Abstract
Compounds present in Hafnia alvei cell-free culture supernatant cumulatively negatively influence the early stage of biofilm development by Salmonella enterica serovar Enteritidis on stainless steel while they also reduce the overall metabolic activity of S. Enteritidis planktonic cells. Although acylhomoserine lactones (AHLs) were detected among these compounds, the use of several synthetic AHLs was not able to affect the initial stage of biofilm formation by this pathogen.
Biofilms are groups of bacteria encased in a self-produced extracellular matrix (5, 6). Biofilms formed on stainless steel (SS) surfaces in food-processing areas are of great importance since they may lead to food spoilage and transmission of diseases (2, 16). This sessile mode of life allows bacteria to enjoy a number of advantages, such as increased resistance to antimicrobial agents (9, 12). Notably, it is widely accepted that bacteria (both planktonic and biofilm cells) communicate by releasing and sensing signaling compounds in a process commonly known as quorum sensing (13, 18, 24).
Salmonella enterica serovar Enteritidis is one of the most important bacterial pathogens worldwide (7, 17). Hafnia alvei are frequent psychrotrophic members of the Enterobacteriaceae community in meat products, playing a role in their spoilage, while they have been shown to be capable of producing signaling compounds (3). In this study, in order to determine any possible influence of compounds produced by H. alvei on the biofilm-forming ability of S. Enteritidis, the latter was left to develop biofilm on SS surfaces in the presence of conditioned medium obtained after the growth of the former. Biofilm formation was assessed directly by detaching cells and enumerating them and, also, indirectly by automated conductance measurements.
Growth media and biofilm development.
To produce biofilms, individual sterile SS coupons (3 by 0.8 by 0.1 cm, type AISI-304; Halyvourgiki, Inc., Athens, Greece) were placed vertically in glass test tubes (length, 10 cm, and diameter, 1.5 cm) containing 3.5 ml of growth medium in such a way that the upper part of their metallic surface was exposed to the air-liquid interface, since this interface provides a selectively advantageous niche for Salmonella biofilm formation (11). The growth media used for biofilm development were based on brain heart infusion (BHI) broth (LAB M; International Diagnostics Group Plc., Bury, Lancashire, United Kingdom) which contained three different concentrations (0, 20, and 50%) of H. alvei strain 718 (3) cell-free culture supernatant (CFS) in such a way that identical amounts of BHI nutrients were contained in the three test media (Table 1). CFS was prepared as previously described by Surette and Bassler (25). Growth media were inoculated with Salmonella enterica subsp. enterica serovar Enteritidis phage type 4 strain P167807 so as to yield an initial bacterial population of ca. 107 CFU ml−1. Inoculated tubes were then incubated statically at 18°C, while biofilm development was evaluated at 12, 24, 48, and 72 h of incubation. It should be noted that all three test media had the same pH at the beginning of incubation (thus, the pH values of BHI broth and CFS were both adjusted to 6.8 before autoclaving or before filtration, respectively).
TABLE 1.
Percentages of BHI and H. alvei CFS contained in the three growth media tested for S. Enteritidis biofilm development on SS coupons
| Growth medium specification | % (vol/vol) of indicated ingredient |
||
|---|---|---|---|
| Full-strength BHI (37 g liter−1) | Double-strength BHI (74 g liter−1) | H. alvei CFS | |
| 0% CFS | 100 | 0 | 0 |
| 20% CFS | 60 | 20 | 20 |
| 50% CFS | 0 | 50 | 50 |
Effect of H. alvei CFS on Salmonella biofilm development. (i) Bead vortexing and agar plating assessment method.
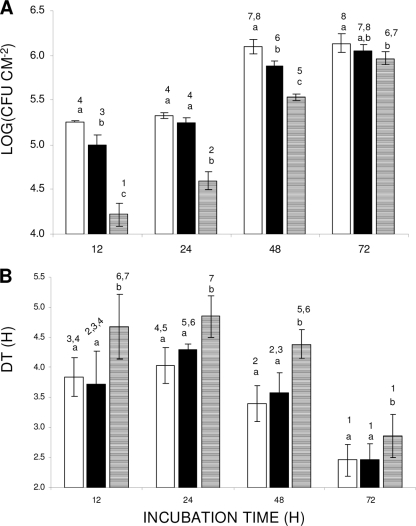
The bead vortexing and agar plating method of assessing the effect of H. alvei CFS on S. Enterica biofilm development was based on the detachment of strongly attached/biofilm cells from the surface of SS coupons through bead vortexing and subsequent enumeration of released bacteria by plating on tryptone soy agar (TSA; LAB M) as previously described (11). The results are presented in Fig. 1A. Regarding incubation time, it can be observed that biofilm formation (log CFU cm−2) increases progressively as incubation time increases, independent of the growth medium composition. However, for the same incubation period (12 to 72 h), the composition of the growth medium (viz., the concentration of H. alvei CFS) significantly (P < 0.05) influenced the quantity of strongly attached/biofilm cells recovered. Specifically, incubation of coupons in 50% CFS resulted in about 1 log reduction in the number of cells after the first 24 h of incubation compared to the results when coupons were incubated in BHI broth containing 0% or 20% CFS. It should be noted that this inhibitory effect was maintained even after the CFS was heated at 100°C for 10 min, which clearly indicates that the origin of the inhibition is not enzymatic. This difference tended to be minimized as incubation time increased to 72 h.
FIG. 1.
(A) Strongly attached/biofilm S. Enteritidis cells (log CFU cm−2) on SS coupons. Coupons were incubated at 18°C for up to 72 h in growth media containing 0% (□), 20% (▪), or 50% (▤) H. alvei CFS. (B) Conductance detection times (DTs; h) corresponding to data presented in panel A. The bars represent the mean values ± standard deviations. Mean values sharing at least one common number shown above the bars are not significantly different at a P value of <0.05. Within a group with the same incubation period (12 to 72 h; x axis), mean values sharing at least one common letter are not significantly different at a P value of <0.05.
(ii) Conductance assessment method.
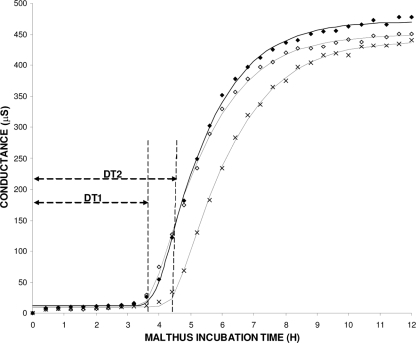
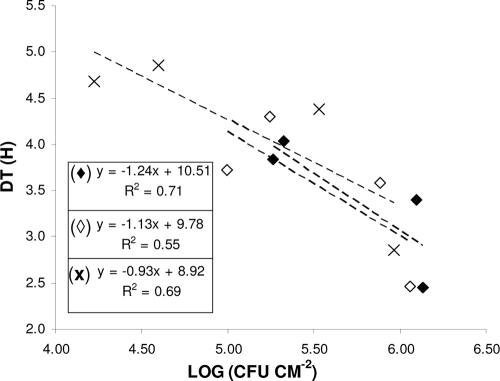
Conductance measurements were also used, in order to indirectly quantify strongly attached/biofilm bacteria on SS coupons, using a Malthus 2000 instrument (Radiometer International, Copenhagen, Denmark) as previously described (4, 8, 10). Representative data on changes in conductance of BHI broth in Malthus tubes are shown in Fig. 2. In our experiment, the Baranyi and Roberts growth model was fitted (using DMFit version 2.1; Institute of Food Research, Norwich Research Park, United Kingdom [http://www.ifr.ac.uk/Safety/DMfit/default.html]) to the conductance data, producing sigmoidal curves typical of microbial growth (1). The model parameters were then used to precisely calculate the detection times (DTs; h). DT is apparently a function of the initial microorganism population in the Malthus tubes (inoculum, being here the initial concentration of strongly attached/biofilm bacteria on coupons), the growth kinetics of the microorganism, and the properties of the conductance medium (22). Thus, for the given test protocol, DT correlates linearly with the initial concentration of strongly attached/biofilm bacteria to the coupons, i.e., a shorter DT suggests a higher level of biofilm formation (Fig. 3). The results of DT calculation are depicted in Fig. 1B. In agreement with the agar plating data, when biofilm incubation was done in 50% CFS, the DTs observed were significantly (P < 0.05) higher than the DTs observed when biofilm development was done in 0% or 20% CFS.
FIG. 2.
Typical changes for broth conductance (μS) versus time (h). Each Malthus tube contained an SS coupon which had previously been incubated for 12 h in growth medium containing 0% (⧫), 20% (⋄), or 50% (×) H. alvei CFS. Sigmoidal curves represent the fitting of the three data series with the model of Baranyi and Roberts (R2 > 0.99). The detection times (DTs; h) calculated by the model are also depicted. Similar DTs were recorded for 0 and 20% CFS (DT1); they were shorter than the DT recorded in the case of 50% CFS (DT2).
FIG. 3.
Linear regression plots between biofilm counts (log CFU cm−2) and corresponding conductance detection times (DTs; h). For biofilm formation, SS coupons were incubated at 18°C for up to 72 h in growth media containing 0% (⧫), 20% (⋄), or 50% (×) H. alvei CFS. Data points represent mean values. For greater clarity, error bars are not included. The mathematical equations of the three regression plots, together with their relevant regression coefficients (R2), are also shown.
Metabolic activity of Salmonella planktonic cells in the presence of H. alvei CFS.
The metabolic activities of S. Enteritidis planktonic cells at 18 and 37°C in the presence of H. alvei CFS compounds were indirectly monitored by conductance measurements, as previously described (19). The Malthus DT is defined as the time interval between the start of conductance monitoring and the beginning of the acceleration phase of conductance values. Obviously, a slower DT is an indication of increased microbial metabolism (22). The metabolic activity of the culture can also be inferred by the steepness of the slope after detection (the greater the activity, the steeper the slope). It can be observed (Table 2) that for both incubation temperatures, the presence of 50% CFS resulted in significant (P < 0.05) increases in the DTs recorded, which was also accompanied by significant decreases (P < 0.05) in the slopes of conductance curves (expressed as maximum slope rates of conductance changes; μS h−1). Undoubtedly, these results demonstrate significant reductions of the metabolic activities of the Salmonella planktonic cells due to the CFS compounds. In order to shed light on the physiological mechanism of the observed inhibition, the major volatile compounds and organic acids contained in the supernatants of the three growth media (0, 20, or 50% CFS) after 12 h of incubation at 18°C were identified by using gas chromatography-mass spectrometry and high-performance liquid chromatography, respectively, as previously described (15, 23). No significant differences in the S. Enteritidis metabolic profiles (as expressed by 53 volatile compounds and 17 organic acids identified in supernatants) in the three cases (results not shown) were revealed.
TABLE 2.
Effect of H. alvei CFS on the metabolic activity of S. Enteritidis planktonic cells at 18°C and 37°C as evaluated by conductance response parametersa
| Growth medium | DT (h) |
MSrCC (μS h−1) |
||
|---|---|---|---|---|
| 18°C | 37°C | 18°C | 37°C | |
| 0% CFS | 22.88 A ± 1.08 | 4.66 A ± 0.08 | 15.85 A ± 2.34 | 171.52 A ± 18.81 |
| 50% CFS | 26.98 B ± 1.45 | 5.29 B ± 0.15 | 9.80 B ± 1.60 | 100.87 B ± 14.68 |
DTs (h) and maximum slope rates of conductance changes (MSrCC; μS h−1) were determined by fitting conductance curves with the model of Baranyi and Roberts. Data are mean values ± standard deviations. Within the same column, means with different letters are statistically significantly different (Student's t test, P < 0.05).
AHL detection by TLC.
At each sampling time (12, 24, 48, and 72 h), the three growth media (Table 1) were tested for the presence of autoinducers by thin-layer chromatography (TLC), as previously described (21). For the autoinducer bioassay, the acylhomoserine lactone (AHL) reporter strain Agrobacterium tumefaciens A136 (pCF218, pCF372) was used. TLC analysis indicated the presence of AHL in the extracts of media containing H. alvei CFS (20 and 50%) during the whole incubation period (representative results are shown in Fig. 4). As expected, AHLs were absent from the supernatants of media without CFS. Notably, the heating of CFS at 100°C for 10 min was not efficient to abolish autoinducer detection (results not shown).
FIG. 4.
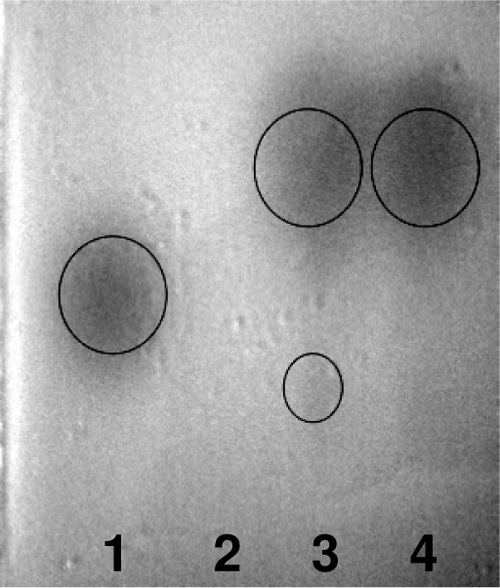
Characteristic TLC profiles of AHLs in extracts from the three growth media at 12 h of incubation. Chromatographs were developed using the reporter strain A. tumefaciens A136. Lanes: 1, C6-HSL standard; 2, 0% CFS; 3, 50% CFS; 4, 20% CFS.
Early biofilm development in the presence of synthetic AHLs.
In order to investigate whether the cause of the observed inhibition in the early stage of Salmonella biofilm development was the AHL compounds present in the CFS, biofilm development was tested in the presence of various commercial synthetic AHLs (all purchased from Sigma-Aldrich). Thus, SS coupons were incubated at 18°C in BHI broth (3.5 ml) inoculated with Salmonella (ca. 107 CFU ml−1), which also contained (i) 10 μmol liter−1 or (ii) 100 μmol liter−1 of N-3-oxo-hexanoyl-l-homoserine lactone (3-oxo-C6-HSL, being the major AHL produced by H. alvei 718) (3) or (iii) a mixture of N-butyryl-dl-homoserine lactone (C4-HSL), 3-oxo-C6-HSL, N-octanoyl-dl-homoserine lactone (C8-HSL), and N-dodecanoyl-dl-homoserine lactone (C12-HSL). The final concentration in BHI broth of each HSL when was provided in mixture (case iii) was adjusted to 10 μmol liter−1. The numbers of strongly attached/biofilm cells were evaluated at 6 and 12 h of incubation by using the bead vortexing and agar plating method, as previously described (11). Under the experimental conditions applied here, the addition of synthetic AHLs in BHI broth did not significantly alter the numbers of strongly attached/biofilm cells recovered (results not shown).
Understanding the mechanisms of interspecies interactions between bacteria isolated from food-processing environments and how these may influence biofilm formation and dispersal is of special interest (14). In sum, the present results suggest that the biofilm-forming potential of S. Enteritidis can be modulated “somehow” by compounds produced by H. alvei. Interestingly, these compounds also reduce the metabolic activity of the pathogen's planktonic cells, and this probably accounts for the biofilm formation decline observed. Although AHLs were detected among these compounds, our results showed that various synthetic compounds of this chemical nature are not able to influence S. Enteritidis biofilm formation. Nevertheless, the present findings may assist in a deeper understanding of the physiological mechanism of biofilm development by this pathogenic bacterium in multispecies environments like those probably encountered in real food-processing areas.
Acknowledgments
This work was partly financially supported by the European Union project (ProSafeBeef) within the 6th Framework Programme (ref. Food-CT-2006-36241). E. D. Giaouris would like to acknowledge the Greek State Scholarships Foundation (IKY) for providing him a fellowship for postdoctoral studies.
We are grateful to Vicky Blana for her valuable advice regarding TLC and synthetic AHLs.
Footnotes
Published ahead of print on 22 January 2010.
REFERENCES
- 1.Baranyi, J., and T. A. Roberts. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277-294. [DOI] [PubMed] [Google Scholar]
- 2.Brooks, J. D., and S. H. Flint. 2008. Biofilms in the food industry: problems and potential solutions. Int. J. Food Sci. Technol. 43:2163-2176. [Google Scholar]
- 3.Bruhn, J. B., A. B. Christensen, L. R. Flodgaard, K. F. Nielsen, T. O. Larsen, M. Givskov, and L. Gram. 2004. Presence of acylated homoserine lactones (AHLs) and AHL-producing bacteria in meat and potential role of AHL in spoilage of meat. Appl. Environ. Microbiol. 70:4293-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chorianopoulos, N. G., E. D. Giaouris, P. N. Skandamis, S. A. Haroutounian, and G.-J. E. Nychas. 2008. Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid-base sanitizers. J. Appl. Microbiol. 104:1586-1596. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 6.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EFSA-ECDC. 2007. The community summary report on trends and sources of zoonoses, zoonotic agents, antimicrobial resistance and foodborne outbreaks in the European Union in 2006. EFSA J. 130:2-352. [Google Scholar]
- 8.Flint, S. H., J. D. Brooks, and P. J. Bremer. 1997. Use of the Malthus conductance growth analyser to determine numbers of thermophilic streptococci on stainless steel. J. Appl. Microbiol. 83:335-339. [DOI] [PubMed] [Google Scholar]
- 9.Gawande, P. V., and A. A. Bhagwat. 2002. Inoculation onto solid surfaces protects Salmonella spp. during acid challenge: a model study using polyethersulfone membranes. Appl. Environ. Microbiol. 68:86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giaouris, E., N. Chorianopoulos, and G.-J. E. Nychas. 2005. Effect of temperature, pH, and water activity on biofilm formation by Salmonella enterica Enteritidis PT4 on stainless steel surfaces as indicated by the bead vortexing method and conductance measurements. J. Food Prot. 68:2149-2154. [DOI] [PubMed] [Google Scholar]
- 11.Giaouris, E., and G.-J. E. Nychas. 2006. The adherence of Salmonella Enteritidis PT4 to stainless steel: the importance of the air-liquid interface and nutrient availability. Food Microbiol. 23:747-752. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert, P., D. G. Allison, and A. J. McBain. 2002. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J. Appl. Microbiol. 92:98S-110S. [PubMed] [Google Scholar]
- 13.Gobbetti, M., M. De Angelis, R. Di Cagno, F. Minervini, and A. Limitone. 2007. Cell-cell communication in food related bacteria. Int. J. Food Microbiol. 120:34-45. [DOI] [PubMed] [Google Scholar]
- 14.James, G. A., L. Beaudette, and J. W. Costerton. 1995. Interspecies bacterial interactions in biofilms. J. Ind. Microbiol. Biotechnol. 15:257-262. [Google Scholar]
- 15.Kandylis, P., A. Goula, and A. A. Koutinas. 2008. Corn starch gel for yeast cell entrapment. A view for catalysis of wine fermentation. J. Agric. Food Chem. 56:12037-12045. [DOI] [PubMed] [Google Scholar]
- 16.Kusumaningrum, H. D., G. Riboldi, W. C. Hazeleger, and R. R. Beumer. 2003. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int. J. Food Microbiol. 85:227-236. [DOI] [PubMed] [Google Scholar]
- 17.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Breese, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 19.Nychas, G.-J. E., D. Dourou, P. Skandamis, K. Koutsoumanis, J. Sofos, and J. Baranyi. 2009. Effect of microbial cell free meat extract on the growth of spoilage bacteria. J. Appl. Microbiol. 107:1819-1829. [DOI] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. U. S. A. 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silley, P., and S. Forsythe. 1996. Impedance microbiology: a rapid change for microbiologists. J. Appl. Bacteriol. 80:233-243. [DOI] [PubMed] [Google Scholar]
- 23.Skandamis, P. N., and G.-J. E. Nychas. 2002. Preservation of fresh meat with active and modified atmosphere packaging conditions. Int. J. Food Microbiol. 79:35-45. [DOI] [PubMed] [Google Scholar]
- 24.Smith, J. L., P. M. Fratamico, and J. S. Novak. 2004. Quorum sensing: a primer for food microbiologists. J. Food Prot. 67:1053-1070. [DOI] [PubMed] [Google Scholar]
- 25.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]