Abstract
Thermal inactivation experiments were carried out to assess the utility of a recently optimized phage amplification assay to accurately enumerate viable Mycobacterium avium subsp. paratuberculosis cells in milk. Ultra-heat-treated (UHT) whole milk was spiked with large numbers of M. avium subsp. paratuberculosis organisms (106 to 107 CFU/ml) and dispensed in 100-μl aliquots in thin-walled 200-μl PCR tubes. A Primus 96 advanced thermal cycler (Peqlab, Erlangen, Germany) was used to achieve the following time and temperature treatments: (i) 63°C for 3, 6, and 9 min; (ii) 68°C for 20, 40, and 60 s; and (iii) 72°C for 5, 10, 15, and 25 s. After thermal stress, the number of surviving M. avium subsp. paratuberculosis cells was assessed by both phage amplification assay and culture on Herrold's egg yolk medium (HEYM). A high correlation between PFU/ml and CFU/ml counts was observed for both unheated (r2 = 0.943) and heated (r2 = 0.971) M. avium subsp. paratuberculosis cells. D and z values obtained using the two types of counts were not significantly different (P > 0.05). The D68°C, mean D63°C, and D72°C for four M. avium subsp. paratuberculosis strains were 81.8, 9.8, and 4.2 s, respectively, yielding a mean z value of 6.9°C. Complete inactivation of 106 to 107 CFU of M. avium subsp. paratuberculosis/ml milk was not observed for any of the time-temperature combinations studied; 5.2- to 6.6-log10 reductions in numbers were achieved depending on the temperature and time. Nonlinear thermal inactivation kinetics were consistently observed for this bacterium. This study confirms that the optimized phage assay can be employed in place of conventional culture on HEYM to speed up the acquisition of results (48 h instead of a minimum of 6 weeks) for inactivation experiments involving M. avium subsp. paratuberculosis-spiked samples.
Due to the possible association of Mycobacterium avium subsp. paratuberculosis, the causative agent of Johne's disease in cattle, with Crohn's disease in humans, the consumption of milk and dairy products contaminated with this pathogenic bacterium has been suggested as a possible source of infection for humans (18). So far, the presence of viable M. avium subsp. paratuberculosis cells has been reported for pasteurized cows' milk (6, 14, 23) and various cheeses (1, 4, 19). However, the rapid detection of viable M. avium subsp. paratuberculosis cells in food remains problematic. Culture is considered the gold standard method of demonstrating the viability of M. avium subsp. paratuberculosis cells, yet this approach is far from perfect and is not really appropriate for risk assessment purposes. First, M. avium subsp. paratuberculosis is a fastidious, slow-growing bacterium requiring a long incubation period before producing visible colonies (4 to 6 weeks minimum). Second, there is no selective growth medium for M. avium subsp. paratuberculosis, and chemical decontamination is required before plating samples on solid Herrold's egg yolk medium (HEYM). This decontamination step, which aims to inactivate the competitive microflora, is often not totally effective, and cultures can be overgrown quickly by non-acid-fast bacteria during incubation. Third, the decontamination step has been demonstrated to have adverse effects on M. avium subsp. paratuberculosis viability (5). This extends the time required for primary isolation (to up to 20 weeks) and undoubtedly underestimates the number of cells originally present in the sample.
Recently, we reported an optimization of the conditions of a commercially available phage amplification assay involving D29 mycobacteriophage (FASTPlaqueTB assay; Biotec Laboratories, Ipswich, United Kingdom) to permit accurate enumeration of M. avium subsp. paratuberculosis cells in milk (7). The main advantage of using phage amplification to detect M. avium subsp. paratuberculosis is that the number of viable cells can be estimated quickly, within 24 to 48 h, based on the count of plaques produced when D29-infected cells burst on a lawn of M. smegmatis indicator cells in an agar plate. Moreover, there is no need to carry out chemical decontamination of the sample before the phage assay because the D29 phage will infect only viable mycobacterial cells, and thus the detection sensitivity of the test is enhanced. For these reasons, the optimized phage amplification method may be used to speed up the acquisition of results during inactivation experiments involving samples artificially spiked with M. avium subsp. paratuberculosis.
So far, the optimized phage amplification assay has been applied for the detection of viable M. avium subsp. paratuberculosis cells in spiked broth and milk samples. However, the performance of the test in assessing the viability of M. avium subsp. paratuberculosis cells subjected to physical or chemical treatments, which are likely to comprise mixtures of viable cells, injured/stressed cells, and dead cells, still needed to be investigated. For this reason, thermal inactivation experiments were carried out in order to assess the utility of this optimized phage assay for use instead of conventional culture for research involving artificially spiked milk samples. The main objectives of this study were to evaluate the correlation between colony and plaque counts for heat-treated M. avium subsp. paratuberculosis and to demonstrate a quicker acquisition of accurate results than that obtainable by culture.
MATERIALS AND METHODS
Bacterial strains.
Two type strains (ATCC 19698 and NCTC 8578) and two milk isolates (796PSS and 806R [14]) of M. avium subsp. paratuberculosis were included in this study. M. avium subsp. paratuberculosis strains were grown in a shaker incubator for 4 to 6 weeks at 37°C to stationary phase in Middlebrook 7H9 broth containing 10% oleic acid-albumin-dextrose-catalase (OADC) supplement (both from Difco) and 2 μg/ml mycobactin J (Synbiotics Europe SAS, Lyon, France). Before being used to spike milk for heat inactivation experiments, broth cultures were declumped by being vortexed (three times for 2 min) with five 3-mm sterile glass beads (VWR International Ltd., England). Mycobacterium smegmatis mc2 155 (originally a gift from Ruth McNerney, London School of Hygiene and Tropical Medicine [LSHTM]) was cultured to stationary phase (2 to 3 days at 37°C) in Middlebrook 7H9 broth containing 10% OADC for use as sensor cells in the optimized phage assay.
Heat treatments.
Ultra-heat-treated (UHT) milk purchased from a local supermarket was spiked with 106 to 107 CFU of M. avium subsp. paratuberculosis per ml and dispensed in 100-μl aliquots into strips of thin-walled 200-μl PCR tubes. A Primus 96 advanced thermal cycler (Peqlab, Erlangen, Germany) was programmed to achieve the following time and temperature treatments: (i) 63°C for 3, 6, and 9 min; (ii) 68°C for 20, 40, and 60 s; and (iii) 72°C for 5, 10, 15, and 25 s. In order to mimic as closely as possible the temperature profile of the laboratory-scale high-temperature, short-time (HTST) pasteurizer (8), each heat treatment included (i) equilibration of the spiked milk at 8°C for 2 min, (ii) heating at a rate of 1.2°C per s, (iii) holding for the desired time, and (iv) cooling on ice. The maximum cooling rate achievable on the thermal cycler was too low compared to that obtained with Franklin HTST plates, so when the thermal cycler display indicated that the holding time was complete, milk samples were immediately transferred to an ice bath for 2 min to facilitate more rapid cooling. Heating experiments were replicated twice with each of the four M. avium subsp. paratuberculosis strains at each of the three temperatures, 63, 68, and 72°C.
Enumeration of M. avium subsp. paratuberculosis cells.
Viable M. avium subsp. paratuberculosis cells were enumerated by both the optimized phage assay and a conventional culture method, as described below. For unheated samples and samples heated for the first heating time at each temperature, 100 μl of sample was appropriately diluted in 7H9 broth plus 10% OADC. For longer heating times at each temperature, 10 100-μl samples that had been heated simultaneously were combined to give a sample volume of 1 ml for testing to improve detection sensitivity. Before being processed through the phage assay, all milk samples (heated and unheated) were centrifuged (16,000 × g for 15 min) to remove milk components known to be inhibitory to the phage assay. The milk pellet was washed once in phosphate-buffered saline, pH 7.4, and resuspended initially in 1 ml 7H9 broth plus 10% OADC. A 100-μl portion of each sample (or appropriate dilutions in 7H9 broth plus 10% OADC, depending on heating time and the estimated number of survivors) was spread on HEYM containing 2 μg/ml mycobactin J for colony count (CFU/ml) determination. The remaining sample (900 μl), with the addition of CaCl2 to a final concentration of 2 mM, was incubated overnight at 37°C and processed through the optimized phage assay as described by Foddai et al. (7). Briefly, 100 μl D29 mycobacteriophage (108 PFU/ml) (originally a gift from Ruth McNerney, LSHTM) was added to the sample before incubation at 37°C for 2 h. Viricide (100 μl of 10 mM ferrous ammonium sulfate) was then added, and the sample was mixed thoroughly and incubated for 5 min at room temperature to inactivate extraneous phage before the addition of 5 ml 7H9 broth plus 10% OADC plus 2 mM CaCl2. The sample was returned to the incubator at 37°C for a further 1.5 h before being plated with 1 ml M. smegmatis mc2 155 culture (108 CFU/ml) and 5 ml molten 7H9 agar. Plaque counts (PFU/ml) were available the next day. Corresponding colony counts (CFU/ml) were obtained after incubating HEYM plates (wrapped in Duraseal laboratory sealing film) at 37°C for 4 to 6 weeks.
Determination of thermal D and z values.
D values (decimal reduction time; the time required at a particular temperature to inactivate a 1-log10 concentration of bacteria) for all four M. avium subsp. paratuberculosis strains, at 63, 68, and 72°C, were calculated from the slope of the regression line obtained by plotting the log10 survivors/ml versus the time of heat exposure at the three test temperatures. z values (the temperature change [°C] required to reduce or increase the D value by 1 log10) for the M. avium subsp. paratuberculosis strains were determined by plotting log10 D values versus the heating temperatures.
Statistical analysis.
The correlation between plaque and colony counts produced from the heated and unheated samples was assessed using linear regression analysis (Microsoft Excel). The statistical significance of differences between colony and plaque counts was assessed using a paired t test (GraphPad Instat 3; GraphPad Prism, La Jolla, CA); differences with P values of <0.05 were considered significant.
RESULTS
Comparison of plaque and colony counts.
Plaque counts (PFU/ml) obtained for samples processed through the phage assay were available within 48 h. This allowed for rapid assessment and enumeration of surviving M. avium subsp. paratuberculosis cells rather than having to wait 4 to 6 weeks for the corresponding colony count (CFU/ml) by conventional culture on HEYM. The optimized phage assay was able to detect smaller numbers of viable cells due to the larger sample volume tested than that used for culture (900 μl and 100 μl, respectively). For 7 of 100 samples, no colonies were observed on HEYM, whereas corresponding PFU counts indicated the presence of between 1 and 10 viable M. avium subsp. paratuberculosis cells per ml in these samples.
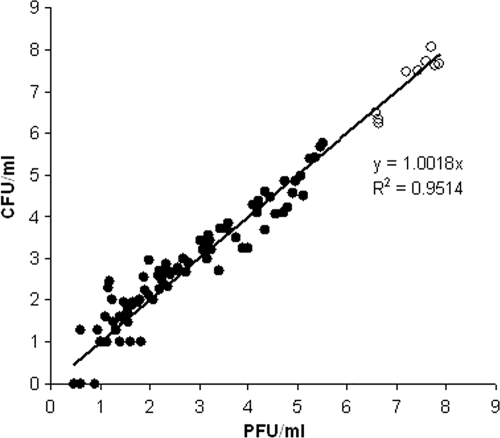
The relationship between the PFU and CFU counts produced from both heated and unheated cells is shown in Fig. 1. A high correlation between plaque (PFU/ml) and colony (CFU/ml) counts obtained for samples before (r2 = 0.943) and after (r2 = 0.971) thermal stress was observed during this study. There was found to be no significant difference between corresponding PFU and CFU counts produced by either unheated or heated M. avium subsp. paratuberculosis cells (paired t test; P = 0.47 and P = 0.73, respectively). This proved that the heat treatment did not affect the sensitivity of M. avium subsp. paratuberculosis cells to infection by mycobacteriophage D29.
FIG. 1.
Relationship between colony and plaque counts for unheated (empty circles) and heated (solid circles) M. avium subsp. paratuberculosis cells.
Thermal D and z values for M. avium subsp. paratuberculosis.
The D and z values calculated for the four strains of M. avium subsp. paratuberculosis heated in milk at 63, 68, and 72°C are shown in Table 1. Differences in D and z values among the four strains of M. avium subsp. paratuberculosis were not significant (P = 0.99 and P = 0.63, respectively). Mean D63°C, D68°C, and D72°C values based on the results produced using the optimized phage assay were 81.8 ± 3.2, 9.8 ± 0.7, and 4.2 ± 0.8 s, respectively, resulting in a mean z value of 6.9 ± 0.5°C. Comparison of corresponding D and z values calculated based on the results produced from the culture method showed that the difference was not significant (P > 0.05).
TABLE 1.
Thermal D and z values for four strains of M. avium subsp. paratuberculosis in milk, calculated from plaque (PFU) and colony (CFU) countsa
| Strain | Count type | Mean D value (s) at: |
Mean z value (°C) | ||
|---|---|---|---|---|---|
| 63°C | 68°C | 72°C | |||
| NCTC 8578 | PFU | 79.9 | 8.6 | 4.4 | 7.1 |
| CFU | 74.8 | 8.6 | 2.9 | 6.3 | |
| ATCC 19698 | PFU | 83.5 | 9.8 | 3.0 | 6.2 |
| CFU | 70.5 | 10.1 | 4.6 | 7.5 | |
| 806R | PFU | 78.5 | 10.5 | 4.7 | 7.3 |
| CFU | 74.8 | 10.6 | 4.9 | 7.5 | |
| 796PSS | PFU | 85.5 | 10.2 | 4.7 | 7.1 |
| CFU | 95.2 | 11.6 | 5.3 | 7.1 | |
| All | PFU | 81.8 ± 3.2 | 9.8 ± 0.7 | 4.2 ± 0.8 | 6.9 ± 0.5 |
| CFU | 78.8 ± 11.1 | 10.2 ± 1.1 | 4.4 ± 1.1 | 7.1 ± 0.6 | |
For each individual strain, the D value represents the mean for two independent experiments. D and z data for all strains in total are reported as means ± standard deviations.
Thermal inactivation curves.
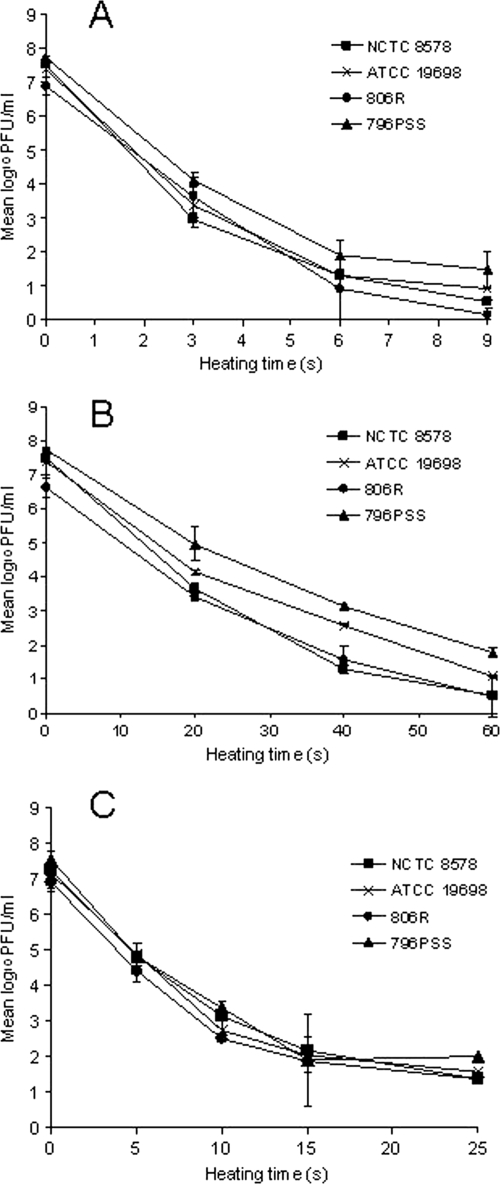
Incomplete inactivation was recorded for all four strains of M. avium subsp. paratuberculosis under all three time-temperature conditions studied. The thermal inactivation curves were consistently concave and showed a progressive log10 reduction in viable cells followed by significant tailing (Fig. 2A, B, and C). The mean log10 reductions observed for each M. avium subsp. paratuberculosis strain after the longest heating times at all three temperatures are summarized in Table 2. The mean log10 reductions in viable M. avium subsp. paratuberculosis cells heated at 72°C for 15 and 25 s were not significantly different, at 5.6 ± 0.1 and 5.2 ± 0.3 log10, respectively (P > 0.05). A larger reduction in the number of viable cells was recorded for samples processed under the two lower time-temperature conditions; mean log10 reductions at 68°C for 60 s and 63°C for 9 min were 6.3 ± 0.4 and 6.6 ± 0.3 log10, respectively.
FIG. 2.
Inactivation kinetics of four strains of M. avium subsp. paratuberculosis heated in milk at 63°C (A), 68°C (B), and 72°C (C), obtained using the phage assay. Each value is the mean of two independent observations ± standard error.
TABLE 2.
Mean log10 reductions calculated for four strains of M. avium subsp. paratuberculosis heated in milk under different time-temperature conditions
| Heat treatment conditions | Log10 reduction in M. avium subsp. paratuberculosisa |
Mean log10 reduction ± SD | |||
|---|---|---|---|---|---|
| NCTC 8578 | ATCC 19698 | 806R | 796PSS | ||
| 72°C, 15 s | 5.9 | 5.6 | 5.5 | 5.5 | 5.6 ± 0.1 |
| 72°C, 25 s | 5.1 | 5.1 | 5.0 | 5.6 | 5.2 ± 0.3 |
| 68°C, 60 s | 7.0 | 6.3 | 6.0 | 5.8 | 6.3 ± 0.5 |
| 63°C, 9 min | 7.0 | 6.5 | 6.8 | 6.3 | 6.6 ± 0.3 |
Each value represents the mean of two independent observations.
DISCUSSION
Many studies have been carried out to assess the impact of pasteurization processes (both the standard holder method—63.5°C for 30 min—and the HTST method—71.7°C for 15 s) on the viability of M. avium subsp. paratuberculosis in milk (3, 10-13, 17, 22, 25, 26, 28-30). Most of these studies confirmed that M. avium subsp. paratuberculosis is more resistant to heat than other Mycobacterium spp. and that HTST pasteurization results in a significant reduction in the number of viable M. avium subsp. paratuberculosis cells (a >4-log10 reduction is generally reported), but some reported the detection of small numbers of surviving M. avium subsp. paratuberculosis cells in some pasteurized milk samples (10, 11). Results of subsequent surveys of commercially pasteurized milk in the United Kingdom, Czech Republic, and United States also tend to indicate that a small number of M. avium subsp. paratuberculosis cells may occasionally survive HTST pasteurization (1, 6, 14), although this remains a contentious subject (2).
The lack of concordance between the results of different pasteurization studies is due in part to different approaches to culturing M. avium subsp. paratuberculosis after heat treatment. In previous heat inactivation studies, culture on HEYM or in automated/semiautomated broth culture systems, such as Bactec 12B, MGIT (Becton Dickinson), or ESP (Trek Diagnostic Systems), has been used to assess the presence of surviving M. avium subsp. paratuberculosis cells. However, the detection of this pathogenic bacterium based on culture takes a long time to generate results due to the slow growth of the microorganism in both solid (4 to 6 weeks minimum) and liquid (up to 40 days) growth media. In the present study, we evaluated the potential utility of a recently optimized phage amplification assay (7) for the rapid detection (within 48 h) of viable cells surviving pasteurization carried out under laboratory conditions. The method has been applied successfully to detect viable M. avium subsp. paratuberculosis cells in broth or milk spiked with cells cultivated up to stationary phase. However, Grant et al. (10) reported that heated M. avium subsp. paratuberculosis cells require a longer incubation time than unheated cells for growth on solid medium, which would be indicative of the presence of sublethally injured cells in heat-treated milk samples. These cells should have the ability, under appropriate conditions, to repair injury and become competent cells again. To demonstrate that the optimized phage assay could be employed in place of culture, its validity when applied to samples containing M. avium subsp. paratuberculosis cells that are not in optimal metabolic condition would first need to be tested. For this reason, this study evaluated the correlation between PFU and CFU counts obtained for M. avium subsp. paratuberculosis cells heated in milk at pasteurization temperatures.
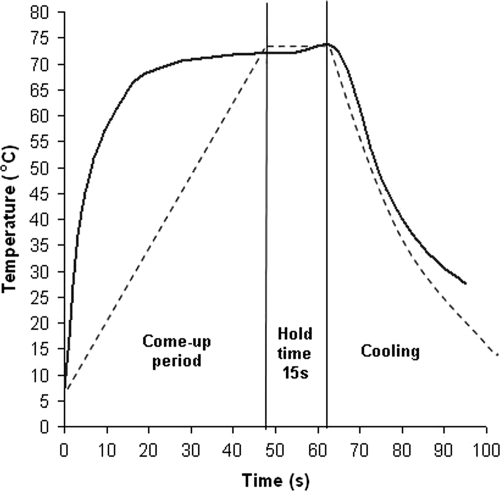
UHT milk samples spiked with M. avium subsp. paratuberculosis at high levels (>106 cells/ml) were heat treated in 100-μl aliquots in thin-walled 200-μl PCR tubes in order to take advantage of the efficient thermal transfer characteristics of a thermal cycler heat block and to attain pasteurization temperature as quickly as possible. This thermal cycler approach was inspired by Gumber and Whittington (16), who used a thermal cycler to simulate temperature flux variations that M. avium subsp. paratuberculosis may be exposed to in the environment. For the pasteurization experiments, the heating rate of the thermal cycler was set to 1.2°C/s to achieve a “come-up” time to 72°C of ca. 50 s (Fig. 3), similar to the time for Franklin HTST plates (developed to simulate commercial HTST pasteurizer conditions [8]), which were used in earlier pasteurization studies by Grant et al. (10, 11). While we acknowledge that a thermal cycler is not a conventional means of simulating milk pasteurization, we contend that the results we obtained validate its use because they agree very favorably with results of numerous previous pasteurization studies.
FIG. 3.
Heating profiles for milk in Franklin HTST pasteurizer units (solid line), developed for lab-scale simulation of commercial pasteurization by Franklin (8) and used in previous studies by Grant et al. (10, 11), and in thin-walled PCR tubes in a Primus 96 thermal cycler (dashed line).
The results of this study confirm that the optimized phage assay permits rapid and accurate enumeration of viable M. avium subsp. paratuberculosis cells in milk. The main advantages of the phage assay over culture on HEYM are as follows: first, survivor cells can be enumerated within 48 h (instead of in 4 to 6 weeks); and second, due to the larger sample volume tested (1 ml), the phage assay is able to detect smaller numbers of viable cells in milk (minimum detection limit of 1 PFU/ml rather than 10 CFU/ml for culture). This was clearly demonstrated in the case of seven heated milk samples where no colonies were observed on HEYM but corresponding PFU counts indicated the presence of 1 to 10 viable M. avium subsp. paratuberculosis cells per ml. A high correlation (r2 = 0.9464) between plaque and colony counts produced from both heated and unheated cells was recorded. This result confirms that heated M. avium subsp. paratuberculosis cells can be detected and accurately enumerated using the optimized phage assay. Since D29 phage would be expected to require host cells in a fully competent metabolic state in order to replicate, this finding suggests that M. avium subsp. paratuberculosis cells surviving heat treatment were in this state when subjected to the phage assay. One of the most probable explanations is that the overnight incubation in 7H9 Middlebrook broth at 37°C (see Materials and Methods) after heating, but before the phage assay commenced, aided in the resuscitation of heat-injured cells. Incomplete inactivation of 106 to 107 CFU of M. avium subsp. paratuberculosis/ml was consistently recorded after heating samples under three different time-temperature conditions, once more suggesting that M. avium subsp. paratuberculosis could survive pasteurization if present in raw milk in large numbers. These results are consistent with the findings of heat inactivation experiments previously carried out at Queen's University of Belfast (10-13). The mean D values calculated for the four M. avium subsp. paratuberculosis strains in this study are similar to those previously reported for M. avium subsp. paratuberculosis heated in milk (D71°C = 11.67 s, D68°C = 21.8 s [30], D63°C = 96 to 150 s [21]). The z value obtained in this study is similar to one reported previously (7.11°C [30]), which confirms that M. avium subsp. paratuberculosis is more heat resistant than Mycobacterium bovis, previously reported to have a z value in milk of 4.8 to 5.2°C (20) and to be inactivated completely after heat treatment at 70°C for 10 s (24), unlike M. avium subsp. paratuberculosis in the present study.
The factors contributing to survival of M. avium subsp. paratuberculosis during heating have not been elucidated fully but may include its thick cell wall (rich in lipids), in addition to the tendency of this bacterium to exist as large clumps of cells. Clumped cells could be less susceptible to heat treatment than individual cells (10, 12). Bacteria inside the clumps could be protected because of slow heat penetration (27), although this possibility has been refuted (2). All broth cultures used in this study were thoroughly declumped by being vortexed with glass beads before being added to the milk and processed by heat treatment. The primary purpose of this step was to reduce as much as possible the presence of large clumps, which are known to affect the correlation between PFU and CFU counts; however, it should also have ensured that single cells, not clumps, were being heat treated. The results obtained during this study suggest that single-cell or small-clump suspensions of M. avium subsp. paratuberculosis can survive pasteurization treatments and also exhibit nonlinear thermal inactivation kinetics (tailing), a phenomenon previously considered an artifact and attributed to the presence of clumped cells (15). Interestingly, it was recently reported that old (stationary-phase) cultures of both Mycobacterium marinum and M. bovis BCG contained spores, which led to tailed survivor curves when cultures were exposed to wet heat at 65°C for 15 min (9). Is M. avium subsp. paratuberculosis also able to form spores, and could the 4- to 6-week-old broth cultures used to spike milk in this study have contained spores, resulting in nonlinear thermal inactivation? This possibility merits further investigation.
It must be emphasized that the experiments reported here were not designed to determine the ability of commercial HTST milk pasteurization to inactivate M. avium subsp. paratuberculosis. For many reasons, continuous-flow HTST pasteurization as applied to commercial products cannot be simulated adequately under laboratory conditions, and direct comparison to the real situation may not be possible considering the fact that the wild strains may be less or more heat tolerant than field isolates or type strains used in the laboratory. Rather, the objective of this research was to validate the optimized phage amplification assay and its use for the rapid detection and enumeration of viable M. avium subsp. paratuberculosis cells after heat treatment. Our results clearly demonstrate that the method could be employed in place of conventional culture to speed up the acquisition of results during inactivation experiments involving spiked milk samples. The reported study was concerned with thermal inactivation. We have also monitored the effect of Sorgene disinfectant (chemical stress) on the viability of M. avium subsp. paratuberculosis by using the optimized phage assay, with similar results. The optimized phage assay may thus have applications for antibiotic susceptibility testing of this bacterium.
Acknowledgments
Antonio Foddai was the recipient of a Master and Back Studentship from the Sardinian government during this study.
Footnotes
Published ahead of print on 22 January 2010.
REFERENCES
- 1.Ayele, W. Y., P. Svastova, P. Roubal, M. Bartos, and I. Pavlik. 2005. Mycobacterium avium subspecies paratuberculosis cultured from locally and commercially pasteurized cow's milk in the Czech Republic. Appl. Environ. Microbiol. 71:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerf, O., M. Griffiths, and F. Aziza. 2007. Assessment of the prevalence of Mycobacterium avium subsp. paratuberculosis in commercially pasteurized milk. Foodborne Pathog. Dis. 4:433-447. [DOI] [PubMed] [Google Scholar]
- 3.Chiodini, R. J., and J. Hermon-Taylor. 1993. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurization. J. Vet. Diagn. Invest. 5:629-631. [DOI] [PubMed] [Google Scholar]
- 4.Clark, D. L., Jr., J. L. Anderson, J. J. Koziczkowski, and J. L. E. Ellingson. 2006. Detection of Mycobacterium avium subspecies paratuberculosis genetic components in retail cheese curds purchased in Wisconsin and Minnesota by PCR. Mol. Cell. Probes 20:197-202. [DOI] [PubMed] [Google Scholar]
- 5.Dundee, L., I. R. Grant, H. J. Ball, and M. T. Rowe. 2001. Comparative evaluation of four decontamination protocols for the isolation of Mycobacterium avium subsp. paratuberculosis from milk. Lett. Appl. Microbiol. 33:173-177. [DOI] [PubMed] [Google Scholar]
- 6.Ellingson, J. L., J. L. Anderson, J. J. Koziczkowsk, R. P. Radcliff, S. J. Sloan, S. E. Allen, and N. M. Sullivan. 2005. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J. Food Prot. 68:966-972. [DOI] [PubMed] [Google Scholar]
- 7.Foddai, A., C. T. Elliott, and I. R. Grant. 2009. Optimization of a phage amplification assay to permit accurate enumeration of viable Mycobacterium avium subsp. paratuberculosis cells. Appl. Environ. Microbiol. 75:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin, J. G. 1965. A simple laboratory-scale HTST milk pasteurizer. J. Dairy Res. 32:281-289. [Google Scholar]
- 9.Ghosh, J., P. Larsson, B. Singh, B. M. F. Pettersson, N. M. Islam, S. N. Sarkar, S. Dasgupta, and L. A. Kirsebom. 2009. Sporulation in mycobacteria. Proc. Natl. Acad. Sci. USA 106:10781-10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant, I. R., H. J. Ball, S. D. Neill, and M. T. Rowe. 1996. Inactivation of Mycobacterium paratuberculosis in cows' milk at pasteurization temperatures. Appl. Environ. Microbiol. 62:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant, I. R., H. J. Ball, and M. T. Rowe. 1998. Effect of high-temperature, short-time (HTST) pasteurization on milk containing low numbers of Mycobacterium paratuberculosis. Lett. Appl. Microbiol. 26:166-170. [DOI] [PubMed] [Google Scholar]
- 12.Grant, I. R., H. J. Ball, and M. T. Rowe. 1999. Effect of higher pasteurization temperatures, and longer holding times at 72°C, on the inactivation of Mycobacterium paratuberculosis in milk. Lett. Appl. Microbiol. 28:461-465. [DOI] [PubMed] [Google Scholar]
- 13.Grant, I. R., E. I. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial-scale high-temperature, short-time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant, I. R., H. J. Ball, and M. T. Rowe. 2002. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 68:2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant, I. R., M. T. Rowe, L. Dundee, and E. Hitchings. 2001. Mycobacterium avium subsp. paratuberculosis: its incidence, heat resistance and detection in milk and dairy products. Int. J. Food Technol. 54:2-13. [Google Scholar]
- 16.Gumber, S., and R. J. Whittington. 2009. Analysis of the growth pattern, survival and proteome of Mycobacterium avium subsp. paratuberculosis following exposure to heat. Vet. Microbiol. 136:82-90. [DOI] [PubMed] [Google Scholar]
- 17.Hammer, P., C. Kiesner, H. G. Walte, K. Knappstein, and P. Teufel. 2002. Heat resistance of Mycobacterium avium subsp. paratuberculosis in raw milk. Kieler Milchwirtsch. Forschungsber. 54:275-303. [Google Scholar]
- 18.Hermon-Taylor, J., and T. Bull. 2002. Crohn's disease caused by Mycobacterium avium subspecies paratuberculosis: a public health tragedy whose resolution is long overdue. J. Med. Microbiol. 51:3-6. [DOI] [PubMed] [Google Scholar]
- 19.Ikonomopoulos, J., I. Pavlik, M. Bartos, P. Svastova, W. Y. Ayele, P. Roubal, J. Lukas, N. Cook, and M. Gazouli. 2007. Detection of Mycobacterium avium subsp. paratuberculosis in retail cheeses from Greece and the Czech Republic. Appl. Environ. Microbiol. 71:8934-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kells, H. R., and S. A. Lear. 1960. Thermal death time curve of Mycobacterium tuberculosis var. bovis in artificially infected milk. Appl. Microbiol. 8:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keswani, J., and J. F. Frank. 1998. Thermal inactivation of Mycobacterium paratuberculosis in milk. J. Food Prot. 61:974-978. [DOI] [PubMed] [Google Scholar]
- 22.McDonald, W. L., K. J. O'Riley, C. J. Schroen, and R. J. Condron. 2005. Heat inactivation of Mycobacterium avium subsp. paratuberculosis in milk. Appl. Environ. Microbiol. 71:1785-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millar, D., J. Ford, J. Sanderson, S. Withey, M. Tizard, T. Doran, and J. Hermon-Taylor. 1996. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows' milk in England and Wales. Appl. Environ. Microbiol. 62:3446-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlas, M. 1990. Thermoresistance of mycobacteria. Acta Vet. Brno 59:65-71. [Google Scholar]
- 25.Pearce, L. E., H. T. Truong, R. A. Crawford, G. F. Yates, S. Cavaignac, and G. W. de Lisle. 2001. Effect of turbulent-flow pasteurization on survival of Mycobacterium avium subsp. paratuberculosis added to raw milk. Appl. Environ. Microbiol. 67:3964-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rademaker, J. L., M. M. Vissers, and M. C. Te Giffel. 2007. Effective heat inactivation of Mycobacterium avium subsp. paratuberculosis in raw milk contaminated with naturally infected feces. Appl. Environ. Microbiol. 73:4185-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe, M. T., I. R. Grant, L. Dundee, and H. J. Ball. 2002. Heat resistance of Mycobacterium avium subsp. paratuberculosis in milk. Irish J. Agric. Food Res. 39:203-208. [Google Scholar]
- 28.Stabel, J. R., E. M. Steadham, and C. A. Bolin. 1997. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl. Environ. Microbiol. 63:4975-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stabel, J. R., and A. Lambertz. 2004. Efficacy of pasteurization conditions for the inactivation of Mycobacterium avium subsp. paratuberculosis in milk. J. Food Prot. 67:2719-2726. [DOI] [PubMed] [Google Scholar]
- 30.Sung, N., and M. T. Collins. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]