Abstract
To estimate the contribution of uncultured bacterial groups to fiber degradation, we attempted to retrieve both ecological and functional information on uncultured groups in the rumen. Among previously reported uncultured bacteria, fiber-associated groups U2 and U3, belonging to the low-GC Gram-positive bacterial group, were targeted. PCR primers and fluorescence in situ hybridization (FISH) probe targeting 16S rRNA genes or rRNA were designed and used to monitor the distribution of targets. The population size of group U2 in the rumen was as high as 1.87%, while that of group U3 was only 0.03%. Strong fluorescence signals were observed from group U2 cells attached to plant fibers in the rumen. These findings indicate the ecological significance of group U2 in the rumen. We succeeded in enriching group U2 using rumen-incubated rice straw as the inoculum followed by incubation in an appropriate medium with an agent inhibitory for Gram-negative bacteria. Consequently, we successfully isolated two strains, designated B76 and R-25, belonging to group U2. Both strains were Gram-positive short rods or cocci that were 0.5 to 0.8 μm in size. Strain B76 possessed xylanase and α-l-arabinofuranosidase activity. In particular, the xylanase activity of strain B76 was higher than that of xylanolytic Butyrivibrio fibrisolvens H17c grown on cellobiose. Strain R-25 showed an α-l-arabinofuranosidase activity higher than that of strain B76. These results suggest that strains B76 and R-25 contribute to hemicellulose degradation in the rumen.
Ruminants can utilize plant fiber as an energy source with the aid of a symbiotic relationship with microbes in the rumen. The rumen is a complex microbial ecosystem comprised of bacteria (1010 to 1011 per ml), protozoa (104 to 106 per ml), and fungi (103 to 106 per ml) (8, 23, 39). Of the rumen microbes, bacteria are considered to be primarily responsible for the biological degradation of plant fiber, due to their high fibrolytic activity and large biomass in the rumen. In order to determine the mechanism of plant fiber degradation in the rumen, numerous studies have been performed on both the physiological and ecological characteristics of rumen bacteria (16, 27, 36). In particular, various aspects of bacterial attachment to feed particles have been investigated (19, 21, 25), because attachment to plant fiber is a critical step in initiating fiber degradation (20).
Recent advances in molecular techniques have allowed recognition of a predominance of uncultured bacteria in the rumen (6, 24, 33). The majority (77%) of fiber-associated community members are uncultured bacteria, although 17% of cloned bacterial 16S rRNA gene sequences were classified as known fibrolytic species, such as Fibrobacter succinogenes and Butyrivibrio fibrisolvens (12). These findings clearly indicate the possibility for involvement of uncultured bacteria in ruminal fiber degradation. Through the phylogenetic analysis of fiber-associated community members, the unidentified bacterial groups were detected and designated uncultured group 2 (U2) and uncultured group 3 (U3). However, their roles in plant fiber digestion have yet to be determined.
The predominance of uncultured bacteria has also been pointed out in other environments (26). Recently, new strategies for cultivation have been introduced to resolve the problem of the bacteria being unculturable. Sait et al. (28) reported that culturing with a polymeric growth substrate and longer incubation time was effective for the isolation of previously uncultured bacteria from soil. Cultivation on low-nutrient medium, using increased incubation times, with simulated natural environments or using a membrane as a solid support for growth has apparently led to improvements in bacterial cultivation (7, 31). On the other hand, the majority of rumen bacteria have yet to be isolated (10) despite great efforts toward the isolation of rumen bacterial strains over the past 50 years. Considering the ecological significance of uncultured rumen bacteria, it is important to cultivate and characterize these bacteria to fully understand the ecology of fiber digestion.
In the present study, molecular monitoring tools were developed to obtain ecological information on target uncultured bacterial groups in the rumen. Previously uncultured rumen bacteria were then isolated and characterized to retrieve functional information.
MATERIALS AND METHODS
Phylogenetic analysis and primer design.
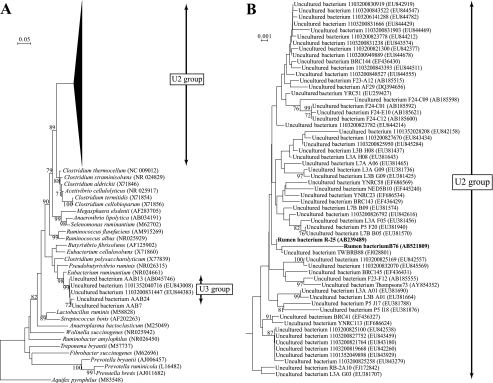
In order to design PCR primers targeting groups U2 and U3, phylogenetic analysis of the uncultured groups was carried out. All currently available 16S rRNA gene sequences belonging to groups U2 and U3 were downloaded from the GenBank database. As a reference for phylogenetic position, 27 sequences of 16S rRNA genes for known species belonging to various phyla were included in the analysis. The sequences were aligned with the Clustal X 2.0.1 multiple sequence alignment software (35) and imported into the MEGA 4.0 software (34). A phylogenetic tree was constructed using the neighbor-joining method with the Kimura two-parameter model in the MEGA software. The robustness of tree topology was tested by bootstrap analysis (1,000 replicates). Sequences within the clusters of groups U2 and U3 (Fig. 1) were separately aligned and compared to each other. PCR primers were designed to satisfy specificity and amplification of all sequences belonging to respective target groups. The primer sequences are shown in Table 1 with the corresponding target site, annealing temperature, and extension time.
FIG. 1.
Phylogenetic placement of 16S rRNA gene sequences of targeted groups U2 and U3. (A) Within a respective target group, each sequence showed >97% similarity. (B) Based on 97% sequence similarity, 59 sequences, including newly isolated strains B76 and R-25, shown in bold, were classified into group U2.
TABLE 1.
PCR primer sequences used in this study
| Target | Sequence (5′-3′) | Target positiona | Annealing temp (°C) | Extension time (s) | Reference |
|---|---|---|---|---|---|
| Group U2 | FW, CTAGGTGTAGGGGGTATC | 834 | 60 | 18 | This study |
| RV, GCTGCCCTCTGTCGTTG | 1259 | ||||
| Group U3 | FW, TTTGAAACTGTCTAGCTAGAGTA | 64 | 64 | 26 | This study |
| RV, CATCACTGCTCTGCTTCC | 1253 | ||||
| F. succinogenes | FW, GGTATGGGATGAGCTTGC | 219 | 60 | 18 | Koike et al. (13) |
| RV, GCCTGCCCCTGAACTATC | 654 | ||||
| Total bacteria | FW, CCTACGGGAGGCAGCAG | 341 | 60 | 10 | Koike et al. (13) |
| RV, ATTACCGCGGCTGCTGG | 534 |
The positions are based on the nucleotide position in Escherichia coli.
Animals and sampling.
All procedures for animal experiments were preapproved by the Animal Care and Welfare Committee of Hokkaido University.
(i) Rumen digesta.
Three sheep (average body weight, 61.3 ± 5.8 kg [mean ± standard deviation]) and four dry Holstein cows (average body weight, 879.5 ± 29.6 kg) were used in the present study. The animals had been fitted with a ruminal cannula. Sheep were fed orchardgrass hay (1.5 kg/day) and commercial formula feed for sheep (300 g/day; Ram 76ME; Mercian, Tokyo, Japan). The diet composition for cows was corn silage (35 kg/day), grass silage (20 kg/day), orchardgrass hay (3 kg/day), and commercial formula feed for cows (1.5 kg/day; New Lead 18; Hokuren, Sapporo, Japan). Sheep were fed once daily at 0900, while cows were fed five times (0300, 0600, 1800, 2100, and 2400) per day. The animals had free access to a mineral block and water.
Sampling was carried out before the morning feeding at 0600 for cows and 0900 for sheep. Rumen contents were collected from five locations in the rumen of each animal, and the subsamples were composited into one sample. In order to separate liquid and solid fractions, the composite sample was squeezed by hand through eight layers of cheesecloth. The liquid fraction was squeezed by hand again through a nylon mesh (45-μm pore size). The solid was washed with saline three times at 38°C and then squeezed by hand to remove excess saline. The samples were placed in 50-ml polypropylene tubes and stored at −80°C.
(ii) In situ-incubated hay.
Stems of orchardgrass hay were ground through a 1-mm screen and enclosed in a nylon bag (10 by 15 cm, with a 45-μm pore size). The bags were placed into the rumen of three sheep immediately prior to feeding. The sheep and their feeding regimen were the same as those mentioned above. Five nylon bags for each incubation time were placed into the rumen of each sheep. One bag at each interval was removed from the rumen of each sheep at 10 min and at 6, 12, 24, and 48 h. After removal from the rumen, the bags were rinsed thoroughly in water (38°C) and then stored at −80°C.
DNA extraction.
DNA was extracted from the samples by the bead beating method as previously described (11). Briefly, each sample (0.35 g) was mixed with 0.35 ml of Tris-EDTA buffer (pH 8.0) and 0.7 ml of Tris-buffered phenol (pH 8.0) in a 2-ml tube containing 0.25 g of glass beads (diameter, 425 to 600 μm; Sigma Chemical, St. Louis, MO). After adding 40 μl of 10% SDS, the tubes were shaken three times for 2 min with 2 min of incubation on ice between shaking sessions. Tubes were centrifuged at 16,000 × g for 5 min. The supernatant was purified by hydroxyapatite chromatography (Bio-Gel HTP hydroxyapatite gel; Bio-Rad, Hercules, CA) followed by gel filtration (MicroSpin S-200 HR columns; GE Healthcare, Tokyo, Japan). Purified DNA was quantified by fluorescence on a DyNA Quant 200 fluorometer (Hoefer Pharmacia Biotech, San Francisco, CA) and adjusted to 10 ng/μl.
Validation of real-time PCR assays.
Plasmid DNA to be used as the PCR standard was obtained by PCR cloning using the primer sets shown in Table 1, as previously described (13). As a standard plasmid for groups U2 and U3, the plasmids containing the respective target DNA fragments obtained in a previous study (12) were employed. The concentration of the plasmid was quantified by using a DyNA Quant 200 fluorometer. The copy number of each standard plasmid was calculated using the molecular weight of the nucleic acids and the length (in base pairs) of the cloned standard plasmid. A 10-fold dilution series ranging from 1 to 109 copies was prepared for each target. To assess the sensitivity and accuracy of assays, the quantification range was determined using the serial dilutions of standard plasmid as the template. Recovery of the target DNA added to the complex DNA was evaluated by spiking different quantities of standard plasmid into the rumen DNA, i.e., 1.0 × 104 to 1.0 × 107 copies of standard plasmid were added to the DNA extracted from the rumen fluid and then quantified by real-time PCR. Recovery was expressed as a percentage of the target quantified to the target added. The assay reproducibility was assessed by determining inter- and intra-assay variation with five replicates.
Real-time PCR was performed with a LightCycler system and the FirstStart DNA Master SYBR green I kit (Roche, Penzberg, Germany). The optimal amplification conditions for each primer set were obtained in 3.75 mM MgCl2 with 0.5 μM of each primer with the combination of annealing temperature and extension time reported in Table 1. As template, 10 ng of DNA was used for PCR. The temperature program consisted of denaturation at 95°C for 10 min, followed by 40 cycles consisting of 94°C for 15 s, annealing for 5 s, and extension at 72°C for the times shown in Table 1. The 10-fold dilution series of the standard plasmid for the respective target was run along with the samples. Amplification of each sample was performed in duplicate. Quantification was based on standard curves obtained from the amplification profile of known concentrations of the standard plasmid for the respective target. The assay values for group U2, group U3, and F. succinogenes were normalized to that of total bacteria. The normalized assay values were analyzed statistically with Student's t test at a significance level of 0.05.
FISH.
A 16S rRNA-targeted oligonucleotide probe (5′-TCCTTCCTCCGCGTTGTC-3′) for visualization of bacteria belonging to group U2 was designed. The specificity of the probe was checked with the Probe Match tool of RDP II (http://rdp.cme.msu.edu/index.jsp). The specificity was also checked manually using the multiple alignment data to confirm a 100% match with all sequences belonging to the U2 group. The 5′ end of the oligonucleotide probe was labeled with Cy3. The procedure for fluorescence in situ hybridization (FISH) was the same as that described by Shinkai and Kobayashi (29). The optimal hybridization stringency was determined by increasing the formamide concentration in the hybridization buffer in increments of 5% from 0 to 70% (18). Strong fluorescence signals were obtained in buffer containing 35% formamide. Pure cultures of group U2 strains (see below) and the hay stem incubated in the rumen (mentioned above) were employed for FISH detection. In order to reduce autofluorescence of the plant material, the samples incubated in the rumen were treated with toluidine blue O (29). Total bacteria were detected by staining with 4′,6′-diamidino-2-phenylindole (DAPI; 1.5 μg/ml) in Vectashield H-1200 mounting medium (Vector Laboratories, Burlingame, CA). The samples were examined with a BX 51 microscope (Olympus, Tokyo, Japan) equipped with a BX-URA2 universal reflected light illuminator (Olympus) and a cooled charge-coupled-device camera (CoolSNAP; Roper Scientific Photometrics, Tucson, AZ).
Enrichment process.
Rice straw or cellulose powder was used as fibrous substrate. Each powder was enclosed in separate nylon bags and then incubated in the rumen of the above-mentioned sheep for 6 h. After incubation, the bags were removed from the rumen and immediately shipped to the laboratory. One-half of the samples incubated in the rumen was inoculated into medium to enrich group U2, while the rest of the samples were used to quantify group U2 by a real-time PCR assay. In order to inhibit the growth of Gram-negative bacteria, phenylethyl alcohol (0.5%, final vol/vol) (5) was added to RGC broth (22) with a modification of the carbon source (glucose and cellobiose were replaced with rice straw). The composition of the medium (per liter) was as follows: mineral solution I, 75 ml; mineral solution II, 75 ml; clarified rumen fluid, 400 ml; resazurin (0.1%), 1 ml; NaHCO3 (8%), 50 ml; l-cysteine hydrochloride, 0.5 g; ball-milled rice straw (2.5%), 200 ml; distilled water, 200 ml. Mineral solutions I and II were as described by Bryant and Burkey (2). The medium was incubated at 39°C for 5 days following the Hungate technique of strict anaerobic culturing (9). In order to monitor the proportion of group U2 during enrichment, the culture was sampled at 1 day and 5 days after incubation, and population sizes of total bacteria and group U2 in the medium were monitored by real-time PCR assays.
Isolation of group U2 strains.
For the isolation of strains belonging to group U2, 5-day cultures were used as the inoculum. The enrichment was serially diluted in a dilution solution for anaerobes (22). To prepare roll tubes, dilutions from 10−6 to 10−4 in a volume of 0.2 ml were transferred into 4.8 ml of RGC agar medium containing rice straw as the sole carbon source. Roll tubes were incubated at 39°C for up to 14 days. Clearly isolated colonies were picked to RGC slant medium containing glucose and cellobiose as the carbon sources. After growth on the slant medium, the isolates were transferred into RGC broth and incubated until mid-log phase. DNA was extracted from the culture using PrepMan Ultra reagent (Applied Biosystems, Foster City, CA) according to the manufacturer's recommendations. PCR screening was performed on DNA from the isolates using group U2-specific primers (Table 1). PCR was conducted using GoTaq DNA polymerase (Promega, Tokyo, Japan). A reaction mixture containing 0.4 μM of each primer, 0.2 mM each deoxyribonucleotide triphosphate, 1× Green GoTaq buffer, 1.25 U of GoTaq DNA polymerase, and 1.0 μl of template DNA in a total volume of 10 μl was prepared. The temperature program consisted of denaturation at 94°C for 2 min, followed by 40 cycles consisting of 94°C for 30 s, 60°C for 30 s, and extension at 72°C for 30 s, with a final extension at 72°C for 7 min. The correct size of PCR product was verified on an agarose gel. For isolates showing positive amplification by group U2-specific PCR, the 16S rRNA gene (ca. 1,500 bp) was amplified using the bacterial universal primers 27F (5′-AGAGTTTGATCCTGGCTCAGA-3′) and 1525R (5′-AAGGAGGTGWTCCARCC-3′). PCR was performed with 25 pmol of each primer and an ExTaq PCR kit (Takara Bio, Otsu, Japan) using a final reaction mixture volume of 25 μl. The cycle conditions consisted of denaturation at 94°C for 5 min, followed by 35 cycles consisting of 94°C for 30 s, 58°C for 30 s, and extension at 72°C for 1.5 min with a final extension at 72°C for 7 min. PCR products were cloned with a pGEM-T Easy vector system (Promega). Sequencing was performed on both strands (primers SP6, 530F, and T7) by Takara Bio Inc. (Otsu, Japan). The sequences obtained were analyzed phylogenetically as described above. Two isolates belonging to group U2 were obtained and named strains B76 and R-25.
Enzyme assays.
B. fibrisolvens H17c has been considered as the representative for hemicellulolytic strains in the rumen, and this strain was employed as a reference strain for the enzyme assays. Isolated strains and B. fibrisolvens H17c were grown in RGC medium containing cellobiose as the sole carbon source to mid-log phase, and the cells were then harvested by centrifugation (12,000 × g, 4°C, 15 min). The resulting cell pellets were washed twice with 50 mM sodium phosphate buffer (pH 6.8) and resuspended in 2% of the original volume in the same buffer. The cell suspensions were sonicated 10 times for 1 min with 1 min of incubation on ice between each sonication period. These crude cell extracts were centrifuged (12,000 × g, 4°C, 15 min), and the supernatants were employed for enzyme assays. The activities of carboxymethylcellulase (CMCase) and xylanase were determined by monitoring the increase in reducing sugar formation from the substrates using dinitrosalicylic acid reagents as described by Cotta (4). Carboxymethylcellulose and oat spelt xylan were dissolved in 50 mM sodium phosphate buffer (pH 6.8) at 1% (wt/vol) and used as substrates. The activities of β-glucosidase, β-xylosidase, α-l-arabinofuranosidase, cellobiohydrolase, and acetylesterase were determined using p-nitrophenyl β-d-glucopyranoside, p-nitrophenyl β-d-xylopyranoside, p-nitrophenyl α-l-arabinofuranoside, p-nitrophenyl β-d-cellobioside, and p-nitrophenyl acetic acid as substrates, respectively (37). Protein concentrations in the samples were measured with the Bio-Rad protein assay kit (Bio-Rad, Tokyo, Japan). One unit of enzyme activity was defined as 1 nmol of sugar or p-nitrophenol formed per minute by mg of protein.
Nucleotide sequence accession numbers.
The B76 and R-25 nucleotide sequences have been deposited in GenBank under the accession numbers AB521809 (strain B76) and AB239489 (strain R-25).
RESULTS
Validation of real-time PCR assays for groups U2 and U3.
A summary of the validation of real-time PCR assays is shown in Table 2. The values for F. succinogenes and total bacteria were obtained from a previous report (13). Linear regression values for threshold cycle versus quantity of the standard for groups U2 and U3 were 0.9979 and 0.9984, respectively (Table 2). The lowest copy number plotted on the regression for the assay for groups U2 and U3 is 100 copies, which corresponds to the minimum detection limit for the target in the pure culture. DNA recoveries, determined by comparison between added and recovered copy numbers of the target, were 103.1% (group U2) and 104.8% (group U3). The intra-assay variations for groups U2 and U3 were 1.73% and 3.85%, respectively. The interassay variations for groups U2 and U3 were 13.78% and 6.04%, respectively (Table 2).
TABLE 2.
Summary of the validation of real-time PCR assays
| Target | Range (log copies of target DNA)a | R2b | Recovery (%)c | % Variation (CV)d |
|
|---|---|---|---|---|---|
| Intra-assay | Interassay | ||||
| Group U2 | 2-9 | 0.9979 | 103.1 ± 8.1 | 1.73 | 13.78 |
| Group U3 | 2-9 | 0.9984 | 104.8 ± 8.5 | 3.85 | 6.04 |
| F. succinogenes | 1-9 | 1.0000 | 82.4 ± 8.4 | 7.50 | 11.60 |
| Total bacteria | 4-9 | 0.9982 | NDe | 8.03 | 11.00 |
The quantification range was determined using serial dilutions of standard plasmid for each target.
Regression of the standard curve obtained from the relationship between quantities of standard and the threshold cycle.
Recovery was calculated as the percentage of target quantified against target added to DNA extracted from rumen fluid.
The coefficient of variation (CV) was calculated from repeat quantifications using the same sample (intra-assay) and for repeat DNA extraction and quantifications separately (interassay).
ND, not determined.
Molecular monitoring of groups U2 and U3 in the rumen.
Distributions of group U2, group U3, and F. succinogenes in different fractions of rumen digesta are shown in Table 3. Irrespective of sample fraction and animal species (either sheep or cattle), the population size of F. succinogenes was the highest, followed by groups U2 and U3. In particular, group U3 showed a quite low population size compared to group U2 and F. succinogenes. F. succinogenes was detected at a similar level in the solid and liquid fractions, while the population size of group U2 was significantly higher in the solid fraction (Table 3).
TABLE 3.
Distribution of group U2, group U3, and F. succinogenes in different fractions of rumen digesta
| Target | % of total bacteriaa |
|||
|---|---|---|---|---|
| Sheep |
Cattle |
|||
| Solid fraction | Liquid fraction | Solid fraction | Liquid fraction | |
| Group U2 | 0.98 ± 0.27** | 0.64 ± 0.15 | 1.87 ± 0.77* | 0.96 ± 0.12 |
| Group U3 | 0.02 ± 0.02 | 0.03 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| F. succinogenes | 4.61 ± 0.84 | 3.98 ± 0.78 | 6.07 ± 4.13 | 5.17 ± 0.85 |
**, P = 0.04 versus liquid fraction of sheep; *, P = 0.06 versus liquid fraction of cattle.
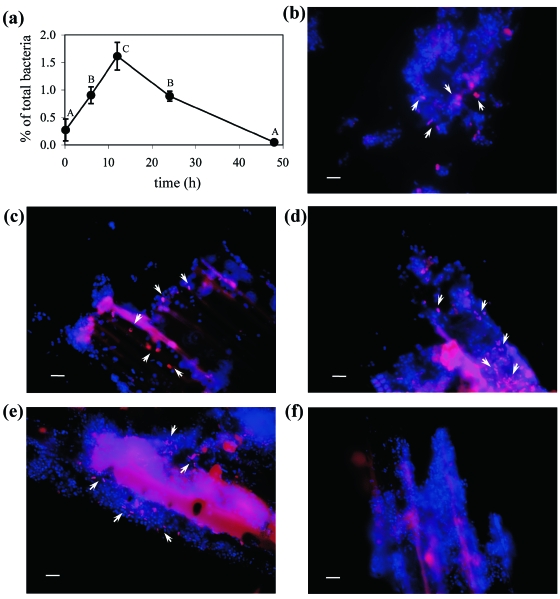
Changes in population size of group U2 on hay incubated in the rumen of three sheep are shown in Fig. 2a. The quantification value for each incubation time was obtained from one bag from each sheep, i.e., each value corresponds to the average of three bags. After 10 min of incubation, the proportion of group U2 in the total bacteria was 0.3%, increasing to 0.9% at 6 h (Fig. 2a). The proportion of group U2 increased linearly up to 12 h of incubation and reached 1.6%. After 12 h, a linear decrease was observed, and the proportion decreased to 0.05% after 48 h of incubation. We also confirmed population changes of group U2 in the same sample by FISH (Fig. 2b to f). Fluorescence signals of group U2 cells from in situ-incubated hay gradually increased until 24 h, but no clear signals were detected after 48 h of incubation. The morphology of the fluorescing cells was short rod to coccoid (Fig. 2 and 3) and resembled the shape of isolates belonging to group U2 described below.
FIG. 2.
Molecular monitoring of group U2 associated with orchardgrass hay incubated in the rumen. (a) The time course of population size of group U2 was determined by real-time PCR from 10 min to 48 h of incubation. Error bars represent standard deviations for real-time PCR quantification of the samples from three sheep. A, B, and C on the graph indicate significant differences (P < 0.01) as determined by Student's t test. (b to f) Fluorescence images show FISH detection of group U2 cells after 10 min (b), 6 h (c), 12 h (d), 24 h (e), and 48 h (f) of ruminal incubation. In the FISH images, target cells were stained red (Cy3), while the other bacteria were stained blue (DAPI). Arrows indicate the target cells. Bars, 5 μm.
FIG. 3.
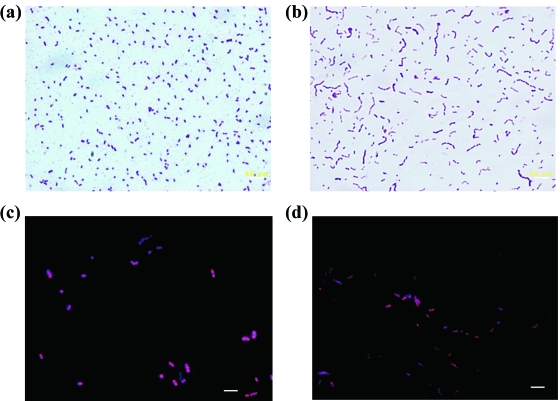
Morphology and Gram staining of pure cultures of strain B76 (a) and strain R-25 (b) grown in RGC broth and detection of fluorescence signals from strains B76 (c) and R-25 cells (d). Bars, 5 μm.
Isolation and partial characterization of group U2 strains.
Through the enrichment process, the relative abundance of group U2 significantly increased (Table 4). In the original rumen digesta, the abundance of group U2 was 0.36% of total bacteria. After incubation of rice straw in the rumen, we obtained an inoculum having a higher proportion (1.12%) of group U2. A further increase in group U2 abundance was achieved using a conventional culturing technique for anaerobic bacteria in medium containing phenylethyl alcohol as an agent inhibitory to Gram-negative bacteria. Consequently, the proportion of group U2 in the final culture was 2.95%, which was an almost 8.2-fold enrichment in group U2 (Table 4). More than 300 picked colonies were tested for PCR screening of group U2, and two isolates showed a positive signal. Sequence similarity analysis of ∼1,500 16S rRNA gene nucleotides showed that both strains had ≥97% similarity with 16S rRNA gene sequences belonging to group U2. We designated these two strains B76 and R-25. The sequence similarity of 16S rRNA genes between strains B76 and R-25 was 97.7% (1,451/1,485 nucleotides). Strain B76 had the closest match (97.5%; 1,481/1,519 nucleotides) with uncultured rumen bacterium clone F23-F12 (AB185555) retrieved from cattle rumen (24), while strain R-25 showed the highest similarity (99.7%; 1,479/1,485 nucleotides) with uncultured rumen bacterium clone TWBRB88 (FJ028801) obtained from the rumen of Taiwan water buffalo (published in the GenBank database). Phylogenetic analysis of 16S rRNA genes confirmed that strains B76 and R-25 fall into the cluster of group U2 (Fig. 1).
TABLE 4.
Real-time PCR quantification of group U2 and total bacteria during the enrichment processa
| Sample source | Group U2 (×108 copies/g) | Total (×1011 copies/g) | % of total |
|---|---|---|---|
| Rumen digesta | 4.82 ± 0.08 | 1.34 ± 0.11 | 0.36 ± 0.03 A |
| Inoculum | 34.20 ± 0.59 | 3.07 ± 0.25 | 1.12 ± 0.10 B |
| 1-day culture | 7.55 ± 0.13 | 0.50 ± 0.04 | 1.53 ± 0.12 C |
| 5-day culture | 9.78 ± 0.17 | 0.33 ± 0.03 | 2.95 ± 0.19 D |
Within a column, means followed by different capital letters differed significantly (P < 0.05).
The morphology and Gram staining of strains B76 and R-25 are shown in Fig. 3. Both strains were Gram-positive, 0.5- to 0.8-μm short rods to cocci. Strain B76 was found as single cells and diplococci, while strain R-25 tended to be chains of 5 to 10 cells. Strong FISH signals were observed from the mid-log phase of pure cultures of both strains (Fig. 3c and d).
The fibrolytic enzyme activities of strains B76 and R-25 are shown in Table 5. Strains B76 and R-25 did not grow on xylan or cellulose, while they grew in the medium containing glucose, cellobiose, or xylose (data not shown). Both strains showed better growth on cellobiose medium than on glucose or xylose medium. Therefore, the culture grown on cellobiose was employed for the enzyme assay. Strain B76 possessed xylanase and arabinofuranosidase activities. In particular, the xylanase activity of this strain was higher than that of xylanolytic B. fibrisolvens H17c when cultured on cellobiose. Strain R-25 showed CMCase, xylanase, and α-l-arabinofuranosidase activity. The activities of CMCase and xylanase were weak, while α-l-arabinofuranosidase activity was higher than that of strain B76 and B. fibrisolvens H17c.
TABLE 5.
Fibrolytic enzyme activities of newly isolated strains B76 and R-25 belonging to the U2 group
| Strain | Enzyme activity (U/mg of protein)a |
||||
|---|---|---|---|---|---|
| Carboxymethylcellulase | Xylanase | Cellobiohydrolase | α-l-Arabinofuranosidase | β-Glucosidase | |
| B76 | ND | 48.8 A | ND | 9.3 B | ND |
| R-25 | 3.1 | 1.2 C | ND | 19.5 A | ND |
| B. fibrisolvens H17c | 2.3 | 14.3 B | 2.4 | 5.0 B | 1.2 |
One unit of enzyme activity was defined as 1 nmol of sugar or p-nitrophenol formed per min. Within a column, means followed by different capital letters differed significantly (P < 0.05). ND, not detected. The activities of β-xylosidase and acetylesterase were not detected in the two newly isolated strains.
DISCUSSION
In the last decade, a predominance of uncultured bacteria has been demonstrated in various environments (26), and rumen microbiota are not exceptional (6, 24, 33). We explored the fiber-associated bacterial community in the rumen of sheep and determined that the majority (77%) of community members had less than 97% identity with 16S rRNA gene sequences of known bacteria (12). To estimate the contribution of uncultured groups to fiber degradation, we attempted to retrieve both ecological and functional information on uncultured bacterial groups in the rumen.
We successfully developed real-time PCR assays for groups U2 and U3 (Table 2). The reliability of the assays was comparable to other real-time PCR assays for rumen bacteria (13), ensuring precise quantification of the target groups. In addition to PCR-based quantification, a FISH probe for group U2 was designed to visualize uncultured bacteria in the rumen. The oligonucleotide probe designed had no mismatches with the 16S rRNA sequence of the target, while it had two mismatches in the central portion of the hybridization region with Clostridium thermocellum, which is the closest nontarget. We confirmed that the FISH probe for group U2 did not give a fluorescence signal in pure cultures of C. thermocellum ATCC 27405 (data not shown). Amann et al. (1) reported that a single mismatch in the central portion of the hybridization region was sufficient to ensure specificity. Burrell et al. (3) achieved specific FISH detection of the target by using oligonucleotide probes having two mismatches with nontarget sequences. Therefore, the probe we designed for group U2 with two mismatches with a nontarget is considered to be specific to the target.
The relative abundance of group U2 was as high as 1.87%, while that of group U3 was only 0.03% (Table 3). Stevenson and Weimer (32) quantified 13 representative ruminal species in the rumen of cattle. They reported that only two species (Prevotella bryantii and Prevotella ruminicola) accounted for >1% of total bacteria; the abundance of another seven species ranged from 0.135 to 0.835%, while that of the remaining four species was <0.03%. Therefore, the abundance of group U2 was reasonably comparable to that of representative ruminal species, while group U3 seemed to be a minor group in the rumen. A higher proportion of group U2 was observed in the solid fraction of rumen contents collected from sheep and cattle (Table 3). This result was expected, because group U2 was originally detected as a fiber-associated bacterial group in sheep rumen (12). Further quantitative and qualitative monitoring of group U2 was carried out for hay incubated in the rumen. The abundance of group U2 in hay incubated in the rumen increased with incubation time and reached a maximum after 12 h of incubation (Fig. 2a). Therefore, group U2 might be able to proliferate and maintain its population size on plant fiber. Similar population changes of the representative fibrolytic rumen bacteria were observed in the previous study (11), i.e., the population sizes of F. succinogenes, Ruminococcus flavefaciens, and Ruminococcus albus on ruminally incubated hay peaked at 24 h or 48 h, and then they decreased to 20% of the respective maximum levels at 96 h. The dynamics of fiber-degrading populations can be explained by the changes in the fiber substrate. The representative fibrolytic species and group U2 probably utilize potentially digestible substrates of plant fiber for their proliferation, and then the population size of these bacteria may decline, accompanied by a decreased digestible fraction of plant fiber. FISH analysis supported the data from the real-time PCR assay, i.e., the number of cells showing strong signal increased with incubation time (Fig. 2b to f). Since rRNA is needed for protein synthesis, FISH signals detected by an rRNA-targeted oligonucleotide probe reflect the protein biosynthetic activity of the target cell (1). The reasonable proportions and strong FISH signals of group U2 grown on plant fiber suggest that this group plays a role in plant fiber digestion in the rumen.
Cultivation of previously uncultured bacteria is a direct approach to obtain functional information on these bacteria, and culturing attempts have been made for this purpose for bacteria from various environments (31, 41). We employed an enrichment process to increase the chance of obtaining group U2 strains. To evaluate the efficacy of the process, the relative abundance of group U2 was monitored by real-time PCR. In a preliminary study, we observed that the abundance of group U2 was higher in rice straw that had been incubated in the rumen than in other samples. Therefore, we hypothesized that group U2 might prefer rice straw as a growth substrate and used it as the sole carbon source through the enrichment process. Comprehensive analysis of the rumen bacterial ecosystem revealed that Gram-negative bacteria occupied around 50% of the ecosystem (6). Therefore, a growth inhibitor for Gram-negative bacteria seemed likely to be useful to enrich group U2 strains belonging to the low-GC Gram-positive phylum. In the present trial, 70% of isolated strains were Gram positive (data not shown), suggesting the efficacy of phenylethyl alcohol in inhibiting the growth of Gram-negative bacteria. We succeeded in enriching group U2 using rice straw incubated first in the rumen followed by incubation in the appropriate medium supplemented with phenylethyl alcohol. Consequently, we successfully obtained two strains belonging to group U2 (Fig. 1 and 3). The information from culture-independent approaches was essential to succeed in the isolation of previously uncultured bacteria, i.e., phylogenetic information was needed for the selection of the Gram-positive target, and both assessment of enrichment and PCR screening of the previously uncultured target were provided by culture-independent molecular techniques. Thus, the combination of culture-dependent and culture-independent approaches is useful for isolation of previously uncultured bacteria.
The newly isolated strains B76 and R-25 had high similarity in 16S rRNA gene sequences (97.7%); however, their morphology and enzyme activity profiles differed (Fig. 3; Table 5). Phenotypic and genetic diversities have been reported within other rumen bacterial species, such as Butyrivibrio spp. (14, 38) and Prevotella spp. (40). Differences in digestive ability for several plant fibers were observed among strains of F. succinogenes (30), R. flavefaciens, and R. albus (15). As indicated for these ruminal species, there might be phenotypic variation among the strains belonging to group U2. Among the fibrolytic enzymes, the xylanase activity of strain B76 and α-l-arabinofuranosidase activity of strain R-25 were higher than those of the representative hemicellulolytic B. fibrisolvens H17c (Table 5). These findings indicate that these two strains may be members of the fiber-degrading community in the rumen. Based on molecular monitoring of group U2 on hay incubated in the rumen, this group seems to grow on plant fiber (Fig. 2). Plant fiber is a complex matrix of polymers, which is mainly composed of cellulose and hemicellulose. Cellulose fibrils are embedded in a matrix of hemicellulose, and hemicellulose must be degraded before cellulose can be effectively degraded by cellulolytic bacteria (17). Xylanse and α-l-arabinofuranosidase attack the xylan backbone and arabinose side chain of xylan, respectively, and they play a significant role in hemicellulose degradation. Therefore, group U2 having the activity of these enzymes may contribute to ruminal fiber degradation via hemicellulose reduction.
This is the first report providing ecological and physiological information on strains belonging to previously uncultured bacterial group U2 in the rumen. We demonstrated that a combination of culture-dependent and culture-independent approaches facilitates the isolation of previously uncultured bacteria from the rumen. The U2 group was detected at a proportion comparable to representative rumen bacterial species, such as F. succinogenes, R. flavefacien, and Prevotella spp. We confirmed that the abundance of group U2 increased with incubation time on hay incubated in the rumen. In addition, the newly isolated strains belonging to group U2 possessed xylanase and α-l-arabinofuranosidase activity. These data suggest the possible involvement of this group in plant fiber digestion.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research (number 18880002 to S.K. and numbers 15580231 and 17380157 to Y.K.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This study was supported in part by the national projects “Special Coordination Funds for Promoting Science and Technology” of the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Published ahead of print on 29 January 2010.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryant, M. P., and L. A. Burkey. 1953. Cultural methods and some characteristics of some of the more numerous groups of bacteria in the bovine rumen. J. Dairy Sci. 36:205-217. [Google Scholar]
- 3.Burrell, P. C., C. O'Sullivan, H. Song, W. P. Clarke, and L. L. Blackall. 2004. Identification, detection, and spatial resolution of Clostridium populations responsible for cellulose degradation in a methanogenic landfill leachate bioreactor. Appl. Environ. Microbiol. 70:2414-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotta, M. A. 1988. Amylolytic activity of selected species of ruminal bacteria. Appl. Environ. Microbiol. 54:772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell, V. R., Jr., E. O. Hill, and W. A. Altemeier. 1964. Use of phenethyl alcohol in media for isolation of anaerobic bacteria. J. Bacteriol. 88:1811-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards, J. E., N. R. McEwan, A. J. Travis, and R. J. Wallace. 2004. 16S rDNA library-based analysis of ruminal bacterial diversity. Antonie Van Leeuwenhoek 86:263-281. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari, B. C., T. Winsley, M. Gillings, and S. Binnerup. 2008. Cultivation previously uncultured soil bacteria using a soil substrate membrane system. Nat. Protoc. 3:1261-1269. [DOI] [PubMed] [Google Scholar]
- 8.Hespell, R. B., D. E. Akin, and B. A. Dehority. 1997. Bacteria, fungi, and protozoa of the rumen, p. 59-141. In R. I. Mackie, B. A. White, and R. E. Isaacson (ed.), Gastrointestinal microbiology, vol. 2. Chapman and Hall, New York, NY.
- 9.Hungate, R. E. 1950. The anaerobic mesophilic cellulolytic bacteria. Bacteriol. Rev. 14:1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi, Y. 2006. Inclusion of novel bacteria in rumen microbiology: need for basic and applied science. Anim. Sci. J. 77:375-385. [Google Scholar]
- 11.Koike, S., J. Pan, Y. Kobayashi, and K. Tanaka. 2003. Kinetics of in sacco fiber-attachment of representative ruminal cellulolytic bacteria monitored by competitive PCR. J. Dairy. Sci. 86:1429-1435. [DOI] [PubMed] [Google Scholar]
- 12.Koike, S., S. Yoshitani, Y. Kobayashi, and K. Tanaka. 2003. Phylogenetic analysis of fiber-associated rumen bacterial community and PCR detection of uncultured bacteria. FEMS Microbiol. Lett. 229:23-30. [DOI] [PubMed] [Google Scholar]
- 13.Koike, S., H. Yabuki, and Y. Kobayashi. 2007. Validation and application of real-time polymerase chain reaction assays for representative rumen bacteria. Anim. Sci. J. 78:135-141. [Google Scholar]
- 14.Kopečný, J., M. Zorec, J. Mrázek, Y. Kobayashi, and R. Marinšek-Logar. 2003. Butyrivibrio hungatei sp. nov. and Pseudobutyrivibrio xylanovorans sp. nov., butyrate producing bacteria from the rumen. Int. J. Syst. Evol. Microbiol. 53:201-209. [DOI] [PubMed] [Google Scholar]
- 15.Krause, D. O., R. J. Bunch, W. J. Smith, and C. S. McSweeney. 1999. Diversity of Ruminococcus strains: a survey of genetic polymorphisms and plant digestibility. J. Appl. Microbiol. 86:487-495. [Google Scholar]
- 16.Latham, M. J., B. E. Brooker, G. L. Pettipher, and P. J. Harris. 1978. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl. Environ. Microbiol. 35:1166-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leschine, S. B. 1995. Cellulose degradation in anaerobic environments. Annu. Rev. Microbiol. 49:399-426. [DOI] [PubMed] [Google Scholar]
- 18.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst. Appl. Microbiol. 1:593-600. [Google Scholar]
- 19.Minato, H., and T. Suto. 1978. Technique for fractionation of bacteria in rumen microbial ecosystem. II. Attachment of bacteria isolated from bovine rumen to cellulose powder in vitro and elution of bacteria attached therefrom. J. Gen. Appl. Microbiol. 24:1-16. [Google Scholar]
- 20.Miron, J., D. Ben-Ghedalia, and M. Morrison. 2001. Adhesion mechanisms of rumen cellulolytic bacteria. J. Dairy Sci. 84:1294-1309. [DOI] [PubMed] [Google Scholar]
- 21.Mosoni, P., G. Fonty, and P. Gouet. 1997. Competition between ruminal cellulolytic bacteria for adhesion to cellulose. Curr. Microbiol. 35:44-47. [DOI] [PubMed] [Google Scholar]
- 22.Ogimoto, K., and S. Imai. 1981. Atlas of rumen microbiology. Japan Scientific Society Press, Tokyo, Japan.
- 23.Orpin, C. G., and K. N. Joblin. 1997. The rumen anaerobic fungi, p. 140-195. In P. N. Hobson and C. S. Stewart (ed.), The rumen microbial ecosystem. Blackie Academic and Professional Publishers, London, United Kingdom.
- 24.Ozutsumi, Y., K. Tajima, A. Takenaka, and H. Itabashi. 2005. The effect of protozoa on the composition of rumen bacteria in cattle using 16S rRNA gene clone libraries. Biosci. Biotechnol. Biochem. 69:499-506. [DOI] [PubMed] [Google Scholar]
- 25.Pegden, R. S., M. A. Larson, R. J. Grant, and M. Morrison. 1998. Adherence of the Gram-positive bacterium Ruminococcus albus to cellulose and identification of a novel form of cellulose-binding protein which belongs to the Pil family of proteins. J. Bacteriol. 180:5921-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rappé, M. S., and S. J. Giovannoni. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57:369-394. [DOI] [PubMed] [Google Scholar]
- 27.Rincon, M. T., T. Čepeljnik, J. C. Martin, R. Lamed, Y. Barak, E. A. Bayer, and H. J. Flint. 2005. Unconventional mode of attachment of the Ruminococcus flavefaciens cellulosome to the cell surface. J. Bacteriol. 187:7569-7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sait, M., P. Hugenholtz, and P. H. Janssen. 2002. Cultivation of globally distributed soil bacterium from phylogenetic lineages previously only detected in cultivation-independent surveys. Environ. Microbiol. 4:654-666. [DOI] [PubMed] [Google Scholar]
- 29.Shinkai, T., and Y. Kobayashi. 2007. Localization of ruminal cellulolytic bacteria on plant fibrous materials as determined by fluorescence in situ hybridization and real-time PCR. Appl. Environ. Microbiol. 73:1646-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinkai, T., R. Ohji, N. Matsumoto, and Y. Kobayashi. 2009. Fibrolytic capabilities of ruminal bacterium Fibrobacter succinogenes in relation to its phylogenetic grouping. FEMS Microbiol. Lett. 294:183-190. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson, B. S., S. A. Eichorst, J. T. Wertz, T. M. Schmidt, and J. A. Breznak. 2004. New strategies for cultivation and detection of previously uncultured microbes. Appl. Environ. Microbiol. 70:4748-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson, D. M., and P. J. Weimer. 2007. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 75:165-174. [DOI] [PubMed] [Google Scholar]
- 33.Tajima, K., R. I. Aminov, T. Nagamine, K. Ogata, M. Nakamura, H. Matsui, and Y. Benno. 1999. Rumen bacterial diversity as determined by sequence analysis of 16S rDNA libraries. FEMS Microbiol. Ecol. 29:159-169. [Google Scholar]
- 34.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 35.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varel, V. H., and B. A. Dehority. 1989. Ruminal cellulolytic bacteria and protozoa from bison, cattle-bison hybrids, and cattle fed three alfalfa-corn diets. Appl. Environ. Microbiol. 55:148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitehead, T. R., and R. B. Hespell. 1990. The genes for three xylan-degrading activities from Bacteroides ovatus are clustered in a 3.8-kilobase region. J. Bacteriol. 172:2408-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willems, A., M. Amat-Marco, and M. D. Collins. 1996. Phylogenetic analysis of Butyrivibrio strains reveals three distinct groups of species within the Clostridium subphylum of the gram-positive bacteria. Int. J. Syst. Evol. Bacteriol. 46:195-199. [DOI] [PubMed] [Google Scholar]
- 39.Williams, A. G., and G. S. Coleman. 1997. The rumen protozoa. In P. N. Hobson and C. S. Stewart (ed.), The rumen microbial ecosystem, p. 73-139. Blackie Academic and Professional Publishers, London, United Kingdom.
- 40.Wood, J., K. P. Scott, G. Avgustin, C. J. Newbold, and H. J. Flint. 1998. Estimation of the relative abundance of different Bacteroides and Prevotella ribotypes in gut samples by restriction enzyme profiling PCR-amplified 16S rRNA gene sequences. Appl. Environ. Microbiol. 64:3683-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zengler, K., G. Toledo, M. Rappé, J. Elkins, E. J. Mathur, J. M. Short, and M. Keller. 2002. Cultivating the uncultured. Proc. Natl. Acad. Sci. U. S. A. 99:15681-15686. [DOI] [PMC free article] [PubMed] [Google Scholar]