tube and bath sonicated for 30 s. The beads were pelleted by centrifugation at 14,000 × g for 4 min, and the clear supernatant was carefully discarded. One milliliter of 50 mM HEPES (pH 7.35) was added to the bead pellet. Beads were resuspended by vortexing for 10 s and were repelleted by centrifugation at 14,000 × g for 4 min. The supernatant was again discarded, and 800 μl of fresh HEPES (pH 7.35) buffer was added. Beads were made monodispersed by vortexing for 30 s followed by sonication for 30 s.
Separate solutions of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and sulfo-N-hydroxysuccinimide (s-NHS) were prepared at 50 mg/ml in HEPES (pH 7.35) buffer immediately before use. One hundred microliters of EDC solution was added to the monodispersed beads, followed closely by 100 μl s-NHS solution. The beads were covered with foil and placed on a circular tube rotator for 20 min at room temperature to form the s-NHS ester. The beads were centrifuged as before, and the supernatant was discarded. Beads were resuspended in 1 ml of PBS (100 mM phosphate) and vortexed for 10 s. The beads were again centrifuged, and the supernatant was discarded. The beads were finally resuspended in 1 ml of PBS and vortexed for 30 s, followed by 15 s of sonication to create monodisperse particles, followed immediately by the conjugation reaction.
Conjugation of polysaccharide to activated beads.
For each vial of Bio-Plex beads (catalog no. 171-506028; 1.25 × 107 beads/ml; Bio-Rad, Hercules, CA), 220 μg of amino-polysaccharide was required. The requisite volume of polysaccharide solution was added to the monodispersed beads and placed on a circular tube rotator for no less than 3 h at room temperature to allow stable amide bonds to form between the amino-polysaccharide and the active ester. The unreacted ester hydrolyzed to regenerate the carboxyl. The unconjugated polysaccharide was removed by pelleting the beads at 14,000 × g for 4 min, adding 1 ml of PBS, and vortexing for 10 s to resuspend the beads (avoid sonication after the antigen has been conjugated). The wash process was repeated at least three times. The conjugated beads were finally resuspended at a concentration of 8.33 × 106 beads/ml in PBS.
Bead Vi polysaccharide immunoassay.
In a 96-well flat-bottom plate (Becton Dickinson catalog no. 353911), human serum was diluted in sterile-filtered human serum diluent (1× PBS, 1% [wt/vol] BSA, 5% [vol/vol] goat serum, 0.05% [vol/vol] Tween 20, 0.1% [vol/vol] Kathon). Day 0 serum samples were diluted 1:50, 1:400, and 1:3,200, while day 28 samples were diluted 1:200, 1:1,600, and 1:12,800. The standard curve sample was diluted beginning at 1:200 (1 EU/ml concentration) followed by eight additional 3-fold dilutions (2-fold dilutions in some assays). Using a 96-well filter bottom plate (MABVN-1250; Millipore), all wells were prewetted with 100 μl of filtered sterilized wash buffer (1× PBS, 0.05% [vol/vol] Tween 20, 0.1% [vol/vol] Kathon) and then buffer was removed by vacuum filtration and 5,000 beads (50 μl per well) diluted in human serum diluent were added. After removal of liquid by vacuum filtration, each well was washed with 100 μl wash buffer and removed by vacuum filtration, and this was repeated for a total of two washes. Fifty microliters of human serum dilutions (samples, standards, controls) was added to a 96-well filter bottom plate that contained the Vi-conjugated beads. After the plate was covered with a plate sealer and aluminum foil, the plate was incubated for 3 h on a plate shaker (300 rpm). After incubation, assay liquid was removed via vacuum filtration, and each well was washed with 100 μl of wash buffer; this was repeated for a total of three washes. Following the final wash, the wash buffer was removed by vacuum filtration, and 50 μl of anti-human IgG-R-phycoerythrin (R-PE) (2040-09; Southern Biotech), diluted 1:5,000 in human serum diluent, was added to each well. The plate was again covered with a plate sealer and foil and incubated on a plate shaker for 30 min (300 rpm). After incubation with anti-human IgG-R-PE, liquid was removed via vacuum filtration, and each well washed with 100 μl of wash buffer; this was repeated for a total of three washes. One hundred microliters of 2% paraformaldehyde (in PBS) was added to each well and then the plate was covered with a plate sealer and foil and incubated on the plate shaker for 15 min. The assay plate was read on a Bio-Plex instrument (Bio-Plex protein array system, catalog no. 171-000005; Bio-Rad, Hercules, CA). Prior to each run, the Bio-Plex machine was calibrated with Bio-Plex calibration beads (Bio-Rad catalog no. 171000205) according to the manufacturer's recommendations. Standard curves (five-parameter logistic [5PL]) were generated by Bio-Plex software for the anti-Vi serum standard, and anti-Vi IgG concentrations (EU/ml) were calculated for the unknowns by comparison to the standard curve.
Total IgG bead immunoassay.
A Beadlyte human IgG, IgA, IgM kit (catalog no. 48-302; Millipore, Billerica, MA) was used according to the manufacturer's instructions except that goat anti-human IgG-R-PE (catalog no. 2040-09; Southern Biotech, Birmingham, AL) was used as the detection reagent. The IgG standard curve and fluorescence values generated using this kit were used to estimate the anti-Vi IgG concentration in our anti-Vi serum standard.
Statistics.
All statistical analysis was performed in GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). A P value of ≤0.05 was considered significant. Specific tests performed are indicated in the figure legends. All standard curves and sample anti-Vi IgG concentrations were calculated after subtraction of the background signal measured in diluent controls from the standards and samples.
RESULTS
Development of a bead immunoassay to measure Vi polysaccharide-specific antibodies.
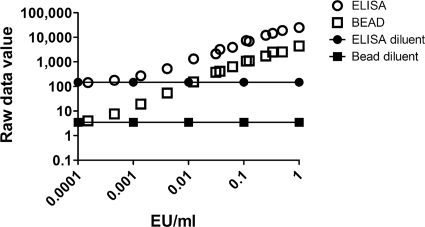
A bead immunoassay utilizing Vi polysaccharide conjugated to fluorescent beads (see Materials and Methods for Vi conjugation and bead assay technical details) was developed to determine if a bead assay would provide an alternative to ELISA for the measurement of Vi-specific antibodies. A standard prepared from pooled serum samples that had been identified using a screening ELISA as having high anti-Vi IgG responses was used to compare the performance of the Vi bead assay to an ELISA utilizing a fluorescent alkaline phosphatase substrate. In both the ELISA and the Vi bead assay, the anti-Vi standard serum was tested using dilutions beginning with 1 EU/ml and ending with 0.000152 EU/ml. As a negative control, a diluent control was included with each assay. Both assays provided dose response raw data signals with the ELISA producing values higher than those of the bead assay when testing the same standard dilution (Fig. 1). However, the ELISA platform had a diluent control value (i.e., background) that was >42-fold greater than the diluent control value for the bead assay (147.9 relative light units [RLU] versus 3.5 median fluorescence intensity [MFI] units, respectively) (Fig. 1). Both assays generated a standard curve with high R2 values, with the ELISA standard curve having an R2 of 0.967 (fifth-order polynomial), and the bead assay standard curve had an R2 value of 0.899 (fifth-order polynomial) (Fig. 1). The standard-curve raw data values generated with the ELISA correlated extremely well with the raw data values generated with the bead assay (Pearson's r = 0.989; 95% confidence interval = 0.965 to 0.996) (data not shown) when comparing raw data values for standard dilutions ranging from 1.0 EU/ml to 0.000152416 EU/ml. Descriptive statistics for raw data values from Fig. 1 are provided in Table 1.
FIG. 1.
Comparison of anti-Vi IgG standard curves using the ELISA or bead assay. A human serum standard (pooled) containing anti-Vi IgG (200 ELISA units/ml in undiluted serum) was tested over various dilutions by ELISA or bead assay, and the raw data values from each assay platform were plotted against the anti-Vi IgG EU/ml in the diluted serum. The average values for sample diluent controls included in each assay are indicated. The results shown in Fig. 1 represent a total of 32 standard curves generated using ELISA and 46 standard curves generated using the bead assay.
TABLE 1.
Descriptive statistics for standard curve raw data values generated using ELISA or bead assaya
| Standard EU/ml | Value generated using: |
|||||||
|---|---|---|---|---|---|---|---|---|
| ELISA |
Bead assay |
|||||||
| Avg | Median | SD | 95% confidence interval of the mean | Avg | Median | SD | 95% confidence interval of the mean | |
| 1 | 25,024.8 | 25,131.0 | 2,819.6 | 24,047.86-26,001.67 | 4,464.4 | 4,209.5 | 1,270.0 | 4,080.34-4,848.53 |
| 0.5 | 18,979.3 | 19,420.0 | 1,682.7 | 18,259.60-9,698.97 | 2,554.7 | 2,501.7 | 395.3 | 2,238.41-2,871.06 |
| 0.333333 | 14,610.3 | 15,472.0 | 3,007.3 | 12,833.17-16,387.47 | 2,528.4 | 2,390.8 | 686.1 | 2,313.03-2,743.67 |
| 0.25 | 12,155.5 | 11,667.0 | 2,032.1 | 11,286.32-13,024.58 | 1,720.1 | 1,636.8 | 270.4 | 1,503.77-1,936.43 |
| 0.125 | 6,893.3 | 6,730.0 | 1,897.1 | 6,081.94-7,704.73 | 1,101.6 | 1,095.4 | 196.9 | 944.03-1,259.15 |
| 0.111111 | 7,389.0 | 7,431.0 | 2,051.9 | 6,176.40-8,601.51 | 1,079.7 | 964.1 | 460.7 | 936.97-1,222.49 |
| 0.0625 | 3,903.7 | 3,851.0 | 1,131.0 | 3,419.99-4,387.44 | 631.8 | 639.3 | 172.3 | 493.95-769.67 |
| 0.037037 | 3,209.8 | 3,370.0 | 681.8 | 2,806.84-3,612.71 | 412.2 | 386.9 | 156.4 | 363.72-460.68 |
| 0.03125 | 2,115.2 | 2,171.0 | 591.1 | 1,862.34-2,367.99 | 378.3 | 384.0 | 106.0 | 293.46-463.14 |
| 0.012346 | 1,326.6 | 1,463.0 | 305.8 | 1,145.91-1,507.36 | 153.2 | 144.9 | 60.8 | 134.39-172.07 |
| 0.004115 | 530.4 | 580.0 | 130.6 | 453.17-607.56 | 54.3 | 47.0 | 21.4 | 47.64-60.90 |
| 0.001372 | 270.1 | 282.0 | 64.9 | 231.81-308.46 | 19.2 | 17.0 | 8.8 | 16.51-21.94 |
| 0.000457 | 178.3 | 187.0 | 38.8 | 155.34-201.21 | 7.6 | 7.0 | 2.8 | 6.76-8.49 |
| 0.000152 | 143.1 | 153.0 | 33.7 | 123.19-162.99 | 4.0 | 4.0 | 1.1 | 3.67-4.33 |
| 0 | 147.9 | 154.6 | 48.1 | 131.25-164.61 | 3.5 | 3.0 | 2.6 | 2.75-4.22 |
Descriptive statistics for standard curves in Fig. 1 from a total of 32 standard curves generated using ELISA and for 46 standard curves generated using the bead assay.
To evaluate the performance of the standard curves generated by ELISA or the bead assay, we performed a “standard recovery” calculation as described by others (4, 13, 38). The standard recovery calculation uses the known concentration for each point of the standard curve (i.e., the expected concentration) and the observed concentration for each point of the standard curve (calculated by entering the raw data value for each dilution of the standard curve into the standard curve equation) and calculates the observed concentration as a percentage of the expected concentration (observed concentration/expected concentration × 100%). This approach is incorporated into multiplex bead (Luminex and Bio-Plex) assay reports as “(Obs/Exp) × 100,” The standard recovery calculation was performed for each standard curve used to compare the ELISA to the bead assay (Table 2) and demonstrated that standard curves generated with the ELISA or the bead assay accurately measured the expected concentration with an average standard recoveries of 101.9% for ELISA (average of all points of the standard curve) and 100.5% for the bead assay (Table 2). However, evaluation of the standard recovery results for individual concentrations of the standard curves demonstrated greater variability at low concentrations of the standard curves generated using ELISA than that of those generated using the bead assay (Table 2). With the 0.000457 anti-Vi IgG EU/ml standard, the ELISA standard curve had an observed anti-Vi IgG EU/ml concentration that was 127.8% of the expected concentration (95% confidence interval of 102% to 153%), while the bead standard curve provided an observed anti-Vi IgG EU/ml concentration that was 101.1% of the expected concentration (95% confidence interval 94%-108.3%). The 0.000152 anti-Vi IgG EU/ml standard provided similar results, with the ELISA standard curve providing an observed anti-Vi IgG EU/ml concentration that was 167.9% of the expected concentration (95% confidence interval of 65% to 272%), while the bead standard curve provided an observed anti-Vi IgG EU/ml concentration that was 135% of the expected concentration (95% confidence interval of 106.7% to 163.4%) (Table 2).
TABLE 2.
Standard recovery evaluation of standard curves generated using ELISA or bead assaya
| Standard EU/ml | Value (%) generated using: |
|||||||
|---|---|---|---|---|---|---|---|---|
| ELISA |
Bead assay |
|||||||
| Avg | Median | SD | 95% confidence interval of the mean | Avg | Median | SD | 95% confidence interval of the mean | |
| 1 | 75.4 | 89.0 | 26.7 | 66-85 | 84.5 | 100.0 | 25.0 | 76.9-92.0 |
| 0.5 | 90.4 | 100.0 | 13.4 | 85-96 | 110.8 | 100.0 | 41.1 | 77.9-143.7 |
| 0.333333 | 73.5 | 72.3 | 24.4 | 59-88 | 89.6 | 100.0 | 13.7 | 85.3-93.9 |
| 0.25 | 100.0 | 100.0 | 0.0 | 100 | 94.8 | 100.0 | 12.8 | 84.6-105.0 |
| 0.125 | 100.0 | 100.0 | 0.0 | 100 | 100.0 | 100.0 | 0.0 | 100.0-100.0 |
| 0.111111 | 99.2 | 100.0 | 2.7 | 98-101 | 98.8 | 100.0 | 5.4 | 97.1-100.5 |
| 0.0625 | 100.0 | 100.0 | 0.0 | 100 | 100.0 | 100.0 | 0.0 | 100 |
| 0.037037 | 99.9 | 99.9 | 0.1 | 100 | 99.1 | 100.0 | 5.7 | 97.3-100.9 |
| 0.03125 | 98.8 | 100.0 | 5.5 | 96-101 | 96.7 | 100.0 | 8.2 | 90.1-103.2 |
| 0.012346 | 100.9 | 101.5 | 1.6 | 100-102 | 100.1 | 100.0 | 1.3 | 99.7-100.5 |
| 0.004115 | 95.1 | 93.4 | 11.8 | 88-102 | 100.0 | 99.9 | 7.6 | 97.7-102.4 |
| 0.001372 | 97.5 | 101.3 | 23.7 | 84-112 | 96.8 | 97.7 | 10.7 | 93.5-100.1 |
| 0.000457 | 127.8 | 124.0 | 36.8 | 102-153 | 101.1 | 97.7 | 23.2 | 94.0-108.3 |
| 0.000152 | 167.9 | 134.2 | 106.5 | 64-272 | 135.0 | 112.7 | 84.3 | 106.7-163.4 |
Standard recovery (back calculation of standard curves) was used to evaluate the performance of the ELISA and bead assay by using data from Fig. 1. A total of 32 ELISA standard curves and 46 bead assay standard curves were evaluated. To perform the “standard recovery” calculation, the standard-curve raw data values (diluent background values subtracted) are used to generate a standard curve using a five-parameter polynomial curve fitting (GraphPad Prism). The standard-curve equation is then used to calculate the observed EU/ml for each standard dilution, and the result is expressed as a percentage of the expected value (i.e., the assigned EU/ml value of the standard dilution). A standard deviation of 0% and a 95% confidence interval of 100% indicate that all values for that standard dilution had an observed EU/ml that was 100% of the expected value.
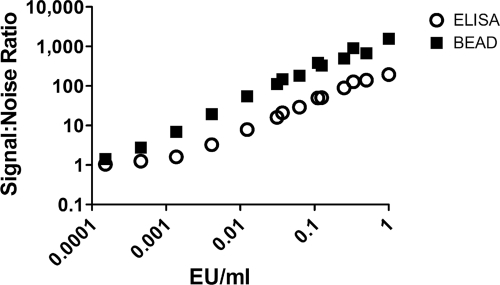
The signal-to-noise ratio is an important parameter for any laboratory assay. The signal-to-noise ratio was calculated for the ELISA and the bead assay by dividing the raw data values for each serum standard dilution by the raw data value for the diluent control. The bead assay provided a signal-to-noise ratio that was greater than that produced by the ELISA (Fig. 2).
FIG. 2.
The Vi bead assay provides a superior signal-to-noise ratio versus ELISA. The signal-to-noise ratio was calculated for the Vi standard curve raw data for each assay platform by dividing the raw data value at each standard concentration by the raw data for the diluent control. Results shown in Fig. 2 represent a total of 32 standard curves generated using ELISA and 46 standard curves generated using the bead assay.
Use of bead immunoassay to measure serum IgG anti-Vi polysaccharide responses before and after vaccination with Vi polysaccharide.
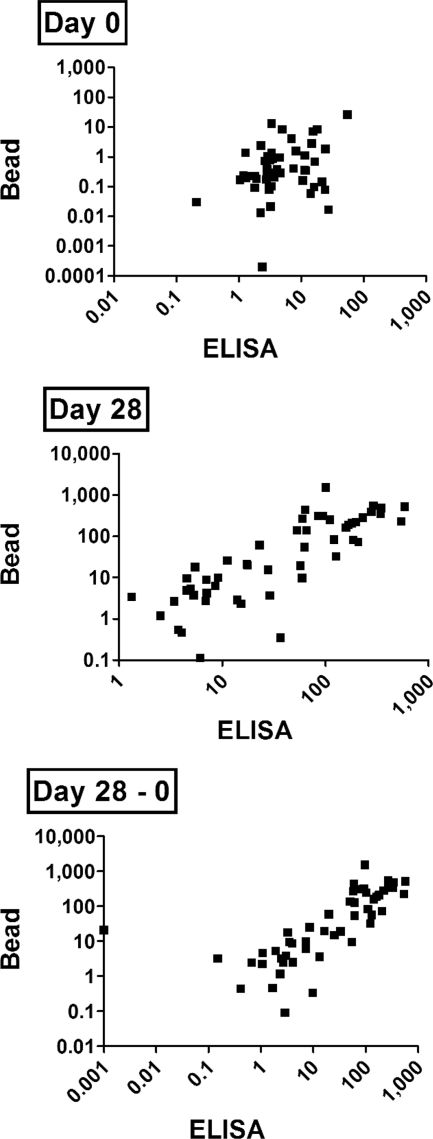
The Vi ELISA and bead assay were used in parallel to measure anti-Vi IgG levels in serum samples from 51 subjects that had been vaccinated intramuscularly with the Vi polysaccharide vaccine to determine if the Vi bead assay performed as well as or better or worse than ELISA. Paired day 0 (prevaccination) and day 28 (postvaccination) serum samples from each subject were tested. In addition to measuring anti-Vi IgG EU/ml in day 0 and day 28 serum samples, we also calculated “response to vaccination” by calculating the change in anti-Vi IgG EU/ml responses due to vaccination by subtracting the day 0 anti-Vi IgG EU/ml value from the day 28 anti-Vi IgG EU/ml value. Results from the bead assay and ELISA correlated for both day 0 (P = 0.049; Spearman's r = 0.277) and day 28 (P < 0.0001; Spearman's r = 0.843) values as well as the “day 28 − day 0” value (value calculated by subtracting day 0 anti-Vi IgG EU/ml from day 28 anti-Vi IgG EU/ml) (P < 0.0001; Spearman's r = 0.847) (Fig. 3). Our results suggest that the bead Vi assay is comparable to ELISA for the measurement of baseline (i.e., prevaccination) and postvaccination serum anti-Vi IgG responses and for calculating response to vaccination (i.e., day 28 − day 0).
FIG. 3.
Measurement of anti-Vi IgG in serum samples from vaccinated subjects (ELISA versus bead). Anti-Vi IgG EU/ml was measured in 51 serum samples collected before vaccination with Vi (day 0) or 28 days after vaccination (day 28). Response to vaccination was calculated by subtracting the day 0 anti-Vi IgG EU/ml from the day 28 value (day 28 − day 0).
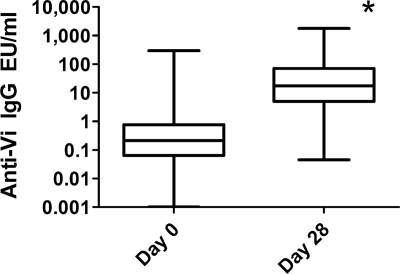
The Vi bead assay was then used to measure serum anti-Vi IgG EU/ml in day 0 and day 28 serum samples collected from 996 individuals that participated in a research study to evaluate the genetics of host response to the Vi polysaccharide vaccines (P. P. Majumder, H. F. Staats, N. Sarkar-Roy, B. Varma, T. Ghosh, K. Narayanasamy, C. Whisnant, J. Stephenson, and D. Wagener, unpublished data). Day 0 serum samples had a median anti-Vi IgG EU/ml response of 0.21 anti-Vi IgG EU/ml, while day 28 serum samples had a median anti-Vi IgG EU/ml of 17.34 anti-Vi IgG EU/ml (Fig. 4). The day 28 values were significantly increased compared to the day 0 values (P < 0.0001; two-tailed Mann-Whitney U test).
FIG. 4.
Serum anti-Vi IgG EU/ml before and after vaccination with Vi polysaccharide, as measured with the Vi bead assay. Serum samples from 996 participants were collected before (day 0) and after (day 28) vaccination with the Vi polysaccharide vaccine and tested for the presence of anti-Vi IgG with a bead immunoassay. The box represents the median and the 25th and 75th percentiles, while the whiskers the represent the largest and smallest values. *, day 28 values significantly increased compared to the day 0 values (P < 0.0001; two-tailed Mann-WhitneyU test).
QC of the Vi bead assay.
Quality control (QC) measures were utilized to ensure consistent performance of the assay over time. To test serum samples from 996 participants, 168 assays were performed over 203 days in two batches (first batch from 7 January 2008 to 7 February 2008, and second batch from 3 April 2008 to 28 July 2008). The standard curve and a control sample were monitored during each assay to evaluate performance of the assay. The standard curve consisted of the standard serum sample tested at dilutions of 1:200 through 1:1,312,200 with 3-fold serial dilutions. Standard recovery calculations (described above) were used to evaluate the performance of each standard dilution by calculating the “observed” concentration. To accept the standard curve, the average observed concentration for the dilutions of the standard curve must be between 80 and 120% of the expected concentration. Of the 40 assays performed during the first batch, four failed the standard curve QC and were repeated (all repeated assays passed the standard curve QC). Of the 128 assays performed in the second batch, all standard curves performed as expected, and no assays were rejected due to poor performance of the standard curve.
A control sample was also tested with each assay. The control sample had an expected anti-Vi IgG EU/ml value of 200. At the completion of all assays performed during each batch, the measured anti-Vi IgG EU/ml value of the controls included on each plate was used to calculate the average and standard deviation of all controls. Any assay that had a control sample measure outside 2 standard deviations of the average was considered unacceptable, and those assays were repeated. Of the 40 assays performed during the first batch, two assays had control readings outside the acceptable range and were subsequently repeated. Of the 128 assays performed in the second batch, seven assays were repeated due to control samples falling outside the accepted range. The control value for all assays that passed QC measures from the first batch had an anti-Vi IgG EU/ml value of 207.0 ± 51.2 (standard deviation) while the control value for all assays that passed QC from the second batch had an anti-Vi IgG EU/ml of 206.36 ± 24.66 (standard deviation). If we combine the values from all assays performed that passed the QC rules, the anti-Vi IgG EU/ml value for our control sample would measure 206.52 ± 31.88.
Estimation of protective levels of anti-Vi polysaccharide serum IgG.
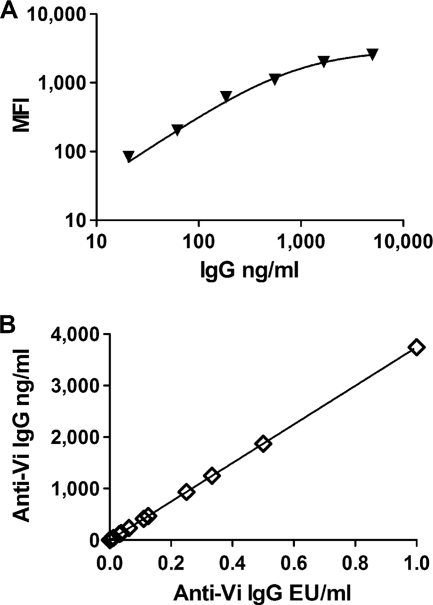
Although our assay performed reproducibly and allowed us to measure serum anti-Vi IgG responses before and after vaccination, we had no information regarding the ability of the antibody responses to protect against Salmonella serotype Typhi infection. As mentioned above, others have determined that serum anti-Vi IgG responses of 1 μg/ml represents a protective serum antibody response based on protection against Salmonella serotype Typhi infection and that 1 μg/ml serum anti-Vi IgG is now considered a protective immune response (17, 20, 31). We therefore performed an experiment that would allow us to estimate the level of anti-Vi IgG in the serum of study participants when expressed as μg/ml. In this experiment, we utilized a bead-based immunoassay kit that measured total IgG to generate a standard curve that plotted IgG versus the assay raw data (MFI units) (Fig. 5A). The MFI units for the Vi bead assay standards that had MFI units that fell within the linear range of the MFI units in the total IgG standard curve were used to estimate the IgG ng/ml values for our anti-Vi EU/ml standard curve used in bead immunoassay (Fig. 5B). Using this information, we estimated that 1 μg/ml (i.e., protective anti-Vi IgG serum antibody level) was equal to 0.267 anti-Vi IgG EU/ml. Evaluation of our day 0 and day 28 anti-Vi IgG EU/ml allowed us to estimate that ∼45% of participants had protective serum levels of anti-Vi IgG at day 0 and ∼98% had protective serum levels of anti-Vi IgG at day 28 (Table 3). These results suggest that some individuals living in areas where Salmonella serotype Typhi is endemic have protective levels of anti-Vi IgG without vaccination and that the Vi vaccine is effective at inducing protective levels of serum anti-Vi IgG.
FIG. 5.
Estimation of anti-Vi IgG μg/ml. (A) A human immunoglobulin isotyping kit (catalog no. 48-302; Millipore, Billerica, MA) was performed according to manufacturer's instructions except that the anti-human IgG-rPE detection reagent utilized in the Vi bead assay was used as the detection reagent. (B) MFI values for the anti-Vi IgG standard curves that were within range of the total IgG standard curve were used to estimate the median IgG concentration of the Vi-specific IgG in the standard. This was used to plot anti-Vi IgG EU/ml versus anti-Vi IgG ng/ml to estimate protective levels of anti-Vi IgG (i.e., 1 μg/ml).
TABLE 3.
Estimation of protective levels of serum anti-Vi IgG before and after vaccination with Vi polysaccharide vaccinea
| Day | No. of total participants | No. of participants with: |
% with protective anti-Vi IgG level (≥1 μg/ml) | |
|---|---|---|---|---|
| <1 μg/ml anti-Vi IgG | >1 μg/ml anti-Vi IgG | |||
| 0 | 996 | 549 | 447 | 44.9 |
| 28 | 996 | 23 | 973 | 97.7 |
The Vi bead assay was used to measure serum anti-Vi IgG at day 0 (before vaccination) and 28 days after vaccination with the Vi polysaccharide vaccine. By comparing the anti-Vi IgG RLU to the total IgG standard curve, we estimated the number of participants with protective (i.e., 1 μg/ml) levels of serum anti-Vi IgG before and after vaccination.
DISCUSSION
In this study, we have developed a bead immunoassay for use in the measurement of serum anti-Salmonella enterica serotype Typhi Vi polysaccharide IgG responses in vaccinated individuals. The bead assay performed comparably to an ELISA for the measurement of serum anti-Vi IgG in subjects before and after vaccination with the Vi polysaccharide vaccine.
Our Vi polysaccharide bead assay is similar in design to other bead assays that utilize polysaccharide antigens covalently conjugated to fluorescent assay beads (5, 8, 23, 27, 34, 35). In our study, due to a much lower background signal, the Vi bead assay provided a signal to noise ratio at each standard dilution that was superior to the signal to noise ratio obtained by ELISA. In agreement with our observation, others have reported that a bead assay developed to measure antibodies specific for polysaccharide antigens from Neisseria meningitidis had a lower background signal and therefore a greater dynamic range than an ELISA used for comparison (27).
Results generated with bead immunoassays have been reported to correlate well with the results generated using ELISA (R2 ranges are 0.716 to 0.9779 [8], 0.9083 to 0.9625 [35], and 0.7471 to 0.9372 [23]). In our study, despite strongly significant correlations between the bead assay and ELISA for day 28 measurements (P < 0.0001) and response-to-vaccination calculations (i.e., day 28 − day 0; P < 0.0001), day 0 serum results demonstrated increased variability between the two assays although the correlation was significant at P < 0.05 (Fig. 3). This may possibly be explained by the superior sensitivity of the bead assay since the median bead assay value for day 0 samples was 0.316 anti-Vi IgG EU/ml, while the median ELISA value for day 0 samples was 3.44 anti-Vi IgG EU/ml, a 10.9-fold difference. The higher standard recovery values at the low end of the standard curve with ELISA (Table 2) may contribute to this observation. However, with day 28 samples (i.e., postvaccination samples with higher responses), the results generated with the bead assay or ELISA were less divergent with the median anti-Vi IgG EU/ml value of 26.1 for the bead assay and 53.1 for ELISA (only a 2-fold difference). Despite other reports indicating that polysaccharide antigens used as ELISA coating antigens provided inconsistent results (3, 15, 16, 26, 33), our data suggest that the Vi polysaccharide used as an ELISA coating antigen provided consistent results (as long as the serum contained elevated levels of anti-Vi antibody) and are supported by previous publications that utilize Vi polysaccharide as the coating antigen in ELISA (7, 19, 21). It seems possible that the purity of the Vi polysaccharide, the type of ELISA reagents utilized (ELISA plates, coating buffers, wash buffers, etc.) (40, 43), and the level of anti-Vi antibodies in the serum assayed may influence the observed performance of ELISA used to measure anti-Vi serum antibodies.
QC guidelines should be established for any assay to ensure that the assay works reproducibly over time. For our Vi bead assay, we evaluated the performance of both the standard curve and control samples as measures of acceptable performance. Of 168 assays performed, a total of 13 (7.7%) were repeated due to the assays failing our QC guidelines. The interassay coefficient of variation for our control sample was 15.44% and is similar to what others have reported for bead immunoassays (9 to 27% [8]) and similar to what has been reported as acceptable performance for ELISA (37).
The evaluation of the induction of vaccine-induced anti-Vi antibodies is difficult in areas where S. enterica serotype Typhi is endemic. Indeed, several studies have reported that 19 to 58% of subjects that live in areas where it is endemic and are involved in Vi polysaccharide vaccine studies exhibit protective levels (1 μg/ml) of anti-Vi antibodies without vaccination (17, 20, 31). This is in agreement with our observation that approximately 45% of the participants in our study had protective levels of serum anti-Vi IgG before vaccination. Vaccination with Vi polysaccharide was effective in our study, since approximately 98% of study participants had protective levels of serum anti-Vi IgG 28 days after vaccination. However, as mentioned above, since this study was performed in an area where Salmonella serotype Typhi is endemic, it is impossible to determine if the increase in serum anti-Vi IgG measured 28 days after vaccination is due to vaccination or if environmental exposure to S. enterica serotype Typhi during the performance of the study contributed to the increased serum anti-Vi IgG responses observed at day 28. Our estimation of 98% of subjects having protective levels of anti-Vi IgG when measured 28 days postvaccination should not be confused with “seroconversion” as discussed in many studies. For example, seroconversion is often defined as an at least 4-fold increase in antibody response after vaccination compared to that before vaccination (18, 31). In our study, subjects may have had a prevaccination anti-Vi IgG response of <1 μg/ml that increased to >1 μg/ml postvaccination, although the increase in antibody response may not have met the seroconversion criteria of increasing at least 4-fold after vaccination. Others have reported that 85 to 95% of subjects vaccinated with Vi polysaccharide develop protective levels of serum anti-Vi (18, 42) or serum agglutinating antibody (9). Therefore, the pre- and postvaccination serum antibody titers observed in our study as measured with a bead immunoassay are in agreement with the vaccine-induced serum antibody responses measured by ELISA or tube agglutination assays. In conclusion, measurement of Vi polysaccharide-specific serum IgG with a bead immunoassay may provide a consistent, reproducible immunoassay method. A potential benefit of the bead assay, compared to ELISA, is its potential to be used in a multiplex assay to measure antibodies specific for more than one antigen in a single assay (5, 8, 23, 27, 34, 35).
Acknowledgments
This work was supported by NIH contract HHSN266200400067C.
Footnotes
Published ahead of print on 27 January 2010.
REFERENCES
- 1.Acharya, I. L., C. U. Lowe, R. Thapa, V. L. Gurubacharya, M. B. Shrestha, M. Cadoz, D. Schulz, J. Armand, D. A. Bryla, B. Trollfors, et al. 1987. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi: a preliminary report. N. Engl. J. Med. 317:1101. [DOI] [PubMed] [Google Scholar]
- 2.Acosta, C. J., C. M. Galindo, R. L. Ochiai, M. C. Danovaro-Holliday, A. L. Page, V. D. Thiem, J. K. Park, E. Park, H. Koo, X. Y. Wang, R. Abu-Elyazeed, M. Ali, M. J. Albert, B. Ivanoff, T. Pang, Z. Y. Xu, and J. D. Clemens. 2004. The role of epidemiology in the introduction of vi polysaccharide typhoid fever vaccines in Asia. J. Health Popul. Nutr. 22:240-245. [PubMed] [Google Scholar]
- 3.Barra, A., D. Schulz, P. Aucouturier, and J. L. Preud'homme. 1988. Measurement of anti-Haemophilus influenzae type b capsular polysaccharide antibodies by ELISA. J. Immunol. Methods 115:111-117. [DOI] [PubMed] [Google Scholar]
- 4.Biagini, R. E., D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. Striley, V. Semenova, E. Steward-Clark, K. Stamey, A. E. Freeman, C. P. Quinn, and J. E. Snawder. 2004. Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxins. Clin. Diagn. Lab. Immunol. 11:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biagini, R. E., S. A. Schlottmann, D. L. Sammons, J. P. Smith, J. C. Snawder, C. A. F. Striley, B. A. MacKenzie, and D. N. Weissman. 2003. Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin. Diagn. Lab. Immunol. 10:744-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradney, C. P., G. D. Sempowski, H.-X. Liao, B. F. Haynes, and H. F. Staats. 2002. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J. Virol. 76:517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canh, D. G., F. Y. Lin, V. D. Thiem, D. D. Trach, N. D. Trong, N. D. Mao, S. Hunt, R. Schneerson, J. B. Robbins, C. Chu, J. Shiloach, D. A. Bryla, M. C. Bonnet, D. Schulz, and S. C. Szu. 2004. Effect of dosage on immunogenicity of a Vi conjugate vaccine injected twice into 2- to 5-year-old Vietnamese children. Infect. Immun. 72:6586-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Voer, R. M., F. R. van der Klis, C. W. Engels, G. T. Rijkers, E. A. Sanders, and G. A. Berbers. 2008. Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin. Vaccine Immunol. 15:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dizer, U., L. Gorenek, O. Guner, T. Pehlivan, V. Ozguven, and A. Pahsa. 2002. Assessment of the antibody response in 110 healthy individuals who have been subject to Vi capsular polysaccharide vaccine. Vaccine 20:3052-3054. [DOI] [PubMed] [Google Scholar]
- 10.Engels, E. A., M. E. Falagas, J. Lau, and M. L. Bennish. 1998. Typhoid fever vaccines: a meta-analysis of studies on efficacy and toxicity. BMJ 316:110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engels, E. A., and J. Lau. 2000. Vaccines for preventing typhoid fever. Cochrane Database Syst. Rev. 2:CD001261. [DOI] [PubMed] [Google Scholar]
- 12.Ferry, B. L., S. A. Misbah, P. Stephens, Z. Sherrell, H. Lythgoe, E. Bateman, C. Banner, J. Jones, N. Groome, and H. M. Chapel. 2004. Development of an anti-Salmonella typhi Vi ELISA: assessment of immunocompetence in healthy donors. Clin. Exp. Immunol. 136:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Findlay, J. W., and R. F. Dillard. 2007. Appropriate calibration curve fitting in ligand binding assays. AAPS J. 9:E260-E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser, A., M. Paul, E. Goldberg, C. J. Acosta, and L. Leibovici. 2007. Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine 25:7848-7857. [DOI] [PubMed] [Google Scholar]
- 15.Hardy, E., M. Ohlin, and M. Llano. 1994. Enhanced ELISA sensitivity using TCA for efficient coating of biologically active lipopolysaccharides or lipid A to the solid phase. J. Immunol. Methods 176:111-116. [DOI] [PubMed] [Google Scholar]
- 16.Jauho, E. S., U. Boas, C. Wiuff, K. Wredstrom, B. Pedersen, L. O. Andresen, P. M. Heegaard, and M. H. Jakobsen. 2000. New technology for regiospecific covalent coupling of polysaccharide antigens in ELISA for serological detection. J. Immunol. Methods 242:133-143. [DOI] [PubMed] [Google Scholar]
- 17.Keddy, K. H., K. P. Klugman, C. F. Hansford, C. Blondeau, and N. N. Bouveret le Cam. 1999. Persistence of antibodies to the Salmonella typhi Vi capsular polysaccharide vaccine in South African school children ten years after immunization. Vaccine 17:110-113. [DOI] [PubMed] [Google Scholar]
- 18.Keitel, W. A., N. L. Bond, J. M. Zahradnik, T. A. Cramton, and J. B. Robbins. 1994. Clinical and serological responses following primary and booster immunization with Salmonella typhi Vi capsular polysaccharide vaccines. Vaccine 12:195-199. [DOI] [PubMed] [Google Scholar]
- 19.Klugman, K. P., I. T. Gilbertson, H. J. Koornhof, J. B. Robbins, R. Schneerson, D. Schulz, M. Cadoz, and J. Armand. 1987. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet ii:1165-1169. [DOI] [PubMed] [Google Scholar]
- 20.Klugman, K. P., H. J. Koornhof, J. B. Robbins, and N. N. Le Cam. 1996. Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine 14:435-438. [DOI] [PubMed] [Google Scholar]
- 21.Kossaczka, Z., F. Y. Lin, V. A. Ho, N. T. Thuy, P. Van Bay, T. C. Thanh, H. B. Khiem, D. D. Trach, A. Karpas, S. Hunt, D. A. Bryla, R. Schneerson, J. B. Robbins, and S. C. Szu. 1999. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect. Immun. 67:5806-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroon, F. P., J. T. van Dissel, E. Ravensbergen, P. H. Nibbering, and R. van Furth. 1999. Impaired antibody response after immunization of HIV-infected individuals with the polysaccharide vaccine against Salmonella typhi (Typhim-Vi). Vaccine 17:2941-2945. [DOI] [PubMed] [Google Scholar]
- 23.Lal, G., P. Balmer, H. Joseph, M. Dawson, and R. Borrow. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin. Diagn. Lab. Immunol. 11:272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lees, A., B. L. Nelson, and J. J. Mond. 1996. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine 14:190-198. [DOI] [PubMed] [Google Scholar]
- 25.Lin, F. Y., V. A. Ho, H. B. Khiem, D. D. Trach, P. V. Bay, T. C. Thanh, Z. Kossaczka, D. A. Bryla, J. Shiloach, J. B. Robbins, R. Schneerson, and S. C. Szu. 2001. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N. Engl. J. Med. 344:1263-1269. [DOI] [PubMed] [Google Scholar]
- 26.Losonsky, G. A., C. Ferreccio, K. L. Kotloff, S. Kaintuck, J. B. Robbins, and M. M. Levine. 1987. Development and evaluation of an enzyme-linked immunosorbent assay for serum Vi antibodies for detection of chronic Salmonella typhi carriers. J. Clin. Microbiol. 25:2266-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins, T. B., T. D. Jaskowski, A. Tebo, and H. R. Hill. 2009. Development of a multiplexed fluorescent immunoassay for the quantitation of antibody responses to four Neisseria meningitidis serogroups. J. Immunol. Methods 342:98-105. [DOI] [PubMed] [Google Scholar]
- 28.McGowen, A. L., L. P. Hale, C. P. Shelburne, S. N. Abraham, and H. F. Staats. 2009. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine 27:3544-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLachlan, J. B., C. P. Shelburne, J. P. Hart, S. V. Pizzo, R. Goyal, R. Brooking-Dixon, H. F. Staats, and S. N. Abraham. 2008. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat. Med. 14:536-541. [DOI] [PubMed] [Google Scholar]
- 30.Nordone, S. K., J. W. Peacock, S. M. Kirwan, and H. F. Staats. 2006. Capric acid and hydroxypropylmethylcellulose increase the immunogenicity of nasally administered peptide vaccines. AIDS Res. Hum. Retroviruses 22:558-568. [DOI] [PubMed] [Google Scholar]
- 31.Panchanathan, V., S. Kumar, W. Yeap, S. Devi, R. Ismail, S. Sarijan, S. M. Sam, Z. Jusoh, S. Nordin, D. Leboulleux, and T. Pang. 2001. Comparison of safety and immunogenicity of a Vi polysaccharide typhoid vaccine with a whole-cell killed vaccine in Malaysian Air Force recruits. Bull. World Health Organ. 79:811-817. [PMC free article] [PubMed] [Google Scholar]
- 32.Parry, C. M., T. T. Hien, G. Dougan, N. J. White, and J. J. Farrar. 2002. Typhoid fever. N. Engl. J. Med. 347:1770-1782. [DOI] [PubMed] [Google Scholar]
- 33.Peterfi, Z., and B. Kocsis. 2000. Comparison of blocking agents for an ELISA for LPS. J. Immunoassay 21:341-354. [DOI] [PubMed] [Google Scholar]
- 34.Pickering, J. W., T. B. Martins, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589-596. [DOI] [PubMed] [Google Scholar]
- 35.Pickering, J. W., T. B. Martins, M. C. Schroder, and H. R. Hill. 2002. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for auantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae type b. Clin. Diagn. Lab. Immunol. 9:872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins, J. B., and R. Schneerson. 2004. Future vaccine development at NICHD. Ann. N. Y. Acad. Sci. 1038:49-59. [DOI] [PubMed] [Google Scholar]
- 37.Selinsky, C. L., V. D. Whitlow, L. R. Smith, D. C. Kaslow, and H. M. Horton. 2007. Qualification and performance characteristics of a quantitative enzyme-linked immunosorbent assay for human IgG antibodies to anthrax lethal factor antigen. Biologicals 35:123-129. [DOI] [PubMed] [Google Scholar]
- 38.Skogstrand, K., P. Thorsen, B. Norgaard-Pedersen, D. E. Schendel, L. C. Sorensen, and D. M. Hougaard. 2005. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin. Chem. 51:1854-1866. [DOI] [PubMed] [Google Scholar]
- 39.Whitaker, J. A., C. Franco-Paredes, C. del Rio, and S. Edupuganti. 2009. Rethinking typhoid fever vaccines: implications for travelers and people living in highly endemics areas. J. Travel Med. 16:46-52. [DOI] [PubMed] [Google Scholar]
- 40.Wood, W. G. 1991. “Matrix effects” in immunoassays. Scand. J. Clin. Lab. Invest. Suppl. 205:105-112. [PubMed] [Google Scholar]
- 41.World Health Organization. 2003. Background document: the diagnosis, treatment and prevention of typhoid fever. WHO/V&B/03.07. http://www.who.int/vaccines-documents/DocsPDF03/www740.pdf.
- 42.World Health Organization. 2008. Typhoid vaccines: WHO position paper. Wkly. Epidemiol. Rec. 83:49-59. [PubMed] [Google Scholar]
- 43.Yoshida, H., Y. Imafuku, and T. Nagai. 2004. Matrix effects in clinical immunoassays and the effect of preheating and cooling analytical samples. Clin. Chem. Lab. Med. 42:51-56. [DOI] [PubMed] [Google Scholar]