Abstract
The cyclic AMP (cAMP) pathway plays a central role in the growth, differentiation, and virulence of pathogenic fungi, including Cryptococcus neoformans. Three upstream signaling regulators of adenylyl cyclase (Cac1), Ras, Aca1, and Gpa1, have been demonstrated to control the cAMP pathway in C. neoformans, but their functional relationship remains elusive. We performed a genome-wide transcriptome analysis with a DNA microarray using the ras1Δ, gpa1Δ, cac1Δ, aca1Δ, and pka1Δ pka2Δ mutants. The aca1Δ, gpa1Δ, cac1Δ, and pka1Δ pka2Δ mutants displayed similar transcriptome patterns, whereas the ras1Δ mutant exhibited transcriptome patterns distinct from those of the wild type and the cAMP mutants. Interestingly, a number of environmental stress response genes are modulated differentially in the ras1Δ and cAMP mutants. In fact, the Ras signaling pathway was found to be involved in osmotic and genotoxic stress responses and the maintenance of cell wall integrity via the Cdc24-dependent signaling pathway. Notably, the Ras and cAMP mutants exhibited hypersensitivity to a polyene drug, amphotericin B, without showing effects on ergosterol biosynthesis, which suggested a novel method of antifungal combination therapy. Among the cAMP-dependent gene products that we characterized, two small heat shock proteins, Hsp12 and Hsp122, were found to be involved in the polyene antifungal drug susceptibility of C. neoformans.
The cyclic AMP (cAMP) signaling pathway plays a central role in the regulation of the growth, differentiation, and virulence of human pathogenic fungi (3, 11, 41, 42, 53). Central signaling components of the cAMP signaling pathway are conserved evolutionarily among organisms from yeasts to mammals, although some variations in the regulatory mechanism exist. The cAMP signaling pathway in Saccharomyces cerevisiae has been well characterized. In response to certain environmental cues, the Cyr1/Cdc35 adenylyl cyclase produces cAMP, which subsequently binds to the protein kinase A (PKA) regulatory subunit (Bcy1) that represses activation of the catalytic subunits of PKA (Tpk1/Tpk2/Tpk3) under normal conditions (50, 56, 69). The cAMP-bound Bcy1 is released from the Tpk proteins, which then activate or repress downstream transcription factors, such as Flo8, to respond to incoming signaling cues (50). cAMP signaling is regulated negatively by feedback inhibition from high-affinity and low-affinity phosphodiesterases, Pde2 and Pde1, respectively (60, 77). The cAMP signaling pathway modulates cellular growth, stress sensitivity, and morphological differentiation, including pseudohyphal and invasive growth of S. cerevisiae (16, 70).
Two upstream signaling branches transfer environmental signaling to the yeast adenylyl cyclase. One branch includes a heterotrimeric GTP-binding protein (G protein), consisting of a Gα subunit (Gpa2) and inhibitory Gβγ-like kelch repeat proteins (Gpb1/Gpb2 and Gpg1), and the associated G protein-coupled receptor (GPCR). The seven-transmembrane-domain GPCR Gpr1 undergoes a conformational change and allows dissociation of Gpa2 from Gpb1/Gpb2 and Gpg1 subunits in response to certain environmental signals, such as glucose addition to glucose-starved cells (28, 48). The GTP-bound Gα subunit binds to and activates Cyr1/Cdc35. The other branch includes a small G protein such as Ras and a cyclase-associated protein such as CAP (also known as Srv2). S. cerevisiae contains two Ras proteins, Ras1 and Ras2, which are critical for cellular growth control (70). Ras proteins are controlled positively by the Cdc25 guanine nucleotide exchange factor (GEF), which accelerates the replacement of GDP with GTP (57), and negatively by Ira1/Ira2 GTPase-activating protein, which enhances the intrinsic GTPase activity of Ras (67). Ras directly activates Cdc35 adenylyl cyclase in association with CAP and subsequently controls PKA (21, 22, 25, 70).
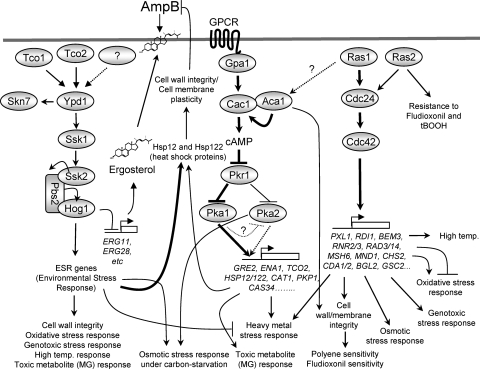
In opportunistic pathogenic fungi that are distributed worldwide, including Aspergillus fumigatus, Candida albicans, and Cryptococcus neoformans, the Ras and cAMP signaling pathways are evolutionarily conserved, and yet significant functional and structural divergence has been observed (3, 11, 42, 53, 58, 68, 73). In C. neoformans, which causes life-threatening fungal meningitis in both immunocompetent and immunocompromised individuals (34, 43), the cAMP signaling pathway is critical for the production of two major virulence factors, the antiphagocytic polysaccharide capsule and the antioxidant melanin pigment, and for sexual differentiation, which is important for the dissemination of infectious spores. C. neoformans contains a single adenylyl cyclase, Cac1 (4). cAMP signaling from Cac1 is bifurcated into two PKA catalytic subunits, Pka1 and Pka2, whose activation is inhibited by the regulatory subunit Pkr1 (9, 30). Notably, Pka1 plays a predominant role in cAMP signaling in the serotype A C. neoformans H99 strain background, whereas Pka2 does so in the serotype D C. neoformans JEC21 strain (30). Interestingly, the low-affinity phosphodiesterase Pde1, but not the high-affinity phosphodiesterase Pde2, plays a key role in negatively regulating the cAMP pathway in C. neoformans (29) (see Fig. 1A).
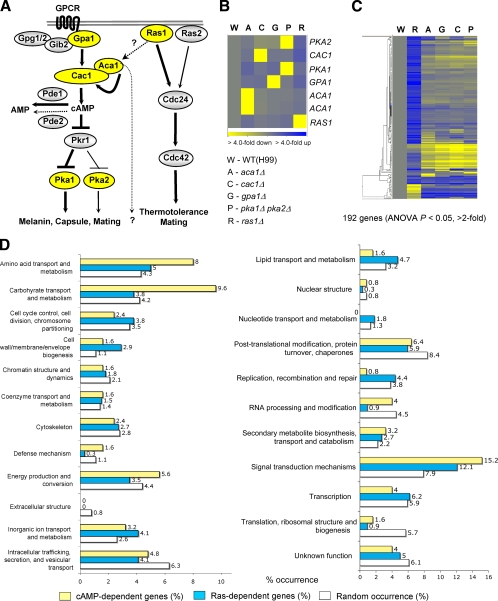
Fig. 1.
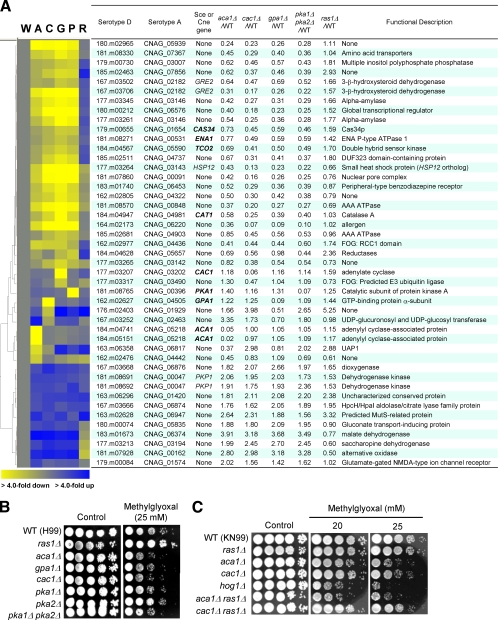
Genome-wide identification of genes regulated by the Ras and cAMP signaling pathways and their functional categories. (A) Schematic diagram summarizing the known signaling components of the Ras and cAMP signaling pathways in C. neoformans. (B and C) The degree of change (n-fold) in gene expression is illustrated by color coding (see the color bar scale at the bottom of the graph in panel B). (B) Relative expression levels of RAS1, ACA1, GPA1, CAC1, PKA1, and PKA2 genes in the ras1Δ (YSB51), aca1Δ (YSB6), gpa1Δ (YSB83), cac1Δ (YSB42), and pka1Δ pka2Δ (YSB200) mutant backgrounds compared to the WT strain (H99). (C) Hierarchical clustering analysis of 192 genes which are significantly up- or downregulated, by >2-fold (P < 0.05; ANOVA), in at least one of the mutant strains indicated in panel B. (D) Functional categories of genes differentially regulated by the Ras and cAMP pathways. Genes showing expression patterns in the ras1Δ (YSB51), aca1Δ (YSB6), gpa1Δ (YSB83), cac1Δ (YSB42), and pka1Δ pka2Δ (YSB200) mutants significantly different (P < 0.05; ANOVA) from those in the WT were functionally categorized based on KOG functional descriptions (http://www.ncbi.nlm.nih.gov/COG/). Bars indicate the following: yellow, percentages of cAMP-dependent genes; blue, percentages of Ras-dependent genes; white, random occurrence rates for genes in each KOG functional category in the whole C. neoformans genome.
Two major upstream signaling regulators of the Cac1 adenylyl cyclase, Aca1 (adenylyl cyclase-associated protein 1, a yeast CAP ortholog) and the Gα subunit protein (Gpa1, a yeast Gpa2 ortholog), have been identified in C. neoformans (2, 9) (see Fig. 1A). Aca1 physically interacts with Cac1 and controls the induction of cAMP, but not the basal levels of cAMP, to govern most cAMP-dependent phenotypes (9). In response to certain environmental signals, including exogenous methionine, the Gpr4 GPCR undergoes conformational changes and releases its C-terminally bound Gα subunit, Gpa1, which subsequently activates Cac1 (2, 78). However, inhibitory Gβ-like kelch repeat proteins such as Gpb1/Gpb2 have not been found in C. neoformans. Instead, Gpa1 physically interacts with Gβ-like protein/RACK1 homolog Gib2, which also interacts with Gpg1/Gpg2 (49). Gib2 regulates cAMP signaling positively but is essential for cellular growth, unlike other cAMP signaling components, and also interacts with protein kinase C (PKC), indicating that Gib2 is a multifunctional protein (49). Gpa1 is negatively regulated by a regulator of G protein signaling (RGS) protein, Crg2, which is also involved in G protein signaling in the pheromone response Cpk1 mitogen-activated protein kinase (MAPK) pathway (63, 79).
Two Ras proteins, Ras1 and Ras2, were discovered in C. neoformans and play both shared and distinct roles (1, 19, 75). Ras1 is a major C. neoformans Ras protein that supports high-temperature growth and invasive growth, which are essential for survival and proliferation inside the host, and promotes sexual differentiation (1). Although the ras2Δ mutant does not have any discernible phenotypes, overexpression of RAS2 partly suppresses most ras1 mutant phenotypes (75). In C. neoformans, as in S. cerevisiae, the disruption of both RAS1 and RAS2 genes affects cellular viability at all temperatures, indicating that Ras proteins are essential for normal cellular growth. Ras signaling is bifurcated into two signaling branches in C. neoformans. To govern thermotolerance and actin cytoskeleton regulation, Ras signaling is mediated through a GEF protein, Cdc24, and a Rho-like GTPase, Cdc42 (47). In contrast, Ras-dependent regulation of invasive growth and mating is mediated through the cAMP signaling pathway (1, 76). However, whether Ras1 directly interacts with the Cac1 adenylyl cyclase is not clear, because Cac1 does not harbor the leucine-rich repeat domain that is a binding site for GTP-bound Ras in S. cerevisiae (65). Since the adenylyl cyclase-cyclase-associated protein complex may provide a second Ras-binding site for adenylyl cyclase activation, as can be observed in S. cerevisiae (64), it is still possible that Ras1 may interact with the Aca1-Cac1 complex for adenylyl cyclase activation in C. neoformans. Taken together, these data demonstrate that the C. neoformans cAMP signaling pathway is controlled by three different upstream regulators, Ras1, Gpa1, and Aca1.
Regardless of the presence of the common upstream regulators of the Cac1 adenylyl cyclase, a functional connection between each signaling component and the target genes regulated in C. neoformans remains elusive. Hence, we performed a genome-wide transcriptome analysis of ras1Δ, gpa1Δ, aca1Δ, cac1Δ, and pka1Δ pka2Δ mutants by using a DNA microarray approach to compare the downstream networks of the Ras-, Aca1-, and Gpa1-dependent signaling pathways with those of the Cac1/PKA signaling pathway. Here, we show that the Aca1- and Gpa1-dependent cAMP/PKA signaling pathway exhibited unique transcriptome patterns that were distinct from those of the Ras1 signaling pathway. In this study, we not only identified a number of Ras1- and cAMP-dependent genes but also discovered novel characteristics of the cAMP and Ras pathways, which include involvement in diverse stress responses and susceptibility to antifungal drugs, such as amphotericin B (AmpB). Therefore, this study not only furthers understanding of the role of Ras and cAMP pathways in C. neoformans but also provides useful information for the development of novel antifungal therapy.
MATERIALS AND METHODS
Strains and growth conditions.
The C. neoformans strains and primers used in this study are listed in Table S1 in the supplemental material, and the strains were cultured in YPD (yeast extract-peptone-dextrose) medium unless otherwise indicated. l-DOPA (l-3,4-dihydroxyphenylalanine) or Niger seed medium for melanin production and agar-based Dulbecco's modified Eagle's medium for capsule production were all as described previously (2, 9, 27, 30).
DNA microarray and data analysis.
Total RNAs for DNA microarray analysis were isolated as follows. The wild-type (WT) strain H99 and the corresponding ras1Δ (YSB51), aca1Δ (YSB6), gpa1Δ (YSB83), cac1Δ (YSB42), and pka1Δ pka2Δ (YSB200) mutant strains were grown in 50 ml YPD medium at 30°C for 16 h. Five milliliters of the overnight culture was inoculated into 100 ml of fresh YPD medium and further incubated for 4 to 5 h at 30°C until it reached an optical density at 600 nm of approximately 1.0. Then the culture was rapidly frozen in liquid nitrogen and lyophilized overnight. Three independent cultures of each strain were prepared as biological replicates for total RNA isolation for the DNA microarray. Total RNAs were isolated by using TRIzol reagent as described previously (38). To provide a control, total RNAs prepared from the WT strain and the ras1Δ, gpa1Δ, aca1Δ, cac1Δ, and pka1Δ pka2Δ mutant strains grown under the conditions described above were pooled to yield reference RNAs.
cDNA synthesis and labeling were performed by using AffinityScript reverse transcriptase (Stratagene) and Cy5/Cy3 labeling agents (Amersham) as described previously (38). The cDNAs prepared from pooled reference RNAs were mixed with Cy3 as a control, and the cDNAs prepared from each test RNA (corresponding to each experimental condition) were mixed with Cy5. For microarray analysis, C. neoformans serotype D 70-mer oligonucleotide microarray slides containing 7,936 spots (Duke University) were used. The microarray slides were prehybridized, hybridized with Cy3/Cy5-labeled cDNAs, and washed as described previously (38). Three independent DNA microarrays with three independent biological replicates were analyzed.
After hybridization and washing, the microarray slides were scanned by a GenePix 4000B scanner (Axon Instruments) and the scanned images were analyzed with GenePix Pro software (version 4.0) by using GenePix Array List (GAL) files (http://genomeold.wustl.edu/activity/ma/cneoformans/array_spec.cgi). For array data analysis, we used the serotype A gene identification database (for the H99 strain) that has mapping data for the corresponding 70-mer oligonucleotide sequences printed on the array slides as described previously (38). Among a total of 6,980 genes in the H99 strain, 6,302 genes were matched to 6,756 spots (including multiple spots for a single gene) on the JEC21 gene chip with an E value of 10−6 (90% coverage) by blastn searching. By using the serotype A gene sequences, each S. cerevisiae gene name or identification number listed in the tables in the supplemental data was assigned by blastp searching (E value cutoff, 10−6). For hierarchical and statistical analyses, data transported from GenePix software were analyzed with Accuity software by employing LOWESS (locally weighted scatterplot smoothing) normalization, reliable gene filtering (>95% filtering), hierarchical clustering, zero transformation, and analysis of variance (ANOVA; P < 0.05) with the use of Microsoft Excel software. The functional category for each C. neoformans H99 gene was assigned using the NCBI KOG (eukaryotic orthologous group) database (http://www.ncbi.nlm.nih.gov/COG/grace/shokog.cgi). The frequency of C. neoformans genes of each functional category among all C. neoformans genes assigned to KOG categories (the percent random occurrence of genes of each category) was calculated as follows: (total number of C. neoformans genes in each KOG functional category) × 100/(total number of C. neoformans genes listed in all KOG categories). The frequency of Ras- or cAMP-dependent genes of each functional category among all Ras- or cAMP-dependent genes assigned to KOG categories was calculated as follows: (total number of Ras- or cAMP-dependent genes in each KOG functional category) × 100/(total number of Ras- or cAMP-dependent genes listed in all KOG categories). If the occurrence of Ras- or cAMP-dependent genes in a category was at least 1.5-fold higher than the corresponding random occurrence of C. neoformans genes in the category, we defined the category as being overrepresented in the pathway.
Disruption of cAMP signaling-dependent genes.
For gene disruption, information on genomic DNA (exon and intron) structures for each gene was obtained from the serotype A C. neoformans genome database (http://www.broadinstitute.org/annotation/genome/cryptococcus_neoformans/MultiHome.html). The genes GRE2 (H99 gene identification number CNAG_02182.2 [hereinafter, only the five digits represented by X in CNAG_XXXXX.2 are given for H99 gene identification numbers]), PKP1 (00047), HSP12 (03143), and HSP122 (01446) in the C. neoformans serotype A H99 strain were deleted by overlap PCR or double-joint PCR with split markers and biolistic transformation as described previously (10, 17, 37). Primers for the generation of the 5′ and 3′ flanking regions of each gene and the dominant selectable nourseothricin resistance marker (NAT, encoding nourseothricin acetyltransferase) are described in Table S1 in the supplemental material. Gold microcarrier beads (0.8 to ∼1.2 μm [Bioworld Inc.] or 0.6 μm [Bio-Rad]) were coated with gel-extracted deletion cassettes produced by overlap PCR, and strain H99 was biolistically transformed with the coated beads. The hsp12Δ hsp122Δ double mutant was constructed by introducing the hsp122Δ::NEO allele into the hsp12Δ mutant via biolistic transformation. Stable transformants selected on YPD medium containing nourseothricin and/or G418 were subjected to an initial screening by diagnostic PCR with primers listed in Table S1 in the supplemental material. Positive mutants were further checked by Southern blot analysis using gene-specific probes (see Fig. S2 to S4 in the supplemental material) prepared by using primers listed in Table S1 in the supplemental material.
Ergosterol assay.
Ergosterol contents in the WT and each mutant strain were measured as described previously (6, 38). Each experiment was performed in duplicate with three independent cultures of each strain.
Stress and antifungal drug sensitivity tests.
For stress and drug sensitivity tests, cells grown overnight at 30°C in YPD medium were washed, serially diluted 10-fold (yielding 1- to 104-fold dilutions) in distilled water, and spotted (in a volume of 3 μl) onto solid YPD agar medium containing the following: the concentrations of NaCl and KCl indicated below for the osmotic stress sensitivity test; hydrogen peroxide (H2O2), menadione, diamide [diazenedicarboxylic acid bis(N,N-dimethylamide)], and tert-butyl hydroperoxide (tBOOH) for the oxidative stress sensitivity test; hydroxyurea (HU) and methyl methanesulfonate (MMS) for the DNA-damaging agent sensitivity test; methylglyoxal (MG) for the toxic-metabolite sensitivity test; cadmium sulfate (CdSO4) for the heavy-metal stress test; and AmpB, fluconazole, ketoconazole, itraconazole, and fludioxonil for the antifungal drug test. To test UV sensitivity, cells were spotted onto solid YPD medium and placed in a UV cross-linker (UVP CX-2000) at energy levels between 200 and 400 J/m2. Then the spotted cells were incubated at 30°C for 2 to 4 days and photographed.
Assay for capsule and melanin production.
Qualitative visualization and quantitative measurement of capsule and melanin production were performed as described previously (9).
Microarray data accession number.
The whole-microarray data generated by this study were submitted to the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE17338.
RESULTS
Comparative transcriptome analysis of C. neoformans ras1Δ, aca1Δ, gpa1Δ, cac1Δ, and pka1Δ pka2Δ mutants.
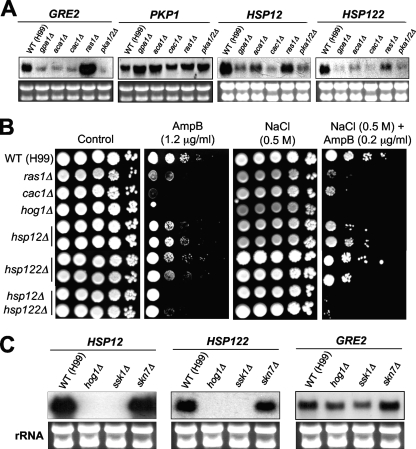
To compare the downstream signaling networks of Ras1-, Aca1-, and Gpa1-dependent signaling pathways, we performed a comparative transcriptome analysis of the serotype A WT strain (H99) and ras1Δ, aca1Δ, gpa1Δ, cac1Δ, and pka1Δ pka2Δ mutants by employing a DNA microarray approach as described in Materials and Methods. For basic validation of our array quality, we checked expression levels of the RAS1, ACA1, GPA1, CAC1, PKA1, and PKA2 genes in our array data. The expression levels of RAS1, ACA1, GPA1, CAC1, PKA1, and PKA2 in the corresponding mutants were very low compared to those in the WT strain (ratios of expression in the mutants to expression in the WT were 0.08, 0.03, 0.09, 0.06, 0.07, and 0.12, respectively), as expected (Fig. 1B; see also Table S2 in the supplemental material). To further confirm the quality of the array, we performed Northern blot analysis for some of the cAMP-dependent genes identified by this microarray, including GRE2, PKP1, HSP12, and HSP122. All of the genes exhibited expected expression patterns, which were highly similar to those in the DNA microarray data, as depicted below (see Fig. 8A). Taken together, all these data supported the quality of the array.
Fig. 8.
The heat shock proteins, Hsp12 and Hsp122, are partly involved in polyene sensitivity and is coregulated by the cAMP and HOG signaling pathways. (A) Northern blot analysis showing basal expression levels of GRE2, PKP1, HSP12, and HSP122 in the WT H99 strain and the ras1Δ (YSB51), aca1Δ (YSB6), gpa1Δ (YSB83), cac1Δ (YSB42), and pka1Δ pka2Δ (YSB200) mutants. (B) C. neoformans strains (the WT H99 strain and the ras1Δ [YSB53], cac1Δ [YSB42], hog1Δ [YSB64], hsp12Δ [YSB599 and YSB600], hsp122Δ [YSB603 and YSB604], and hsp12Δ hsp122Δ [YSB757 and YSB758] mutant strains) were grown overnight at 30°C in liquid YPD medium, serially diluted 10-fold (yielding 1- to 104-fold dilutions), spotted (in 3-μl volumes) onto YPD agar containing the indicated concentration of AmpB with or without osmotic stress (0.5 M NaCl), incubated at 30°C for 72 h, and photographed. (C) Northern blot analysis showed basal expression levels of HSP12, HSP122, and GRE2 in the WT H99 strain and the hog1Δ (YSB64), ssk1Δ (YSB261), and skn7Δ (YSB349) mutants.
Among a total of 7,936 genes monitored by this DNA microarray, 565 genes exhibited differential expression patterns in the Ras and cAMP mutants at statistically significant levels (P < 0.05; ANOVA) compared to expression in the WT strain. Among these, 192 genes exhibited more than twofold induction or reduction (Fig. 1C). The hierarchical clustering analysis of the Ras- or cAMP-dependent genes revealed several important facts. First, the transcriptome patterns governed by the Ras1 signaling pathway were distinct from those controlled by the cAMP/PKA signaling pathway (Fig. 1C). The statistical analysis indicated that basal expression levels of a total of 400 genes changed significantly in the ras1Δ mutant compared to the WT but that expression levels of 132 genes changed significantly in the aca1Δ, gpa1Δ, cac1Δ, and pka1Δ pka2Δ mutants (see Fig. S1A and B in the supplemental material). Besides the number of such genes, the expression patterns of a majority of the Ras1-dependent genes distinguished them from the cAMP-dependent genes (Fig. 1C; see also Fig. S1 in the supplemental material), which indicated that the Ras1 signaling pathway is largely independent of the cAMP signaling pathway in C. neoformans. Second, the aca1Δ and gpa1Δ mutants showed transcriptome patterns similar to those of the cac1Δ and pka1Δ pka2Δ mutants, suggesting that Aca1 and Gpa1 are the two major signaling modulators of the cAMP signaling pathway (see Fig. S1B in the supplemental material). However, there was a small group of genes whose expression was differentially regulated between the aca1Δ and gpa1Δ mutants. This finding indicates that Aca1 and Gpa1 may have other, minor signaling branches (see Fig. S1B in the supplemental material). As expected, the cac1Δ mutant exhibited transcriptome patterns almost identical to those of the pka1Δ pka2Δ mutant, further suggesting that Pka1 and Pka2 are necessary and sufficient protein kinases downstream of the adenylyl cyclase in C. neoformans (see Fig. S1B in the supplemental material).
The genes regulated by the Ras and cAMP signaling pathways encompass a wide variety of cellular functions (Fig. 1D). Genes involved in signal transduction mechanisms (15.2%), carbohydrate transport and metabolism (9.6%), and amino acid transport and metabolism (8.0%) were overrepresented among the cAMP signaling-dependent genes. These findings were expected since the cAMP pathway is a central signal transduction cascade that regulates the growth, differentiation, and virulence of C. neoformans and is known to sense glucose and amino acids (9, 78). Similarly, genes involved in signal transduction mechanisms (12.1%) were most overrepresented in the ras1Δ mutant (Fig. 1D). However, genes involved in cell wall/membrane/envelope biogenesis (2.9%) were also overrepresented in the Ras pathway, in contrast to the cAMP pathway, which implies that Ras1 may be involved in the maintenance of cell wall integrity.
Among the Ras- and cAMP-dependent genes, a significant proportion were found to be regulated by environmental stress (Fig. 2; see also Fig. S1C in the supplemental material). Our prior transcriptome analysis revealed a number of environmental stress response (ESR) genes, which were defined as genes whose expression is up- or downregulated more than twofold in C. neoformans in response to at least one stress condition, such as osmotic or oxidative stress or antifungal drug (fludioxonil) treatment (38). Among the 1,959 ESR genes, 109 genes were found to be regulated in response to all stresses and were named common stress response (CSR) genes (38). Interestingly, our present array analysis revealed that a subset of the ESR genes (a total of 225 ESR genes) exhibited significant changes in expression levels in either the ras1Δ or cAMP mutants compared to the WT strain (P < 0.05; ANOVA) (Fig. 2; see also Fig. S1C and Table S4 in the supplemental material). Among these, 86 ESR genes showed more than twofold induction or reduction in the mutants (see Fig. S1C in the supplemental material). Furthermore, a total of 55 CSR genes were found to be differentially regulated (P < 0.05; ANOVA), and 31 of these genes exhibited more than twofold induction or reduction in the mutants (see Fig. S1C in the supplemental material). The majority of the Ras or cAMP pathway-dependent ESR and CSR genes did not have significant homology to any other genes (see Table S6 in the supplemental material). Nevertheless, these results implicated the Ras and cAMP signaling pathways in diverse stress responses of C. neoformans.
Fig. 2.
Identification of Ras1 signaling-dependent genes in C. neoformans. (A) Relative expression profiles of Ras-dependent genes from C. neoformans, S. cerevisiae, or Schizosaccharomyces pombe which have been identified or characterized and show more than twofold induction or repression in the ras1Δ mutant compared to the WT strain. (B) Relative expression profiles of Ras-dependent genes in C. neoformans which do not have any orthologs or known functions in S. cerevisiae or S. pombe and show more than threefold induction or repression in the ras1Δ mutant compared to the WT strain. In both panels, the degree of change (n-fold) is illustrated by color coding (see the color bar scales at the bottoms of the graphs), and the exact value for each gene is indicated in the table to the right of the hierarchical clustering diagram. Abbreviations are as follows: ESR-up and ESR-down, significant up- and downregulation (more than twofold), respectively, of ESR genes; OxR, oxidative stress response; OsR, osmotic stress response; FxR, fludioxoniol response; ER, endoplasmic reticulum; and ACK, activated Cdc42-associated kinase.
The Ras signaling pathway controls the osmotic stress response and is required for maintenance of cell wall integrity in C. neoformans.
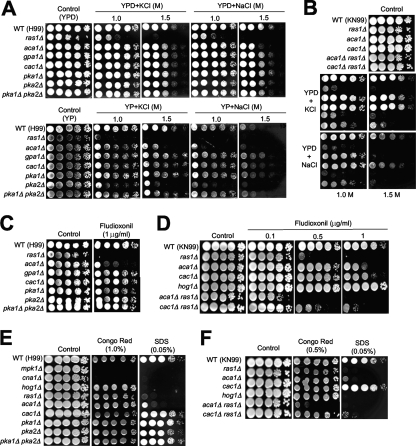
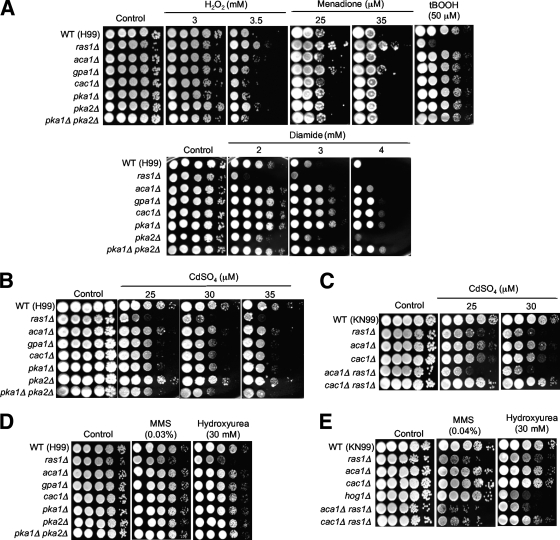
To address the roles of the Ras and cAMP pathways in the stress responses of C. neoformans, we monitored the susceptibilities of the Ras and cAMP mutants to diverse stress-inducing agents. We found that the ras1Δ mutation affected a variety of stress responses, besides conferring the known susceptibility of the ras1Δ mutant to high temperature. Notably, the ras1Δ mutant, but not the aca1Δ, gpa1Δ, cac1Δ, pka1Δ, and pka1Δ pka2Δ mutants, exhibited increased susceptibility to hyperosmotic stress imposed by high KCl and NaCl concentrations under both glucose-rich conditions (YPD medium) and conditions of glucose starvation (yeast extract-peptone medium) (Fig. 3A). This observed osmosensitive phenotype of the ras1Δ mutant was even more pronounced than those of the hog1Δ and ena1Δ mutants of C. neoformans, which show hyperosmosensitivity only under conditions of glucose starvation (38). Interestingly, the aca1Δ mutant also showed hyperosmosensitivity (particularly in the presence of Na+ salt rather than K+ salt), albeit to a lesser extent than the ras1Δ mutant (Fig. 3A). The ras1Δ aca1Δ double mutant showed even greater susceptibility to NaCl, indicating that Ras1 and Aca1 independently contribute to resistance to Na+ salt stress (Fig. 3B).
Fig. 3.
The Ras1 signaling pathway controls the osmotic stress response and is required for maintaining cell wall integrity in C. neoformans. Each C. neoformans strain indicated below was grown overnight at 30°C in liquid YPD medium, serially diluted 10-fold (yielding 1- to 104-fold dilutions), and spotted (in 3-μl volumes) onto yeast extract-peptone (YP) or YPD agar containing the indicated concentrations of either NaCl or KCl (A and B), fludioxonil (C and D), and Congo red and SDS (E and F). Cells were incubated at 30°C for 72 h and photographed. (A, C, and E) The WT H99 strain (MATα) and the ras1Δ (YSB53), aca1Δ (YSB6), gpa1Δ (YSB83), cac1Δ (YSB42), pka1Δ (YSB188), pka2Δ (YSB194), and pka1Δ pka2Δ (YSB200) mutant strains were used. In addition, the mpk1Δ (KK3), cna1Δ (KK1), and hog1Δ (YSB64) mutants were used for the analysis in panel E. (B, D, and F) The WT KN99 strain (MATa) and the ras1Δ (YSB73), cac1Δ (YSB183), aca1Δ (YSB176), hog1Δ (YSB81), aca1Δ ras1Δ (YSB175), and cac1Δ ras1Δ (YSB187) mutant stains were used.
Another interesting finding was the involvement of Pka2 in the osmostress response under glucose starvation conditions (Fig. 3A). In serotype A C. neoformans (the H99 strain background), Pka1 plays a major role in controlling the cAMP pathway whereas Pka2 plays only a minor role (9, 30). Here, we found that the pka2Δ mutant is hypersensitive to osmotic shock only under conditions of glucose starvation (Fig. 3A). Interestingly, the hyperosmosensitivity of the pka2Δ mutant is abolished by additional disruption of PKA1 (Fig. 3A), which indicates that Pka1 and Pka2 may play opposing roles in the osmostress response of C. neoformans under glucose starvation conditions. All the independently constructed pka2Δ mutants of MATα H99 and MATa KN99 strains exhibited the same osmosensitivity phenotypes (data not shown).
To address whether the increased osmosensitivity of the ras1Δ mutant may result from decreased cell wall integrity, we tested the fludioxonil sensitivities of the ras1Δ and aca1Δ mutants; C. neoformans mutants with effects on cell wall integrity, such as cells carrying the cna1Δ mutation in the Ca2+-calmodulin/calcineurin pathway and cells harboring the mpk1Δ mutation in the PKC/Mpk1 MAPK pathway, exhibit hypersensitivity to fludioxonil (40). This antifungal drug hyperactivates the high-osmolarity glycerol (HOG)-dependent pathway, increases the intracellular glycerol content, and eventually arrests the cell cycle (40). Osmotic stress and fludioxonil treatment generate similar genome-wide remodeling patterns in C. neoformans (38). Like the cna1Δ and mpk1Δ mutants, the ras1Δ mutant displayed hypersensitivity to fludioxonil (Fig. 3C). The aca1Δ ras1Δ mutant exhibited even greater susceptibility to fludioxonil (Fig. 3D), which indicated that Aca1 and Ras1 may independently contribute to the maintenance of cell wall integrity. To further support this possibility, we tested the susceptibilities of the mutants to cell wall-destabilizing agents, such as Congo red and sodium dodecyl sulfate (SDS). As expected, the mpk1Δ and cna1Δ mutants exhibited hypersensitivity to both Congo red and SDS (Fig. 3E). In contrast, the ras1Δ, aca1Δ, and hog1Δ mutants exhibited greater susceptibility to SDS than the WT (Fig. 3E). Although the ras1Δ mutant did not display significant hypersensitivity to Congo red, the aca1Δ ras1Δ double mutant showed hypersensitivity to Congo red (Fig. 3F), further indicating that Aca1 and Ras1 play redundant or independent roles in maintaining cell wall integrity. Taken together, these findings demonstrate that the Ras1 signaling pathway is required for cell wall integrity in C. neoformans, which may explain the increased osmosensitivity of the ras1Δ mutant.
The Ras1 signaling pathway is required for oxidative and genotoxic stress responses in C. neoformans.
Since the ESR and CSR genes were defined as genes regulated during response to antifungal drug treatment, osmotic stress, and/or oxidative stress, we also examined the oxidative stress sensitivities of the ras1Δ and cAMP mutants by using a variety of reactive oxygen species (ROS) and oxidative agents inducing different cellular responses. In cells, toxic superoxide radicals are rapidly converted into hydrogen peroxide (H2O2) by superoxide dismutases. Either H2O2 is detoxified into H2O by catalases, or it modifies thiol groups of target proteins (as in the formation of sulfenic/sulfinic acids and disulfide bonds), which can be detoxified (reduced back to thiols) by thioredoxin, glutaredoxin, or sulfiredoxin systems. In contrast, an alkyl hydroperoxide, such as tBOOH, is detoxified by thioredoxin- or glutathione-dependent thiol peroxidases. Diamide is an exogenous oxidant reacting with thiol groups of proteins and glutathione, which generates disulfide bonds or protein-glutathionyl bonds. It is known that cellular responses to diamide and H2O2 are quite different (see reference 44 for a review).
The ras1Δ mutant was not sensitive to oxidative stress exerted by H2O2 and a superoxide (O2−) generator, menadione, and indeed displayed slightly increased resistance to the oxidative damage agents compared to the WT (Fig. 4A). In contrast, the gpa1Δ, cac1Δ, pka1Δ, pka2Δ, and pka1Δ pka2Δ mutants showed almost WT levels of sensitivity to H2O2 and menadione (Fig. 4A). Notably, however, the ras1Δ mutant was found to be extremely sensitive to diamide and tBOOH, whereas the gpa1Δ, cac1Δ, pka1Δ, and pka1Δ pka2Δ mutants, but not the pka2Δ mutant, exhibited hyperresistance to diamide (Fig. 4A). In contrast to the ras1Δ mutant, the hog1Δ mutant was highly sensitive to H2O2 (10, 38) but resistant to diamide (data not shown). These data indicate that Ras1, cAMP, and Hog1 pathways independently control oxidative stress responses of C. neoformans.
Fig. 4.
The Ras1 signaling pathway is involved in oxidative and genotoxic stress responses in C. neoformans. Each C. neoformans strain indicated below was grown overnight at 30°C in liquid YPD medium, serially diluted 10-fold (yielding 1- to 104-fold dilutions), and spotted (in 3-μl volumes) onto YPD agar containing the indicated concentrations of hydrogen peroxide (H2O2), the superoxide generator menadione, diamide, and tBOOH (A), cadmium sulfate (B and C), and HU and MMS (D and E). Cells were incubated at 30°C for 72 h and photographed. (A, B, and D) The WT H99 strain (MATα) and the ras1Δ (YSB53), aca1Δ (YSB6), gpa1Δ (YSB83), cac1Δ (YSB42), pka1Δ (YSB188), pka2Δ (YSB194), and pka1Δ pka2Δ (YSB200) mutant strains were used. (C and E) The WT KN99 strain (MATa) and the ras1Δ (YSB73), cac1Δ (YSB183), aca1Δ (YSB176), hog1Δ (YSB81), aca1Δ ras1Δ (YSB175), and cac1Δ ras1Δ (YSB187) mutant stains were used.
In response to the heavy metal cadmium, the ras1Δ mutant showed hypersusceptibility (Fig. 4B), which is also opposite the phenotype of the hog1Δ mutant, which is hyperresistant to cadmium (38). Interestingly, the gpa1Δ, cac1Δ, pka1Δ, and pka1Δ pka2Δ mutants also showed greater cadmium sensitivity than the WT strain, to the same extents. The aca1Δ mutant was slightly more sensitive to cadmium treatment than the WT but less sensitive than the other cAMP mutants (Fig. 4B). This finding indicated that the cAMP pathway plays some role in the heavy-metal stress response. Additional mutation of the RAS1 gene did not increase the cadmium sensitivity of the cac1Δ mutant and, indeed, slightly restored cadmium resistance to the cac1Δ mutant (Fig. 4C), suggesting that Ras1 may share a signaling pathway and cross talk with the cAMP pathway to control resistance against the heavy metal cadmium.
Besides having a role as an oxidative stress agent, diamide is known to be a radiosensitizer, which can delay or impair DNA repair after DNA-damaging treatment (genotoxic stress), such as X-irradiation (13). Cadmium is also a genotoxic agent (15). Therefore, we further tested the role of Ras1 in genotoxic stress response by using other well-known DNA-damaging agents, such as HU and MMS. HU inhibits DNA replication by blocking ribonucleotide reductase, whereas MMS is a DNA-alkylating agent that can induce mutagenesis by causing mispairs that block DNA replication (39, 45). Both agents can damage chromosomal DNA by inducing DNA double-strand breaks. The ras1Δ mutant, but not any of the cAMP mutants, was highly susceptible to the two genotoxic agents (Fig. 4D). The aca1Δ ras1Δ double mutant was more susceptible to both MMS and HU than each single mutant (Fig. 4E), suggesting that Aca1 may play a minor role in the genotoxic stress response. Interestingly, the hog1Δ mutant exhibited increased resistance to HU but not to MMS (Fig. 4D). These data indicate that the Ras signaling pathway is uniquely involved in oxidative and genotoxic stress responses, which are independent of the HOG pathway.
Ras1-dependent stress control is mediated mainly by the Cdc24-dependent signaling pathway.
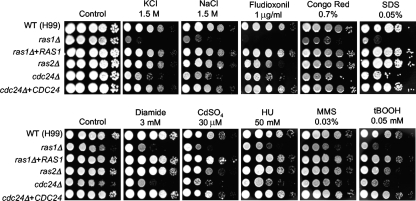
Based on the results described above, the cAMP and HOG signaling pathways did not appear to be involved in regulation of the stress-regulatory function of the Ras1 signaling pathway. Recently, Nichols et al. identified a GEF, Cdc24, which mediates thermotolerance downstream of Ras1 (47). Therefore, we examined whether the function of Ras1 in diverse stress responses is mediated by the Cdc24-dependent signaling pathway (Fig. 5). The ras1Δ mutant strain (CBN45) that was independently constructed by researchers in the Andrew Alspaugh laboratory exhibited the same stress-related phenotypes as the ras1Δ mutants that we constructed with either a MATα or a MATa background, and the ras1Δ RAS1 complemented strain exhibited WT stress phenotypes, which corroborated the role of Ras1 in stress response (Fig. 5). Here, we found that the cdc24Δ mutant exhibited stress response phenotypes that are almost identical to those of the ras1Δ mutants. The cdc24Δ mutant was as hypersensitive to diamide, cadmium, HU, MMS, and tBOOH as the ras1Δ mutant (Fig. 5), which suggested that the oxidative and genotoxic stress responses by Ras1 were mediated by Cdc24. During exposure to osmotic stress (1.5 M NaCl or KCl), fludioxonil, Congo red, or SDS, the cdc24Δ mutant exhibited greater susceptibility than the WT and the corresponding complemented strain but was more resistant than the ras1Δ mutant. These data indicated the function of Ras1 in stress response to be mediated mainly by Cdc24 but also controlled by an unknown regulator(s). Interestingly, although the ras2Δ mutant showed mostly WT levels of stress resistance, it exhibited slightly increased sensitivities to fludioxonil and tBOOH (Fig. 5), which indicated that Ras2 may play a minor role in stress response.
Fig. 5.
The Ras1-mediated stress response is controlled by the Cdc24-dependent signaling pathway in C. neoformans. C. neoformans strains (the WT H99 strain, the ras1Δ mutant [CBN45], the ras1Δ RAS1 complemented strain [CBN64], the ras2Δ mutant [MWC12], the cdc24Δ mutant [CBN32], and the cdc24Δ CDC24 complemented strain [CBN33]) were grown overnight at 30°C in liquid YPD medium, serially diluted 10-fold (yielding 1- to 104-fold dilutions), and spotted (in 3-μl volumes) onto YPD agar containing the indicated concentrations of either NaCl or KCl, fludioxonil, Congo red and SDS, H2O2, menadione, tBOOH, cadmium sulfate, HU, and MMS. Cells were incubated at 30°C for 72 h and photographed.
Identification of the Ras- or cAMP-dependent genes in C. neoformans.
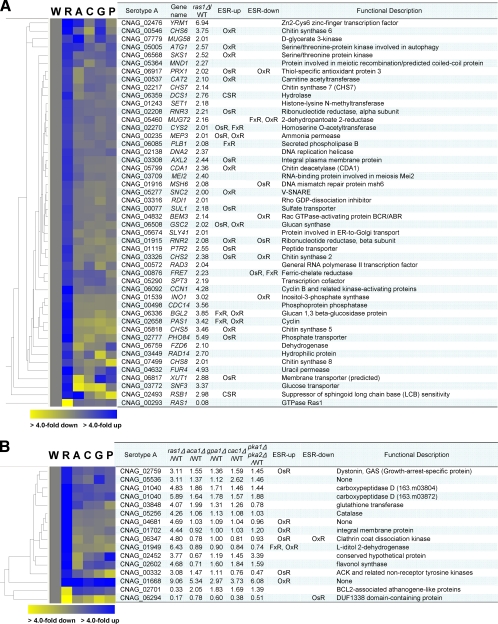
Next, we further investigated individual Ras1- and cAMP-dependent genes identified by our transcriptome analysis. Among the 161 selected Ras-dependent genes (those identified by using a cutoff of twofold up- or downregulation), a majority (101 genes [63%]) do not have any orthologs in other fungi (see Table S4 in the supplemental material), which indicated that C. neoformans contains a unique set of Ras-dependent genes. Among the evolutionarily conserved Ras-dependent genes, three genes, PXL1 (identification number 03907), RDI1 (03316), and BEM3 (04832), whose orthologs are known to be involved in the regulation of Rho-GTPase Cdc42 in S. cerevisiae, were notable since the Ras1-Cdc24 signaling pathway has been reported to be controlled by one of three Cdc42 homologs in C. neoformans (47). RDI1 and BEM3 encode a Rho-GDP dissociation inhibitor and a Rho-GTPase-activating protein, respectively (52, 80). Rdi1 plays a key role in controlling membrane localization and the normal function of Cdc42 and is required for full virulence of C. neoformans (52, 80). Notably, in agreement with the role of Ras1 in the genotoxic stress response of C. neoformans (Fig. 4), a number of genes involved in the regulation of DNA damage repair were identified as Ras-dependent genes. These include RNR2 and RNR3 (identification numbers 01915 and 02208; encoding ribonucleotide-diphosphate reductase), RAD3 (00572; encoding DNA helicase, a subunit of nucleotide excision repair factor 3), RAD14 (03449; encoding a subunit of nucleotide excision repair factor 1), MSH6 (01916; encoding a protein required for mismatch repair), MND1 (05364; encoding a protein required for recombination and repair of DNA double-strand breaks), and DNA2 (02138; encoding ATP-dependent nuclease). Finally, several genes, CHS2 (03326; encoding chitin synthase 2), CHS5 (05818), CHS6 (00546), CHS7 (02217), CHS8 (07499), CDA1 (05799; encoding chitin deacetylase 1), BGL2 (06336; encoding glucan 1,3-β-glucosidase), and GSC2 (06508; encoding glucan synthase), potentially involved in governing cell wall integrity were also identified as Ras-dependent genes (see Table S4 in the supplemental material), which further supported the role of Ras1 in maintaining the cell wall integrity of C. neoformans.
The statistical comparison of transcriptome data obtained from the cAMP mutants (the aca1Δ, gpa1Δ, cac1Δ, and pka1Δ pka2Δ strains) with that obtained from the WT strain (P < 0.05; ANOVA) identified 163 genes (Fig. 1C). Among these, 38 genes other than CAC1, ACA1, PKA1, and GPA1 exhibited more than twofold induction or reduction in the cAMP mutants (Fig. 6). A majority of the cAMP-dependent genes (31 genes [81%]) do not have any known function in C. neoformans or orthologs in S. cerevisiae, which indicated that, similar to the C. neoformans Ras-dependent genes, the cAMP-dependent genes in C. neoformans are a unique set. This observation further corroborates the finding that C. neoformans cAMP mutants have unique phenotypic characteristics that have not been observed in other fungi. Five cAMP-dependent genes (GRE2, ENA1, HSP12, CAT1, and PKP1) in C. neoformans appear to be evolutionarily conserved in other fungi. Interestingly, the GRE2, ENA1, and HSP12 genes in S. cerevisiae are known to be transcriptionally regulated by environmental stress. It has been reported recently that, in C. neoformans, Ena1 not only controls osmotic stress under carbon starvation conditions (38) but also is required for survival in alkaline pH and for in vivo virulence (35). GRE2 (gene de respuesta a estrés [stress-responsive gene]), a homolog of the mammalian 3-β-hydroxysteroid dehydrogenase gene, is strongly induced in response to a variety of stresses, including osmotic and oxidative stresses, upon binding of the HOG-dependent Sko1 transcription factor to CRE (cAMP response element) in the promoter region in S. cerevisiae (23, 55). The heat shock protein encoded by HSP12 (03143) is a small hydrophilic protein whose expression is also induced by diverse stresses and regulated by both HOG and cAMP signaling pathways (71).
Fig. 6.
Identification of cAMP signaling-dependent genes in C. neoformans. (A) Relative expression profiles of cAMP-dependent genes which show more than twofold induction or repression in the aca1Δ, gpa1Δ, cac1Δ, or pka1Δ pka2Δ mutant compared to the WT strain. The degree of change (n-fold) is illustrated by color coding (see the color bar scale at the bottom of the graph), and the exact value for each gene is indicated in the table to the right of the hierarchical clustering diagram. Sce, S. cerevisiae; Cne, C. neoformans; NMDA, N-methyl-d-aspartate. (B and C) C. neoformans strains (the WT H99 strain and the corresponding ras1Δ [YSB53], aca1Δ [YSB6], gpa1Δ [YSB83], cac1Δ [YSB42], pka1Δ [YSB188], pka2Δ [YSB194], and pka1Δ pka2Δ [YSB200] mutant strains [B] and the WT KN99 strain and the corresponding ras1Δ [YSB73], cac1Δ [YSB183], aca1Δ [YSB176], hog1Δ [YSB81], aca1Δ ras1Δ [YSB175], and cac1Δ ras1Δ [YSB187] mutant strains [C]) were grown overnight at 30°C in liquid YPD medium, serially diluted 10-fold (yielding 1- to 104-fold dilutions), and spotted (in 3-μl volumes) onto YPD agar containing the indicated concentrations of MG. Cells were incubated at 30°C for 72 h and photographed.
Another interesting observation was that TCO2 (05590) was downregulated in the aca1Δ, gpa1Δ, cac1Δ, and pka1Δ pka2Δ mutants but slightly upregulated in the ras1Δ mutant (Fig. 6A). Tco2 is a double-hybrid histidine kinase that is uniquely found in C. neoformans and is one of the major sensor kinases upstream of the Cryptococcus HOG pathway. As Tco2 is a mostly positive upstream regulator of the HOG pathway, it is involved in resistance to a toxic metabolite, MG. Indeed, our MG susceptibility assay showed that the cAMP mutants that have reduced levels of Tco2, but not the ras1Δ mutant, were hypersensitive to MG treatment (Fig. 6B and C).
Inhibition of the Ras and cAMP signaling pathways increased polyene sensitivity.
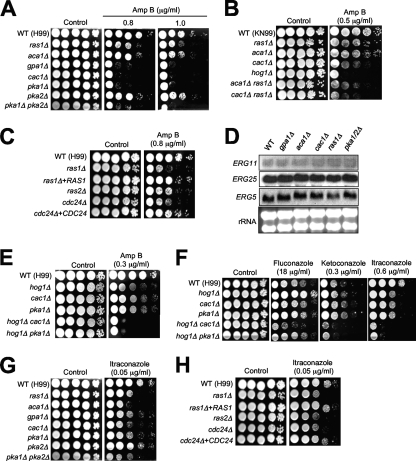
Gre2 is involved in the regulation of ergosterol biosynthesis genes, including ERG6, ERG10, and ERG19/MVD1 (74). Furthermore, GRE2 is reported to be one of six genes in S. cerevisiae whose expression increases with resistance to AmpB (5). Therefore, we examined whether the C. neoformans Ras and cAMP mutants are more susceptible to AmpB treatment than the WT (Fig. 7). The ras1Δ mutant showed greater susceptibility to AmpB than the WT, and the aca1Δ mutant exhibited slightly greater AmpB susceptibility than the WT (Fig. 7A). The ras1Δ aca1Δ double mutant exhibited greater AmpB sensitivity than each single mutant (Fig. 7B), indicating that Ras1 and Aca1 redundantly or independently control AmpB sensitivity. Cdc24 appears to work downstream of Ras1 for regulation of polyene drug resistance since the cdc24Δ mutant was as hypersensitive to AmpB as the ras1Δ mutant (Fig. 7C). Interestingly, the ras2Δ mutant was also slightly more sensitive to AmpB than the WT, indicating that both Ras proteins control the resistance of C. neoformans to polyene drugs.
Fig. 7.
Perturbation of the Ras and cAMP signaling pathways increases polyene drug sensitivity independently of ergosterol biosynthesis. (A, B, C, E, F, G, and H) Each C. neoformans strain indicated below was grown overnight at 30°C in liquid YPD medium, serially diluted 10-fold (yielding 1- to 104-fold dilutions), and spotted (in 3-μl volumes) onto YPD agar containing the indicated concentrations of AmpB, fluconazole, ketoconazole, or itraconazole. Cells were incubated at 30°C for 72 h and photographed. (D) Verification of transcriptional activation of ERG5, ERG25, and ERG11 in the Ras and cAMP pathway mutants by Northern blot analysis. (A, E, F, and G) The WT H99 strain and the ras1Δ (YSB53), aca1Δ (YSB6), gpa1Δ (YSB83), cac1Δ (YSB42), pka1Δ (YSB188), pka2Δ (YSB194), pka1Δ pka2Δ (YSB200), hog1Δ (YSB64), hog1Δ cac1Δ (YSB156), and hog1Δ pka1Δ (YSB112) mutant strains were used. (B) The WT KN99 strain (MATa) and the ras1Δ (YSB73), cac1Δ (YSB183), aca1Δ (YSB176), hog1Δ (YSB81), aca1Δ ras1Δ (YSB175), and cac1Δ ras1Δ (YSB187) mutant stains were used. (C and H) The WT H99 strain, the ras1Δ mutant (CBN45), the ras1Δ RAS1 complemented strain (CBN64), the ras2Δ mutant (MWC12), the cdc24Δ mutant (CBN32), and the cdc24Δ CDC24 complemented strain (CBN33) were used.
Notably, the gpa1Δ and cac1Δ mutants showed much greater AmpB sensitivity than the WT and even than the ras1Δ or aca1Δ mutant (Fig. 7A). The pka1Δ mutant, but not the pka2Δ mutant, showed increased susceptibility to AmpB (Fig. 7A). Pka1 is downstream of the Cac1 adenylyl cyclase, strongly indicating that the Gpa1-Cac1-Pka1 signaling cascade is one of the signaling circuits that control polyene drug sensitivity. The ras1Δ cac1Δ double mutant exhibited even greater AmpB susceptibility than either single mutant (Fig. 7B), indicating that the Ras and Gpa1-Cac1-Pka1 pathways are independently involved in AmpB susceptibility. Independently derived ras1Δ and ras1Δ cac1Δ mutants of the KN99 strain with the MATa background exhibited the same phenotypes (data not shown).
To address whether the involvement of the Ras and cAMP pathways in polyene sensitivity is related to the levels of ergosterol biosynthesis, we checked expression levels of ergosterol biosynthesis genes in the mutants by using our array data. Interestingly, none of the ergosterol biosynthesis genes, except ERG3 and ERG25 (which exhibited changes of less than twofold), exhibited significant expression changes in the ras1Δ, aca1Δ, gpa1Δ, cac1Δ, or pka1Δ pka2Δ mutant compared to the WT (see Tables S3 and S5 in the supplemental material). Northern blot analysis showed that expression levels of the ERG25 gene in the mutants were not significantly different from the level in the WT (Fig. 7D). The ERG3 transcript was rarely detectable by Northern blot analysis (data not shown). We also monitored ERG5 expression patterns, although they did not fulfill the statistical ANOVA criteria, because ERG5 appeared to be slightly induced (1.4- to ∼1.5-fold) in the cAMP mutants. Expression levels of ERG5 in the mutants were almost equivalent to the level in the WT, although the ras1Δ mutant appeared to have slightly lower ERG5 expression than the WT (Fig. 7D). We also assayed cellular ergosterol contents in the Ras and cAMP mutants and found that they were not significantly increased in these mutants compared to the content in the WT but that the hog1Δ mutant had increased ergosterol content (data not shown), as reported previously (38). Furthermore, expression levels of ERG11 in the ras1Δ and cAMP mutants were not significantly different from the level in the WT (Fig. 7D). In support of this finding, the gpa1Δ, cac1Δ, pka1Δ, pka2Δ, and pka1Δ pka2Δ mutants were nearly as resistant to fluconazole (which targets the fungal cytochrome P450 enzyme 14α-demethylase [Erg11] and inhibits the conversion of lanosterol to ergosterol) as the WT strain (data not shown). These data indicated that the Ras and cAMP signaling pathways independently influence polyene sensitivity without affecting ergosterol biosynthesis.
We recently reported that the HOG pathway controls ergosterol biosynthesis in C. neoformans under unstressed conditions and that the HOG pathway mutants are hypersensitive to AmpB but hyperresistant to fluconazole because of the increased cellular ergosterol contents of the mutants (38). Therefore, it is easily conceivable that the HOG and cAMP pathways influence polyene sensitivity in different manners. In support of this possibility, we found that the hog1Δ cac1Δ and hog1Δ pka1Δ double mutants were even more sensitive to AmpB than the hog1Δ, cac1Δ, or pka1Δ single mutant (Fig. 7E). Unexpectedly, the hog1Δ cac1Δ double mutant also exhibited hypersensitivity to various azole drugs, such as fluconazole, ketoconazole, and itraconazole (Fig. 7F). Interestingly, the ras1Δ, aca1Δ, gpa1Δ, cac1Δ, and pka1Δ mutants all showed increased sensitivity to itraconazole (Fig. 7G). Furthermore, the ras2Δ and cdc24Δ mutants were as hypersensitive to itraconazole as the ras1Δ mutant (Fig. 7H). Taken together, these data indicate that the HOG pathway and the cAMP signaling pathway independently control polyene and azole drug susceptibility.
Characterization of the cAMP-dependent genes in C. neoformans.
Among the cAMP-dependent genes identified by our array analysis, we decided to further characterize the roles of the genes that showed significant expression changes (>2.0-fold; P < 0.05 [ANOVA]) and are evolutionarily conserved in other fungi but whose functions in C. neoformans have not been studied. These included GRE2, HSP12, and PKP1 (Fig. 6A). Interestingly, C. neoformans contains another HSP12 homolog (01446), which exhibited expression patterns similar to those of HSP12 based upon our array data (see Table S3 in the supplemental material). Therefore, we named this gene HSP122. First, we confirmed the gene expression patterns by Northern blot analysis to verify DNA microarray data (Fig. 8A). Our array showed that the expression of both HSP12 and HSP122 was highly repressed (up to eightfold) in the cac1Δ, gpa1Δ, and pka1Δ pka2Δ mutants but that it was slightly repressed (up to twofold) in the aca1Δ and ras1Δ mutants. Northern blot data also confirmed the array data (Fig. 8A). Notably, both HSP12 and HSP122 were highly expressed in the WT C. neoformans strain even under unstressed, glucose-rich conditions (Fig. 8A). In S. cerevisiae, HSP12 is not expressed under unstressed, glucose-rich conditions but is induced in response to environmental stresses (51, 66). Based on microarray data, GRE2 expression was greatly reduced (3- to 6-fold) in the cAMP mutants whereas it was minimally induced (1.5-fold) in the ras1Δ mutant. Northern blot analysis confirmed the expression patterns of GRE2 (Fig. 8A). In contrast to the expression of HSP12, HSP122, and GRE2, PKP1 expression was slightly induced (1.5- to 2-fold) in both the ras1Δ and cAMP mutants based on microarray data. Northern blotting results demonstrated that PKP1 expression in the gpa1Δ, aca1Δ, and pka1Δ pka2Δ mutants was slightly induced compared to that in the WT, which was in agreement with the array data (Fig. 8A). Interestingly, however, PKP1 induction in the cac1Δ mutant did not appear to be obvious, indicating that Gpa1 or Aca1 may regulate Pka1 to repress PKP1 by bypassing Cac1. Taken together, these findings indicate that HSP12 and GRE2 are positively regulated but that PKP1 is negative regulated by the cAMP signaling pathway.
We constructed gre2Δ, pkp1Δ, hsp12Δ, and hsp122Δ mutants as described in Materials and Methods and analyzed their phenotypes to identify the functions of the corresponding genes in connection with the Ras and cAMP pathways. To address whether Hsp12 and Hsp122 play redundant roles, the hsp12Δ hsp122Δ double mutant was also constructed. For each gene, at least two independent mutants with the H99 strain background were constructed and characterized. First, we examined the abilities of the mutants to produce two virulence factors, capsule and melanin, known to be controlled by the cAMP pathway in C. neoformans. None of the mutants exhibited any significant changes in capsule or melanin production levels compared to the WT strain (see Fig. S6 in the supplemental material).
Next, we addressed the roles of the cAMP-dependent genes in diverse stress responses and the antifungal drug susceptibility of C. neoformans, since in this study we discovered the involvement of the cAMP pathway in these features (see Fig. S7 to S9 in the supplemental material). Similar to the cAMP mutants, all of the hsp12Δ, hsp122Δ, gre2Δ, and pkp1Δ mutants showed WT levels of resistance to osmotic shock (1.5 M NaCl or KCl), cell wall/membrane stress (Congo red or SDS), oxidative stress (H2O2, diamide, or menadione), genotoxic stress (HU or MMS), and fludioxonil. Furthermore, all of the gre2Δ, hsp12Δ, hsp122Δ, and pkp1Δ mutants exhibited WT levels of sensitivity to MG and azole drugs. In response to cadmium, the gre2Δ, pkp1Δ, hsp12Δ, and hsp122Δ mutants exhibited slightly greater sensitivity than the WT strain (see Fig. S8 and S9 in the supplemental material). Most notably, the hsp12Δ and hsp122Δ mutants exhibited greater susceptibility to AmpB than the WT (Fig. 8B). The hypersensitivity of the hsp12Δ and hsp122Δ mutants to AmpB appeared to be even more obvious under minor osmotic conditions (in the presence of 0.5 M NaCl) (Fig. 8B). The hsp12Δ hsp122Δ double mutant showed greater AmpB sensitivity than each of the hsp12Δ and hsp122Δ single mutants and even than the cac1Δ mutant (Fig. 8B). These data indicate that Hsp12 and Hsp122 play redundant roles in defending against AmpB and that decreased expression of HSP12 and HSP122 may contribute to hypersensitivity of the cAMP pathway mutants to the polyene drug. In contrast, the gre2Δ and pkp1Δ mutants exhibited WT levels of AmpB sensitivity under normal or osmotic conditions (see Fig. S7 in the supplemental material).
To further characterize the regulatory mechanism of Hsp12 and Hsp122, we performed Northern blot analysis to address whether the HOG pathway modulates expression of the HSP12 and HSP122 genes. Interestingly, our previous study showed that both HSP12 and HSP12 may also be controlled by the HOG pathway because their expression levels are considerably low in the hog1Δ and ssk1Δ mutants but not in the skn7Δ mutant based on our previous microarray data (38). To confirm this, we performed Northern blot analysis and found that expression levels of both HSP12 and HSP122 were very high in the WT and the skn7Δ mutant but were undetectable in the hog1Δ and ssk1Δ mutants (Fig. 8E). In S. cerevisiae, GRE2 is positively regulated by the HOG pathway (55). C. neoformans GRE2 was also found to be a HOG-dependent gene. Basal expression levels of GRE2 were slightly reduced in the hog1Δ and ssk1Δ mutants but not in the skn7Δ mutant (Fig. 8E). All these data strongly indicate that HSP12, HSP122, and GRE2 are coregulated by the cAMP and HOG signaling pathways.
DISCUSSION
In this study, we performed a transcriptome analysis of the C. neoformans ras1Δ, gpa1Δ, aca1Δ, cac1Δ, and pka1Δ pka2Δ mutants compared to the WT strain to elucidate the functional connections among the upstream regulators of the cAMP signaling pathway, including Ras, Aca1 cyclase-associated protein, and the Gpa1 Gα subunit, and identify their downstream target genes. The pathway is crucial for modulating the growth, differentiation, morphology, and virulence of C. neoformans. In general, the ras1Δ mutant exhibited transcriptome patterns distinct from those of the other cAMP mutants as well as the WT strain, whereas the aca1Δ, gpa1Δ, cac1Δ, and pka1Δ pka2Δ mutants showed mostly similar transcriptome patterns. These results strongly indicate that the Ras1 signaling pathway is mainly independent of the Aca1- or Gpa1-dependent cAMP/PKA signaling pathways. Genes regulated by the Ras signaling pathway include those involved in signal transduction and cell wall/membrane/envelope biogenesis, whereas genes regulated by the cAMP signaling pathway include those involved in signal transduction mechanisms, carbohydrate transport and metabolism, and amino acid transport and metabolism. Furthermore, we have found several important features of the Ras and cAMP signaling pathways that have not been reported previously. First, a number of C. neoformans ESR and CSR genes (38) were modulated by the Ras and cAMP signaling pathways. Here, we provided experimental evidence that the Ras and cAMP signaling pathways were indeed involved in controlling diverse stress responses. Second, the Ras and cAMP signaling pathways were involved in resistance and susceptibility to polyene and azole drugs, which provides an opportunity to develop a novel antifungal combination therapy. The proposed model for the roles of the Ras and cAMP signaling pathways in stress response is summarized in Fig. 9.
Fig. 9.
Proposed model for the regulatory mechanisms of the Ras and cAMP signaling pathways in stress response and antifungal drug susceptibility in C. neoformans. The Ras1-dependent signaling pathway promotes cell survival under diverse environmental stresses, such as cell wall destabilization, osmotic shock, oxidative and genotoxic stresses, and high temperature, in a manner mediated by Cdc24 and Cdc42. Ras1 appears to affect resistance to antifungal drugs by maintaining cell wall and membrane integrity. Along with Ras1, Ras2 plays some minor role in controlling antifungal and oxidative stress sensitivity. The cAMP signaling pathway is involved in responses to stress from heavy metals and toxic metabolites. Notably, small heat shock proteins, Hsp12 and Hsp122, are positively regulated by the cAMP signaling pathway and appears to affect resistance against AmpB treatment. However, expression of HSP12 and HSP122 is much more significantly regulated by the HOG pathway.
Roles of the Ras1 and cAMP signaling pathways in stress responses of C. neoformans.
This study gives the first report of the involvement of the Ras1 signaling pathway in the osmotic stress response and the oxidative/genotoxic stress response of C. neoformans. The data presented in this study suggested that the role of Ras1 in stress response was mainly independent from the HOG and PKC/Mpk1 pathways, which are central stress-activated signaling pathways in C. neoformans (7, 36, 38). Previously, we have shown that the HOG pathway plays an important role in controlling the osmotic stress response by modulating the expression of the ENA1 ATPase Na+/K+ efflux pump, which is critical for long-term adaptation to hyperosmotic shock (38). However, the regulatory mechanism of Ras1 for osmotic stress response appeared to be distinct from that of the HOG pathway for two reasons. First, the ras1Δ mutant showed more dramatic osmosensitivity than the HOG mutants, exhibiting sensitivity even under glucose-rich conditions whereas the HOG and ena1Δ mutants exhibited significant osmosensitivity only under glucose starvation conditions (38). Second, basal expression levels of ENA1 were not reduced but indeed slightly increased in the ras1Δ mutant. Therefore, it is conceivable that osmosensitivity of the ras1Δ mutant may result from other indirect reasons, such as a cell wall integrity defect. Maintaining cell wall integrity is likely to be important for cells to survive under high osmotic pressure. This hypothesis was further confirmed by the findings that the ras1Δ mutant was hypersensitive not only to fludioxonil, which is extremely lethal to cells having cell wall integrity defects, such as those with the mpk1Δ mutation in the PKC pathway and the cna1Δ mutation in the calmodulin/calcineurin pathway (8, 40), but also to well-known cell wall integrity destabilizers, such as Congo red and SDS. In addition, genes required for maintaining cell wall integrity, CHS2, CHS5, CHS6, CHS7, CHS8, CDA1, BGL2, and GSC2, were found to be Ras-dependent genes. CHS1 (03099), CDA2 (01230), and CSR2 (07636; encoding chitin synthase regulator 2) were also differentially regulated in the ras1Δ mutant, although the differences in expression were less than twofold (see Table S4 in the supplemental material). C. neoformans encodes eight chitin synthases (Chs1 to Chs8) and three chitin synthase regulators (Csr1 to Csr3). Among these, Chs3 and Csr2 are required mainly for maintaining cell wall integrity and chitosan production (14). However, the chs5Δ, chs6Δ, chs7Δ, and chs8Δ mutants show increased thermosensitivity at 40°C, albeit to a lesser extent than the chs3Δ mutant, indicating that the corresponding chitin synthases may play a role in maintaining cell wall integrity at high temperature (14). Therefore, it is possible that some chitin synthases and regulators play redundant roles in chitin production and the maintenance of cell wall integrity. Deletions of combinations of these genes would answer this question. C. neoformans encodes three functional chitin deacetylases (Cda1 to Cda3) that play redundant roles in converting chitin to chitosan and maintaining the cell wall integrity of C. neoformans (12). Similarly, Ras1 controls oxidative and genotoxic stress responses in a HOG-independent manner, since the ras1Δ mutant exhibited oxidative and genotoxic stress response patterns clearly distinct from those of the hog1Δ mutant.
Similar to the Ras signaling pathway, the PKC pathway controls thermotolerance, cell wall integrity, and osmotic and oxidative stress responses. However, for the following reasons, the two pathways appear to work independently to control stress response. First, the pkc1Δ mutant exhibited more severe defects in maintaining cell wall integrity than the ras1Δ mutant (24) (Fig. 3E). Second, the pkc1Δ mutant was hypersensitive to both H2O2 and diamide whereas the ras1Δ mutant was hypersensitive only to diamide (Fig. 4). Nevertheless, the possibility that the Ras and PKC signaling pathways share cross talk needs further study in the future.
We suggested that the Cdc24-Cdc42 signaling pathway may work downstream of Ras1 to control diverse stress responses. Previously, it has been shown that the thermosensitivity of the C. neoformans ras1Δ mutant results from alterations in actin polarization and that, therefore, the ras1Δ mutant arrests as unbudded cells (1, 75). Recently, it has been reported that a GEF protein, Cdc24, mediates the thermotolerance governed by Ras1 in C. neoformans by directly interacting with Ras1 and that the overexpression of CDC24 restores thermotolerance to the ras1Δ mutant, indicating that Cdc24 works downstream of Ras1 in C. neoformans (47). Nichols and coworkers also demonstrated that the overexpression of a Rho-like GTPase, Cdc42, restores thermotolerance to the cdc24Δ and ras1Δ mutants, indicating that Cdc42 works downstream of Cdc24 (47). Similarly, the S. cerevisiae ras2Δ mutant exhibits temperature-sensitive growth defects accompanied by delocalized Cdc42 and a depolarized actin cytoskeleton (31). In S. cerevisiae, however, the overexpression of Cdc42 is unable to suppress the thermosensitivity of the ras2Δ mutant (31), further indicating that the C. neoformans Ras1 signaling pathway operates differently from the S. cerevisiae Ras1 signaling pathway. It is highly likely that aberrant actin cytoskeleton regulation may cause impaired cell wall integrity. The finding of genes involved in Cdc42 regulation to be Ras-dependent genes, such as PXL1, RDI1, and BEM3, by our transcriptome analysis further suggests that Ras1 regulates stress responses in a Cdc24-Cdc42-dependent manner.
The cAMP pathway was not as significantly involved in the general stress response of C. neoformans as the Ras1 or HOG pathways but played some roles in responding to a heavy metal (cadmium) and a toxic metabolite (MG). The role of Pka2 in the osmotic stress response under glucose starvation conditions is rather unexpected. Thus far, Pka2 has been considered to be a minor PKA downstream of the cAMP pathway in the serotype A H99 strain background, although it plays a major role in the serotype D C. neoformans JEC21 background (9, 30). Notably, deletion of PKA1 restored WT osmosensitivity to the pka2Δ mutant, indicating that Pka1 and Pka2 may have opposing roles under certain environmental conditions. The functional relationships between Pka1 and Pka2 need to be further elucidated in a future study.
Role of Aca1 in the stress response of C. neoformans.
We demonstrated that the aca1Δ mutant exhibited transcriptome patterns similar to those of the cac1Δ mutant, which supported the previous finding that Cac1 is the major downstream effector of Aca1 (9). However, a minor group of genes was found to be specifically Aca1 regulated, indicating that Aca1 signaling may bifurcate into the Cac1/Pka1 pathway and another unknown signaling pathway. The unknown signaling pathway appeared to be implicated in the maintenance of cell wall integrity, potentially through actin cytoskeleton regulation. The aca1Δ mutant exhibited hypersensitivity to osmotic shock, fludioxonil, and SDS, and such phenotypes are generally found in mutants defective in cell wall integrity. In S. cerevisiae, CAP (an Aca1 ortholog, also known as Srv2) plays an important role in connecting Ras signaling to actin cytoskeleton regulation since it has both Ras/adenylyl cyclase-binding sites at the N terminus and F-actin/G-actin-binding sites between the middle region and the C terminus (33). Therefore, the S. cerevisiae ras2Δ mutant is rescued from thermosensitivity by the overexpression of CAP (22, 25). Recently, Gourlay and Ayscough provided further evidence for a functional link between Ras and CAP in S. cerevisiae (26). Actin stabilization triggers aggregation of the adenylyl cyclase complex, including GTP-bound active Ras and CAP, with F-actin, which hyperactivates the cAMP signaling pathway so as to elevate ROS levels and results in apoptosis (26). All of these data indicate that Ras and CAP have a positive relationship in regulating the actin cytoskeleton and activating the cAMP signaling pathway in the model yeast. In C. neoformans, however, the Aca1-specific pathway that mediates stress response appears to be mainly independent from Ras1 and cAMP pathways for the following reasons. First, none of the stress phenotypes observed in the aca1Δ mutant were observed in the other cAMP mutants, such as the gpa1Δ, cac1Δ, and pka1Δ mutants. Second, the aca1Δ ras1Δ double mutant always exhibited more severe stress phenotypes than each single mutant, which implies that Aca1 and Ras1 control cell wall integrity independently. However, it remains possible that Aca1 and Ras1 may functionally interact to maintain the cell wall integrity of C. neoformans. To obtain further insight into this matter, characterization of Aca1-specific target genes, such as those with identification numbers 04442 and 06817 (UAP1, encoding purine permease) and 06213, 02463, and 02584 (see Table S5 in the supplemental material), is necessary in future studies.
Identification of Ras- and cAMP-dependent genes in C. neoformans.
Among the cAMP-dependent genes identified by our array analysis, we functionally characterized GRE2, PKP1, HSP12, and HSP122. We found that Gre2, Pkp1, Hsp12, and Hsp122 are weakly involved in resistance to heavy metal. Notably, the hsp12Δ and hsp122Δ mutants showed increased polyene sensitivity, which is one of the cAMP-dependent phenotypes that we discovered in this study. Hsp12 and Hsp122 are orthologous to the small heat shock protein Hsp12 in S. cerevisiae and are predicted to comprise 83 and 70 amino acids, respectively. S. cerevisiae has two small heat shock proteins, Hsp26 and Hsp12, the former of which was not found in C. neoformans. Interestingly, our study indicated that the regulatory mechanism of the small heat shock proteins in C. neoformans was distinct from that in S. cerevisiae. In S. cerevisiae, HSP12 expression is undetectable under unstressed, glucose-rich conditions but is highly induced in response to a wide range of environmental stresses, such as heat or cold shock, osmotic shock, desiccation, and exposure to alcohol or oxidative damage agents (51, 59, 66, 71, 72). Interestingly, even a very low concentration of glucose (0.005%) can repress HSP12 expression (18). In C. neoformans, however, HSP12 and HSP122 were highly expressed even under unstressed conditions with abundant glucose (2%). Furthermore, S. cerevisiae HSP12 is regulated negatively by the cAMP signaling pathway but positively by the HOG pathway (51, 71). Basal and induced levels of HSP12 expression in S. cerevisiae are increased by inhibition of the cAMP signaling pathway, which is in stark contrast to those in C. neoformans, in which basal levels of HSP12 and HSP122 expression were significantly reduced by mutations affecting cAMP signaling. The common finding for S. cerevisiae and C. neoformans HSP12 genes is that both genes are regulated positively by the HOG pathway. In fact, the finding that the expression of HSP12 and HSP122 in C. neoformans was nearly shut down in the HOG pathway mutants strongly indicates that the HOG pathway plays a major role in regulating the expression of HSP12 in the pathogen.
Although Gre2 and Pkp1 appeared to be dispensable for the regulation of a number of cAMP signaling-dependent phenotypes based on analyses of corresponding deletion mutants, it is still possible that they may play some role in the cAMP pathway. A similar case is that of the cAMP-regulated gene SMG1, which encodes a multicopy suppressor of gpa1 mutant phenotypes (such as melanin deficiency) and is one of the genes whose expression is repressed in the gpa1Δ mutant (20, 54). Unexpectedly, however, the smg1Δ mutant exhibits WT levels of melanin (49), indicating that the role of Smg1 may overlap with those of other signaling components in the regulation of the cAMP signaling pathway. Therefore, whether overexpression of GRE2 or PKP1 may suppress some of the phenotypes of the cAMP pathway mutants needs to be addressed in future studies.
Roles of the Ras and cAMP pathways in antifungal drug susceptibility of C. neoformans and novel combination antifungal therapeutic methods.
One of the key findings of this study was that the Ras and cAMP signaling pathways control the polyene drug susceptibility of C. neoformans. Both Ras1 and Ras2 appear to be involved in polyene susceptibility by using Cdc24 as a downstream effector. Interestingly, the ras1Δ aca1Δ mutant was also hypersensitive to AmpB, indicating that Ras1 and Aca1 may play minor roles in susceptibility to polyene drugs. It may be possible that the perturbation of actin cytoskeleton regulation and cell wall integrity by ras1 and aca1 mutations renders the cell more susceptible to polyene drugs.
The cAMP signaling pathway was even more significantly involved in polyene sensitivity than the Ras signaling pathway. Mutation of the GPA1, CAC1, and PKA1 genes rendered C. neoformans cells hypersensitive to polyene drugs, such as AmpB. We recently reported that perturbation of the HOG pathway also renders C. neoformans cells hypersensitive to AmpB (38). However, the cAMP and HOG pathways appear to work differently for modulation of polyene drug susceptibility. Inhibition of the HOG pathway, but not the cAMP pathway, increases ergosterol biosynthesis, which enhances polyene drug susceptibility and azole drug resistance (38). Furthermore, the hog1Δ mutant exhibited greater sensitivity to AmpB than the cAMP mutants.
Hypersensitivity of the cAMP mutants to the polyene drug appeared to be partly contributed by decreased expression of the heat shock proteins Hsp12 and Hsp122. In S. cerevisiae, Hsp12 plays a role in stabilizing the plasma membrane as a cell wall plasticizer and water replacement molecule (59, 62) and therefore is involved in maintaining the cell wall integrity of S. cerevisiae under stressful conditions (61). The S. cerevisiae hsp12Δ mutant is unable to grow in the presence of a cell wall destabilizer, Congo red (46). Perturbation of the cAMP signaling pathway reduces basal levels of Hsp12 and Hsp122 expression, which subsequently weakens cell wall integrity and membrane plasticity of C. neoformans. Similarly, repressed expression of HSP12 and HSP122 may be partly responsible for the hypersensitivity of the HOG pathway mutants to polyene drugs. However, it is still possible that other factors, except ergosterol biosynthesis, may affect resistance of the cAMP mutants to polyene drugs. In support of this, the hog1Δ cac1Δ and hog1Δ pka1Δ double mutants exhibited even greater polyene drug sensitivity than each single mutant, which indicates that the HOG and cAMP pathways play independent roles in polyene drug susceptibility. Notably, the double mutation of the HOG1 and CAC1 genes renders C. neoformans cells hypersensitive to azole drugs, including fluconazole, ketoconazole, and itraconazole, for unknown reasons. In any case, modulation of each of the Ras, cAMP/PKA, and HOG signaling pathways (or a combination of them) may provide a novel antifungal therapeutic approach in combination with polyene and azole drugs. Simultaneous inhibition of the cAMP and HOG pathways and treatment with AmpB may be one of the most powerful combination therapies for the treatment of cryptococcosis.
Comparison of our transcriptome analysis with others.
In addition to this study, two transcriptome analyses of the cAMP signaling pathway have been independently performed by others (32, 54). Pukkila-Worley and colleagues performed a comparative transcriptome analysis by using the gpa1Δ mutant and discovered a number of Gpa1-dependent genes, including SMG1 and LAC2 (encoding laccase 2) (54). Hu and colleagues used serial analysis of gene expression (SAGE) to examine the transcriptomes of the pka1Δ and pkr1Δ mutants and discovered a number of Pka1- or Pkr1-dependent genes, including OVA1 (encoding an OV-16 antigen precursor) (32). Similar to us, Hu et al. found that the cAMP signaling pathway modulates a number of stress response genes, including HSP122, in C. neoformans. However, we found that the lists of cAMP-dependent genes identified by others overlapped only minimally with that identified in this study. The discrepancy among the findings of this and other studies may result from the following factors. First, the mutant strain sets for the comparative transcriptome analyses were different. We used a greater number of cAMP signaling mutants (gpa1Δ, aca1Δ, cac1Δ, pka1Δ pka2Δ, and ras1Δ mutants) than others. Therefore, the number and kind of genes that fulfilled the statistical analysis criteria may be different from those in other studies. Second, the RNA isolation conditions were different. Pukkila-Worley et al. isolated RNAs from cells that were grown in minimal yeast nitrogen base medium with or without glucose and incubated for 1 h. Hu et al. used low-iron medium, which is a capsule-inducing medium, for total RNA isolation for SAGE. In contrast, we isolated RNAs from strains that were synchronized by being grown to the logarithmic phase in YPD medium. Second, the transcriptome analysis methodologies were different. Pukkila-Worley et al. used microarray chips spotted with 111 known C. neoformans cDNAs or 6,144 PCR products generated from the H99 strain, and Hu et al. used SAGE. In contrast, we used serotype D 70-mer oligonucleotide microarray slides. Therefore, some of the cAMP-dependent genes identified by others, including SMG1 and OVA1, were not identified by our study. At the same time, however, some of the cAMP-dependent genes, such as GRE2, PKP1, and HSP12, which were identified by our array and checked by Northern blot analysis were not discovered by others. These factors indicate that both the previous studies and the present study are complementary to one another in identifying the cAMP signaling-dependent genes.
In conclusion, our comparative transcriptome analysis revealed novel roles for the Ras and cAMP signaling pathways in C. neoformans in both stress response and antifungal drug susceptibility. This study provides a unique opportunity to develop novel antifungal therapeutic methods by modulating signal transduction cascades.
Supplementary Material
ACKNOWLEDGMENTS
We thank Andrew Alspaugh for kindly providing C. neoformans ras1Δ, ras2Δ, and cdc24Δ mutant strains.
This work was supported by National Research Foundation of Korea (NRF) grants (no. 2009-0058681 and no. 2009-0063344) funded by the Korean government (MEST). This work was also supported in part by grants RO1 AI50438 and R21 AI70230 from the NIH/NIAID (to J.H.).
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/cgi/content/full/9/3/360/DC1.
Published ahead of print on 22 January 2010.
REFERENCES
- 1.Alspaugh J. A., Cavallo L. M., Perfect J. R., Heitman J. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352–365 [DOI] [PubMed] [Google Scholar]
- 2.Alspaugh J. A., Perfect J. R., Heitman J. 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 11:3206–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alspaugh J. A., Perfect J. R., Heitman J. 1998. Signal transduction pathways regulating differentiation and pathogenicity of Cryptococcus neoformans. Fungal Genet. Biol. 25:1–14 [DOI] [PubMed] [Google Scholar]
- 4.Alspaugh J. A., Pukkila-Worley R., Harashima T., Cavallo L. M., Funnell D., Cox G. M., Perfect J. R., Kronstad J. W., Heitman J. 2002. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J. B., Sirjusingh C., Syed N., Lafayette S. 2009. Gene expression and evolution of antifungal drug resistance. Antimicrob. Agents Chemother. 53:1931–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arthington-Skaggs B. A., Jradi H., Desai T., Morrison C. J. 1999. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 37:3332–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahn Y. S. 2008. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot. Cell 7:2017–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahn Y. S., Geunes-Boyer S., Heitman J. 2007. Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress-activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryot. Cell 6:2278–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahn Y. S., Hicks J. K., Giles S. S., Cox G. M., Heitman J. 2004. Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade. Eukaryot. Cell 3:1476–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahn Y. S., Kojima K., Cox G. M., Heitman J. 2005. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell 16:2285–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahn Y. S., Xue C., Idnurm A., Rutherford J. C., Heitman J., Cardenas M. E. 2007. Sensing the environment: lessons from fungi. Nat. Rev. Microbiol. 5:57–69 [DOI] [PubMed] [Google Scholar]
- 12.Baker L. G., Specht C. A., Donlin M. J., Lodge J. K. 2007. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 6:855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker M. A., Hagner B. A. 1990. Diamide induced shift in protein and glutathione thiol: disulfide status delays DNA rejoining after X-irradiation of human cancer cells. Biochim. Biophys. Acta 1037:39–47 [DOI] [PubMed] [Google Scholar]
- 14.Banks I. R., Specht C. A., Donlin M. J., Gerik K. J., Levitz S. M., Lodge J. K. 2005. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 4:1902–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertin G., Averbeck D. 2006. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88:1549–1559 [DOI] [PubMed] [Google Scholar]
- 16.Buck J., Sinclair M. L., Schapal L., Cann M. J., Levin L. R. 1999. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. U. S. A. 96:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson R. C., Blankenship J. R., Kraus P. R., de Jesus Berrios M., Hull C. M., D'Souza C., Wang P., Heitman J. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607–2615 [DOI] [PubMed] [Google Scholar]
- 18.de Groot E., Bebelman J. P., Mager W. H., Planta R. J. 2000. Very low amounts of glucose cause repression of the stress-responsive gene HSP12 in Saccharomyces cerevisiae. Microbiology 146Pt. 2:367–375 [DOI] [PubMed] [Google Scholar]
- 19.D'Souza C. A., Alspaugh J. A., Yue C., Harashima T., Cox G. M., Perfect J. R., Heitman J. 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21:3179–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan W., Kraus P. R., Boily M. J., Heitman J. 2005. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot. Cell 4:1420–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedor-Chaiken M., Deschenes R. J., Broach J. R. 1990. SRV2, a gene required for RAS activation of adenylate cyclase in yeast. Cell 61:329–340 [DOI] [PubMed] [Google Scholar]
- 22.Field J., Vojtek A., Ballester R., Bolger G., Colicelli J., Ferguson K., Gerst J., Kataoka T., Michaeli T., Powers S., et al. 1990. Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell 61:319–327 [DOI] [PubMed] [Google Scholar]
- 23.Garay-Arroyo A., Covarrubias A. A. 1999. Three genes whose expression is induced by stress in Saccharomyces cerevisiae. Yeast 15:879–892 [DOI] [PubMed] [Google Scholar]
- 24.Gerik K. J., Bhimireddy S. R., Ryerse J. S., Specht C. A., Lodge J. K. 2008. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot. Cell 7:1685–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerst J. E., Ferguson K., Vojtek A., Wigler M., Field J. 1991. CAP is a bifunctional component of the Saccharomyces cerevisiae adenylyl cyclase complex. Mol. Cell. Biol. 11:1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gourlay C. W., Ayscough K. R. 2006. Actin-induced hyperactivation of the Ras signaling pathway leads to apoptosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 26:6487–6501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granger D. L., Perfect J. R., Durack D. T. 1985. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J. Clin. Invest. 76:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harashima T., Heitman J. 2002. The Gα protein Gpa2 controls yeast differentiation by interacting with kelch repeat proteins that mimic Gβ subunits. Mol. Cell 10:163–173 [DOI] [PubMed] [Google Scholar]
- 29.Hicks J. K., Bahn Y. S., Heitman J. 2005. Pde1 phosphodiesterase modulates cyclic AMP levels through a protein kinase A-mediated negative feedback loop in Cryptococcus neoformans. Eukaryot. Cell 4:1971–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks J. K., D'Souza C. A., Cox G. M., Heitman J. 2004. Cyclic AMP-dependent protein kinase catalytic subunits have divergent roles in virulence factor production in two varieties of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 3:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho J., Bretscher A. 2001. Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol. Biol. Cell 12:1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu G., Steen B. R., Lian T., Sham A. P., Tam N., Tangen K. L., Kronstad J. W. 2007. Transcriptional regulation by protein kinase A in Cryptococcus neoformans. PLoS Pathog. 3:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubberstey A. V., Mottillo E. P. 2002. Cyclase-associated proteins: CAPacity for linking signal transduction and actin polymerization. FASEB J. 16:487–499 [DOI] [PubMed] [Google Scholar]
- 34.Idnurm A., Bahn Y. S., Nielsen K., Lin X., Fraser J. A., Heitman J. 2005. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 3:753–764 [DOI] [PubMed] [Google Scholar]
- 35.Idnurm A., Walton F. J., Floyd A., Reedy J. L., Heitman J. 2009. Identification of ENA1 as a virulence gene of the human pathogenic fungus Cryptococcus neoformans through signature-tagged insertional mutagenesis. Eukaryot. Cell 8:315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung K. W., Bahn Y. S. 2009. The stress-activated signaling (SAS) pathways of a human fungal pathogen, Cryptococcus neoformans. Mycobiology 37:161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M. S., Kim S. Y., Yun J. K., Lee Y. W., Bahn Y. S. 2009. An efficient gene disruption method in Cryptococcus neoformans by double-joint PCR with NAT-split markers. Biochem. Biophys. Res. Commun. 390:983–988 [DOI] [PubMed] [Google Scholar]
- 38.Ko Y. J., Yu Y. M., Kim G. B., Lee G. W., Maeng P. J., Kim S. S., Floyd A., Heitman J., Bahn Y. S. 2009. Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot. Cell 8:1197–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koc A., Wheeler L. J., Mathews C. K., Merrill G. F. 2004. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J. Biol. Chem. 279:223–230 [DOI] [PubMed] [Google Scholar]
- 40.Kojima K., Bahn Y. S., Heitman J. 2006. Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology 152:591–604 [DOI] [PubMed] [Google Scholar]
- 41.Langfelder K., Streibel M., Jahn B., Haase G., Brakhage A. A. 2003. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38:143–158 [DOI] [PubMed] [Google Scholar]
- 42.Lengeler K. B., Davidson R. C., D'Souza C., Harashima T., Shen W. C., Wang P., Pan X., Waugh M., Heitman J. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin X., Heitman J. 2006. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 60:69–105 [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Mirabal H. R., Winther J. R. 2008. Redox characteristics of the eukaryotic cytosol. Biochim. Biophys. Acta 1783:629–640 [DOI] [PubMed] [Google Scholar]
- 45.Lundin C., North M., Erixon K., Walters K., Jenssen D., Goldman A. S., Helleday T. 2005. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 33:3799–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motshwene P., Karreman R., Kgari G., Brandt W., Lindsey G. 2004. LEA (late embryonic abundant)-like protein Hsp12 (heat-shock protein 12) is present in the cell wall and enhances the barotolerance of the yeast Saccharomyces cerevisiae. Biochem. J. 377:769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichols C. B., Perfect Z. H., Alspaugh J. A. 2007. A Ras1-Cdc24 signal transduction pathway mediates thermotolerance in the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 63:1118–1130 [DOI] [PubMed] [Google Scholar]
- 48.Niranjan T., Guo X., Victor J., Lu A., Hirsch J. P. 2007. Kelch repeat protein interacts with the yeast Gα subunit Gpa2p at a site that couples receptor binding to guanine nucleotide exchange. J. Biol. Chem. 282:24231–24238 [DOI] [PubMed] [Google Scholar]
- 49.Palmer D. A., Thompson J. K., Li L., Prat A., Wang P. 2006. Gib2, a novel G-like/RACK1 homolog, functions as a Gβ subunit in cAMP signaling and is essential in Cryptococcus neoformans. J. Biol. Chem. 281:32596–32605 [DOI] [PubMed] [Google Scholar]
- 50.Pan X., Heitman J. 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4874–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Praekelt U. M., Meacock P. A. 1990. HSP12, a new small heat shock gene of Saccharomyces cerevisiae: analysis of structure, regulation and function. Mol. Gen. Genet. 223:97–106 [DOI] [PubMed] [Google Scholar]
- 52.Price M. S., Nichols C. B., Alspaugh J. A. 2008. The Cryptococcus neoformans Rho-GDP dissociation inhibitor mediates intracellular survival and virulence. Infect. Immun. 76:5729–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pukkila-Worley R., Alspaugh J. A. 2004. Cyclic AMP signaling in Cryptococcus neoformans. FEMS Yeast Res. 4:361–367 [DOI] [PubMed] [Google Scholar]
- 54.Pukkila-Worley R., Gerrald Q. D., Kraus P. R., Boily M. J., Davis M. J., Giles S. S., Cox G. M., Heitman J., Alspaugh J. A. 2005. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot. Cell 4:190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rep M., Proft M., Remize F., Tamas M., Serrano R., Thevelein J. M., Hohmann S. 2001. The Saccharomyces cerevisiae Sko1p transcription factor mediates HOG pathway-dependent osmotic regulation of a set of genes encoding enzymes implicated in protection from oxidative damage. Mol. Microbiol. 40:1067–1083 [DOI] [PubMed] [Google Scholar]
- 56.Robertson L. S., Fink G. R. 1998. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. U. S. A. 95:13783–13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson L. C., Gibbs J. B., Marshall M. S., Sigal I. S., Tatchell K. 1987. CDC25: a component of the RAS-adenylate cyclase pathway in Saccharomyces cerevisiae. Science 235:1218–1221 [DOI] [PubMed] [Google Scholar]
- 58.Rolland F., Winderickx J., Thevelein J. M. 2002. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2:183–201 [DOI] [PubMed] [Google Scholar]
- 59.Sales K., Brandt W., Rumbak E., Lindsey G. 2000. The LEA-like protein HSP12 in Saccharomyces cerevisiae has a plasma membrane location and protects membranes against desiccation and ethanol-induced stress. Biochim. Biophys. Acta 1463:267–278 [DOI] [PubMed] [Google Scholar]
- 60.Sass P., Field J., Nikawa J., Toda T., Wigler M. 1986. Cloning and characterization of the high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 83:9303–9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shamrock V. J., Duval J. F., Lindsey G. G., Gaboriaud F. 2009. The role of the heat shock protein Hsp12p in the dynamic response of Saccharomyces cerevisiae to the addition of Congo red. FEMS Yeast Res. 9:391–399 [DOI] [PubMed] [Google Scholar]
- 62.Shamrock V. J., Lindsey G. G. 2008. A compensatory increase in trehalose synthesis in response to desiccation stress in Saccharomyces cerevisiae cells lacking the heat shock protein Hsp12p. Can. J. Microbiol. 54:559–568 [DOI] [PubMed] [Google Scholar]
- 63.Shen G., Wang Y. L., Whittington A., Li L., Wang P. 2008. The RGS protein Crg2 regulates pheromone and cyclic AMP signaling in Cryptococcus neoformans. Eukaryot. Cell 7:1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shima F., Okada T., Kido M., Sen H., Tanaka Y., Tamada M., Hu C. D., Yamawaki-Kataoka Y., Kariya K., Kataoka T. 2000. Association of yeast adenylyl cyclase with cyclase-associated protein CAP forms a second Ras-binding site which mediates its Ras-dependent activation. Mol. Cell. Biol. 20:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shima F., Yamawaki-Kataoka Y., Yanagihara C., Tamada M., Okada T., Kariya K., Kataoka T. 1997. Effect of association with adenylyl cyclase-associated protein on the interaction of yeast adenylyl cyclase with Ras protein. Mol. Cell. Biol. 17:1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siderius M., Rots E., Mager W. H. 1997. High-osmolarity signalling in Saccharomyces cerevisiae is modulated in a carbon-source-dependent fashion. Microbiology 143Pt. 10:3241–3250 [DOI] [PubMed] [Google Scholar]
- 67.Tanaka K., Nakafuku M., Satoh T., Marshall M. S., Gibbs J. B., Matsumoto K., Kaziro Y., Toh-e A. 1990. S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian ras GTPase activating protein. Cell 60:803–807 [DOI] [PubMed] [Google Scholar]
- 68.Thevelein J. M., de Winde J. H. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904–918 [DOI] [PubMed] [Google Scholar]
- 69.Toda T., Cameron S., Sass P., Zoller M., Wigler M. 1987. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50:277–287 [DOI] [PubMed] [Google Scholar]
- 70.Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. 1985. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40:27–36 [DOI] [PubMed] [Google Scholar]
- 71.Varela J. C., Praekelt U. M., Meacock P. A., Planta R. J., Mager W. H. 1995. The Saccharomyces cerevisiae HSP12 gene is activated by the high-osmolarity glycerol pathway and negatively regulated by protein kinase A. Mol. Cell. Biol. 15:6232–6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varela J. C., van Beekvelt C., Planta R. J., Mager W. H. 1992. Osmostress-induced changes in yeast gene expression. Mol. Microbiol. 6:2183–2190 [DOI] [PubMed] [Google Scholar]
- 73.Wang P., Heitman J. 1999. Signal transduction cascades regulating mating, filamentation, and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 2:358–362 [DOI] [PubMed] [Google Scholar]
- 74.Warringer J., Blomberg A. 2006. Involvement of yeast YOL151W/GRE2 in ergosterol metabolism. Yeast 23:389–398 [DOI] [PubMed] [Google Scholar]
- 75.Waugh M. S., Nichols C. B., DeCesare C. M., Cox G. M., Heitman J., Alspaugh J. A. 2002. Ras1 and Ras2 contribute shared and unique roles in physiology and virulence of Cryptococcus neoformans. Microbiology 148:191–201 [DOI] [PubMed] [Google Scholar]
- 76.Waugh M. S., Vallim M. A., Heitman J., Alspaugh J. A. 2003. Ras1 controls pheromone expression and response during mating in Cryptococcus neoformans. Fungal Genet. Biol. 38:110–121 [DOI] [PubMed] [Google Scholar]
- 77.Wilson R. B., Tatchell K. 1988. SRA5 encodes the low-Km cyclic AMP phosphodiesterase of Saccharomyces cerevisiae. Mol. Cell. Biol. 8:505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xue C., Bahn Y. S., Cox G. M., Heitman J. 2006. G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol. Biol. Cell 17:667–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xue C., Hsueh Y. P., Chen L., Heitman J. 2008. The RGS protein Crg2 regulates both pheromone and cAMP signalling in Cryptococcus neoformans. Mol. Microbiol. 70:379–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng Y., Cerione R., Bender A. 1994. Control of the yeast bud-site assembly GTPase Cdc42. Catalysis of guanine nucleotide exchange by Cdc24 and stimulation of GTPase activity by Bem3. J. Biol. Chem. 269:2369–2372 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.