Abstract
It is now recognized that membranes are not simple physical barriers but represent a complex and dynamic environment that affects membrane protein structures and their functions. Recent data emphasize the role of membranes in sensing temperature changes, and it has been shown that the physical state of the plasma membrane influences the expression of a variety of genes such as heat shock genes. It has been widely shown that minor alterations in lipid membranes are critically involved in the conversion of signals from the environment to the transcriptional activation of heat shock genes. Previously, we have proposed that the composition, molecular arrangement, and physical state of lipid membranes and their organization have crucial roles in cellular responses during stress caused by physical and chemical factors as well as in pathological states. Here, we show that transformation of Salmonella enterica serovar Typhimurium LT2 (Salmonella Typhimurium) with a heterologous Δ12-desaturase (or with its trans-membrane regions) causes major changes in the pathogen's membrane dynamic. In addition, this pathogen is strongly impaired in the synthesis of major stress proteins (heat shock proteins) under heat shock. These data support the hypothesis that the perception of temperature in Salmonella is strictly controlled by membrane order and by a specific membrane lipid/protein ratio that ultimately causes transcriptional activation of heat shock genes. These results represent a previously unrecognized mode of sensing temperature variation used by this pathogen at the onset of infection.
The heat shock response, one of the most studied cellular homeostatic mechanisms, is involved in the maintenance of cell functionality during stress (25, 60). The heat shock response is also elicited by pathogens at the onset of infection as a result of exposure to environmental stresses such as a heat shock or during macrophage infection (25, 55). In addition, the heat shock response is severely altered as a result of several chronic diseases, cancer, apoptosis, and the aging process (3, 27, 45). The resulting protection from different types of stresses is due to the transcriptional activation of heat shock genes (29, 54). Further, moderate heat (or stress) treatment induces transient acquisition of thermotolerance, even in the absence of denatured proteins (40, 51), due to the accumulation of heat shock proteins (HSPs) (35). These proteins are not only essential under a variety of stress conditions, such as heat, drought, salinity, osmotic shock, membrane shearing, ischemia, etc. (11, 23, 55, 67), but are also crucial under physiological conditions for protein folding, translocation, mRNA splicing, nucleic acid and protein syntheses, mitochondrial electron transport, and photosynthesis, for example (4, 24, 25, 39). Members of the group of small HSPs (sHSPs) have also been shown to antagonize heat-induced membrane hyperfluidization and to stabilize the bilayer state under stress conditions (40, 54, 59).
According to the conventional model, during stress, the accumulation of denatured proteins within cells has been considered the primary signal that induces heat shock gene transcription (29). Activation of heat shock factor (HSF) occurs via the titration of HSPs by the stress-induced formation of unfolded and denatured proteins. In eukaryotic cells, the active HSF translocates into the nucleus, where it trimerizes and binds to heat shock gene promoters prior to undergoing phosphorylation (29). Yet this model does not take into proper consideration the fact that poikilotherms, plants (which constitute more than 95% of all living species on Earth), and microorganisms do not induce HSPs at a genetically determined temperature; instead, their responses vary as their physiological temperatures adjust during temperature changes (15, 50). Furthermore, this model does not explain how very moderate temperature increments during temperature acclimation, which are unlikely to cause protein damage in vivo, could lead to the strong induction of HSPs and to the establishment of thermotolerance (40). Further, in humans it is well known that HSPs are present at abnormal levels in several chronic diseases (6, 13, 17, 19, 56), and their synthesis decreases with aging (57). However, there is no evidence of a modification of the kinetics or accumulation of denatured proteins in these cases or during seasonal temperature acclimation that could explain the changes observed in heat shock gene transcription.
Recent research has focused on an alternative, but not exclusive, mechanism by which cells perceive a stress via a membrane primary sensor that is translated into a signal that activates heat shock mRNA transcription (2, 7, 20, 40, 64). This alternative model proposes that the cellular temperature-sensing mechanism is not related to denaturation of proteins during heat shock but is intimately associated with the lipid composition and membrane physical state (MPS) of the cell membrane at the time of stress (2, 30, 59). We along with others have shown that under physiological temperatures, membrane lipid fluidity, regulated by the environmental temperature (or by diet in mammals and birds), is controlled by lipid unsaturation, the protein-lipid ratio, composition of lipid molecular species, etc., which determine the temperature at which heat shock genes are transcribed (10, 49, 60). This model suggests that subtle changes in lipid composition and the MPS trigger selectively stress signaling responses via specific chemical interactions of boundary lipids with membrane proteins (18, 20, 64). According to our most recent studies, in addition to overall membrane hyperfluidization (2), distinct reorganization of cholesterol-rich plasma membrane microdomains is required for the generation and transmission of stress signals to activate Hsp genes via HSF in a murine cell line (30). One of the factors in regulating the MPS is represented by fatty acid desaturases (Δ5, Δ9, Δ12, etc.). These enzymes introduce a double bond in a specific position of long-chain fatty acids (e.g., in position C-12 of oleic acid by Δ12-desaturase) and are conserved across kingdoms. The degree of unsaturation of fatty acids affects physical-chemical properties of membrane phospholipids and associated proteins (64). Desaturases are characterized by the presence of three conserved histidine domains that compose the Fe-binding active centers of the enzymes (26).
Earlier, we showed that at 25°C the addition at of oleic acid (an unsaturated fatty acid [UFA]) or the membrane fluidizer benzyl alcohol (BA) to mycelia of the human fungal pathogen Histoplasma capsulatum caused a strong repression of Hsp70 and Hsp82 mRNA when cells were heat shocked at 37°C (7). Further, we showed that when a Saccharomyces cerevisiae Ole1 lipid mutant defective in Δ9-desaturase activity (a mutant that does not synthesize oleic acid) is complemented with its own Δ9-desaturase sequence, the levels of Hsp70 and Hsp82 gene transcription are contingent on the activity of the promoter used to drive Δ9-desaturase transcription. The complemented strains, depending on the Ole1 promoter used, had different membrane fluid states, as measured by 1,6-diphenyl-1,3,5-hexatriene (DPH) fluorescence anisotropy, that matched with the levels of transcription of heat shock genes (7). Extensive data in the literature show that plasma membrane microdomains (containing specific lipid compositions) are essential for efficient signal transduction (5, 43, 58, 64). In subsequent studies, we have shown that the specificity of expression of stress genes depends on the particular presence and distribution of membrane microdomains (rafts, calveolae, and lipid shells, e.g.) (51, 61, 62). Thus, simple changes in the physical properties of the membrane can cause remarkable alterations in the pattern of heat shock gene expression.
We have approached the study of the role of the MPS during the process of macrophage infection in Salmonella enterica serovar Typhimurium since it is an important human pathogen whose mechanisms of pathogenicity are extensively well studied. Here, we show that in S. Typhimurium a change of the MPS alters the pattern of HSP accumulation, which eventually has profound biological significance for the organism's pathogenicity.
MATERIALS AND METHODS
Bacterial strains, media, growth, and conditions.
S. Typhimurium (kindly provided by R. Rappuoli, Novartis, Siena, Italy) and Escherichia coli TOP10F′ (Invitrogen) were routinely cultured on LB agar or LB broth supplemented with appropriate antibiotics. Transformed Salmonella strains were grown overnight (o.n.) at 30° and 37°C with vigorous shaking. Bacteria were diluted to an optical density at 600 nm (OD600) of 0.07; then aliquots were grown under aerobic and anaerobic conditions in Bijoux tubes at 30° and 37°C without shaking. The growth rate of bacteria was determined at the OD600.
Nucleic acid manipulation.
Genomic DNA extraction, DNA and RNA electrophoresis, hybridization procedures, plasmid isolation, and restriction enzyme analyses were performed according to standard procedures (41). To purify total RNA, bacteria were collected by centrifugation and immediately used for total-RNA extraction as described previously (7).
Bacteria electroporation.
Bacteria grown o.n. were diluted 1:100 in LB medium and grown at 37°C. When the culture reached an OD600 of 0.5 to 0.7, it was incubated at 4°C for 30 min, pelleted, and washed five times with ice-cold 10% (wt/wt) glycerol. After a washing step, the pellet was resuspended in ice-cold 10% (wt/wt) glycerol (125 μl in every 100 ml of the starting culture). Forty microliters of electrocompetent cells was mixed with 1.0 μg of DNA in double-distilled H2O (ddH2O), and then the mixture was added to a prechilled 0.2-cm chilled cuvette (Bio-Rad, Richmond, CA). Bacteria were pulsed using a Bio-Rad gene pulser set at 2.5 kV, 25 μF, and 200 Ω. Immediately after the bacteria were pulsed, 500 μl of LB broth was added, and bacteria were incubated at 37°C for 30 min. Subsequently, 150-μl aliquots were spread on LB agar plates containing 100 μg/μl ampicillin (Amp100) (Sigma-Aldrich) and incubated o.n. at 37°C.
Plasmid construction.
Using Synechocystis PCC6803 genomic DNA as a template and the primers DesA5′ (5′-CCCCCCCATATGACTGCCACGATTCCCCCGTTGACAC-3′) and DesA3′ (5′-CCCCCCCATATGACTAGTTGTCCCAGTATTAAAC-3′), the entire coding sequence of the Synechocystis desA gene coding for a Δ12-fatty acid desaturase, a membrane-bound enzyme (34) (accession number X53508) (CyanoBase [http://www.Kazusa.or.jp/cyano/]), was amplified. Both oligonucleotides carried an NdeI [C(A/T)ATG] restriction site next to the 5′ end. In primer DesA5′ the NdeI site overlaps the ATG start codon of Synechocystis desA. PCR was carried out in 100 μl of 1× PCR buffer containing 200 μM concentration of the deoxynucleoside triphosphates (dNTPs), 50 pmol of each primer, 1.5 mM MgCl2, 100 ng of DNA template, and 2.5 U of Taq polymerase (Perkin Elmer) with an annealing temperature of 55°C for 33 cycles. PCR products were cloned into PCR2.1 vector (Invitrogen) and fully sequenced. One plasmid, named pCR2.1-desA, carrying the correct desA sequence, was digested with NdeI; the resulting 1,072-bp DNA fragment was cloned into the NdeI site of plasmid pNir100 under the transcriptional control of the E. coli PnirB promoter (9, 36) and transformed into E. coli strain TOP10F′. NdeI, PseI, and SpeI single digestion and SpeI/BglII double digestion of recombinants showed that one plasmid carried the desA gene in the correct orientation with respect to the inducible promoter PnirB, and this plasmid was named pΔ12. Plasmid pΔ12 was used to transform a Salmonella strain to generate the strain Stm(pΔ12); a control strain named Stm(pNir) carrying pNir100 plasmid was also constructed.
The Synechocystis Δ12-desaturase contains two transmembrane domains of 45 and 49 amino acids (http://www.cbs.dtu.dk/services/TMHMM/) that allow the enzyme to intercalate into the thylakoid membrane (34). Each domain consists of two α-helices separated by a stretch of 4 or 3 amino acids protruding outside the membrane. The two membrane domains were cloned into pBAD Myc/His vector (Invitrogen) to generate plasmids pBAD-200 and pBAD-212. pBAD-200 carries open reading frame 200 (ORF200) encoding a 59-amino-acid (aa) peptide containing the 45 aa of the first membrane domain (Trp46/His90) of the Δ12-fatty acid desaturase and K41ASKA45 and D91CGHR95 amino acids flanking this domain. pBAD-212 carries ORF212 coding a 63-aa peptide containing 49 aa of the second membrane domain (Ile199/Ile247) of the Δ12-fatty acid desaturase and K194VKLS198 and P248EIRF252 amino acids flanking this domain. Using plasmid pΔ12 containing Synechocystis Δ12-desaturase as a PCR template, ORF200 and ORF212 were amplified with the primer pair 200rev (5′-CCCCCCATGGAAAAAGCGAGCAAAGCCTGGGCTTC-3′) and 200fw (5′-CCCCCAGATCTTCATCAAAGCTTGCGATGGCCACAGTCATGGC-3′) and the pair 212rev (5′-CCCCCCATGGAAAAAGTCAAATTATCCATTGCCGT-3′) and 212fw (5′-CCCCCAGATCTTCATCAAAGCTTGAAACGAATTTCGGGAATGGTG-3′), respectively. Both reverse primers carried the NcoI restriction site (in boldface) that contains an ATG start codon at the 5′ end. Two A nucleotides were also added downstream to the NcoI site to form a GAA codon (Glu) to allow the correct translation of ORF200 and ORF212. Two stop codons were introduced in tandem at the 3′ ends of the amplified products. PCR products were then cloned into the T-vector pCR2.1 (Invitrogen). Recombinant plasmids were used to transform E. coli DH5α. DNA was purified, and sequences were determined using a Sequenase kit, version 2.0 (USB). ORF200 and ORF212 were isolated from recombinant plasmids by enzymatic digestion with NcoI and BglII and cloned into the NcoI/BglII sites of pBAD vector under the transcriptional control of the arabinose-inducible PBAD promoter to yield pBAD-200 and pBAD-212. These two recombinant vectors were then used to transform the S. Typhimurium LT2 wild-type (wt) strain to obtain Stm(pBAD-200) and Stm(pBAD-212). A control strain, Stm(pBAD), carrying the pBAD vector was also constructed.
The same strategy was used to generate plasmid pBAD-Δ12 using the primers DESArev (5′-CCCCCCATGGGAACTGCCACGATTCCCCCGTTGACAC-3′) and DESAfw (5′-CCCCCAGATCTTCAAAGCTTCTAGTTGTCCCAGTATTAAAC-3′). Plasmid pBAD-Δ12 was used to transform Salmonella Typhimurium to generate Stm(pBAD-Δ12).
We used as a primary vector pNir since we wanted to use conditions (anaerobiosis and aerobiosis) encountered by Salmonella during infection in the host. We chose the pBAD system for the internal membrane fragments since this promoter has high transcription efficiency particularly with short peptide fragments and can be easily induced by arabinose. However, we tested both vectors and found very similar results.
DNA probes.
DNA probes for dnaK and ibpB were obtained by PCR amplification using Salmonella genomic DNA as a template with the following primers and conditions: primers dnaKF (5′-CGATTATGGATGGAACGCAGG-3′) and dnaKR (5′-GTGGGTATCACCGTTGGTTGC-3′) with an annealing temperature of 58°C for 30 cycles; primers ibpBF (5′-CCCCACTGCTGCGTCAATGGATC-3′) and ibpBR (5′-GCGGAGCGTTCGTTAATGGCG-3′) with an annealing temperature of 61°C for 35 cycles.
Heterologous expression of Synechocystis desA in Salmonella.
Salmonella strains Stm(pNir) and Stm(pΔ12) were grown o.n. at 30° or 37°C in LB-Amp100 medium with vigorous shaking. After 24 h, cultures were diluted to an OD600 of 0.07 in LB-Amp100 medium containing 10 mg/ml glucose and were then used to fill 7.0-ml Bijoux tubes. Bacteria were grown under anaerobic conditions without shaking to an OD600 of 0.6, harvested by centrifugation, and processed for total RNA purification or protein extraction. Total RNA from Stm(pNir) and Stm(pΔ12) were fractionated on a denaturing gel and blotted onto nylon membrane, and filters were hybridized with a desA probe labeled with [α-32P]dCTP (3,000 Ci/mmol, Amersham) by a High Prime DNA labeling system (Boheringer). Filters were washed twice at 55°C in 0.1× phosphate buffer [50 mM Na/Na2(PO2), pH 7.2, 0.5% (wt/wt) SDS, 1.0 mM EDTA, pH 8.0] for 15 min and analyzed with a Phosphorimager SF (Molecular Dynamics) after a 4-h exposure at room temperature. The level of expression of detected desA mRNA was quantified by NIH ImageJ software, version 1.40, after normalization with 18S rRNA.
Heterologous expression of Δ12-fatty acid desaturase membrane domains in Salmonella.
Stm(pBAD), Stm(pBAD-200), and Stm(pBAD-212) were grown o.n. at 37°C in RM minimal medium (2% Casamino Acids, 1× M9 salts [6.8 g of NA2HPO4, 3 g of KH2PO4, and 1 g of NH4Cl per liter], 0.4% glucose, 1 mM MgCl2, 1% glycerol, and Amp100) to an OD600 of 0.5 to 0.8. Strains were then subcultured at an OD600 of 0.005 in RM minimal medium supplemented with 2% l-(+)-arabinose to allow transcription of ORF200 and ORF212 from the arabinose-inducible PBAD promoter. Bacterial growth rate was monitored spectrophotometrically for 12 h at 30° and at 37°C.
Membrane purification, lipid determination, and Western blotting.
Inner, outer, and total membranes were isolated from Salmonella as described previously (33). Briefly, cells were grown to an OD600 of 0.8 and harvested by centrifugation at 4,000 rpm in a Sorvall SS34 rotor for 10 min at 4°C. The pellet was resuspended in ice-cold 0.75 M sucrose, 10 mM Tris-HCl (pH 7.8) (7 ml per 100 absorbance units of the original culture). Lysozyme was added immediately to a final concentration of 0.1 mg/ml. After a 2-min incubation on ice, samples were slowly diluted with 2 volumes of ice-cold 1.5 mM Na-EDTA (pH 7.5) for 10 min. Spheroplasts were lysed by adding the suspension slowly to 4 volumes of cold water and stirring for 10 min. Intact cells were removed by centrifugation at 1,200 × g for 20 min. The supernatant was centrifuged at 26,000 rpm for 1.5 h in a Sorvall AH627 rotor to yield total membranes that were resuspended in a small volume of 10 mM Tris-HCl (pH 7.5). The lipid content of isolated Salmonella total membrane was determined by gas chromatography using heptadecaonic acid as an internal standard, as described previously (33). Membranes were solubilized in the Laemmli sample buffer and analyzed by Western blotting (41) using a monoclonal antibody against Synechocystis Δ12-desaturase. Protein concentration was measured as described previously (8).
Membrane functionality analysis.
Perturbation of membrane functionality was measured, in vivo, as a function of membrane permeability using the fluorescent dye NPN (1-N-phenylnaphtylamine) as described previously (53). Sixty milliliters of cells was grown at 30°C to an OD600 of 0.8 and then harvested at 4,000 rpm (SS-34 Sorvall rotor) for 10 min at room temperature. Pellets were resuspended in 10 ml of 50 mM HEPES buffer (pH 7.2) and diluted to an OD600 of 0.6 with HEPES buffer. Aliquots (100 μl) were then heat shocked between 30° and 60°C for 15 min. After heat treatment, 900 μl of HEPES buffer (pH 7.2) containing 5 μM NPN (Molecular Probes) was added to each aliquot and incubated for 150 s at 30°C. NPN fluorescence intensities were measured by using a Quanta Master QM-1 fluorescence spectrometer (Photon Technology International, Princeton, NJ) with excitation and emission wavelengths of 350 and 422 nm at 10 and 4 nm slits, respectively.
Membrane fluidity.
The membrane fraction of S. Typhimurium was labeled with diphenylhexatriene (DPH), and the steady-state fluorescence anisotropy was monitored as a function of temperature (7).
Identification of sHSPs in the outer membrane of strain Stm(pΔ12) by mass spectrometry.
To characterize the proteins identified in the gels, a Coomassie blue-stained one-dimensional gel band from Salmonella Stm(pΔ12) outer membrane preparation was cut out (molecular mass, <20 kDa). The gel band was subjected to an in-gel digestion protocol (0.1 mg of trypsin for 7 h at 37°C following reduction with dithiothreitol [DTT] and 10 h alkylation [with iodoacetic amide]) of the Cys sulfhydryl groups. After extraction of the tryptic peptide digest from the gel, purification over a C18 ZipTip was performed, and the resulting unseparated mixture was analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) in a dihydroxybenzoic acid matrix. Based on MALDI analysis, an MS-Fit database search identified two proteins from the mixture: IbpB and IbpA.
DSC.
Thermal analysis of outer membranes was performed using a VP-DSC microcalorimeter (Microcal Inc., Southampton, United Kingdom), which contained matched 0.5-ml tantalum cells. Membranes were suspended in 10 mM TES (2-{[2-hydroxy-l,l-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid, pH 7.0) at a protein concentration of approximately 10 mg/ml. Equal volumes (0.5 ml) of sample and reference buffer were added after deaeration for 30 min at 20 mm Hg. Differential scanning calorimetry (DSC) scans were performed at temperatures ranging from 5° to 110°C using a scanning rate of 60°C/h. Samples were preincubated at the initial temperature for 60 min. After the first heating, a down-scan to 5°C was performed with the same parameters. The sample was kept at this temperature for 60 min before a second heating scan was started (54). Experimental thermograms were analyzed using the Microcal Origin software supplied with the instrument.
Benzyl alcohol treatment of Salmonella strains.
To determine BA toxicity, cells were grown to mid-log phase at 30° or 37°C and then incubated for 30 min in the presence of 5, 10, 20, 40, 50, and 80 mM BA; cells were then washed and serially diluted on LB agar plates or used for MΦ infection.
Heat shock gene expression in Salmonella.
Stm(pNir) and Stm(pΔ12) were grown at 30°C under microaerobic conditions to mid-log phase; aliquots were then heat shocked for 15 min at different temperatures and immediately processed for total-RNA extraction. Expression of heat shock genes was determined by Northern analysis using DNA fragments of dnaK and ibpB as probes. Expression of the dnaK gene was measured in Stm(pNir) treated with BA or heat shocked at 42°C without BA treatment and in Stm(pΔ12) heat shocked at 42°C after anaerobic induction of desA. Reversibility of induction of dnaK gene expression was also tested in bacteria treated with BA and in Stm(pΔ12) grown anaerobically after cultures were shifted for 5, 15, 30, and 60 min in LB broth without BA or in aerobiosis, respectively.
Infection of murine macrophage with Salmonella.
Entry and intracellular persistence into MΦ (cell line J774A.1; ATCC TIB 67 [American Type Culture Collection, Manassas, VA]) of Stm(wt) treated with BA, Stm(pNir), Stm(pΔ12) containing Synechocystis Δ12-fatty acid desaturase under the transcriptional control of E. coli nirB promoter, Stm(pBAD), Stm(pBAD-200), and Stm(pBAD-212) were carried out in 24-well tissue cultures plates (Costar) in triplicate. MΦ were maintained at 37°C in 5% CO2 in low-glucose Dulbecco's modified eagle medium (DMEM; Gibco, Bethesda, MD) supplemented with 10% fetal bovine serum (Gibco) and 2 mM l-glutamine. J774A.1 cells were seeded at a density of 105 cells/well 24 h prior to infection. Stm(wt) treated with BA, Stm(pNir) and Stm(pΔ12) grown under anaerobic conditions (in Bijoux tubes) and Stm(pBAD), Stm(pBAD-200), and Stm(pBAD-212) grown on minimal medium supplemented with 2% arabinose were added to the MΦ monolayer to achieve a multiplicity of infection (MOI) of 10:1. Plates were centrifuged for 2 min to synchronize MΦ infections, which proceeded for 30 min at 37°C. Cultures were washed with phosphate-buffered saline (PBS) to eliminate all noninternalized bacteria and incubated in fresh medium containing 30 μg/ml kanamycin to inhibit extracellular proliferation of bacteria at 37°C in a 5% CO2 atmosphere. Macrophages were lysed at different time points with 0.1% Triton-X (Sigma-Aldrich, St. Louis, MO) to recover bacteria. Serial dilutions were plated on LB agar or on LB agar containing Amp100 and incubated o.n. at 37°C to determine the number of CFU. Survival is expressed as percentage of CFU at each time point (Tx) compared to the number of CFU present at time zero (T0).
RESULTS
Cloning of Synechocystis desA gene and ORF200 and ORF212 transmembrane regions.
In enterobacteria, the nirB gene codes for a nitrate reductase enzyme whose expression is induced by low oxygen tension (36). Under this condition, expression of nirB from its PnirB promoter depends on the anaerobic transcription factor FNR (fumarate and nitrate reduction), a protein which controls transcription of genes involved in anaerobic respiratory metabolism of enterobacteria (46). To investigate the effect on modification of the MPS, plasmid pΔ12 carrying the full-length Synechocystis desA gene (Δ12-desaturase) under the transcriptional control of E. coli PnirB promoter and plasmids pBAD-200 and pBAD-212 containing the two membrane domains of Synechocystis Δ12-desaturase were used to electroporate S. Typhimurium to obtain strains Stm(pΔ12), Stm(pBAD-200), and Stm(pBAD-212), respectively. Control Stm(pNir) and Stm(pBAD) strains, carrying pNir100 and pBAD, respectively, were also constructed.
Synechocystis desA expression in Salmonella.
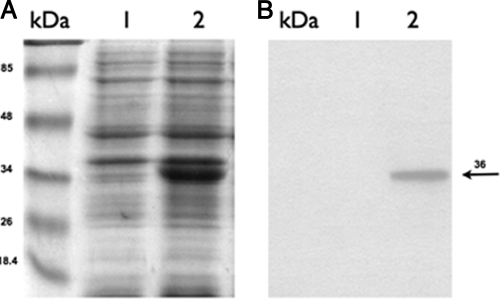
To test the induction of Synechocystis desA from the E. coli PnirB promoter, Stm(pΔ12) and Stm(pNir) were grown under anaerobic conditions at 37°C and then heat shocked for 15 min at 41°, 45°, and 47°C. Total RNAs extracted from Stm(pΔ12) and Stm(pNir) were probed with an internal fragment of the desA gene. The expression of desA was tested up to 47°C since heat shock experiments have been carried out within the temperature range of 37° to 47°C (Fig. 1). Under these conditions, desA was strongly expressed in Stm(pΔ12), whereas no hybridization was detected in the Stm(pNir) control strain (Fig. 1), confirming that the desA gene is not present in the S. Typhimurium LT2 strain. Proteins of outer membrane fraction from Stm(pΔ12) and Stm(pNir) grown at 30°C under anaerobic conditions were fractionated by 10% SDS-PAGE (Fig. 2A) and analyzed by Western blotting. Figure 2B shows the presence of the 36-kDa Δ12-desaturase protein expressed at the normal growth temperature of 30°C prior to heat shock. The same result was obtained within the temperature range of 37° to 47°C used in the heat shock experiments (data not shown). DesA protein was revealed using anti-Synechocystis Δ12-desaturase monoclonal antibodies (Fig. 2B).
FIG. 1.
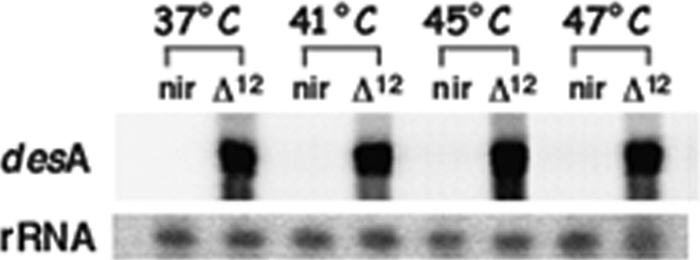
Expression of the desA gene of Synechocystis PCC6803 in S. Typhimurium. desA is strongly expressed in Stm(pΔ12) (Δ12) heat shocked for 15 min at 41°, 45°, and 47°C while it is not expressed in Stm(pNir) (nir)containing the empty vector.
FIG. 2.
SDS-PAGE and Western blotting of S. Typhimurium strains. (A) Proteins of the membrane fraction were separated by SDS-PAGE and stained with Coomassie brilliant blue. (B) Western blot analysis using a monoclonal antibody against Synechocystis Δ12-desaturase shows that it was present in the membrane fraction of Stm(pΔ12). Lanes kDa, protein markers; lanes 1, membrane proteins from Stm(pNir) cells; lanes 2, membrane proteins from Stm(pΔ12). The arrow indicates the position of the Δ12-desaturase, with an apparent molecular mass of 36 kDa.
Effects of expression of heterologous Synechocystis Δ12-fatty acid desaturase on MPS in S. Typhimurium.
Protein and lipid analyses (Table 1) showed that insertion of Synechocystis Δ12-desaturase in the membrane causes a dramatic imbalance within the membrane by shifting the protein/lipid ratio from 100% in Stm(pNir) to approximately 170% (standard error of the mean [SEM], ±8.42%) of the membrane protein/lipid ratio in Stm(pΔ12) (Table 1). The effect of stress conditions on membrane integrity was also determined measuring in vivo membrane leakage as determined by NPN fluorescence due to the insertion of Δ12-desaturase (Fig. 3). Values were obtained by shifting cells from 25°C to a desired temperature for 15 min. Within this period of time cells do not modify their lipid/protein profile since the process of homeoviscous adaptation (the ability to modulate the fluidity of their constituent membrane components to compensate for the direct effects of altered environmental temperature [12]) requires a much longer time (1). It is worth noting that such an effect is very significant in the physiological temperature range of 25° to 40°C while it is not present at higher temperatures. NPN is an uncharged lipophilic dye that is excluded from intact outer membrane and fluoresces weakly in aqueous environments. Its fluorescence increases greatly in nonpolar hydrophobic environments such as the cell membrane. As a result of the outer membrane damage, NPN enters into the phospholipid bilayer, causing the signal to increase (53). In a broad temperature range the overproduction of Δ12-desaturase induced membrane destabilization, which resulted in a permanent “leakiness” of the outer membrane of Stm(pΔ12) cells compared to Stm(pNir) cells. This explains the inducibility of heat shock genes in Stm(pΔ12) cells at a lower, nonstressing temperature.
TABLE 1.
Protein and lipid content and membrane fluiditya
| Strain | Lipid content (mg/ml) | Protein content (mg/ml) | Protein/lipid ratio | Membrane fluidity (mg/ml membrane extract) at: |
|
|---|---|---|---|---|---|
| 30°C | 40°C | ||||
| Stm(pNir) | 0.78 ± 0.16 | 3.94 ± 0.06 | 5.06 | 0.212708 ± 0.02 | 0.165846 ± 0.01 |
| Stm(pΔ12) | 0.74 ± 0.06 | 6.23 ± 0.06 | 8.42 | 0.220125 ± 0.02 | 0.185249 ± 0.01 |
Protein and lipid were purified from cells grown at 30°C. The membrane fraction of S. typhimurium was labeled with DPH, and the steady-state fluorescence anisotropy was monitored as a function of temperature. The values represent the average of four independent extraction/purification procedures.
FIG. 3.
NPN fluorescence of Stm(pNir) and Stm(pΔ12) membranes. Overproduction of Δ12-desaturase induced membrane destabilization, which resulted in a permanent leakiness of the outer membrane of Stm(pΔ12) compared to Stm(pNir). The effect is very significant within the physiological range of 25° to 40°C while it is not present at higher temperatures. Bars represent the standard deviation. All statistical differences were evaluated by a two-tailed Student's t test. P values less than 0.05 were considered statistically significant. All experiments were performed at least in quadruplicates.
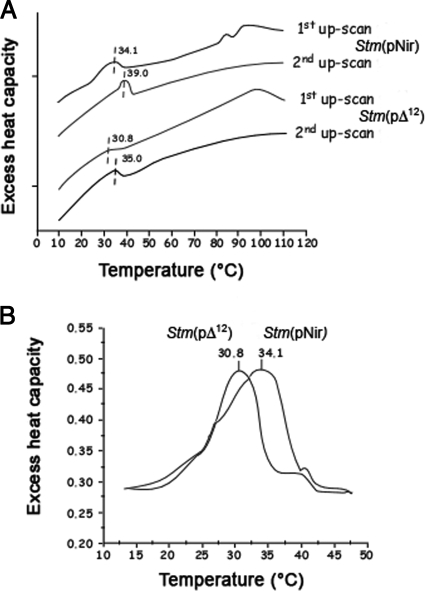
The difference in permeability of the two strains between 25° and 40°C disappears completely at higher heat shock temperatures. Clearly, above 42°to 45°C the heat-induced breakdown of membrane became dominant over the protein/phospholipid imbalance, inducing changes in membrane permeability detected at lower temperatures. The effect of overexpression of Synechocystis Δ12-desaturase on the thermotropic behavior of membranes was confirmed by the thermal analysis of isolated outer membranes by DSC. Thus, the overproduction of Δ12-desaturase lowered the transition temperature of certain lipid domains in the outer membranes of bacteria (Fig. 4). In the temperature range between 10° and 65°C, one major endothermic peak was observed in the first as well as in the second up-scan. The reversible endothermic peaks in the temperature range of 15° to 45°C corresponds to the phase transition of membrane lipids. The midpoints of the phase transition were at 34.1° and 30.8°C for the outer membranes isolated from Stm(pNir) and the Stm(pΔ12) strains, respectively. The endothermic peaks in the high-temperature range were absent in the second up-scan, suggesting that these peaks originated from irreversible protein denaturation. Thus, S. Typhimurium expressing Δ12-desaturase displays a greater permeability of outer membrane, even under nonstressing conditions. It is likely that the unbalanced membrane organization triggered a complex compensatory mechanism, including alteration of the ratio of gel/fluid lipid domains (Fig. 3 and 4).
FIG. 4.
Differential scanning calorimetry of the isolated outer membrane of Stm(pNir) and Stm(pΔ12). (A) The overproduction of Δ12-desaturase lowered the transition temperature of lipid domains in the outer membranes of bacteria. Between 10° and 65°C, one major endothermic peak was observed in the first and in the second up-scans. The reversible endothermic peaks in the temperature range of 15° to 45°C corresponds to the phase transition of membrane lipids. (B) The midpoints of the phase transition were at 34.1° and 30.8°C for outer membranes of Stm(pNir) and of Stm(pΔ12), respectively.
Effect of Δ12-desaturase on membrane fluidity of Salmonella.
The S. Typhimurium genome does not contain any Δ9-fatty acid desaturase (26) (http://genome.wustl.edu/pub/organism/Microbes/Enteric_Bacteria/Salmonella_enterica_serovar_Typhimurium_strain_LT2/map/fpc/); hence, it does not produce oleic acid (18:1), which is the natural substrate for Δ12-fatty acid desaturase. Very likely, S. Typhimurium synthesizes UFAs via independent biosynthetic pathways, as shown in E. coli (70). Wada et al. (66) expressed the Synechocystis sp. strain PCC6803 desA gene in E. coli, which does not contain any fatty acid desaturase (26). The cloned Δ12-desaturase in Stm(pΔ12) caused a significant change in membrane fluidity due to the higher protein/lipid ratio (Table 1) and membrane leakage (Fig. 3). This protein that has been shown to be a membrane-bound enzyme (34) and has no enzymatic activity in Salmonella; thus, its perturbing effect on the MPS is due to its insertion in the membrane (34) rather than to a change in phospholipid composition.
Effect of Δ12-desaturase on Salmonella growth.
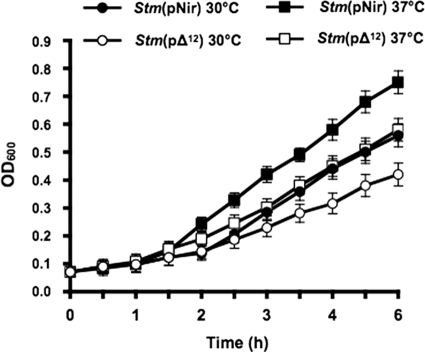
No difference in growth rates of Stm(pΔ12) and Stm(pNir) was seen in log-phase bacteria grown under aerobic conditions at 30° and 37°C (data not shown). A slight difference in anaerobic growth of Stm(pΔ12) and Stm(pNir) was detected at 30° and 37°C (Fig. 5). Similar results were obtained using Stm(pBAD) and Stm(pBAD-Δ12).
FIG. 5.
Bacterial growth. Overnight cultures at 30° and 37°C of Stm(pΔ12) and Stm(pNir) were grown anaerobically in LB medium. A slight difference in growth was detected at these temperatures. Bars represent the standard deviations. All statistical differences were evaluated by a two-tailed Student's t test. P values less than 0.05 were considered statistically significant. All experiments were performed at least in quadruplicate.
Expression of Synechocystis desA membrane domains in Salmonella.
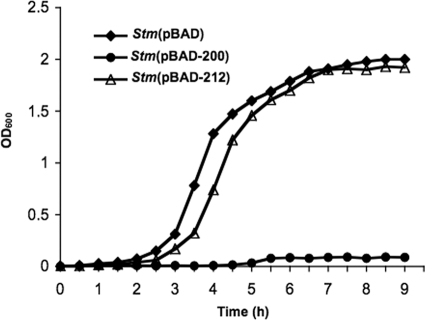
Stm(pBAD-200), Stm(pBAD-212), and control strain Stm(pBAD) were grown in minimal medium containing, as a sole carbon source, either 0.4% glucose or 2% arabinose to induce transcription of ORF200 and ORF212 from the PBAD promoter. No difference in bacterial growth was observed in the presence of glucose at either 30° or 37°C (data not shown). When arabinose replaced glucose, expression of either ORF200 or ORF212 had no significant effect on bacterial growth at 30°C (data not shown). However, expression of ORF200 strongly inhibited growth up to 9 h at 37°C (Fig. 6).
FIG. 6.
Bacterial growth. Overnight cultures of Stm(pBAD-200), Stm(pBAD-212), and Stm(pBAD) grown at 37°C in RM minimal medium containing 0.4% glucose as a sole carbon source were inoculated in fresh RM minimal medium containing 2% arabinose to induce transcription of ORF200 and ORF212 from the PBAD promoter. Expression of ORF200 strongly inhibited growth of Stm(pBAD-200) compared to the control strain Stm(pBAD) up to 9 h; expression of ORF212 had no significant effect on bacterial growth.
Fatty acid composition of membranes isolated from Salmonella inducing ORF200 and ORF212.
In Salmonella expressing either pBAD-200 or pBAD-212, the average fatty acid chain length remained constant while the lipid unsaturation decreased significantly (∼20%) compared to the control (Table 2). We also determined fatty acid composition of Salmonella and of a transformed strain inducing the entire Δ12 [Stm(pΔ12)] with similar results (data not shown). This suggests that the accumulation of either peptide caused a change in membrane fluidity while cells compensated for the increased fluidity by reducing the unsaturation of their membrane lipids. The most prominent effects were the decrease of the 16:1 (more in pBAD-212) and the increase of 18:0 (equally in both strains) fatty acids. Thus, the entire Δ12-desaturase or either transmembrane domain had a similar perturbing effect on membrane fatty acid composition (even in the absence of enzymatic activity).
TABLE 2.
Fatty acid composition of membranes isolated from Stm(pBAD-200), Stm(pBAD-212), and control strain Stm(pBAD)
| Fatty acid | Fatty acid composition (%) in:a |
||||
|---|---|---|---|---|---|
| Stm(pBAD) (control) | Stm(pBAD-200) | Stm(pBAD-200) relative to control | Stm(pBAD-212) | Stm(pBAD-212) relative to control | |
| 14:0 | 4.3 | 4.7 | 9.3 | 4.9 | 14.0 |
| 15:0 | 0.9 | 1.0 | 11.1 | 0.9 | 0.0 |
| 16:0 | 40.3 | 38.7 | −4.0 | 39.8 | −1.2 |
| 16:1 | 21.3 | 17.3 | −18.8 | 15.7 | −26.3 |
| c17:0 | 8.1 | 8.3 | 2.5 | 8.1 | 0.0 |
| 18:0 | 11.3 | 19.4 | 71.7 | 19.2 | 69.9 |
| 18:1 | 0.2 | 0.2 | 0.0 | 0.2 | 0.0 |
| 18:1 v | 13.6 | 10.4 | −23.5 | 11.1 | −18.4 |
| c19:0 | 0.8 | 0.2 | −75.0 | 0.8 | 0.0 |
| Avg fatty acid chain length | 16.64 | 16.61 | 0.0 | 16.73 | 0.5 |
| Double bond index | 0.35 | 0.28 | −20.0 | 0.27 | −22.9 |
Fatty acids were purified from membrane of bacteria grown at 37°C.
Heat shock response in genetically modified Salmonella.
Stm(pΔ12) and Stm(pNir) grown at 30° and 37°C were heat shocked for 15 min at different temperatures, and the expression of heat shock genes was determined by Northern blot analysis using two members of the main families of bacterial HSPs, dnaK (HSP70) and ibpB (HSP17, a member of sHSPs [52]). The pattern of expression of heat shock genes was significantly different in Stm(pΔ12) from that in the control strain Stm(pNir). For example, dnaK was expressed at 30°C in Stm(pΔ12) while it was scarcely detectable in the control strain Stm(pNir), and expression increased after 15 min of heat shock treatment at 37° or 39°C. Further, expression decreased when Stm(pΔ12) was heat shocked at 43°C (Fig. 7A). A change in the pattern of expression of dnaK was more significant when Stm(pΔ12) was grown at 37°C: dnaK was highly expressed at the growth temperature of 37°C (destabilization of the MPS by DesA is more robust at this higher temperature), but when cells were heat shocked between 41° and 47°C, no increase in the expression of dnaK was observed (Fig. 7B and C). The increase in the MPS due to the expression of desA caused, at the physiological growth temperature of 37°C, a reset of the temperature of expression of dnaK and ibpB, as we have shown previously in other prokaryotic and eukaryotic cells (7, 51, 59, 60, 61, 63). In other words, the modified strains have more fluid membranes at 30°C, and this change in fluidity is equivalent to a heat stress so that cells “sense” it as a heat shock. When cells are transferred to a higher temperature (more severe heat shock), e.g., 40°C, membranes tend to be in a physical state that is similar to that at much higher temperatures (membranes acquired an inverted hexagonal nonlamellar HII phase) and can no longer induce HSPs (59, 60). Likewise, expression of the ibpB gene was detectable in Stm(pΔ12) grown at 30°C and absent in control cells (Fig. 8). After a 30-min heat shock at 45°C Stm(pΔ12), ibpB was not transcriptionally induced, and it actually decreased due to the loss of membrane functionality. A normal heat shock response was measured in Stm(pNir) (Fig. 8).
FIG. 7.
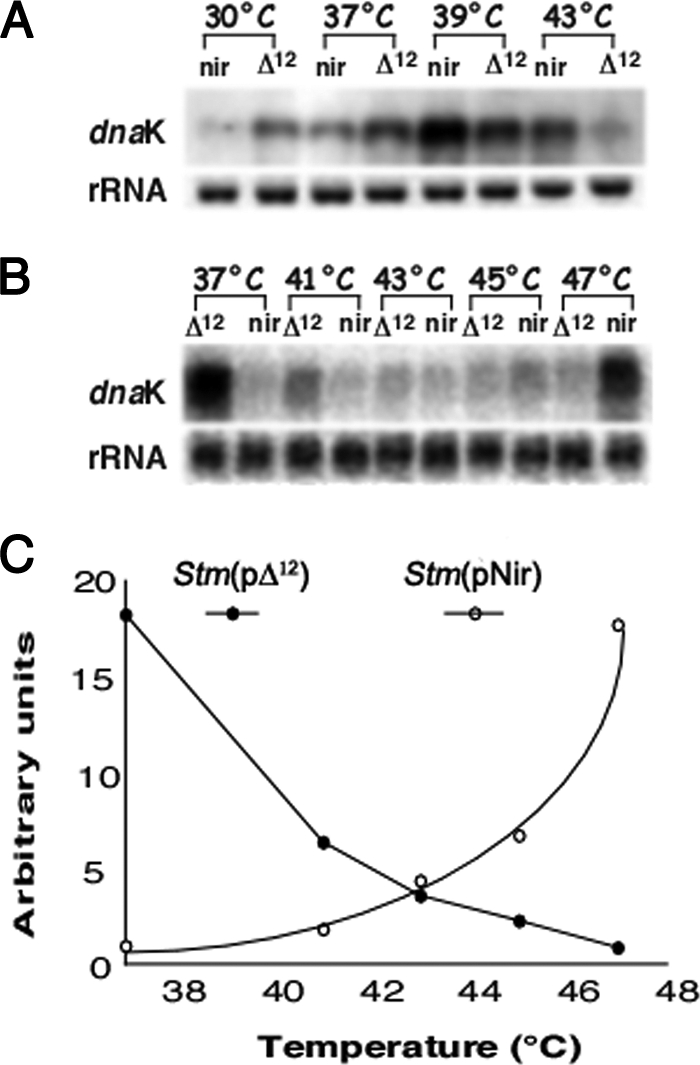
dnaK mRNA expression in Stm(pNir) (nir) and Stm(pΔ12) (Δ12). (A) dnaK was expressed at 30°C in Stm(pΔ12) but was scarcely detectable in the control strain Stm(pNir). dnaK expression increased in Stm(pΔ12) when the strain was heat shocked for 15 min at 37° or 39°C. dnaK expression decreased in Stm(pΔ12) when it was heat shocked at 43°C. (B and C) When Stm(pΔ12) was grown at the non-heat shock temperature of 37°C, dnaK was highly expressed. When cells were heat shocked between 41° and 47°C, dnaK expression was not detectable.
FIG. 8.
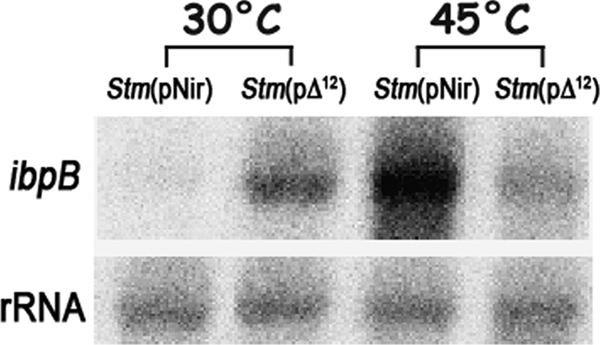
ibpB mRNA expression in Stm(pNir) and Stm(pΔ12). A strong decrease in ibpB expression was observed in Stm(pΔ12) under heat shock conditions compared to Stm(pNir).
Identification of sHSPs on the outer membrane of Stm(pΔ12) strains by mass spectrometry.
A 15% SDS-PAGE analysis of outer membrane proteins revealed a significant accumulation of IbpA/IbpB in strain Stm(pΔ12) grown at 30°C compared to Stm(pNir) (Fig. 9). We showed by Western blot analysis the presence of Δ12-desaturase in the outer membrane fraction of Stm(pΔ12) (Fig. 2). Under these experimental conditions, we could not detect any Δ12-desaturase signal because of the higher percentage of the gel and the reduced running time of the electrophoresis. This protein band was further characterized by mass spectrometry and was found to consist of two heat shock proteins of Salmonella, IbpA and IbpB (members of the sHSP family). Based on MALDI analysis (47), an MS-Fit database search (69) identified two proteins from the mixture. One was heat shock protein IbpB (of S. enterica NCBI 16762514; molecular mass, 16 kDa); this hit identified 30% of the found m/z (mass/charge) values covering 40% of the identified protein. This identification was further confirmed by the post-source decay (PSD) spectrum of MH+ = 961.65 Th, identified by an MS-Tag database search as ITLALAGFR of the above protein. The other protein was shock protein IbpA (of S. enterica NCBI 16762513; molecular mass, 16 kDa). This hit identified an additional 35% of the found m/z values covering 40% of the identified protein. This identification was further confirmed by the PSD spectrum of MH+ = 1124.58 Th, identified by an MS-Tag database search as NFDLSPLYR of the above protein. Thus, Stm(pΔ12) cells display an altered thermal-phase transition profile and greatly elevated permeability in their outer membranes even under nonstressed conditions (Fig. 3). An unbalanced membrane organization (primary event) triggered a complex compensatory mechanism, including alteration of the phase transition temperature of certain lipid domains (68) and the association to the outer membrane of members of the sHSP family, IbpA and IbpB. As a result of the changes in the MPS and membrane leakage, the pattern of expression of HSPs is strongly altered, as we have shown previously in several other prokaryotic and eukaryotic cells (7, 40, 59, 60). This change in the pattern of heat shock response, in turn, affects the proper mechanism of adaptation of the modified strain when it encounters a susceptible host. We have previously shown that when the MPS is altered by genetic modification or chemical treatment (e.g., BA or heptanol), cells try to compensate for such membrane changes by reorganizing the synthesis of membrane phospholipids by inducing specific genes (2; also microarray analysis in progress).
FIG. 9.
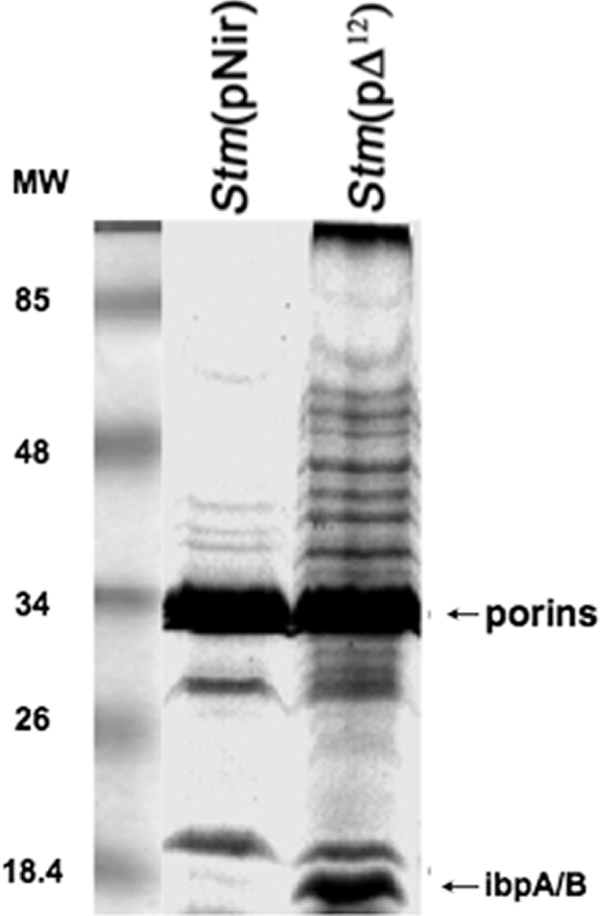
SDS-PAGE analysis of outer membrane proteins stained with Coomassie brilliant blue. A significant accumulation of IbpA and IbpB in strain Stm(pΔ12) grown at 30°C is detectable.
Benzyl alcohol treatment and dnaK gene expression.
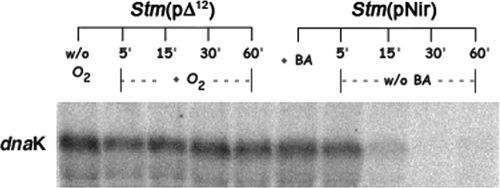
S. Typhimurium was incubated for 30 min with different concentrations (5, 10, 20, 40 50, and 80 mM) of the membrane fluidizer BA (2, 7, 44) and then plated on LB agar. No toxic effect was observed up to 50 mM BA on cell viability (data not shown). We along with other authors had previously shown that BA or heptanol, both membrane fluidizers, did not cause any detectable protein denaturation at concentrations that induced the heat shock response (14, 40) at noninducing heat shock temperatures, implying that the capacity to induce heat shock gene expression was due to their interaction with the membrane (59, 61). Expression of dnaK was determined in Stm(pNir) that was grown at 30°C, incubated for 30 min with 50 mM BA, and then heat shocked at 42°C for 15 min (Fig. 10). Both Stm(pΔ12) and BA-treated Stm(pNir) showed strong dnaK transcription at the growth temperature of 30°C while the gene was not detectable in Stm(pNir) at 30°C. Thus, either BA treatment of Stm(pNir) or expression of Δ12-desaturase in Stm(pΔ12), by altering the MPS, caused a resetting of the temperature of the heat shock response at 30°C. However, the change in dnaK transcription due to BA treatment was reversible since dnaK expression at 30°C returned to a normal level within 15 min after removal of BA from the culture medium (Fig. 11). In contrast, overexpression of dnaK persisted in Stm(pΔ12) at least up to 60 min after the culture was shifted from anaerobic to aerobic conditions (Fig. 11). Further, under anaerobic conditions, a synergistic lethal effect of BA treatment in Stm(pΔ12) expressing desA was also observed (data not shown).
FIG. 10.
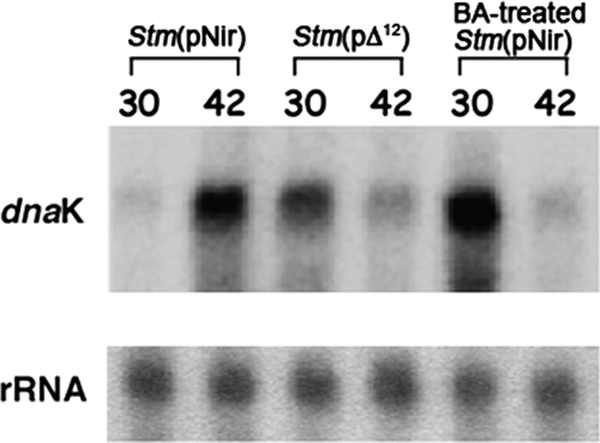
Expression of dnaK in BA-treated Stm(pNir) cells. dnaK expression was absent in Stm(pNir) grown at 30°C under anaerobic conditions and incubated for 30 min with 50 mM BA. Both Stm(pΔ12) and BA-treated Stm(pNir) showed high levels of dnaK expression at 30°C.
FIG. 11.
Reversible expression of dnaK in BA-treated Stm(pNir) cells. dnaK is still detectable in Stm(pΔ12) after a shift to aerobic conditions; in BA-treated Stm(pNir) cells dnaK is no longer present when BA is removed from the medium.
Murine macrophage infection with Salmonella.
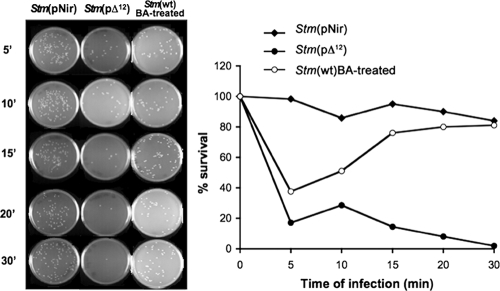
Internalization and intracellular persistence inside MΦ was monitored by infecting MΦ with Stm(wt) after treatment with BA, Stm(pNir), and Salmonella containing the Synechocystis Δ12-desaturase sequence, Stm(pΔ12) (Fig. 12). The efficiency of MΦ infection of Stm(pNir) that was treated for 30 min with 50 mM BA, which was removed by washing, was similar to the efficiency measured with Stm(pΔ12) within the first 5 min of infection. However, within 15 min, BA-treated bacteria recovered the ability to survive and multiply inside MΦ. In addition, the kinetics of cell recovery matched the kinetics of loss of dnaK expression after removal of BA (Fig. 12). In contrast, genetically modified Stm(pΔ12) inducing a low level of dnaK due to the presence of Δ12-desaturase was unable to survive inside MΦ (Fig. 12). A similar failure to infect and persist inside MΦ was observed with Stm(pBAD-200) and Stm(pBAD-212) expressing either the first or second transmembrane domain (data not shown). However, as we have shown in other prokaryotes and eukaryotes, it is the overall pattern of the heat shock response that is severely hampered. Thus, it is the lack of the appropriate accumulation of all HSPs that is of physiological significance.
FIG. 12.
Intracellular persistence inside MΦ of Stm(pNir), BA-treated Stm(wt), and Stm(pΔ12). At different time points, MΦwere lysed, and recovered bacteria were plated on LB agar or LB Amp100 agar with similar plating efficiencies. Survival is expressed as a percentage of the number of CFU at each time point (0, 5, 10, 15, 20, and 30 min) compared to the number of CFU present at time zero. The efficiency of MΦ infection of Salmonella wt treated for 30 min with 50 mM BA within the first 5 min of infection was similar to that measured with Stm(pΔ12). However, within 15 min, BA-treated Stm(wt) recovered the ability to survive and multiply inside MΦ. Genetically modified Salmonella Stm(pΔ12) was unable to survive inside MΦ. Values are representative of three independent experiments, each performed in duplicate. Statistical differences were evaluated by a two-tailed Student's t test. P values less than 0.05 were considered significant.
DISCUSSION
In previous work, we demonstrated that the primary sensor of temperature variations and, in general, of other forms of stresses is localized in the membrane (2, 7, 59). In Synechocystis, Suzuki et al. (48) have shown by systematic disruption of putative genes for histidine kinases and random mutagenesis that Hik33, a membrane-bound protein, is activated during stress and induces the expression of both osmostress-inducible and cold-inducible genes. Recently, we along with others have shown that an abrupt temperature change or an exposure to other forms of stress determines a physical reorganization of lipid and protein membrane components (5, 28, 59, 64) that is followed by a specific gene response designed to, among other things, compensate variations in the MPS (21, 44). We have also shown that there is cross talk between changes in the MPS and transcriptional regulation of genes involved in lipid metabolism and heat shock genes (21, 54, 64). Further, the interactions between certain sHSPs and specific domains of membranes remodel the status of the MPS (overall phase state, lipid order, permeability, etc.) (21, 22, 31). There is evidence that in eukaryotes the role of HSP70 depends on its intracellular, membrane-bound, or extracellular location. While the major action of chaperones in the cytoplasm is to maintain protein folding, HSP70 can inhibit lysosomal membrane permeabilization by interacting with the membrane lipid lyso(bis)phosphatidic acid (32). Further, it has been suggested that HSP70 becomes localized in lipid rafts as a component of signaling or trafficking complexes (10) and also associates with the plasma membrane lipids, where it has immunogenic properties (21, 38, 42, 62).
We have focused our attention on the crucial role of membranes as primary targets of heat stress and have attempted to understand how proper lipid-protein interactions within membranes determine the observed transcriptional regulation of heat shock genes. We suggested that the specificity of gene expression is obtained by the uneven distribution of these membrane domains that precisely sense biological and physical environmental regulating signals and different forms of stresses (64). Membranes are highly dynamic structures that have areas of heterogeneity (domains or assembly of lipid/protein in a nonrandom organization) essential for the MPS and proper functionality. Changes in the MPS due, for example, to a new growth temperature, redetermines the threshold temperature at which HSPs are normally synthesized under stress conditions. Desaturases have been found in all organisms examined, with the exception of some bacteria such as E. coli, which is evolutionarily very close to Salmonella (26). The unsaturation of fatty acids in glycerolipids is essential for the proper functioning of biological membranes. E. coli possesses a type II fatty acid synthase system, and a double bond is introduced into the growing acyl chain at the 10-carbon hydroxydecanoyl-ACP1 intermediate, which is then elongated (70).
Wada et al. (66) purified the Synechocystis desA protein expressed in E. coli and showed that is had Δ12-desaturase activity in the cell homogenate of E. coli. Cells were grown in the presence of 18:1(9) that had been incorporated into the cells and esterified to membrane lipids.
Mass spectrophotometry analyses showed that insertion of Synechocystis Δ12-desaturase in the lipid bilayer of S. Typhimurium membrane caused a dramatic imbalance in the membrane protein/lipid ratio in Stm(pΔ12) compared to the Stm(pNir) strain. Perturbation of membrane functionality was determined by measuring membrane leakage by NPN fluorescence due to the insertion of Δ12-desaturase and membrane destabilization. Such an effect was particularly significant in the physiological temperature range of 25° to 40°C while it was not present at higher temperatures. The effect of the overproduction of SynechocystisΔ12-desaturase on the thermotropic behavior of membranes was confirmed by DSC on isolated outer membranes. We have shown that the overproduction of Δ12-desaturase lowered the transition temperature of certain lipid domains in the outer membranes of bacteria. In the temperature range between 10° and 65°C, one major endothermic peak was observed in the first and in the second up-scans as well. The reversible endothermic peaks in the temperature range of 15° to 45°C correspond to the phase transition of membrane lipids. The midpoints of temperature transition were at 34.1° and 30.8°C for the outer membranes isolated from the Stm(pNir) and the Stm(p 196 12) strains, respectively. Thus, S. Typhimurium expressing Δ12-desaturase displayed greater permeability in the outer membrane, even under nonstressing conditions. It is likely that the unbalanced membrane organization triggered a complex compensatory mechanism, including alteration of the ratio of gel-fluid lipid domains (21).
The induction of Δ12-desaturase and the consequent perturbation of the MPS dramatically reset the optimal temperature of expression of heat shock genes in Salmonella. Stm(pΔ12) inducing Δ12-desaturase expressed dnaK and ibpB at 30° and 37°C, with different patterns of expression when bacteria experienced thermal stress. Further, we identified two sHSPs, IbpA and IbpB, in the membrane fraction of Stm(pΔ12) by mass spectrometry. Thus, overexpression of Δ12-desaturase and its localization in the membrane triggered a complex compensatory mechanism that included alteration of the phase transition temperature of certain lipid domains and the association to the outer membrane of IbpA and IbpB (60, 64). In addition, we demonstrated that the independent expression of two membrane-spanning domains of Synechocystis Δ12-desaturase in Salmonella induced a profound rearrangement of lipid membrane. Therefore, it is likely that the observed behavior of Salmonella in response to the expression of the entire Δ12-desaturase coding sequence is at least partially due to its insertion in the membrane of this pathogen.
However, the effect of perturbation by Δ12-desaturase on the MPS and the heat shock response can be obtained also by using other membrane proteins unrelated to fatty acid metabolism or desaturation, as we have shown by expressing α-crystallin in eukaryotic cells (54; M. Luongo et al., unpublished data). Most cellular functions are highly dependent on the lipid environment that is controlled by proteins in or around membrane. The existence of special lipid regions and lipid rafts has also been used to design specific lipid therapies for the treatment of several human degenerative diseases (16, 65).
Similar changes in the level of expression of dnaK were obtained in Salmonella wt when membrane fluidification was attained by incubating bacteria in the presence of nontoxic concentrations of BA. However, resetting of the heat shock response due to BA treatment was temporary since 15 min after removal of BA from the culture medium dnaK was no longer expressed. In contrast, the overexpression of dnaK observed in Stm(pΔ12) inducing exogenous Δ12-desaturase under anaerobic conditions was stable up to 1 h after the culture was incubated under atmospheric oxygen tension. Similar results were obtained using the Stm(pBAD-Δ12) strain. Further, BA-treated Salmonella initially could not grow inside macrophages, but since the effect of BA on the MPS/heat shock response is reversible within 15 min after BA removal from medium, bacteria eventually recovered and grew normally inside macrophages.
In conclusion, we have shown that in S. Typhimurium the imbalance in the membrane lipid/protein ratio due to overexpression either of Δ12-desaturase or individually expressed membrane regions of this protein caused major changes in the MPS and a significant impairment of the heat shock response. Taken together, these results suggest that in this pathogen the perception of temperature is strictly controlled by membrane order and by a specific membrane lipid/protein ratio that ultimately cause transcriptional activation of heat shock genes. These results represent a novel and previously unrecognized mode of sensing temperature variation in pathogens and a subsequent mechanism of compensation of a microbial infective agent. Further, these effects have profound consequences on the pathogenicity of this microorganism. (37). As shown in the accompanying paper, the capacity to control the level of HSPs allows the possibility to produce pathogens with a reduced capacity to adapt to the host environment at the onset of infection (37).
Acknowledgments
This work was supported in part by grants from MIUR/2009, Italy, and from the Hungarian National Scientific Research Foundation (OTKA NK 68379).
We have no commercial interest or conflict of interest relevant to this study.
We thank Sergio Colonna-Romano for his technical help.
Footnotes
Published ahead of print on 5 February 2010.
REFERENCES
- 1.Alvarez-Ordóñez, A., A. Fernández, M. López, R. Arenas, and A. Bernardo. 2008. Modifications in membrane fatty acid composition of Salmonella typhimurium in response to growth conditions and their effect on heat resistance. Int. J. Food Microbiol. 123:212-219. [DOI] [PubMed] [Google Scholar]
- 2.Balogh, G., I. Horváth, E. Nagy, Z. Hoyk, S. Benkõ, and L. Vígh. 2005. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 272:6077-6086. [DOI] [PubMed] [Google Scholar]
- 3.Beere, H. M. 2004. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 117:2641-2651. [DOI] [PubMed] [Google Scholar]
- 4.Broadley, S. A., and F. U. Hartl. 2008. Mitochondrial stress signaling: a pathway unfolds. Trends Cell Biol. 18:1-4. [DOI] [PubMed] [Google Scholar]
- 5.Broquet, A. H., G. Thomas, J. Masliah, G. Trugnan, and M. Bachelet. 2003. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 278:21601-21606. [DOI] [PubMed] [Google Scholar]
- 6.Calderwood, S. K., M. A. Khaleque, D. B. Sawyer, and D. R. Ciocca. 2006. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sc. 31:164-172. [DOI] [PubMed] [Google Scholar]
- 7.Carratù, L., S. Franceschelli, C. L. Pardini, G. S. Kobayashi, I. Horváth, L. Vigh, and B. Maresca. 1996. Membrane lipid perturbation modifies the set point of heat shock response in yeast. Proc. Nat. Ac. Sci. U. S. A. 93:3870-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. C. 1992. Efficient precipitation and accurate quantitation of detergent-solubilized membrane proteins. Anal. Biochem. 205:22-26. [DOI] [PubMed] [Google Scholar]
- 9.Chatfield, S. N., I. G. Charles, A. J. Makoff, M. D. Oxer, G. Dougan, D. Pickard, D. Slater, and N. F. Fairweather. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Biotechnology (NY) 10:888-892. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Y., D. Bawa, S. Besshoh, J. W. Gurd, and I. R. Brown. 2005. Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J. Neurosci. Res. 81:522-529. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Y., T. S. Voegeli, P. P. Liu, E. G. Noble, and R. W. Currie. 2007. Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflamm. Allergy Drug Targets 6:91-100. [DOI] [PubMed] [Google Scholar]
- 12.Cossins, A. R. 1994. Homeoviscous adaptation of biological membranes and its functional significance, p. 63-76. In A. R. Cossins (ed.), Temperature adaptation of biological membranes. Portland Press, London, United Kingdom.
- 13.Delogu, G., M. Signore, A. Mechelli, and G. Famularo. 2002. Heat shock proteins and their role in heart injury. Curr. Opin. Crit. Care 8:411-416. [DOI] [PubMed] [Google Scholar]
- 14.de Marco, A., L. Vigh, S. Diamant, and P. Goloubinoff. 2005. Native folding of aggregation-prone recombinant proteins in Escherichia coli by osmolytes, plasmid- or benzyl alcohol-overexpressed molecular chaperones. Cell Stress Chaperones 10:329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz, J. T., and G. N. Somero. 1992. The threshold induction temperature of the 90 kDa heat shock protein is subject to acclimatization in eurythermal goby fishes (genus Gillichthys). Proc. Natl. Acad. Sci. U. S. A. 89:3389-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escribá, P. V. 2006. Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol. Med. 12:34-43. [DOI] [PubMed] [Google Scholar]
- 17.Giffard, R. G., L. Xu, H. Zhao, W. Carrico, Y. Ouyang, Y. Qiao, R. Sapolsky, G. Steinberg, B. Hu, and M. A. Yenari. 2004. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J. Exp. Biol. 207:3213-3220. [DOI] [PubMed] [Google Scholar]
- 18.Gyorfy, Z., I. Horváth, G. Balogh, A. Domonkos, E. Duda, B. Maresca, and L. Vígh. 1997. Modulation of lipid unsaturation and membrane fluid state in mammalian cells by stable transformation with the Δ9-desaturase gene of Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 237:362-366. [DOI] [PubMed] [Google Scholar]
- 19.Hooper, P. L., and J. J. Hooper. 2005. Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol. Ther. 7:204-208. [DOI] [PubMed] [Google Scholar]
- 20.Horváth, I., A. Glatz, V. Varvasovszki, Z. Török, T. Pali, G. Balogh, E. Kovács, L. Nádasdi, S. Benkö, F. Joó, and L. Vígh. 1998. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc. Natl. Acad. Sci. U. S. A. 95:3513-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horváth, I., G. Multhoff, A. Sonnleitner, and L. Vígh. 2008. Membrane-associated stress proteins: more than simply chaperones. Biochim. Biophys. Acta 1778:1653-1664. [DOI] [PubMed] [Google Scholar]
- 22.Janoff, A. S., A. Haug, and E. J. McGroarty. 1979. Relationship of growth temperature and thermotropic lipid phase changes in cytoplasmic and outer membranes from Escherichia coli K-12. Biochim. Biophys. Acta 5 55:56-66. [DOI] [PubMed] [Google Scholar]
- 23.Laplante, A. F., V. Moulin, F. A. Auger, J. Landry, H. Li, G. Morrow, R. M. Tanguay, and L. Germain. 1998. Expression of heat shock proteins in mouse skin during wound healing. J. Histochem. Cytochem. 46:1291-1301. [DOI] [PubMed] [Google Scholar]
- 24.Lee, U., I. Rioflorido, S. W. Hong, J. Larkindale, E. R. Waters, and E. Vierling. 2007. The Arabidopsis ClpB/Hsp100 family of proteins: chaperones for stress and chloroplast development. Plant J. 49:115-127. [DOI] [PubMed] [Google Scholar]
- 25.Lindquist, S. 1986. The heat shock response. Annu. Rev. Biochem. 5:1151-1191. [DOI] [PubMed] [Google Scholar]
- 26.Los, D. A., and N. Murata. 1998. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta 1394:3-15. [DOI] [PubMed] [Google Scholar]
- 27.Maytin, E. V. 1992. Differential effects of heat shock and UVB light upon stress protein expression in epidermal keratinocytes. J. Biol. Chem. 267:23189-23196. [PubMed] [Google Scholar]
- 28.Mikami, K., and N. Murata. 2003. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog. Lipid Res. 42:527-543. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto, R. I. 1998. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12:3788-3796. [DOI] [PubMed] [Google Scholar]
- 30.Nagy, E., Z. Balogi, I. Gombos, M. Akerfelt, A. Björkbom, G. Balogh, Z. Török, A. Maslyanko, A. Fiszer-Kierzkowska, K. Lisowska, P. J. Slotte, L. Sistonen, I. Horváth, and L. Vígh. 2007. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc. Natl. Acad. Sci. U. S. A. 104:7945-7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamoto, H., and L. Vigh. 2007. The small heat shock proteins and their clients. Cell. Mol. Life Sci. 64:294-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nylandsted, J., M. Gyrd-Hansen, A. Danielewicz, N. Fehrenbacher, U. Lademann, M. Høyer-Hansen, E. Weber, G. Multhoff, M. Rohde, and M. Jäättelä. 2004. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 200:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osborn, M. J., J. E. Gander, E. Parisi, and J. Carson. 1972. Mechanism of assembly of the outer membrane of Salmonella Typhimurium: isolation and characterization of cytoplasmic and outer membrane J. Biol. Chem. 247:3962-3972. [PubMed] [Google Scholar]
- 34.Panpoom, S., D. A. Los, and N. Murata. 1998. Biochemical characterization of a Δ12 acyl-lipid desaturase after over-expression of the enzyme in Escherichia coli. Biochim. Biophys. Acta 1390:323-332. [DOI] [PubMed] [Google Scholar]
- 35.Parsell, D. A., J. Taulien, and S. Lindquist. 1993. The role of heat-shock proteins in thermotolerance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 339:279-285. [DOI] [PubMed] [Google Scholar]
- 36.Peakman, T., S. Busby, and J. Cole. 1990. Transcriptional control of the cysG gene of Escherichia coli K-12 during aerobic and anaerobic growth. Eur. J. Biochem. 191:325-331. [DOI] [PubMed] [Google Scholar]
- 37.Porta, A., A. Eletto, Z. Török, S. Franceschelli, L. Vígh, A. Glatz, and B. Maresca. 2010. Changes in membrane fluid state and heat shock response cause attenuation in virulence. J. Bacteriol. 192:1999-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosette, C., and M. Karin. 1996. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274:1194-1197. [DOI] [PubMed] [Google Scholar]
- 39.Saibil, H. R. 2008. Chaperone machines in action. Curr. Opin. Struct. Biol. 18:35-42. [DOI] [PubMed] [Google Scholar]
- 40.Saidi, Y., A. Finka, M. Muriset, Z. Bromberg, Y. G. Weiss, F. J. Maathuis, and P. Goloubinoff. 2009. The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 21:2829-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- 42.Schaeffer, E. L., Jr., F. Bassi, and W. F. Gattaz. 2005. Inhibition of phospholipase A2 activity reduces membrane fluidity in rat hippocampus. J. Neural. Transm. 112:641-647. [DOI] [PubMed] [Google Scholar]
- 43.Shaw, A. S. 2006. Lipid rafts: now you see them, now you don't. Nature Immunol. 7:1139-1142. [DOI] [PubMed] [Google Scholar]
- 44.Shigapova, N., Z. Török, G. Balogh, P. Goloubinoff, L. Vigh, and I. Horváth. 2005. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 328:1216-1223. [DOI] [PubMed] [Google Scholar]
- 45.Söti, C., and P. Csermely. 2007. Protein stress and stress proteins: implications in aging and disease. J. Biosci. 32:511-515. [DOI] [PubMed] [Google Scholar]
- 46.Spiro, S., and J. R. Guest. 1987. Regulation and over-expression of the fnr gene of Escherichia coli. J. Gen. Microbiol. 133:3279-3288. [DOI] [PubMed] [Google Scholar]
- 47.Stults, J. T. 1995. Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). Curr. Opin. Struct. Biol. 5:691-698. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki, I., D. A. Los, Y. Kanesaki, K. Mikami, and N. Murata. 2000. The pathway for perception and transduction of low-temperature signals in Synechocystis. EMBO J. 19:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szalontai, B., Y. Nishiyama, Z. Gombos, and N. Murata. 2000. Membrane dynamics as seen by Fourier transform infrared spectroscopy in a cyanobacterium, Synechocystis PCC 6803. The effects of lipid unsaturation and the protein-to-lipid ratio. Biochim. Biophys. Acta 1509:409-419. [DOI] [PubMed] [Google Scholar]
- 50.Tomanek, L., and G. N. Somero. 1999. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J. Exp. Biol. 202:2925-2936. [DOI] [PubMed] [Google Scholar]
- 51.Török, Z., N. M. Tsvetkova, G. Balogh, I. Horváth, E. Nagy, Z. Pénzes, J. Hargitai, O. Bensaude, P. Csermely, J. H. Crowe, B. Maresca, and L. Vigh. 2003. Heat shock protein coinducers with no effect on protein denaturation specifically modulate the membrane lipid phase. Proc. Natl. Acad. Sci. U. S. A. 100:3131-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Török, Z., P. Goloubinoff, I. Horváth, N. M. Tsvetkova, A. Glatz, G. Balogh, V. Varvasovszki, D. A. Los, E. Vierling, J. H. Crowe, and L. Vigh. 2001. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. U. S. A. 98:3098-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsuchido, T., I. Aoki, and M. Takano. 1989. Interaction of the fluorescent dye 1-N-phenylnaphthylamine with Escherichia coli cells during heat stress and recovery from heat stress. J. Gen. Microbiol. 135:1941-1947. [DOI] [PubMed] [Google Scholar]
- 54.Tsvetkova, N. M., I. Horváth, Z. Török, W. F. Wolkers, Z. Balogi, N. Shigapova, L. M. Crowe, F. Tablin, E. Vierling, J. H. Crowe, and L. Vigh. 2002. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. U. S. A. 99:13504-13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Eden, W., and D. B. Young. 1996. Stress proteins in medicine. Marcel Dekker, New York, NY.
- 56.van Eden, W., G. Wick, S. Albani, and I. Cohen. 2007. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann. N. Y. Acad. Sci. 1113:217-237. [DOI] [PubMed] [Google Scholar]
- 57.Verbeke, P., B. F. Clark, and S. I. Rattan. 2000. Modulating cellular aging in vitro: hormetic effects of repeated mild heat stress on protein oxidation and glycation. Exp. Gerontol. 35:787-794. [DOI] [PubMed] [Google Scholar]
- 58.Vereb, G., J. Szöllosi, J. Matkó, P. Nagy, T. Farkas, L. Vigh, L. Mátyus, T. A. Waldmann, and S. Damjanovich. 2003. Dynamic, yet structured: the cell membrane three decades after the Singer-Nicolson model. Proc. Natl. Acad. Sci. U. S. A. 100:8053-8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vigh, L., and B. Maresca. 2002. Dual role of membranes in heat stress: as thermosensors modulate the expression of stress genes and by interacting with stress proteins, re-organize their own lipid order and functionality, p. 173-178. In K. B. Storey and J. M., Storey (ed.), Cell and molecular responses to stresses, vol. 3. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 60.Vigh, L., B. Maresca, and J. L. Harvood. 1998. Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem. Sci. 23:369-3674. [DOI] [PubMed] [Google Scholar]
- 61.Vigh, L., I. Horváth, B. Maresca, and J. L. Harwood. 2007. Can the stress protein response be controlled by “membrane-lipid therapy”? Trends Biochem. Sci. 32:357-363. [DOI] [PubMed] [Google Scholar]
- 62.Vigh, L., H. Nakamoto, J. Landry, A. Gomez-Munoz, J. L. Harwood, and I. Horvath. 2007. Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann. N. Y. Acad. Sci. 1113:40-51. [DOI] [PubMed] [Google Scholar]
- 63.Vigh, L., N. P. Literati, I. Horvath, Z. Török, G. Balogh, A. Glatz, E. Kovács, I. Boros, P. Ferdinándy, B. Farkas, L. Jaszlits, A. Jednákovits, L. Korányi, and B. Maresca. 1997. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat. Med. 3:1150-1154. [DOI] [PubMed] [Google Scholar]
- 64.Vigh, L., P. Escriba, A. Sonnleitner, M. Sonnleitner, S. Piotto, B. Maresca, I. Horváth, and J. L. Harwood. 2005. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog. Lipid Res. 44:303-344. [DOI] [PubMed] [Google Scholar]
- 65.Vögler, O., J. M. Barceló, C. Ribas, and P. V. Escribá. 2008. Membrane interactions of G proteins and other related proteins. Biochim. Biophys. Acta 1778:1640-1652. [DOI] [PubMed] [Google Scholar]
- 66.Wada, H., M. H. Avelange-Macherel, and N. Murata. 1993. The desA gene of the cyanobacterium Synechocystis sp. strain PCC6803 is the structural gene for delta 12 desaturase. J. Bacteriol. 175:6056-6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, W., B. Vinocur, and A. Altman. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1-14. [DOI] [PubMed] [Google Scholar]
- 68.Wilkison, W. O., and R. M. Bell. 1997. sn-Glycerol-3-phosphate acyltransferase from Escherichia coli. Biochim. Biophys. Acta 1348:3-9. [DOI] [PubMed] [Google Scholar]
- 69.Yanagida, M., Y. Miura, K. Yagasaki, M. Taoka, T. Isobe, and N. Takahashi. 2000. Matrix assisted laser desorption/ionization-time of flight-mass spectrometry analysis of proteins detected by anti-phosphotyrosine antibody on two-dimensional-gels of fibroblast cell lysates after tumor necrosis factor-alpha stimulation. Electrophoresis 21:1890-1898. [DOI] [PubMed] [Google Scholar]
- 70.Zhang, Y. M., H. Marrakchi, and C. O. Rock. 2002. The FabR (YijC) transcription factor regulates unsaturated fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 277:15558-15565. [DOI] [PubMed] [Google Scholar]