Abstract
The study was to determine the efficacy of copper-silver ionization against the formation of Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Acinetobacter baumannii in biofilms and planktonic phases. At concentrations below the EPA limits, ionization has potential to control the three waterborne pathogens, in addition to Legionella, in hospital water systems for nosocomial infection control.
Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Acinetobacter baumannii are the waterborne pathogens commonly found in chlorinated potable water and linked to nosocomial infections (2, 6, 12, 15, 20-22). These pathogens exist in both free-flowing planktonic cells and biofilm-associated sessile cells adhering to pipe inner surfaces (14, 23). Pathogens persisting in biofilms are much more resistant to disinfectants than planktonic cells of the same isolate (5, 16, 19). The control of pathogens in biofilms is a challenge to health care facilities for prevention of nosocomial infections.
Copper-silver ionization systems have emerged as a long-term disinfection method for Legionella in hospital water systems (4, 10, 13, 17, 18). Copper and silver ions have demonstrated in vitro efficacy against the waterborne pathogens (9). However, the efficacy against biofilm-associated pathogens has not yet been investigated. Thus, the objective is to evaluate copper and silver ions as a disinfection method against P. aeruginosa, S. maltophilia, and A. baumannii in a model plumbing system that simulates water distribution systems. Our finding may determine if this ionization method can be applied for control of waterborne pathogen colonization in hospital water systems.
Environmental isolates of P. aeruginosa, S. maltophilia, and A. baumannii were selected and prepared as previously described (9). Four liters of bacteria suspension was made to achieve the initial concentration of 3 × 106 CFU/ml for each experiment. The inoculum solution consisted of 4 liters of the bacterial suspension (3 × 106 CFU/ml), 400 liters of dechlorinated tap water, and 1 liter of sterile nutrient supplement solution (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl per liter). The total volume was 405 liters.
A model plumbing system was designed as a partially open system, consisting of four transparent PVC biofilm sampling pipes (Fig. 1). Each experiment was divided into two stages at room temperature: a 14-day inoculation period followed by a 120-hour disinfection. The four-loop system was first inoculated with 405 liters of inoculum solution recirculating through all four loops simultaneously for 14 days at a flow rate of 10 liters/min measured by the flow meter. During the disinfection period, inoculum solutions and disinfectant solution were added into the four individual loops (approximately 100 liters in each loop) and the circulation within each loop was maintained using individual pumps. A 72-h ion maintenance period was selected because we found regrowth of pathogens in both biofilms and planktonic phases within 24 h in prior experiments when the disinfectants were added only at the beginning of the experiment. To overcome this regrowth and better simulate the conditions in the field, we maintained the ion concentrations for the first 72 h at every sampling point and supplied disinfectants, if needed.
FIG. 1.
Model plumbing system.
The copper-silver ions were generated by a commercial ionization system (Liquidator S50; LiquiTech, Inc., Lombard, IL) at concentrations of Cu/Ag targeted at 0.8/0.08, 0.4/0.04, and 0.2/0.02 mg/liter (EPA limits: Cu, 1.3 mg/liter; Ag, 0.1 mg/liter). The ion concentrations were confirmed by an inductively coupled plasma (PerkinElmer, Waltham, MA). Biofilms and water samples were collected at 0, 3, 6, 12, 24, 48, 72, 96, and 120 h. Biofilm samples were taken by swabbing the inner surface of a premeasured section of the sampling pipe using a sterile swab. The swab was vortexed for 1 min in 2 ml sterilized deionized water with 20 μl neutralizer before plating. A 10-ml planktonic sample was collected from each loop, diluted, and plated onto MacConkey's culture medium for enumeration. Each disinfection experiment for an individual pathogen was conducted at least twice for consistency. SPSS v17.0 software was used for calculation of the 95% confidence interval from the mean value (in logarithms) of each data point.
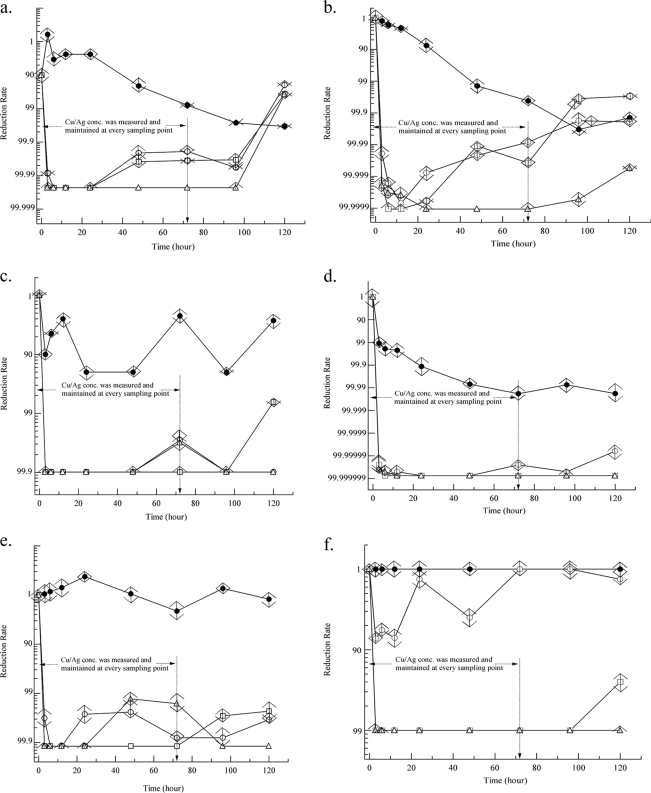
During the first 72 h of the experiment, when the Cu/Ag concentrations were maintained as described previously, all Cu/Ag concentrations tested (0.2/0.02 to 0.8/0.08 mg/liter) achieved complete inactivation of biofilm-associated P. aeruginosa within the first 24 h (Fig. 2a). P. aeruginosa concentrations in both biofilms and planktonic samples reached the baseline level after the 72-h ion maintenance period (Fig. 2a and b). This result suggests that maintaining ion concentrations is successful in controlling P. aeruginosa. Cu/Ag concentrations tested (0.2/0.02 to 0.8/0.08 mg/liter) achieved complete inactivation of biofilms (3-log reduction) and plankton-associated (>6-log reduction) S. maltophilia in 48 h (Fig. 2c and d). Higher Cu/Ag concentrations tested (0.4/0.04 and 0.8/0.08 mg/liter) maintained the reduction even after the 72-h ion maintenance period, unlike the result with P. aeruginosa. The same Cu/Ag concentrations tested achieved 99.9% killing of biofilm-associated A. baumannii in 12 h (Fig. 2e). Only Cu/Ag concentrations of 0.8/0.08 mg/liter maintained complete inactivation in the first 72 h. Cu/Ag concentrations of 0.4/0.04 and 0.8/0.08 mg/liter achieved complete inactivation of plankton-associated A. baumannii in the first 72 h (Fig. 2f). Less than 1 log regrowth was observed with both biofilms and planktonic samples for A. baumannii, unlike the finding for P. aeruginosa. S. maltophilia appears to be more susceptible to copper and silver ions than P. aeruginosa and A. baumannii.
FIG. 2.
Efficacy of copper and silver ions in waterborne-pathogen inactivation. •, control; ○, 0.2/0.02 mg/liter; □, 0.4/0.04 mg/liter; ▵, 0.8/0.08 mg/liter (Cu/Ag). ↕ indicates 95% confidence interval. (a) Cu/Ag ions achieved more than 99.99% reduction of biofilm-associated P. aeruginosa within 24 h. (b) Cu/Ag ions achieved more than 99.999% reduction of plankton-associated P. aeruginosa within 12 h. (c) Cu/Ag ions achieved more than 99.9% reduction of biofilm-associated S. maltophilia within 48 h. (d) Cu/Ag ions achieved more than 99.99999% reduction of plankton-associated S. maltophilia within 72 h. (e) Cu/Ag ions achieved more than 99.9% reduction of biofilm-associated A. baumannii within 12 h. (f) Cu/Ag ion concentrations at 0.4/0.04 and 0.8/0.08 mg/liter achieved more than 99% reduction of plankton-associated A. baumannii within 100 h.
The persistence of waterborne pathogens in the hospital water supply system was responsible for hospital-acquired infections. World Health Organization guidelines recommend that water must not be contaminated by waterborne pathogens in the health care setting during storage, distribution, or handling (1). Disinfecting the water system, targeting these pathogens, can be an option for prevention of waterborne pathogen-related infections.
Our results show that copper-silver ionization is effective in controlling biofilms and plankton-associated waterborne pathogens. Although copper and silver ions were added at the appropriate concentrations initially, the regrowth of the test organisms was observed as described previously. This regrowth may be due to the fact that these metallic ions are attached to the test organisms, remain attached throughout the experiment, and have no further killing effect on other organisms (11). This is indicated by the decrease of ion concentrations in the planktonic phase during the first 72 h of each experiment (data not shown). Thus, it is important to maintain proper ion concentrations when applying this method to water systems. In addition, there are measurable decreases in the control populations (i.e., no disinfectants) of plankton-associated P. aeruginosa and S. maltophilia. We are unable to provide an explanation to describe this observation. It may be because P. aeruginosa and S. maltophilia are more susceptible to the manmade model plumbing system than A. baumannii.
The biofilms and planktonic populations of P. aeruginosa, S. maltophilia, and A. baumannii populations in this study are only 14 days old, much younger than those persisting in the real water distribution system, which may be more resistant to disinfectants. A prospective surveillance should be conducted to validate the efficacy of this procedure in real hospital water systems before it is widely recommended. In addition, copper-silver ionization is a new application to drinking water treatment for Legionella and other waterborne pathogens. Registration of copper-silver ionization in drinking water may be required (8) because of adverse effects on human health (3, 7). Currently, ionization manufacturers may continue to offer the technology before the grace period ends.
In summary, copper-silver ionization is efficacious for control of biofilms and plankton-associated waterborne pathogens in a model plumbing system. Copper-silver ionization may be capable of controlling waterborne pathogens, in addition to Legionella, in the hospital water distribution system.
Acknowledgments
This study was supported in part by National Health Research Institute (NHRI-EX96-9206PC) and National Kaohsiung Normal University, Taiwan.
Footnotes
Published ahead of print on 15 January 2010.
REFERENCES
- 1.Adams, J., J. Bartram, and Y. Chartier (ed.). 2008. Essential environmental health standards in health care. World Health Organization, Geneva, Switzerland.
- 2.Anaissie, E. J., S. R. Penzak, and M. C. Dignani. 2002. The hospital water supply as a source of nosocomial infections: a plea for action. Arch. Intern. Med. 162:1483-1492. [DOI] [PubMed] [Google Scholar]
- 3.Araya, M., B. Chen, L. M. Klevay, J. J. Strain, L. Johnson, P. Robson, W. Shi, F. Nielsen, H. Zhu, M. Olivares, F. Pizarro, and L. T. Haber. 2003. Confirmation of an acute no-observed-adverse-effect and low-observed-adverse-effect level for copper in bottled drinking water in a multi-site international study. Regul. Toxicol. Pharmacol. 38:389-399. [DOI] [PubMed] [Google Scholar]
- 4.Biurrun, A., L. Caballero, C. Pelaz, E. Leon, and A. Gago. 1999. Treatment of a Legionella pneumophila-colonized water distribution system using copper-silver ionization and continuous chlorination. Infect. Control Hosp. Epidemiol. 20:426-428. [DOI] [PubMed] [Google Scholar]
- 5.Cargill, K. L., B. H. Pyle, R. L. Sauer, and G. A. McFeters. 1992. Effects of culture conditions and biofilm formation on the iodine susceptibility of Legionella pneumophila. Can. J. Microbiol. 38:423-429. [DOI] [PubMed] [Google Scholar]
- 6.Emmerson, A. M. 2001. Emerging waterborne infections in health-care settings. Emerg. Infect. Dis. 7:272-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Environmental Protection Agency. 1992. Secondary drinking water regulations: guidance for nuisance chemicals. Report no. EPA 810/K-92-001. Environmental Protection Agency, Washington, DC.
- 8.Environmental Protection Agency. 2007. Pesticide registration: clarification for ion-generating equipment. Report no. EPA-HQ-OPP-2007-0949. Environmental Protection Agency, Washington, DC.
- 9.Huang, H. I., H. Y. Shih, C. M. Lee, T. C. Yang, J. J. Lay, and Y. E. Lin. 2008. In vitro efficacy of copper and silver ions in eradicating Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Acinetobacter baumannii: implications for on-site disinfection for hospital infection control. Water Res. 42:73-80. [DOI] [PubMed] [Google Scholar]
- 10.Lin, Y. S., J. E. Stout, V. L. Yu, and R. D. Vidic. 1998. Disinfection of water distribution systems for Legionella. Semin. Respir. Infect. 13:147-159. [PubMed] [Google Scholar]
- 11.Lin, Y. S. E., R. D. Vidic, J. E. Stout, and V. L. Yu. 1996. Individual and combined effects of copper and silver ions on inactivation of Legionella pneumophila. Water Res. 30:1905-1913. [Google Scholar]
- 12.Merlani, G. M., and P. Francioli. 2003. Established and emerging waterborne nosocomial infections. Curr. Opin. Infect. Dis. 16:343-347. [DOI] [PubMed] [Google Scholar]
- 13.Mietzner, S., R. C. Schwille, A. Farley, E. R. Wald, J. H. Ge, S. J. States, T. Libert, and R. M. Wadowsky. 1997. Efficacy of thermal treatment and copper-silver ionization for controlling Legionella pneumophila in high-volume hot water plumbing systems in hospitals. Am. J. Infect. Control 25:452-457. [DOI] [PubMed] [Google Scholar]
- 14.Percival, S. L. 1998. Review of potable water biofilms in engineered systems. Br. Corrosion J. 33:130-137. [Google Scholar]
- 15.Shin, J. H., E. J. Lee, H. R. Lee, S. M. Ryu, H. R. Kim, C. L. Chang, Y. J. Kim, and J. N. Lee. 2007. Prevalence of non-tuberculous mycobacteria in a hospital environment. J. Hosp. Infect. 65:143-148. [DOI] [PubMed] [Google Scholar]
- 16.Stewart, P. S., J. Rayner, F. Roe, and W. M. Rees. 2001. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl. Microbiol. 91:525-532. [DOI] [PubMed] [Google Scholar]
- 17.Stout, J. E., Y. S. Lin, A. M. Goetz, and R. R. Muder. 1998. Controlling Legionella in hospital water systems: experience with the superheat-and-flush method and copper-silver ionization. Infect. Control Hosp. Epidemiol. 19:911-914. [PubMed] [Google Scholar]
- 18.Stout, J. E., and V. L. Yu. 2003. Experiences of the first 16 hospitals using copper-silver ionization for Legionella control: implications for the evaluation of other disinfection modalities. Infect. Control Hosp. Epidemiol. 24:563-568. [DOI] [PubMed] [Google Scholar]
- 19.Tachikawa, M., M. Tezuka, M. Morita, K. Isogai, and S. Okada. 2005. Evaluation of some halogen biocides using a microbial biofilm system. Water Res. 39:4126-4132. [DOI] [PubMed] [Google Scholar]
- 20.Trautmann, M., T. Michalsky, H. Wiedeck, V. Radosavljevic, and M. Ruhnke. 2001. Tap water colonization with Pseudomonas aeruginosa in a surgical intensive care unit (ICU) and relation to Pseudomonas infections of ICU patients. Infect. Control Hosp. Epidemiol. 22:49-52. [DOI] [PubMed] [Google Scholar]
- 21.Vanden Bergh, M. F. Q., P. E. Verweij, and A. Voss. 1999. Epidemiology of nosocomial fungal infections: invasive aspergillosis and the environment. Diagn. Microbiol. Infect. Dis. 34:221-227. [DOI] [PubMed] [Google Scholar]
- 22.Weber, D. J., W. A. Rutala, C. N. Blanchet, M. Jordan, and M. F. Gergen. 1999. Faucet aerators: a source of patient colonization with Stenotrophomonas maltophilia. Am. J. Infect. Control 27:59-63. [DOI] [PubMed] [Google Scholar]
- 23.Wingender, J., and H. C. Flemming. 2004. Contamination potential of drinking water distribution network biofilms. Water Sci. Technol. 49:277-286. [PubMed] [Google Scholar]