Abstract
Improving vaccine immunogenicity by developing new adjuvant formulations has long been a goal of vaccinologists. It has previously been shown that a natural mix of lysophosphatidylcholine (LPC) from chicken eggs promotes mature dendritic cell (DC) generation in vitro and primes antigen-specific immune responses in mice. In the present study, we dissected the adjuvant potentials of five individual LPC components found in the chicken egg mixture. In vitro analyses of the impact of the individual components on the maturation of human DCs were performed by means of phenotypic analysis, chemokine secretion analysis, and analysis of the ability of mature DC to stimulate T lymphocytes. Two components, C16:0-LPC and C18:0-LPC, were identified to be capable of the upregulation of expression of CD86, HLA-DR, and CD40 on in vitro-cultured monocyte-derived DCs from healthy donors. Both induced the release of chemokines to high concentrations (macrophage inflammatory protein 1, monocyte chemoattractant protein 1) or moderate concentrations (interleukin-8 [IL-8], gamma interferon-inducible protein 10). In addition, C16:0-LPC engaged naïve T cells to produce gamma interferon. This suggests that C16:0-LPC and C18:0-LPC have the capacity to promote, at least in vitro, a Th1-oriented response. The intravenous injection of C16:0-LPC or C18:0-LPC into mice resulted in the detectable secretion of IL-6 and IL-5 in sera. Both LPC components were tested for their capacities to act as adjuvants for two selected immunogens: the hepatitis B virus surface antigen and the hepatitis C virus NS3 helicase. The secretion of specific IgG1 was observed with either or both C16:0-LPC and C18:0-LPC, depending on the immunogen tested, and was observed at an efficiency comparable to that of alum. These data identify C16:0-LPC and C18:0-LPC as the active components of the LPC natural mixture. Although discrepancies between the results of the in vitro and in vivo analyses existed, studies with animals suggest that these components can trigger significant and specific humoral-mediated immunity.
The development of attenuated live vaccines, although often very effective, can be problematic due to safety concerns. Other approaches, based on whole killed organisms or, more recently, their subunit counterparts, meet the safety profiles required by regulatory authorities (22), but they generally need the addition of an adjuvant and/or a delivery system to improve their immunogenicity (1). Aluminum-containing adjuvants (referred as “alum”), identified more than 80 years ago (9), are the most widely used adjuvants and were, until very recently, the only adjuvant that the FDA authorized for use. However, aluminum compounds can have side effects following injection (12), preparations may not be always reproducible (11), and aluminum salts cannot be frozen (3). The field of adjuvant research is remarkably active and has led to the granting of several licenses for novel compounds in recent decades. This includes an oil-in-water emulsion, MF59, incorporated in the influenza vaccine Fluad (Novartis), MonoPhosphoryl Lipid A (GSK-Bio) included in a hepatitis B virus (HBV) vaccine (Fendrix) and a human papillomavirus vaccine (Cervarix), and the cholera toxin B subunit in a whole-cell cholera vaccine (Dukoral, Crucell). In addition, immunopotentiating reconstituted influenza virosomes (IRIVs) have been used as adjuvants in vaccines against hepatitis A (Epaxal; Berna Biotech) and influenza (Inflexal V [Berna Biotech] and Invivac [Solvay]). Fluad and Fendrix are marketed in Europe, and FDA has now authorized the use of Cervarix. Finally, an aminoalkyl glucosaminide phosphate adjuvant related to lipid A, RC529, is licensed in Argentina by Dynavax Europe for vaccination against HBV (Supervax). These novel adjuvants, as well as alum, are potent inducers of humoral immune responses and suit vaccines requiring a Th2-type immune response, but their ability to elicit cellular immune responses is limited. Therefore, new adjuvants that can be easily produced and that are able to stimulate strong Th1-type immune responses while maintaining an acceptable safety profile are needed (18).
The acute-phase response (APR) is a nonspecific physiological alarm of the body in response to infection or trauma that induces dramatic changes in the composition of plasma proteins and lipid metabolism, leading to the increased oxidation of low-density lipoproteins (oxLDLs) (2, 4, 19). oxLDLs were first studied for their effects on atherogenesis (23), but recent work has revealed that oxLDLs favor the differentiation of phenotypically and functionally mature dendritic cells (DCs) from human monocytes (16). By sensing the biochemical composition of lipoprotein particles, the innate immune system can identify various endogenous modified lipids that can be signals modulating the immune response (5). A major constituent of oxLDL is lysophosphatidylcholine (LPC), produced by the oxidation and fragmentation of the polyunsaturated sn-2 fatty acyl residues of phosphatidylcholine (PC), followed by the hydrolysis of shortened fatty acyl residues by lipoprotein-associated enzymes. LPCs can also be generated by the hydrolysis of PC by secretory phospholipase A2 (20). Coutant et al. showed that LPC is an active molecule within oxLDLs that promotes the maturation of DCs from differentiating monocytes that acts, at least partially, through G-protein-coupled receptors (6). Together, these data have identified LPC as a regulator of the efficient induction of phenotypic and functional DC maturation and have thus raised the possibility that LPC could be used as a vaccine adjuvant. Indeed, LPC can initiate both humoral and cellular responses against ovalbumin or egg lysozyme model antigens when it is injected subcutaneously (s.c.) into mice (15). One major concern that requires further evaluation of the value of LPC as an adjuvant is that the LPC used in those studies was a natural mixture extracted from chicken eggs. It contains several LPC molecular components that differ from each other by the fatty acid chain esterified in the sn-1 position. This fatty acyl residue is characterized by the length of the carbohydrate chain and the number of unsaturated bonds.
Since the composition of egg LPC may vary from batch to batch, we investigated whether single molecular components of LPC with a defined fatty acyl residue could retain the adjuvant properties of LPC. In the study described here, we compared the adjuvant properties of several LPC molecules contained in an egg-derived LPC mixture and found two individual LPC components that displayed the capacity to promote DC maturation in vitro. Using two model immunogens derived from hepatitis B and C viruses, we show the capacity of the two LPC components to trigger specific humoral responses, albeit under our experimental conditions, no induction of specific T-cell responses against the model antigens could be observed.
MATERIALS AND METHODS
Materials.
l-alpha-Lysophosphatidylcholines (LPCs) from chicken eggs or soybeans and synthetic LPC components with a defined fatty acid esterified at the sn-1 position (lauric acid, C12:0; myristic acid, C14:0; palmitic acid, C16:0; stearic acid, C18:0; oleic acid, C18:1) were purchased from Avanti Polar Lipids (Alabaster, AL). They were used after dissolution in sterile phosphate-buffered saline (PBS). Alum was obtained from Pierce (Imject alum; Rockford, IL) and is composed of aluminum hydroxide (40 mg/ml) and magnesium hydroxide (40 mg/ml) plus inactive stabilizers. It was mixed at equal volumes with the antigen for 30 min at room temperature, in accordance with the manufacturer's instructions. Lipopolysaccharide (LPS; 1 μg/ml, Escherichia coli) was purchased from Sigma-Aldrich (St. Louis, MO). The LPS (Escherichia coli), poly(I:C), and CpG-ODN1826 used in the inflammatory response experiment were provided as lyophilized products by Cayla-Invivogen (Toulouse, France). They were hydrated in endotoxin-free water and were stored according to the manufacturer's instructions. Recombinant hepatitis B virus surface antigen (HBsAg; adr antigen) was obtained from HyTest (Turku, Finland). Recombinant hepatitis C virus (HCV) NS3 (amino acids 1192 to 1457, genotype 1b) was produced in Escherichia coli, and because of its ease of production, the NS3 helicase domain was more specifically selected. Both recombinant antigens were >95% pure and had endotoxin levels below 150 units per 20 μg protein, as measured by a Limulus amebocyte lysate test (Associates of Cape Cod for HBsAg and Lonza [Basel, Switzerland] for NS3 helicase).
Purification of monocytes and lymphocytes.
Monocytes and lymphocytes were isolated from whole blood collected from healthy donors (Etablissement Français du Sang, Lyon, France), as described previously (6). Briefly, the separation of mononuclear cells from human peripheral blood lymphocytes (PBLs) was performed by two successive centrifugations, one on Ficoll-Hypaque and one on a 50% Percoll solution (both from GE Healthcare, Little Chalfont, United Kingdom). The monocytes were further purified by immunomagnetic depletion (Dynal Biotech, Oslo, Norway) with a cocktail of monoclonal antibodies (MAbs): anti-CD3 (MAb OKT3 clone; ATCC, Manassas, VA); anti-CD19 (MAb 4G7 hybridoma provided by Ron Levy); and anti-CD16 (MAb 3G8), anti-CD56 (MAb NKH1), and anti-glycophorine A (MAb 11E4B7.6) (the last three MAbs were from Beckman Coulter, Fullerton, CA). By using a similar magnetic bead-depletion method, the T lymphocytes were purified from fresh or frozen PBLs with the same MAb mixture, except that the anti-CD3 MAb was replaced by an anti-CD14 MAb (MAb RMO52; Beckman Coulter). Flow cytometric analysis was performed with a FACSCalibur apparatus (BD Biosciences, Franklin Lakes, NJ) and showed that purified monocytes contained more than 90% CD14+ cells and that purified T lymphocytes contained more than 95% CD3+ cells.
Generation and treatment of MoDCs.
Isolated monocytes were cultured in RPMI 1640 medium supplemented with 10% lipoprotein-deficient fetal calf serum (Sigma-Aldrich) plus 2 mM glutamine, 10 mM HEPES, and 40 ng/ml gentamicin (all from Invitrogen, Cergy Pontoise, France). Monocytes plated in 12-well plates were supplemented with 250 U/ml human recombinant interleukin-4 (IL-4) and 40 ng/ml of human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) from Abcys (Paris, France) and were incubated for 6 days at 37°C. On day 5, monocyte-derived dendritic cells (MoDCs) were stimulated for 24 h in the presence of 40 μM LPC or 10 ng/ml LPS as a positive control for 24 h. PBS-treated cells were used as negative controls. The cells were harvested at day 6, and the supernatants were kept frozen.
Phenotype.
Cultured cells were assessed by flow cytometry with a FACSCalibur apparatus to ensure the correct lineage differentiation over time and to analyze the state of maturation of the DCs. On day 6, the MoDCs were phenotyped by using fluorescein isothiocyanate-conjugated anti-CD14, -HLA-DR, -CD80, and -CD54 and phycoerythrin-conjugated anti-CD1a, -CD86, -CD83, and -CD40 (all from Beckman Coulter).
Mixed leukocyte reaction (MLR).
On day 6, the MoDCs were collected, washed, and cultured in 200 μl RPMI 1640 medium supplemented with 2 mM glutamine, 10 mM HEPES, 40 ng/ml gentamicin, and 10% fetal calf serum in flat-bottomed 96-well plates with 2 × 105 allogeneic purified T lymphocytes per well at a DC/T-cell ratio of 1:10. Following culture for 5 days at 37°C, the supernatants were harvested and assessed for the presence of gamma interferon (IFN-γ), IL-5, and IL-13.
Cytokine and chemokine assays.
The concentrations of cytokines or chemokines in the supernatants of MoDCs harvested on day 6 or in the MLR supernatants were determined. The cytokine concentrations were measured by the use of enzyme-linked immunosorbent assay (ELISA) kits purchased from Perbio (Helsingbörg, Sweden) for IL-6, IL-10, and tumor necrosis factor alpha (TNF-α) and from Biosource (Camarillo, CA) for IL-12 p40. The concentrations of chemokine macrophage inflammatory protein 1β (MIP-1β), monocyte chemoattractant protein 1 (MCP-1), IFN-γ-inducible protein 10 (IP-10), and IL-8 and the MLR cytokines IFN-γ, IL-13, and IL-5 were assessed by use of the cytometric bead array (CBA) technology (BD Biosciences).
Mice.
Female C57BL/6N mice (age, 8 weeks) or female BALB/c mice (age, 6 to 7 weeks) from Charles River Laboratories (L'Arbresle, France) were housed at the Plateau de Biologie Experimental de la Souris (Lyon, France) or the Agence Française de Sécurité Sanitaire des Aliments (Lyon, France). The mice were housed in appropriate animal care facilities and handled according to international guidelines required for experiments with animals, and the protocols were approved by an animal ethics committee.
Inflammatory response.
To determine the inflammatory profile induced by the LPC components, C57BL/6N mice under isoflurane anesthesia received intravenous injections (retroorbital) of either 500 nmol of C16:0-LPC or C18:0-LPC or 10 μg to 25 μg of the Toll-like receptor (TLR) activators LPS, poly(I:C), and CpG-ODN1826, which were used as positive controls. The final volume was adjusted to 100 μl with PBS, and mice receiving PBS injections were used as negative controls. Two mice per group were injected at time zero and were killed 2 h, 4 h, or 12 h after the injection. Blood samples were harvested by retroorbital puncture, kept at 4°C for 4 to 30 h, and centrifuged; and the sera were collected and conserved at −20°C until analysis.
Immunization schedules.
The mice received either two s.c. injections (tail base) of 0.5 μg HBsAg adr 2 weeks apart or three s.c. injections of 1 μg NS3 helicase 3 weeks apart. The adjuvants for both treatments were LPC and alum (a single mouse received a total of 2 mg of both aluminum hydroxide and magnesium hydroxide at each injection) to evaluate the in vivo adjuvant potential of LPC. The mice were bled by retroorbital puncture before the first immunization and 8 weeks after the first HBsAg injection or 9 weeks after the first NS3 helicase injection. The samples were kept at 4°C before the collection of sera by centrifugation and were conserved at −20°C until ELISA analysis.
Determination of inflammatory profiles by multiplex assay.
A mouse Milliplex map kit (Millipore, Billerica, MA) was used to detect cytokines in mouse sera, according to the manufacturer's instructions. Standards, quality controls, and samples were mixed with antibody-coated beads (specific for IL-6, IL-1β, TNF-α, IL-12 p40, IFN-γ, or IL-5) on a 96-well filter plate. Following 2 h of incubation under mild agitation, the plates were washed and biotinylated antibodies were added for an additional hour. After a further 30-min incubation in the presence of streptavidin-phycoerythrin conjugates, the wells were washed before the plates were read on a Bio-Plex 100 system (Bio-Rad, Hercules, CA). The data were analyzed with Bio-Plex Manager (version 4.0) software (Bio-Rad). The positive threshold defined for each time point and for each cytokine was two times the mean secretion for the two mice that received the PBS injection.
Detection of antibodies by in-house ELISA.
Ninety-six well plates were coated overnight at 4°C with a sodium azide buffer containing HBsAg or NS3 helicase at 1 μg/ml. The wells were washed three times with PBS-0.1% Tween 20, before the addition of a 3% bovine serum albumin solution (Sigma-Aldrich) for saturation. Dilutions of sera, negative, and positive controls were incubated for 1 h at 37°C. Polyclonal goat anti-mouse IgG-horseradish peroxidase (HRP)-coupled antibody (Dako, Glostrup, Denmark) was added for 1 h at 37°C. The HRP substrate o-phenylenediamine (Sigma-Aldrich) was incubated, and the color reaction was stopped by addition of 0.8 M HCl. The optical density was measured at 490 nm on a Thermo Max microplate reader (Molecular Devices, Sunnyvale, CA), and the values obtained for each mouse before immunization were subtracted from those obtained following immunization. The values were considered positive when they were three times greater than the mean for the preimmunization serum diluted 1/200. Statistical analyses were conducted by a Mann-Whitney test with the values for the individual mice.
RESULTS
Modulation of MoDC phenotypic markers of maturation by C16:0- and C18:0-LPC components.
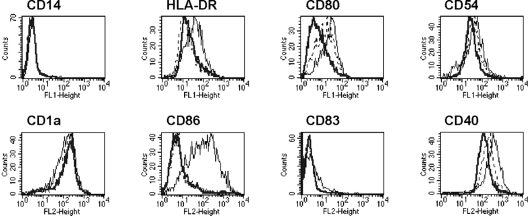
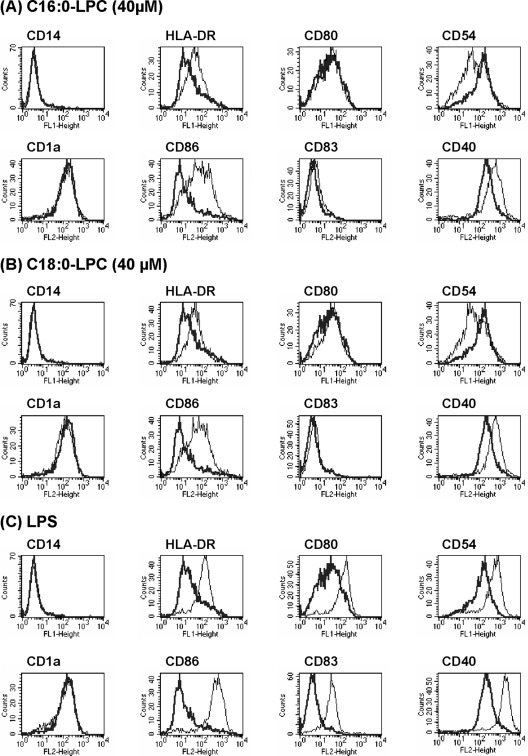
It has been described that the natural mix of LPCs from chicken eggs induces the phenotypic and functional maturation of MoDCs cultured in lipoprotein-deficient serum (6). The capacities of various LPC molecular components to promote DC maturation were investigated. Immature DCs were differentiated from human monocytes with GM-CSF and IL-4 for 5 days, and LPC was then added for the last 24 h of culture. The effects of the LPCs on MoDCs varied according to the fatty acid composition. Indeed, LPC extracted from soybeans did not induce DC maturation, whereas LPC extracted from eggs did (Fig. 1). Egg LPC is richer in saturated fatty acids. According to information from the manufacturer, it is composed of 69% C16:0-LPC, 25% C18:0-LPC, 3% C18:1-LPC, and 3% diverse molecular components, including C14:0-, C16:1-, and C18:2-LPC, and has a saturation/unsaturation (S/U) ratio of 18.4. In contrast, soybean LPC contains 26% C16:0-LPC, 7% C18:0-LPC, 9% C18:1-LPC, 50% C18:2-LPC, 5% C18:3-LPC, and 3% diverse molecular components and has an S/U ratio of 0.52. These data suggest that DC maturation is induced by saturated LPC. This was tested by using the largest range of synthetic LPC commercially available as single defined components: C12:0-, C14:0-, C16:0-, C18:0-, and C18:1-LPCs. Differentiated MoDCs were CD14− and CD1a+. The maturation of the DCs was followed by the expression of several surface markers (HLA-DR, CD-80, CD-54, CD-86, CD-83, and CD-40). As expected, control immature DCs (PBS treated) did not express CD-86 and CD-83, had low to intermediate levels of expression of CD-80 and HLA-DR, and had moderate levels of expression of CD-54 and CD-40 (Fig. 1 and 2A to C, thick lines). Cells treated with LPS, a microbial agent known to efficiently induce DC maturation and used here as a positive control, upregulated HLA-DR, CD-80, CD-54, CD-86, CD-83, and CD-40 (Fig. 2C, thin lines). Cells incubated with C12:0-, C14:0-, and C18:1-LPCs displayed a surface expression profile similar to that of PBS-treated negative control cells (data not shown). In contrast, HLA-DR, CD-86, and CD-40 surface expression was upregulated when the cells were incubated with the natural egg LPC mix, C16:0-LPC, or C18:0-LPC at an optimal concentration of 40 μM (Fig. 1 and 2A and B, respectively). The level of CD-54 expression was increased with LPS but not with C16:0- or C18:0-LPC, for which even a slight decrease was found. Overall, these results show that two single LPC components, C16:0-LPC and C18:0-LPC, are able to induce phenotypically mature DCs.
FIG. 1.
Effect of fatty acid composition of LPC on DC maturation. The phenotypes of day 6 MoDCs stimulated for 24 h with PBS (control, thick line), egg LPC (thin line), or soybean LPC (dashed line) were analyzed by flow cytometry. The results are representative of those of one of three experiments.
FIG. 2.
Mature DC generation induced by LPC single components. The phenotypes of day 6 MoDCs stimulated for 24 h with LPC components (A and B) or LPS (C) were analyzed by flow cytometry. The expression of surface markers on treated cells (thin lines) was compared to that on control cells receiving only PBS (thick lines). The results are representative of those of 1 of 10 experiments.
Cytokine and chemokine secretions driven by single LPC components.
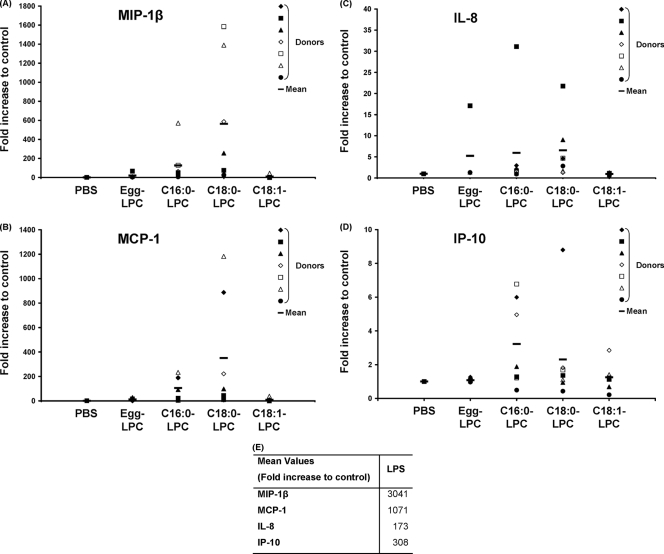
We next analyzed whether C16:0-LPC and C18:0-LPC could induce the release of cytokines or chemokines by treated MoDCs. Culture supernatants collected from day 6 MoDCs were assessed for the presence of representative cytokines and chemokines associated with Th1 or Th2 immune responses: TNF-α, IL-6, IL-10, and IL-12 by ELISA and MIP-1β, MCP-1, IL-8, and IP-10 by use of a cytometric bead array. No significant modification of the levels of TNF-α, IL-6, IL-10, or IL-12 secretion from cells treated with C16:0-LPC, C18:0-LPC, C18:1-LPC, or egg LPC was observed (data not shown). In contrast, we observed notable changes in the levels of production of chemokines in the supernatants of LPC-treated cells (Fig. 3). The mean values normalized to the results for the control (arbitrarily set equal to 1) showed 127- and 564-fold increases in the levels of production of MIP-1β by C16:0-LPC- and C18:0-LPC-treated cells, respectively. A moderate increase of 21-fold was observed after treatment with egg LPC, and only a 9-fold increase was observed with C18:1-LPC (Fig. 3A). MCP-1 secretion was similarly enhanced, with increases of 105- and 351-fold in cultures containing C16:0-LPC and C18:0-LPC, respectively, being noted, whereas a 12-fold increase was noted in the presence of egg LPC and only a 9-fold increase was detected in the presence of C18:1-LPC (Fig. 3B). For these two chemokines, C18:0-LPC was a more potent inducer than C16:0-LPC. Stimulation with C16:0-LPC and C18:0-LPC led to increases in the levels of IL-8 of six- and sevenfold, respectively, and increases in the levels of IP-10 of three- and twofold, respectively (Fig. 3C and D). Egg LPC showed a weaker capacity to induce IL-8 secretion, for which there was a fivefold increase, and no ability to increase the level of IP-10 secretion. IL-8 and IP-10 were not induced by C18:1-LPC treatment of DCs. Altogether, these data show that the two single LPC components, C16:0-LPC and C18:0-LPC, strongly upregulate the production of key Th1 chemokines after a brief 24 h of incubation with MoDCs. These levels of secretion were always greater than those obtained with the egg LPC mix.
FIG. 3.
Chemokines secreted by MoDCs upon stimulation with various LPCs. The supernatants of MoDCs treated with LPC components or PBS (A to D) or with LPS (E) at day 5 for 24 h were collected, and the chemokine concentrations were measured by using the CBA technology. Raw data were normalized to the results for the control (PBS treated), which was arbitrarily set equal to 1. The data for each donor are plotted individually; data for a total of seven donors were assayed independently, and the mean for the donors is indicated by the thick horizontal lines. (A) MIP-1β; (B) MCP-1; (C) IL-8; (D) IP-10. The mean values obtained with LPS-treated cells are indicated separately (E).
Activation induced by single LPC components in MLRs.
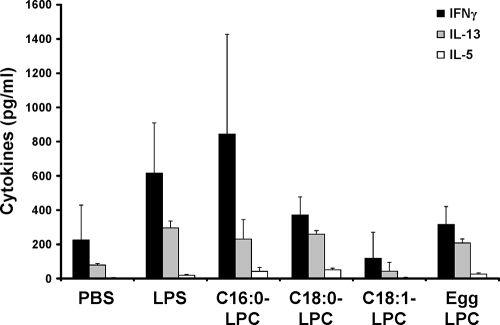
The capacity to stimulate allogeneic T cells is another characteristic of mature DCs. We therefore investigated whether LPC-treated cells showed differences with respect to their functional properties in vitro using an MLR test. Allogeneic T cells were mixed at a 1:10 DC/T-cell ratio and cultured for 5 days. At the end of the coculture, the supernatants were harvested and a fluorescent CBA assay was performed to detect Th1-related (IFN-γ) and Th2-related (IL-13 and IL-5) cytokines. The analysis of the results for several donors indicated that C16:0-LPC-treated DCs shifted T cells toward a Th1 profile, as we observed more IFN-γ and less IL-13 and IL-5 in the culture supernatants (Fig. 4) with C16:0-LPC-treated DCs. The C16:0-LPC-treated DCs induced a Th1 response similar to that induced by DCs stimulated with a low dose of LPS (10 ng/ml). C18:0-LPC- and egg LPC-treated DCs were less efficient at the induction of T-cell activation. DCs treated with the unsaturated LPC (C18:1-LPC) were unable to induce T-cell activation in MLR experiments. These data suggest that the functionality of MoDCs is biased toward a Th1 profile when stimulation is with the C16:0-LPC component or, to a lesser extent, the C18:0-LPC component, in correlation with the chemokine secretion data.
FIG. 4.
Allogeneic T-cell stimulation by LPC-treated cells. MoDCs, treated or not treated with an LPC component for 24 h, were cocultured with allogeneic T cells at a ratio of 1:10 for 5 days. The cell supernatants were assessed for the presence of IFN-γ, IL-13, and IL-5 by CBA assays. Cytokine detection was performed in triplicate, and the means plus standard deviations are represented. The results are representative of those of three separate experiments with sera from different donors.
Inflammatory profile induced by single LPC components in vivo.
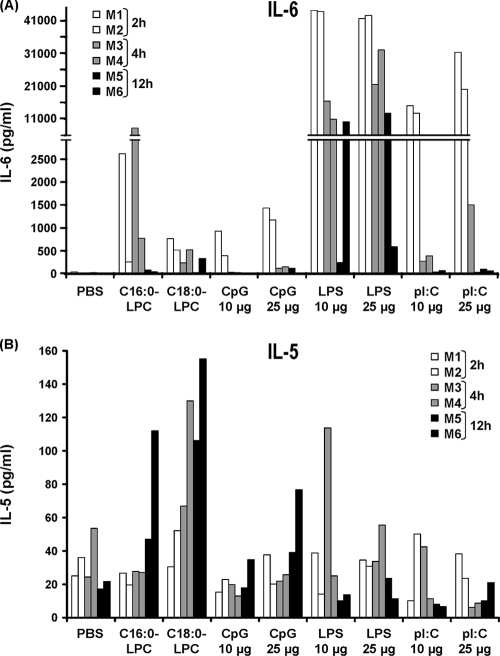
The C16:0- and C18:0-LPC components were found to be potential adjuvants in vitro with human DCs and lymphocytes. Since it is not possible to examine their adjuvant properties in vivo in humans, we evaluated the capacity of LPC to induce cytokine secretion in vivo following intravenous injection in mice. The in vivo cytokine profile directly induced by an adjuvant can provide initial information on the immunological environment likely to be put into place by the adjuvant and likely to influence the orientation of subsequent adaptive immune responses. The levels of IL-6, IL-1β, TNF-α, IL-12, IL-5, and IFN-γ were directly measured in the sera of anesthetized mice following a single intravenous injection of either C16:0-LPC, C18:0-LPC, or positive control TLR activators [i.e., LPS, CpG-ODN1826, and poly(I:C)]. All control activators were injected at two doses, 10 μg and 25 μg.
The LPC components were found to induce the secretion of IL-6 and IL-5. The injection of C16:0-LPC triggered the secretion of IL-6, which was detected in blood as early as after 2 h. The IL-6 level peaked at 4 h (777 and 8,249 pg/ml for the two mice, respectively) and was still detectable 12 h after the injection (Fig. 5A). C18:0-LPC induced the secretion of IL-6, which peaked at 2 h (770 and 521 pg/ml for the two mice, respectively); the level had decreased slightly by 4 h but was still detectable at 12 h after injection. As expected, the injection of LPS (TLR4 ligand) and poly(I:C) (a TLR3 ligand) induced high levels of secretion of IL-6 as early as 2 h postinjection (above 40,000 pg/ml and 12,000 pg/ml for the two mice, respectively). In contrast, the level of IL-6 secretion induced by CpG (a TLR9 ligand) was closer in intensity to that induced by the LPC components. Both LPC components were found to induce IL-5 secretion at similar levels (for the two mice tested in each group, 47 and 112 pg/ml at 12 h, respectively, for C16:0-LPC and 106 and 155 pg/ml at 12 h, respectively, for C18:0-LPC), although the induction of IL-5 secretion was later than that of IL-6 secretion (with a peak at 12 h) (Fig. 5B). This level of secretion was higher than that observed after the injection of the TLR activators LPS, poly(I:C), and CpG, the levels of which were not significantly greater than the positive threshold. In contrast, the TLR activators induced the secretion of TNF-α, IL-1β (only LPS), IL-12 p40, and IFN-γ (data not shown).
FIG. 5.
Inflammatory profile induced by C16:0-LPC and C18:0-LPC. Sera from anesthetized C57BL/6 mice receiving intravenous injections of either C16:0-LPC (500 nmol), C18:0-LPC (500 nmol), CpG-ODN1826 (10 μg and 25 μg), LPS (10 μg and 25 μg), or poly(I:C) (10 μg and 25 μg) were assayed for the presence of cytokines by using a Milliplex map kit. The data for two mice are included per time point; and sera were collected at 2 h (mouse 1 [M1], M2), 4 h (M3, M4), or 12 h (M5, M6). (A) IL-6. The positive thresholds, defined as two times the mean level of secretion for PBS-injected mice, were 45 pg/ml at 2 h, 45 pg/ml at 4 h, and 0 pg/ml at 12 h. (B) IL-5. The positive thresholds were 61 pg/ml at 2 h, 78 pg/ml at 4 h, and 39 pg/ml at 12 h.
In conclusion, both single LPC components were found to induce significant amounts of IL-6 and, to a lesser extent, IL-5 secretion following direct intravenous injection into mice. These preliminary in vivo data point to the abilities of the two LPC components to induce instead a Th2-oriented response, in contrast to the in vitro data, which pointed to a Th1-orientated response.
Capacity of single LPC components to induce humoral responses. (i) HBsAg from HBV.
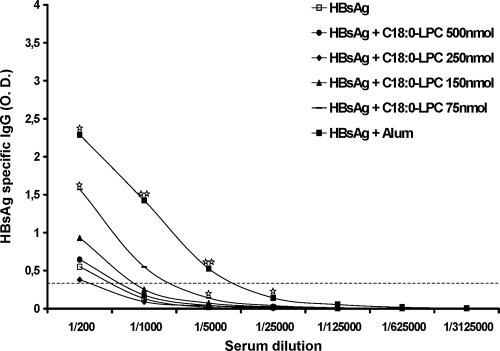
The HBsAg-specific humoral responses elicited by the two LPC components identified were evaluated, because in this model protection is tightly mediated by antibodies. The schedule of immunization of BALB/c mice consisted of two injections of 0.5 μg of HBsAg with LPC as the adjuvant (decreasing doses from 500 to 75 nmol) at a 2-week interval, and the antibody levels were measured 6 weeks after the 2nd injection (Fig. 6). The results obtained with the standard alum adjuvant were used as a benchmark. No induction of specific antibodies could be observed when C16:0-LPC was used (data not shown). In contrast, C18:0-LPC enhanced the production of HBsAg-specific IgG compared with that achieved by the injection of HBsAg alone. This increase was statistically significant for the lowest dose of LPC used, 75 nmol (Fig. 6). Mice receiving alum plus HBsAg demonstrated a significant increase in the level of the HBsAg-specific IgG at 1/1,000 and 1/5,000 dilutions compared with those achieved under the other conditions evaluated. Similar to alum, the secreted Ig was IgG1, while IgG2a was never detected under the conditions used for testing. Thus, the antibody profiles induced in mice, in line with the findings of the analysis of the cytokine profiles in sera described above, point to the capacity of the LPC components to orientate immune responses toward a Th2 profile.
FIG. 6.
Anti-HBsAg humoral response induced in BALB/c mice following immunization with HBsAg combined with C18:0-LPC. Groups of six mice were immunized two times at a 2-week interval with 0.5 μg of HBsAg combined with C18:0-LPC at decreasing doses (from 500 to 75 nmol), and the humoral responses were analyzed by ELISA 6 weeks after the last immunization. The median optical density (O.D.) values (490 nm) for each group at various serum dilutions are represented. The positive threshold was defined as three times the mean for preimmune sera diluted 1/200 for each mouse. One star, P < 0.05 compared with the results for the control group (treatment with HBsAg alone); two stars, P < 0.05 compared with the results for any other group.
(ii) NS3 helicase from HCV.
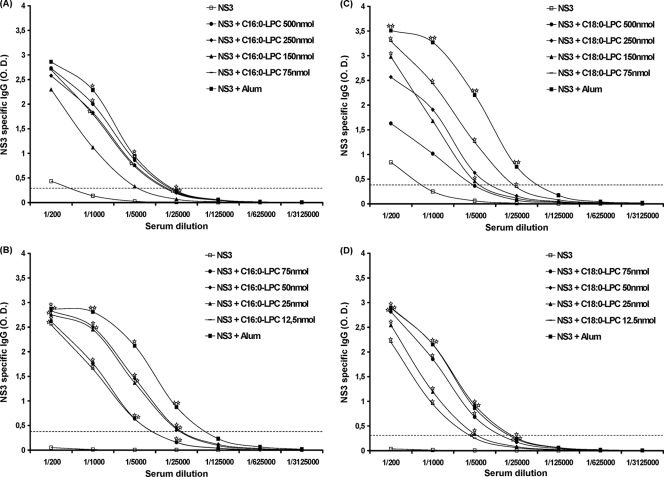
The HCV NS3 protein is the target of a robust and broadly detectable antibody response during natural infection. BALB/c mice were injected three times at 3-week intervals with 1 μg of recombinant NS3 combined with decreasing doses of C16:0- or C18:0-LPC, and the humoral responses were analyzed by ELISA 3 weeks after the last immunization. A clear increase in the level of induction of NS3-specific antibodies was observed with the different doses of C16:0-LPC (75 and 500 nmol) compared with the level of induction achieved by the injection of the NS3 protein alone (Fig. 7A). C16:0-LPC was as efficient as alum (P < 0.05) at both doses. Lower doses (from 75 to 12.5 nmol) were also tested, and Fig. 7B shows that the adjuvant capacity of C16:0-LPC was similar to or lower than that of alum, depending on the dilution evaluated. Even the lowest dose tested (12.5 nmol) showed a significant increase in the adjuvant potential compared with that achieved with the NS3 protein alone.
FIG. 7.
Anti-NS3 humoral response induced in BALB/c mice following immunization with NS3 helicase combined with C16:0-LPC or C18:0-LPC. Groups of six mice were immunized three times at 3-week intervals with 1 μg of NS3 helicase combined with either C16:0-LPC (A and B) or C18:0-LPC (C and D) at decreasing doses from 500 to 75 nmol (A and C) or 75 to 12.5 nmol (B and D). The humoral responses were analyzed by ELISA 3 weeks after the last immunization. The median optical density (O.D.) values (490 nm) for each group at various serum dilutions are represented. The positive threshold was defined as three times the mean for preimmune sera diluted 1/200 for each mouse. One star, P < 0.05 compared with the results for the control group (treatment with NS3 helicase alone); two stars, P < 0.05 compared with the results for any other group.
Similar to C16:0-LPC and as shown in Fig. 7C, C18:0-LPC enhanced NS3-specifc antibody production compared with that achieved with the protein alone at all doses tested in mice. This effect was statistically significant for the two lowest doses tested (75 and 150 nmol) and for all serum dilutions tested for the 75-nmol dose (P < 0.05). The humoral response induced was in the range of that achieved following immunization with alum, although it was lower than that achieved with alum. The adjuvant properties of C18:0-LPC were further analyzed with lower doses: 75 to 12.5 nmol (Fig. 7D). A similar significant increase in the level of antibody production was observed at all doses compared with that achieved with protein alone, although a significantly higher response was observed at 50 nmol than at 12.5 nmol (P < 0.05). This suggests that the optimal adjuvant capacity of C18:0-LPC is reached at 50 nmol. In addition, the humoral responses against HCV NS3 induced by the C16:0- and C18:0-LPC components were maintained beyond the 1/5,000 dilution.
The characterization of Ig subtypes revealed that, similar to HBsAg immunizations, the injection of NS3 helicase with C16:0-LPC or C18:0-LPC as the adjuvant led to the production of IgG1 only (data not shown).
DISCUSSION
The present study focused on the preclinical evaluation of a novel lipid-based adjuvant, LPC. A natural egg-derived mixture of LPC has been shown to promote mature DC generation in vitro and initiate adaptive immune responses in mice (6, 15). The objective of this study was to determine which single molecular LPC component within the natural egg-derived LPC mixture is responsible for the adjuvant properties of this potential novel adjuvant that have been described. We show that two single LPC components, C16:0-LPC and C18:0-LPC, exhibit adjuvant features. These two molecules supported the in vitro maturation of differentiating monocytes into functional DCs, resulting in the release of Th1 chemokines. Following the direct intravenous injection of the two LPC components into mice, the transient secretion of IL-6 and IL-5 was reported. In addition, immunization of the mice with the C18:0-LPC resulted in the significant induction of antibodies specific for the two model antigens tested (HBsAg and HCV NS3), while immunization with C16:0-LPC was a good inducer of antibodies only when it was mixed with HCV NS3. In both cases, the secretion of IgG1 suggested a biased response toward a Th2 profile.
We first showed that a soybean-based LPC mix, mainly composed of unsaturated LPC components, was unable to promote DC maturation, whereas an egg-derived LPC mix, which mostly contained saturated molecules, was able to do so. This result suggests that DC maturation is induced by saturated LPC. When analyzing saturated single LPC components, ranging from the C12:0-LPC to the C18:0-LPC, as well as the unsaturated C18:1-LPC component, we observed that C16:0-LPC and C18:0-LPC were the only single LPC components that gave rise to phenotypically and functionally mature MoDCs in vitro. This finding suggests a relationship between the adjuvant potential of the LPC and both its carbon chain length and its saturation state. Since C16:0-LPC and C18:0-LPC are the most representative components of total LPC in vivo (21), this reinforces the previously proposed concept that a burst of LPC during the APR is an endogenous signal favoring the induction of immune responses.
Cell surface markers of differentiation and maturation were analyzed by flow cytometry. C16:0-LPC and C18:0-LPC induced the upregulation of HLA-DR, CD86, and CD40 surface expression. The others LPC components, especially C18:1-LPC, did not affect the immature phenotype of MoDCs. It is of note that LPC molecules (egg LPC, C16:0-LPC, or C18:0-LPC as a single component) induced a slight decrease in the level of CD54 expression at the surface of MoDCs. CD54, or intercellular adhesion molecule 1 (ICAM-1), is involved in the recruitment of immune cells at the site of inflammation and in the interaction between DC and T cells (7). Whether the downregulation of CD54 by the C16:0- and C18:0-LPC components observed impairs the adjuvant potential of these two molecules, in particular, their capacity to induce T-cell-mediated immunity, remains to be demonstrated.
Analysis of cytokines and chemokines in the culture supernatants of MoDCs treated with LPC showed that C16:0-LPC or C18:0-LPC could trigger the secretion of the Th1 chemokines MIP-1β, MCP-1, IL-8, and IP-10, while it did not influence the secretion of IL-12 p40, IL-6, TNF-α, or IL-10. The induction of chemokines is of great interest since these molecules are responsible for the recruitment of monocytes, neutrophils, and macrophages (24) and are essential for the efficient induction of the immune response (10). Interestingly, chemokine secretion was stronger with the C16:0- or C18:0-LPC individually than with the same dose of egg-derived LPC, probably because the egg LPC contains inactive or even inhibitory compounds mixed with the active LPC. This highlights the importance of working with precisely defined structures of LPC. In this context, it was decided to focus on the C16:0-LPC and C18:0-LPC molecules to further pursue our analysis in vivo.
Analysis of cytokine induction was also done in vivo following the direct injection of the LPC components into mice and was compared with the level induced by various immunomodulators. The inflammatory response induced, in particular, the secretion of IL-6 and IL-5, was documented. This preliminary analysis suggested that, in contrast to in vitro observations, the LPC components may orientate the host response toward a Th2 profile.
The adjuvant effect of LPC was analyzed by the use of immunization procedures with two antigens displaying either a particulate structure, in the case of HBsAg (13), or a structure composed of three isolated domains, in the case of HCV NS3 helicase (14). C16:0-LPC compared favorably with alum for the promotion of NS3-specific antibodies, even at the lowest dose used in the present study (12.5 nmol). Although C18:0-LPC was a little less efficient, it was capable of eliciting humoral responses against NS3 similar to those elicited by alum. Overall, both LPC components showed adjuvant properties at very low doses, which is an important feature for the selection of a safe adjuvant. Although C18:0-LPC could act as an adjuvant for both antigens, the effect on HBsAg was not comparable to that seen on HCV NS3. These differences could be related to the nature of the antigen tested, as HBsAg has a structure that confers an unusually high degree of intrinsic immunogenicity.
Interestingly, a previous study has shown that the subcutaneous injection of C16:0-LPC in the range of 200 to 800 nmol triggered a transient inflammatory response without causing severe adverse events in human volunteers (17). In this context, the weak adjuvant dose attained is of particular interest, because LPC can reach concentrations as high as 150 nmol/ml in the serum of healthy individuals (21). It is tempting to speculate that low doses of LPC could retain inflammatory and adjuvant features and be used safely in the clinic.
The capacity of the LPC components to induce cell-mediated specific immune responses was very difficult to document. Although in vitro analysis with human cells suggested that LPC has the potential to trigger a Th1-oriented response, the results obtained in vivo did rather point to the induction of Th2-biased responses. BALB/c mice were used, and they are indeed a reference for the induction of humoral responses. Nonetheless, we used mice with a C57BL/6 genetic background in order to document the induction of cell-mediated responses using a panel of assays (enzyme-linked immunospot assay for IFN-γ, intracellular staining for IFN-γ, or in vitro cytotoxic T-lymphocyte assays [8]). The failure to detect such responses was surprising, as that finding is in contrast to the in vitro data. To a certain extent, that finding is also in contrast to our observations obtained by the use of LPC from chicken eggs (15). Major differences between these studies that may have contributed to the divergent results observed exist, in particular, the fact that different antigens, mouse strains, and immunization schedules were used. The difficulty with the reconciliation of the in vitro and in vivo data does point to the limitation of the alternative models currently used to evaluate adjuvants and vaccines.
In conclusion, we succeeded in identifying two individual synthetic LPC components, C16:0-LPC and C18:0-LPC, originating from a natural mix of LPCs from chicken eggs that exhibit adjuvant properties. Further characterization is needed to confirm their potential as candidate adjuvants for clinical development, but the fact that these components are precisely defined chemical entities and synthetically available is undoubtedly an interesting feature.
Footnotes
Published ahead of print on 13 January 2010.
REFERENCES
- 1.Aguilar, J. C., and E. G. Rodriguez. 2007. Vaccine adjuvants revisited. Vaccine 25:3752-3762. [DOI] [PubMed] [Google Scholar]
- 2.Baumann, H., and J. Gauldie. 1994. The acute phase response. Immunol. Today 15:74-80. [DOI] [PubMed] [Google Scholar]
- 3.Braun, L. J., A. Tyagi, S. Perkins, J. Carpenter, D. Sylvester, M. Guy, D. Kristensen, and D. Chen. 2009. Development of a freeze-stable formulation for vaccines containing aluminum salt adjuvants. Vaccine 27:72-79. [DOI] [PubMed] [Google Scholar]
- 4.Cabana, V. G., J. N. Siegel, and S. M. Sabesin. 1989. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J. Lipid Res. 30:39-49. [PubMed] [Google Scholar]
- 5.Coutant, F., S. Agaugue, L. Perrin-Cocon, P. Andre, and V. Lotteau. 2004. Sensing environmental lipids by dendritic cell modulates its function. J. Immunol. 172:54-60. [DOI] [PubMed] [Google Scholar]
- 6.Coutant, F., L. Perrin-Cocon, S. Agaugue, T. Delair, P. Andre, and V. Lotteau. 2002. Mature dendritic cell generation promoted by lysophosphatidylcholine. J. Immunol. 169:1688-1695. [DOI] [PubMed] [Google Scholar]
- 7.Dustin, M. L., and T. A. Springer. 1991. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu. Rev. Immunol. 9:27-66. [DOI] [PubMed] [Google Scholar]
- 8.Fournillier, A., E. Gerossier, A. Evlashev, D. Schmitt, B. Simon, L. Chatel, P. Martin, N. Silvestre, J. M. Balloul, R. Barry, and G. Inchauspe. 2007. An accelerated vaccine schedule with a poly-antigenic hepatitis C virus MVA-based candidate vaccine induces potent, long lasting and in vivo cross-reactive T cell responses. Vaccine 25:7339-7353. [DOI] [PubMed] [Google Scholar]
- 9.Glenny, A. T., C. G. Pope, H. Waddington, and U. Wallace. 1926. Immunological notes XVII-XXIV. J. Pathol. Bacteriol. 29:31-40. [Google Scholar]
- 10.Gouwy, M., S. Struyf, P. Proost, and J. Van Damme. 2005. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev. 16:561-580. [DOI] [PubMed] [Google Scholar]
- 11.Gupta, R. K. 1998. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 32:155-172. [DOI] [PubMed] [Google Scholar]
- 12.Lindblad, E. B. 2004. Aluminium adjuvants—in retrospect and prospect. Vaccine 22:3658-3668. [DOI] [PubMed] [Google Scholar]
- 13.Miyanohara, A., A. Toh-e, C. Nozaki, F. Hamada, N. Ohtomo, and K. Matsubara. 1983. Expression of hepatitis B surface antigen gene in yeast. Proc. Natl. Acad. Sci. U. S. A. 80:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penin, F., J. Dubuisson, F. A. Rey, D. Moradpour, and J. M. Pawlotsky. 2004. Structural biology of hepatitis C virus. Hepatology 39:5-19. [DOI] [PubMed] [Google Scholar]
- 15.Perrin-Cocon, L., S. Agaugue, F. Coutant, P. Saint-Mezard, A. Guironnet-Paquet, J. F. Nicolas, P. Andre, and V. Lotteau. 2006. Lysophosphatidylcholine is a natural adjuvant that initiates cellular immune responses. Vaccine 24:1254-1263. [DOI] [PubMed] [Google Scholar]
- 16.Perrin-Cocon, L., F. Coutant, S. Agaugue, S. Deforges, P. Andre, and V. Lotteau. 2001. Oxidized low-density lipoprotein promotes mature dendritic cell transition from differentiating monocyte. J. Immunol. 167:3785-3791. [DOI] [PubMed] [Google Scholar]
- 17.Ryborg, A. K., B. Deleuran, H. Sogaard, and K. Kragballe. 2000. Intracutaneous injection of lysophosphatidylcholine induces skin inflammation and accumulation of leukocytes. Acta Derm. Venereol. 80:242-246. [DOI] [PubMed] [Google Scholar]
- 18.Schijns, V. 2006. Unraveling “the immunologist's dirty little secret.” In V. E. J. C. Schijns and D. T. O'Hagan (ed.), Immunopotentiators in modern vaccines. Elsevier Academic Press, Amsterdam, The Netherlands.
- 19.Steinberg, D. 1997. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 272:20963-20966. [DOI] [PubMed] [Google Scholar]
- 20.Steinbrecher, U. P., and P. H. Pritchard. 1989. Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J. Lipid Res. 30:305-315. [PubMed] [Google Scholar]
- 21.Subbaiah, P. V., C. H. Chen, J. D. Bagdade, and J. J. Albers. 1985. Substrate specificity of plasma lysolecithin acyltransferase and the molecular species of lecithin formed by the reaction. J. Biol. Chem. 260:5308-5314. [PubMed] [Google Scholar]
- 22.Ulmer, J. B., U. Valley, and R. Rappuoli. 2006. Vaccine manufacturing: challenges and solutions. Nat. Biotechnol. 24:1377-1383. [DOI] [PubMed] [Google Scholar]
- 23.Witztum, J. L., and D. Steinberg. 1991. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 88:1785-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]