Abstract
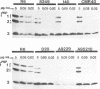
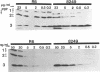
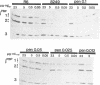
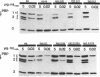
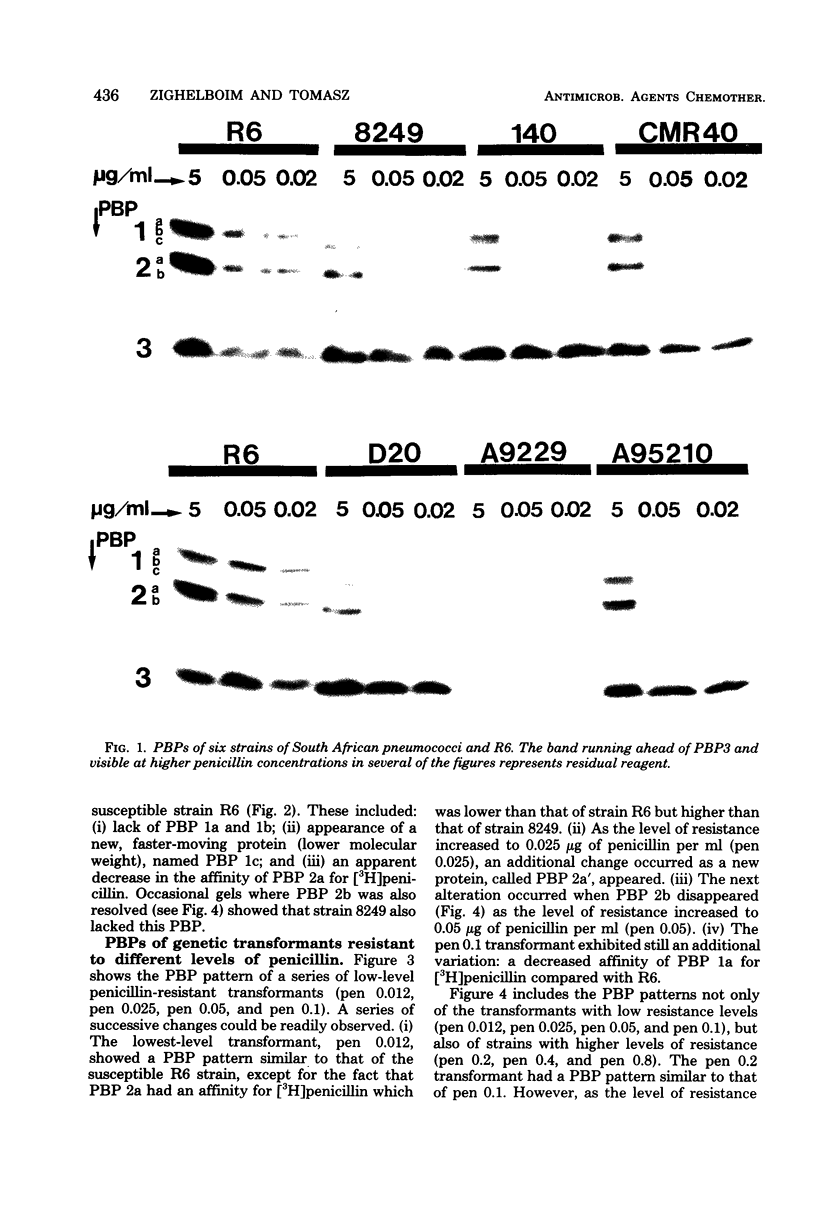
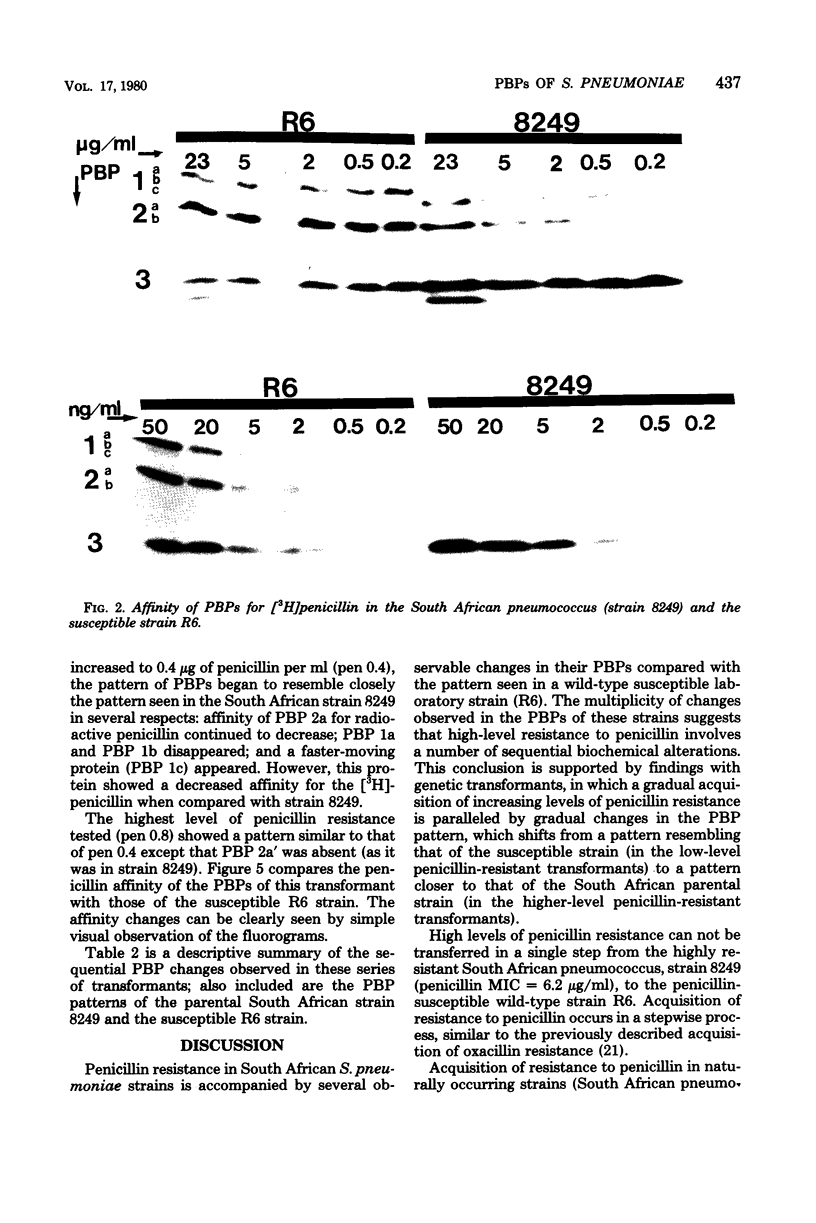
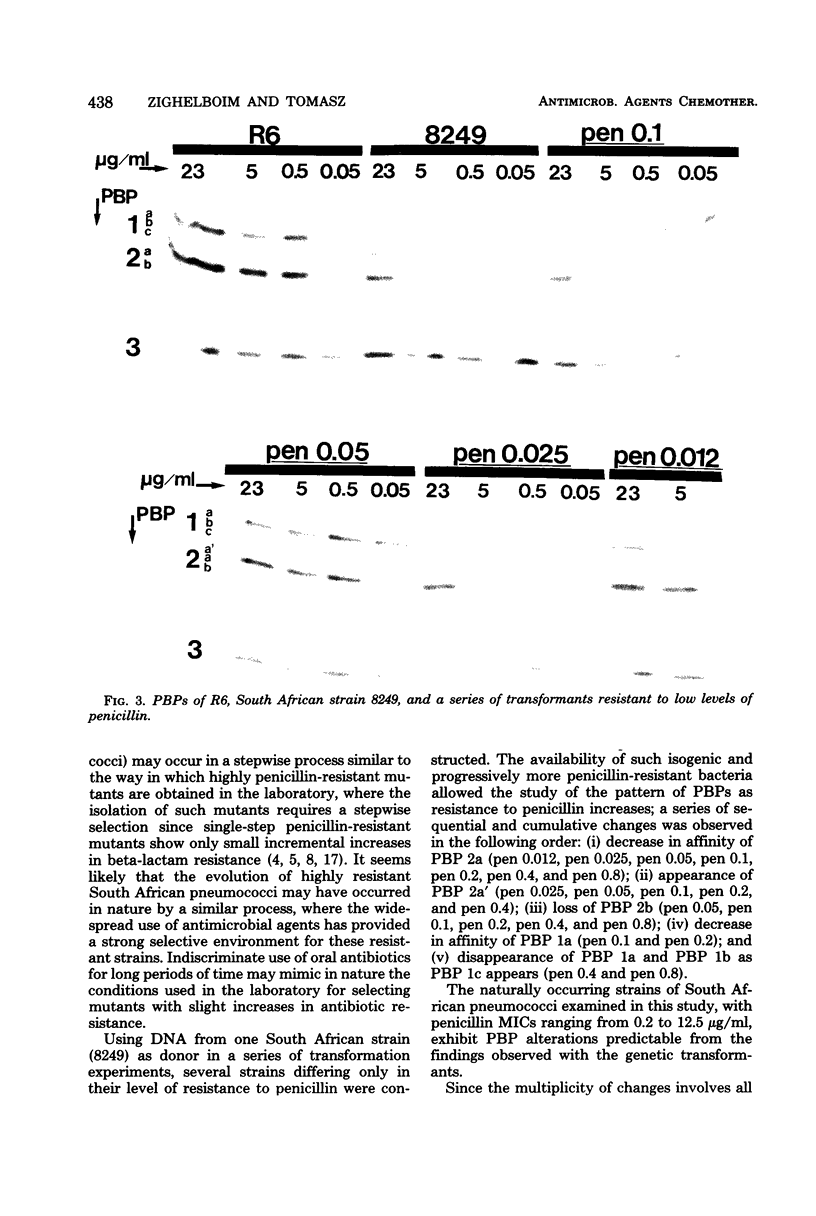
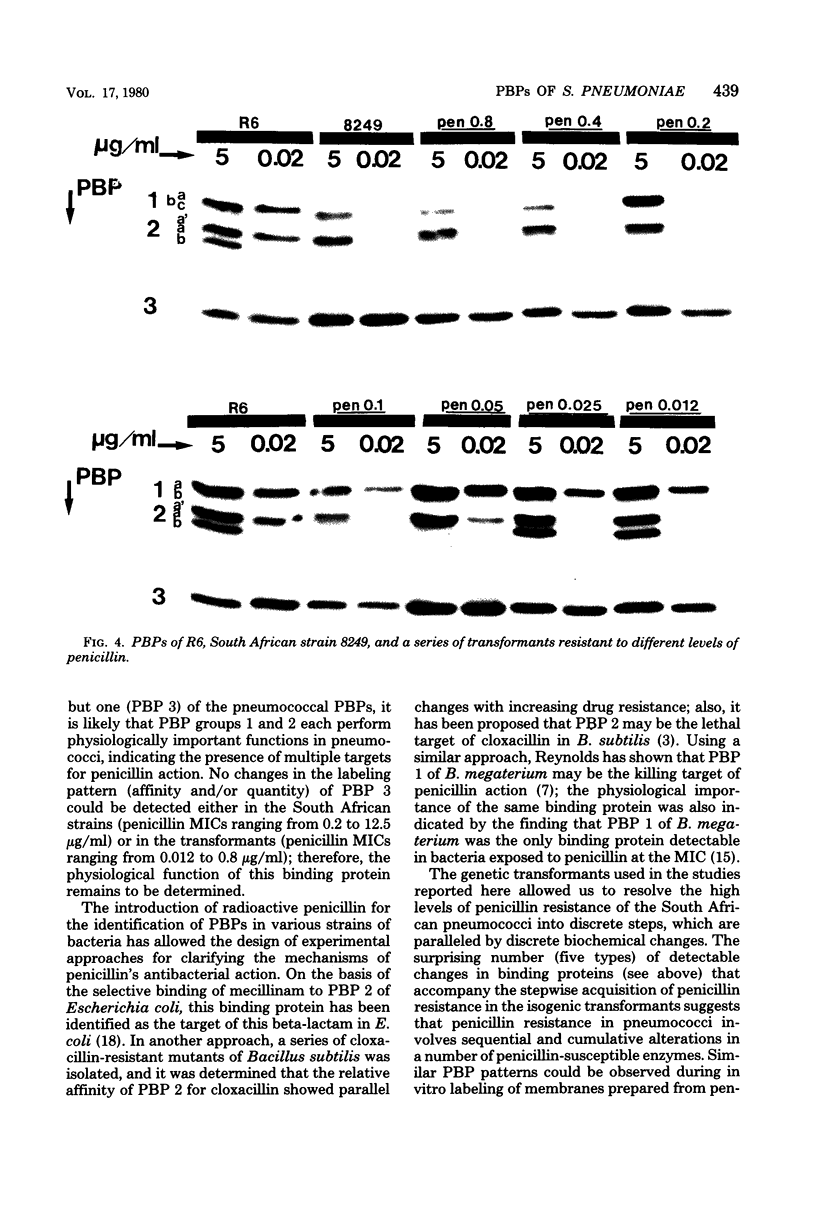
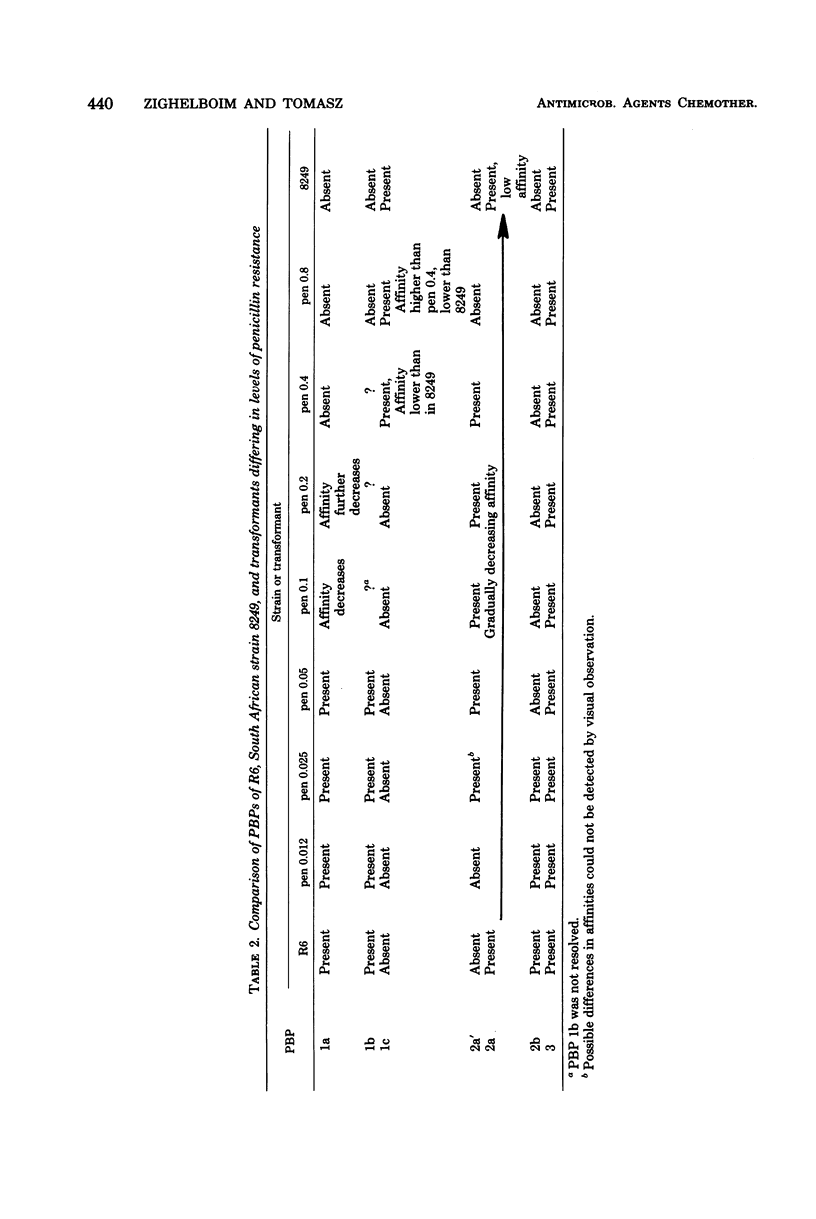
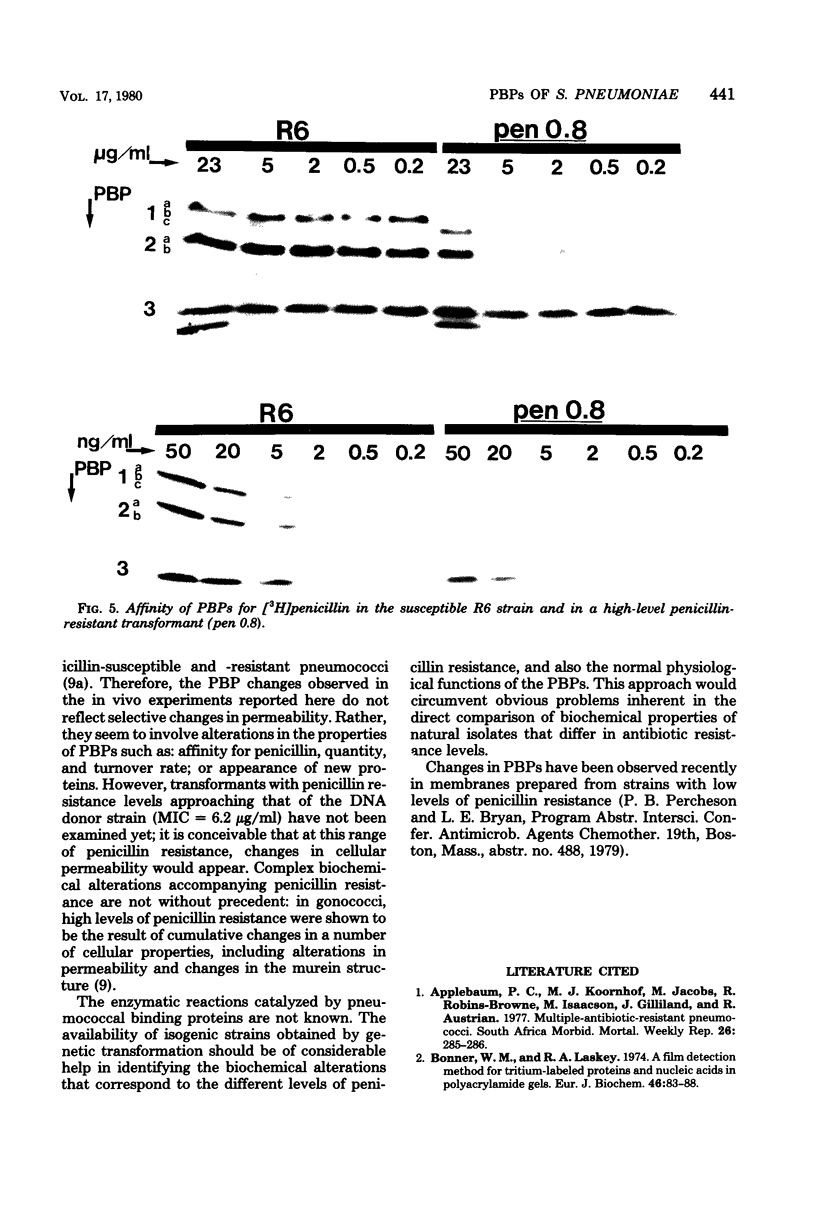
Multiply drug-resistant South African pneumococci (with penicillin minimal inhibitory concentrations ranging from 0.2 to 12.5 microgram/ml) showed several types of major alterations in their penicillin-binding protein (PBP) pattern compared with that of a penicillin-susceptible laboratory strain of Streptococcus pneumoniae (R6; penicillin minimal inhibitory concentration = 0.006 microgram/ml). Genetic transformants were obtained by using South African pneumococcus (strain 8249) deoxyribonucleic acid as donor and the competent cells of strain R6 as recipient; seven classes of transformants with progressively higher penicillin resistance were isolated, and their PBPs were tested. The PBP patterns exhibited a gradual shift from a pattern similar to that of the recipient to a pattern resembling that of the donor strain as the level of penicillin resistance increased.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler L. O., Smiley M. B. Characterization by transformation of an ampicillin-resistant mutant of pneumococcus. J Gen Microbiol. 1970 May;61(2):189–195. doi: 10.1099/00221287-61-2-189. [DOI] [PubMed] [Google Scholar]
- Demerec M. Origin of Bacterial Resistance to Antibiotics. J Bacteriol. 1948 Jul;56(1):63–74. doi: 10.1128/jb.56.1.63-74.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Giles A. F., Reynolds R. E. Bacillus megaterium resistance to cloxacillin accompanied by a compensatory change in penicillin binding proteins. Nature. 1979 Jul 12;280(5718):167–168. doi: 10.1038/280167a0. [DOI] [PubMed] [Google Scholar]
- Gunnison J. B., Fraher M. A., Pelcher E. A., Jasetz E. Penicillin-resistant variants of pneumococci. Appl Microbiol. 1968 Feb;16(2):311–314. doi: 10.1128/am.16.2.311-314.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Tarpay M., Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E., Shepherd S. T., Chase H. A. Identification of the binding protein which may be the target of penicillin action in Bacillus megaterium. Nature. 1978 Feb 9;271(5645):568–570. doi: 10.1038/271568a0. [DOI] [PubMed] [Google Scholar]
- Robins-Brown R. M., Gaspar M. N., Ward J. I., Wachsmuth I. K., Koornhof H. J., Jacobs M. R., Thornsberry C. Resistance mechanisms of multiply resistant pneumococci: antibiotic degradation studies. Antimicrob Agents Chemother. 1979 Mar;15(3):470–474. doi: 10.1128/aac.15.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley T. E., Hotchkiss R. D. Stepwise introduction of transformable penicillin resistance in Pneumococcus. Genetics. 1970 Mar-Apr;64(3):397–408. [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]