Abstract
The Aspergillus fumigatus ΔpmrA (Golgi apparatus Ca2+/Mn2+ P-type ATPase) strain has osmotically suppressible basal growth defects and cationic tolerance associated with increased expression of calcineurin pathway genes. Despite increased β-glucan and chitin content, it is hypersensitive to cell wall inhibitors but remains virulent, suggesting a role for PmrA in cation homeostasis and cell wall integrity.
Calcineurin, a conserved serine/threonine-specific phosphatase, mediates environmental stress adaptation in fungi through the transcription factor Crz1/Prz1/CrzA (4, 6, 9, 10, 12, 16, 22). The P-type ATPases, encoded by PMR1, PMC1, and ENA1, have also been characterized as targets of calcineurin in Saccharomyces cerevisiae (3, 5, 11, 20), but their filamentous fungal counterparts have not been completely investigated. PMR1, encoding the Ca2+/Mn2+ P-type ATPase, localizes to the Golgi apparatus, where it mediates Ca2+ homeostasis and supplies Mn2+ required for protein glycosylation (7). The S. cerevisiae PMR1 mutant secretes misfolded proteins and exhibits defective growth under low-Ca2+ conditions and in the presence of Mn2+ (1, 13, 18, 20). In Candida albicans, PMR1 deletion results in loss of virulence (2). In Aspergillus niger, the ΔpmrA strain exhibits growth retardation on EGTA-containing medium, suggesting the involvement of pmrA in Ca2+ homeostasis in Aspergillus species (27). Ectopic expression of an Aspergillus fumigatus PMR1-like ATPase in S. cerevisiae demonstrated its requirement for Ca2+ and Mn2+ homeostasis (23), but no functional characterization in A. fumigatus was reported. BLAST search using the S. cerevisiae PMR1 sequence revealed 3 open reading frames (ORFs) (AFUA_2G05860, AFUA_G01320, and AFUA_6G06740) with homology to the Pmr1 protein (65%, 30%, and 37% identities, respectively). We constructed the pmrA mutant by deleting the ORF (AFUA_2G05860) with the highest similarity to S. cerevisiae PMR1 (E value, 5.6e−220) and examined its growth, cation homeostasis, cell wall integrity, and pathogenicity.
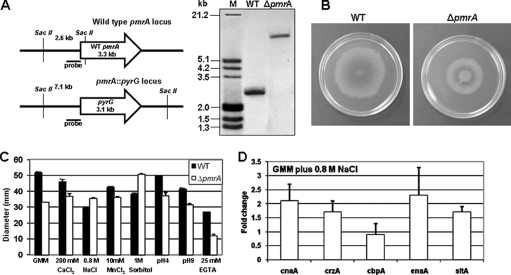
The A. fumigatus ΔpmrA strain was generated by replacing the pmrA ORF with the Aspergillus parasiticus pyrG gene and confirmed by Southern analysis (Fig. 1A). Successful reconstitution of pmrA, employing a construct containing the entire pmrA coding region, including the 2.5-kb upstream sequence and 500-bp downstream flanking sequence, was confirmed by Southern analysis (data not shown). Growth of the ΔpmrA strain was reduced to ∼60% of the wild type (WT) under standard conditions (Fig. 1B), and complementation (not shown) restored radial growth. In the yeasts S. cerevisiae and Schizosaccharomyces pombe, PMR1 functions in Ca2+ sequestration and Ca2+ and Mn2+ tolerance (3, 5); therefore, we tested growth of the ΔpmrA strain in the presence of cations (Fig. 1C). While the WT was sensitive to 200 mM CaCl2, 0.8 M NaCl, and 10 mM MnCl2, the ΔpmrA strain was more tolerant (Fig. 1C). Treatment with 25 mM EGTA revealed greater sensitivity with the ΔpmrA strain (∼60% inhibition) than with the WT (∼50% growth inhibition) (Fig. 1C). Each strain's growth recovered with the addition of 200 mM CaCl2 (data not shown). Interestingly, culture on osmotically stabilized medium (1 M sorbitol) improved growth of the ΔpmrA strain by ∼40%, indicating cell wall instability (Fig. 1C). Growth on sorbitol rescues yeast cell wall integrity mutations by providing an isosmotic environment (3). Since the cation sequestration process can indirectly affect intracellular pH, the strains were grown under acidic and alkaline pH conditions. While the ΔpmrA strain showed tolerance at pH 4.0, the WT showed reduced growth at alkaline pH 9.0. However, the exact role of pmrA in growth under alkaline or acidic pH conditions remains to be established.
Fig. 1.
Construction of the ΔpmrA strain and phenotypic analysis. (A) Schematic representation of the genomic locus of the WT and ΔpmrA strains. The entire coding sequence of the A. fumigatus pmrA gene was replaced with that of the A. parasiticus pyrG gene by homologous recombination. Southern analysis with SacII-digested genomic DNA and the pmrA left flank probe shows the replacement of pmrA with pyrG as a 7.1-kb fragment in the ΔpmrA strain. (B) Culture morphology of the WT and ΔpmrA strains on glucose minimal medium agar (GMM) after 5 days of growth at 37°C. (C) Radial growth of the ΔpmrA strain under different stress conditions, compared to that of the WT strain. A total of 1 × 104 conidia were inoculated onto GMM plates containing different salt concentrations. In addition, growth analysis was also performed on alkaline (pH 9.0) and acidic (pH 4.0) media. Three independent experiments were performed, and the results shown are the means and standard deviations obtained after 4 days of growth at 37°C. (D) RT-PCR expression analysis in response to salt stress. The expression levels of calcineurin catalytic subunit A (cnaA), a calcipressin (cbpA), a Na+ ATPase (enaA), and two zinc finger transcription factors (crzA and sltA) were analyzed after 20 min of exposure to 0.8 M NaCl. Three independent experiments were performed, and the results shown are the means and standard deviations.
In contrast to the S. cerevisiae PMR1 mutant, which exhibits growth sensitivity to Ca2+ and Mn2+ (1, 13, 18, 20), our data revealed cation tolerance in the ΔpmrA strain. This discrepancy prompted us to investigate the expression levels of the major genes of the calcineurin pathway, including cnaA, crzA, and cbpA. For real-time reverse transcription (RT)-PCR analysis, RNA extracted from 15-h cultures incubated for 20 min with 0.8 M NaCl was subjected to first-strand cDNA synthesis. Triplicate RT-PCR assays were performed using 1× iQ SYBR green master mix with two biological replicates, and expression levels were normalized to those for β-tubulin. The 2−ΔΔCT method was used to determine fold changes of expression.
Following 0.8 M NaCl treatment, the expression levels of cnaA and its downstream effector crzA increased 2.1- and 1.7-fold in the ΔpmrA strain, respectively (Fig. 1D). The ΔpmrA strain was tolerant to FK506 treatment (data not shown), which correlates with the increased expression of cnaA. The expression of the endogenous calcineurin inhibitor CbpA (19) was not significantly altered. As the S. cerevisiae Na+/Li+ ATPase encoded by ENA1 and PMR2 is regulated by calcineurin and CRZ1 during alkaline pH, salt, and osmotic stress adaptation (11, 14–16, 18, 21), we analyzed the expression of the A. fumigatus homolog of ENA1 (AFUA_6G03690; enaA) in the ΔpmrA strain after exposure to NaCl. Furthermore, considering the role of the Aspergillus nidulans sltA gene, encoding a C2H2 zinc finger transcription factor, in alleviating sensitivity to salt stress (17), we also studied the expression of the homologous gene (AFUA_1G13050) in the ΔpmrA strain. Increased expression of both enaA (2.3-fold) and sltA (1.7-fold) genes was noted (Fig. 1D), suggesting a role for PmrA in Na+ homeostasis and salt stress adaptation of A. fumigatus. Recently, sltA, which has no identifiable homolog in S. cerevisiae but, like crzA, is involved in cation adaptation and homeostasis, has been shown to positively regulate the transcription of enaA (24). Interestingly, both the sltA and enaA genes contain potential calcineurin-dependent response elements (CDRE) in their promoters (data not shown), implicating the involvement of calcineurin in Na+ homeostasis. Although increased cytosolic Ca2+ and the subsequent activation of calcineurin may be helpful during salt stress, perturbed Ca2+ homeostasis and prolonged calcineurin activity may be harmful. Loss of Ca2+ ATPase activity in the ΔpmrA strain probably results in prolonged Ca2+ signaling, which in turn affects radial growth. Therefore, PmrA likely acts as the major Ca2+ ATPase during salt stress.
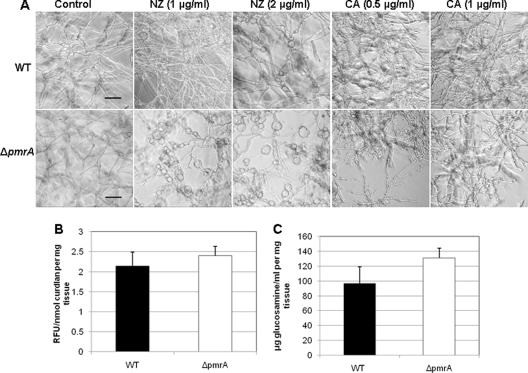
Since deletion of pmrA induced a growth defect which was overcome by addition of sorbitol, indicating a cell wall defect, we examined the susceptibility of the ΔpmrA strain to the cell wall-destabilizing antifungals nikkomycin Z (NZ) and caspofungin (CA). Broth microdilution analysis revealed minimal effective concentrations (MECs) of NZ and CA to be 0.03125 μg/ml and 0.5 μg/ml, respectively, for the ΔpmrA strain. As shown in Fig. 2A, the ΔpmrA strain was more susceptible to nikkomycin Z than the WT, showing drastically swollen hyphae and weakened cell wall architecture. Caspofungin treatment (0.5 to 1 μg/ml) also caused distorted hyphae in the ΔpmrA strain (Fig. 2A). As the enhanced sensitivity of the ΔpmrA strain to cell wall antifungals suggests a role for pmrA in cell wall integrity and possibly biosynthesis of cell wall components, we assayed for total cell wall β-glucan and chitin content. Interestingly, the β-glucan and chitin content increased by 12% and 25%, respectively, in the ΔpmrA strain (Fig. 2B and C). Despite decreased 1,3-β-d-glucan synthase activity, an increase in cell wall α- and β-glucans has been reported for S. pombe (3). A similar increase of glucans was noted in the C. albicans pmr1Δ strain as a reciprocal response to decreased mannans (2).
Fig. 2.
(A) Effect of cell wall inhibitors on the WT and ΔpmrA strains. A total of 103 conidia/ml of the WT and ΔpmrA strains were inoculated onto coverslips immersed in GMM medium and grown in the presence or absence of the indicated concentrations of nikkomycin Z (NZ) or caspofungin (CA) for 16 to 20 h at 37°C. Coverslips were removed and observed by microscopy. Scale bar, 20 μm. (B) β-1,3-Glucan content was quantified using the aniline blue assay as previously described (8, 25, 26), using curdlan, a β-1,3-glucan analog, as the standard. Values are expressed as relative fluorescence units (RFU) per mg of mycelial tissue. (C) Quantification of chitin was performed based on a modified protocol (8) and reported as glucosamine equivalents (μg/ml).
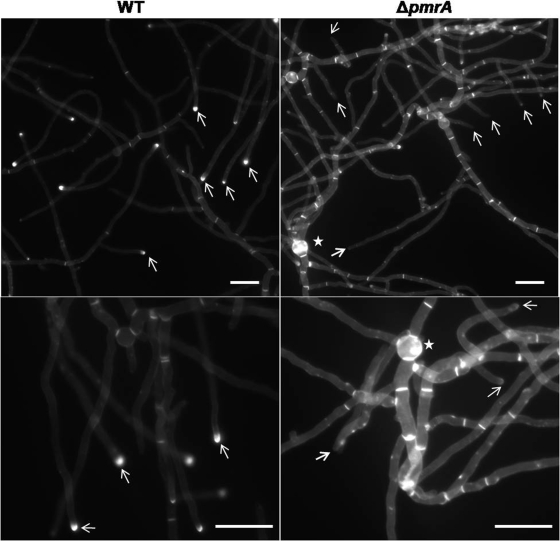
Calcofluor white staining was performed to verify any variations in cell wall morphology and showed aggregation of cell wall polysaccharides in the conidial vesicles and thickened septa in the ΔpmrA strain (Fig. 3). Uniform accumulation of cell wall material was seen at the hyphal tips of the WT strain but not in the ΔpmrA strain, indicating that perturbed cell wall assembly leads to chitin and glucan accumulation at the septum in the ΔpmrA strain.
Fig. 3.
Calcofluor white staining of the WT and ΔpmrA strains. A total of 103 conidia/ml of the WT and ΔpmrA strains were inoculated onto coverslips immersed in GMM medium and grown for 24 h at 37°C. The coverslips were rinsed in sterile water and inverted onto a 200-μl drop of calcofluor white staining solution for 5 min at room temperature. Coverslips were then rinsed twice for 10 min in sterile water and mounted in Cytoseal 60 mounting medium. To ensure that the results of the calcofluor white staining of different samples were appropriately compared, the ultraviolet light exposure time for each sample was set at 25 ms for the WT untreated control, and the strains were exposed for the same lengths of time. Scale bar, 50 μm.
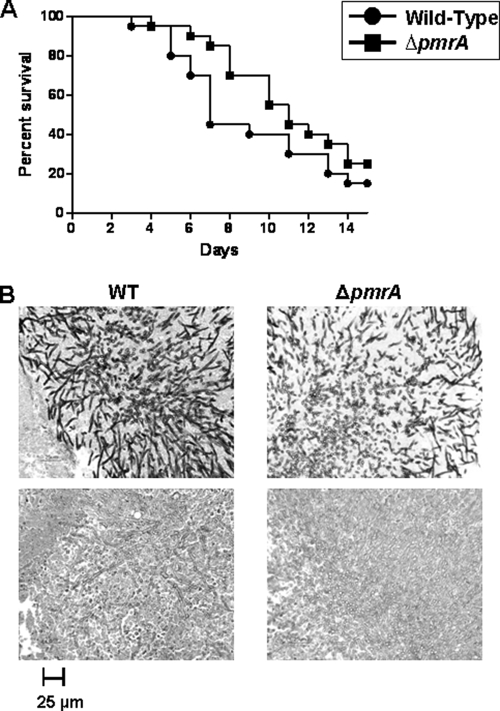
Next, the influence of pmrA deletion on virulence was investigated in an inhalational murine model of invasive pulmonary aspergillosis, as previously described (4, 19, 25). Briefly, 6-week-old CD1 male mice were immunosuppressed with cyclophosphamide and triamcinolone. In a Hinners inhalational chamber, three groups of 20 unanesthetized mice were each exposed by inhalation to 40 ml of an aerosolized suspension of 1 × 109 conidia/ml of the WT and ΔpmrA strains as well as a control diluent. Survival was assessed by Kaplan-Meier analysis and pairwise comparison using the log rank test. Statistical significance was defined as a two-tailed test with a P value of <0.05. Unlike the Candida albicans Δpmr1 strain, which was significantly attenuated in virulence (2), there was no difference in virulence between the WT and ΔpmrA strains (Fig. 4A). Histopathological analysis of lungs harvested from mice euthanized at defined time points and stained with Gomori's methenamine silver and hematoxylin-eosin revealed similar hyphal invasion patterns and extensive inflammation in both the WT and ΔpmrA strains (Fig. 4B). Despite the basal growth defect, different cation sensitivities, increased susceptibility to cell wall antifungals, and increased cell wall chitin and β-glucan, deletion of pmrA did not affect overall pathogenesis. It is possible that a hyphal growth threshold exists between being pathogenic and nonpathogenic. Further studies are required to understand the persistent virulence of the ΔpmrA strain in spite of these aberrancies. In conclusion, we demonstrate that PmrA is involved in hyphal growth, cation homeostasis, and cell wall integrity and likely acts as the major Ca2+ ATPase during salt stress in A. fumigatus.
Fig. 4.
Virulence analysis in a murine inhalational model of invasive aspergillosis. (A) Kaplan-Meier survival curve. At day 14 postinfection, mice infected with the ΔpmrA strain displayed about 70% mortality, which is comparable to that of mice infected with the WT strain (P = 0.1774). (B) Lung histopathology at day 7 postinfection at ×400 magnification. (Top) Gomori's methenamine silver staining demonstrated comparable extensive hyphal proliferation patterns in the lung tissue of mice infected with the WT and ΔpmrA strains. (Bottom) Hematoxylin and eosin staining shows necrosis and inflammation in the tested strains.
Acknowledgments
We thank Eduardo A. Espeso (CSIC, Madrid, Spain) for the annotation of SltA.
N.P. was supported by a Faculty of Medicine-Siriraj Hospital Fellowship, and W.J.S. is supported by a K08 award (A1061149), a Basic Science Faculty Development grant from the American Society for Transplantation, and a Children's Miracle Network grant.
Footnotes
Published ahead of print on 22 January 2010.
REFERENCES
- 1.Antebi A., Fink G. R. 1992. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localized in a novel Golgi-like distribution. Mol. Biol. Cell 3:633–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates S., MacCallum D. M., Bertram G., Munro C. A., Hughes H. B., Buurman E. T., Brown A. J., Odds F. C., Gow N. A. 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280:23408–23415 [DOI] [PubMed] [Google Scholar]
- 3.Cortes J. C., Katoh-Fukui R., Moto K., Ribas J. C., Ishiguro J. 2004. Schizosaccharomyces pombe Pmr1p is essential for cell wall integrity and is required for polarized cell growth and cytokinesis. Eukaryot. Cell 3:1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer R. A., Jr., Perfect B. Z., Pinchai N., Park S., Perlin D. S., Asfaw Y. G., Heitman J., Perfect J. R., Steinbach W. J. 2008. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell 7:1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham K. W., Fink G. R. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cyert M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311:1143–1150 [DOI] [PubMed] [Google Scholar]
- 7.Dürr G., Strayle J., Plemper R., Elbs S., Klee S. K., Catty P., Wolf D. H., Rudolph H. K. 1998. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9:1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortwendel J. R., Juvvadi P. R., Pinchai N., Perfect B. Z., Alspaugh J. A., Perfect J. R., Steinbach W. J. 2009. Differential effects of inhibiting chitin and 1,3-beta-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 53:476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Lopez M. J., et al. 2006. Regulation of salt tolerance by Torulaspora delbrueckii calcineurin target Crz1p. Eukaryot. Cell 5:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama S., Sugiura R., Lu Y., Maeda T., Kawagishi K., Yokoyama M., Tohda H., Giga-Hama Y., Shuntoh H., Kuno T. 2003. Zinc finger protein Prz1 regulates Ca2+ but not Cl− homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J. Biol. Chem. 278:18078–18084 [DOI] [PubMed] [Google Scholar]
- 11.Idnurm A., Walton F. J., Floyd A., Reedy J. L., Heitman J. 2009. Identification of ENA1 as a virulence gene of the human pathogenic fungus Cryptococcus neoformans through signature-tagged insertional mutagenesis. Eukaryot. Cell 8:315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karababa M., Valentino E., Pardini G., Coste A. T., Bille J., Sanglard D. 2006. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59:1429–1451 [DOI] [PubMed] [Google Scholar]
- 13.Lapinskas P. J., Cunningham K. W., Liu X. F, Fink G. R., Culotta V. C. 1995. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15:1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marquez J. A., Serrano R. 1996. Multiple transduction pathways regulate the sodium-extrusion gene PMR2/ENA1 during salt stress in yeast. FEBS Lett. 382:89–92 [DOI] [PubMed] [Google Scholar]
- 15.Mendizabal I., Pascual-Ahuir A., Serrano R., de Larrinoa I. F. 2001. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol. Genet. Genomics 265:801–811 [DOI] [PubMed] [Google Scholar]
- 16.Mendoza I., Rubio F., Rodriguez-Navarro A., Pardo J. M. 1994. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 269:8792–8796 [PubMed] [Google Scholar]
- 17.O'Neil J. D., Bugno M., Stanley M. S., Barham-Morris J. B., Woodcock N. A., Clement D. J., Clipson N. J. W., Whitehead M. P., Fincham D. A., Hooley P. 2002. Cloning of a novel gene encoding a C2H2 zinc finger protein that alleviates sensitivity to abiotic stresses in Aspergillus nidulans. Mycol. Res. 106:491–498 [Google Scholar]
- 18.Park S. Y., Seo S. B., Lee S. J., Na J. G., Kim Y. J. 2001. Mutation in PMR1, a Ca(2+)-ATPase in Golgi, confers salt tolerance in Saccharomyces cerevisiae by inducing expression of PMR2, an Na(+)-ATPase in plasma membrane. J. Biol. Chem. 276:28694–28699 [DOI] [PubMed] [Google Scholar]
- 19.Pinchai N., Perfect B. Z., Juvvadi P. R., Fortwendel J. R., Cramer R. A., Jr., Asfaw Y. G., Heitman J., Perfect J. R., Steinbach W. J. 2009. Aspergillus fumigatus calcipressin CbpA is involved in hyphal growth and calcium homeostasis. Eukaryot. Cell 8:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., LeVitre J., Davidow L. S., Mao J., Moir D. T. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+-ATPase family. Cell 58:133–145 [DOI] [PubMed] [Google Scholar]
- 21.Ruiz A., Arino J. 2007. Function and regulation of the Saccharomyces cerevisiae ENA sodium ATPase system. Eukaryot. Cell 6:2175–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soriani F. M., Malavazi I., da Silva Ferreira M. E., Savoldi M., Von Zeska Kress M. R., de Souza Goldman M. H., Loss O., Bignell E., Goldman G. H. 2008. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol. Microbiol. 67:1274–1291 [DOI] [PubMed] [Google Scholar]
- 23.Soriani F. M., Martins V. P., Magnani T., Tudella V. G., Curti C., Uyemura S. A. 2005. A PMR1-like calcium ATPase of Aspergillus fumigatus: cloning, identification and functional expression in S. cerevisiae. Yeast 22:813–824 [DOI] [PubMed] [Google Scholar]
- 24.Spielvogel A., Findon H., Arst H. N., Araújo-Bazán L., Hernández-Ortíz P., Stahl U., Meyer V., Espeso E. A. 2008. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in Aspergillus nidulans. Biochem. J. 414:419–429 [DOI] [PubMed] [Google Scholar]
- 25.Steinbach W. J., Cramer R. A., Jr., Perfect B. Z., Asfaw Y. G., Sauer T. C., Najvar L. K., Kirkpatrick W. R., Patterson T. F., Benjamin D. K., Jr., Heitman J., Perfect J. R. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinbach W. J., Cramer R. A., Jr., Perfect B. Z., Henn C., Nielsen K., Heitman J., Perfect J. R. 2007. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:2979–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J., Kang H. A., Ko S. M., Chae S. K., Ryu D. D., Kim J. Y. 2001. Cloning of the Aspergillus niger pmrA gene, a homologue of yeast PMR1, and characterization of a pmrA null mutant. FEMS Microbiol. Lett. 199:97–102 [DOI] [PubMed] [Google Scholar]