Abstract
So far attenuation of pathogens has been mainly obtained by chemical or heat treatment of microbial pathogens. Recently, live attenuated strains have been produced by genetic modification. We have previously demonstrated that in several prokaryotes as well as in yeasts and mammalian cells the heat shock response is controlled by the membrane physical state (MPS). We have also shown that in Salmonella enterica serovar Typhimurium LT2 (Salmonella Typhimurium) overexpression of a Δ12-desaturase gene alters the MPS, inducing a sharp impairment of transcription of major heat shock genes and failure of the pathogen to grow inside macrophage (MΦ) (A. Porta et al., J. Bacteriol. 192:1988-1998, 2010). Here, we show that overexpression of a homologous Δ9-desaturase sequence in the highly virulent G217B strain of the human fungal pathogen Histoplasma capsulatum causes loss of its ability to survive and persist within murine MΦ along with the impairment of the heat shock response. When the attenuated strain of H. capsulatum was injected in a mouse model of infection, it did not cause disease. Further, treated mice were protected when challenged with the virulent fungal parental strain. Attenuation of virulence in MΦ of two evolutionarily distant pathogens was obtained by genetic modification of the MPS, suggesting that this is a new method that may be used to produce attenuation or loss of virulence in both other intracellular prokaryotic and eukaryotic pathogens. This new procedure to generate attenuated forms of pathogens may be used eventually to produce a novel class of vaccines based on the genetic manipulation of a pathogen's membrane fluid state and stress response.
So far, a methodology to obtain live attenuated strains has not been generated for fungal pathogens. With respect to parasites, a live attenuated strain of Toxoplasma has been produced. However, this modified strain was used to develop a vaccine that was considered expensive, caused side effects, and had a short shelf life. Furthermore, the authors emphasized that this vaccine might revert to a pathogenic strain and, therefore, was not considered suitable for human use (17). In Leishmania and other parasites, as yet none of the current candidate subunit vaccines has achieved complete protection reproducibly. Attempts to develop an effective vaccine to control leishmaniasis have been shown to be feasible, but no vaccine is in active clinical use (18). The ability to create genetically modified pathogens by eliminating virulence or essential genes is considered a powerful alternative in the development of an effective protective vaccine. The current genetic procedures are based on the use of specific (virulence) genes that may cause attenuation and are species specific. In other words, a given method of attenuation may be effective in a specific organism but not in other pathogens.
We have previously shown in several prokaryotic and eukaryotic cells, including pathogens such as Salmonella (34) and in the human pathogenic fungus Histoplasma capsulatum that the normal temperature threshold of the heat shock response is regulated by the membrane physical state (MPS) (3, 4, 26, 44, 45). We have also shown that the MPS can be modified genetically (by altering the saturated/unsaturated fatty acids ratio [SFA/UFA]) or chemically (by treating cells with membrane fluidizers such as benzyl alcohol [BA], heptanol, or ether), which in turn causes a refined heat shock response (44-46). The possibility to manipulate the MPS genetically allowed us to investigate the effect of reduced levels of heat shock proteins (HSPs) on the virulence of a fungal pathogen at the onset of infection in murine macrophage (MΦ) and in a mouse model of infection.
One of the main functions of HSPs is to refold or protect the folding of proteins under physiological or stress (heat shock) conditions (14, 19). It has been shown that intracellular pathogens, such as Salmonella enterica serovar Typhimurium, Mycobacterium tuberculosis, H. capsulatum, and protozoan parasites, e.g., at the onset of infection of MΦ and/or of other cells stimulate the transcriptional activation of two sets of genes: virulence genes (species specific) and genes involved in the stress response (highly conserved) (1, 13). Virulence genes, induced during host invasion/adaptation, operate in a coordinate fashion and allow pathogens to invade, replicate, and induce disease in the host. Simultaneously, heat shock genes are induced, and their protein products (HSPs) are responsible for the adaptation to the higher temperature (allowing the folding of the pathogen's proteins) and to the hostile conditions present in host cells such as MΦ. In addition, upon host invasion, HSP70 of eukaryotic pathogens and bacterial GroEL (HSP60) are upregulated and become the major antigens, constituting up to 15% of the cell's dry weight (7). These coordinated and generalized genetic responses allow intracellular pathogens to avoid the mechanisms of defense and cell immune response and eventually allow them to induce a disease.
Here, we show that a genetically or chemically modified virulent strain of H. capsulatum unable to induce normal amounts of HSPs lost the ability to survive in murine MΦ, and the attenuated strain did not cause disease in a mouse model of infection. Further, the modified H. capsulatum strain injected in mice induced protection when mice were challenged with the normal virulent strain. These data suggest that this methodology may represent a new procedure to obtain attenuated strains that may be considered to produce a novel class of human and animal vaccines.
MATERIALS AND METHODS
Strains, media, plasmids, and growth conditions.
Escherichia coli TOP10F′ (Invitrogen) cells were routinely cultured on LB agar or LB broth supplemented with 100 μg/μl ampicillin (Amp100; Sigma-Aldrich) at 30° and 37°C. The temperature-susceptible avirulent Downs (ATCC 38904; American Type Culture Collection, Rockville, MD) and the temperature-tolerant virulent G217B (ATCC 26034) strains of H. capsulatum were grown in Histoplasma macrophage medium (HMM) broth or on HMM agarose plates supplemented with 5 mg/ml bovine serum albumin. H. capsulatum strain G217B ura5-23 (a kind gift of W. Goldman, Washington University, St. Louis, MO) (49) was grown in HMM broth supplemented with 0.2 mg/ml uracil (Sigma-Aldrich, St. Louis, MO). H. capsulatum was grown as yeast cells at 34° or 37°C in liquid cultures with gyratory shaking and transferred every 3 days at a 1:25 dilution. Plates were grown at 37°C in a humidified incubator.
DNA manipulation.
Genomic DNA extraction, DNA electrophoresis, plasmid isolation, restriction enzymes analysis, and PCR amplification were performed according to standard procedures (36).
Plasmid construction.
The XbaI-NcoI fragment of the Ole1 promoter of H. capsulatum Downs strain was cloned in Litmus 29 cloning vector to generate pLD plasmid. Total RNA of G217B strain was used for reverse transcriptase (RT) PCR amplification of Ole1 cDNA. A specific 3′ end primer containing an NheI site and an XhoI site at the 5′ end (cDNAF, 5′-CTCGAGCTAGCCGTTATTACATAGAACATC-3′) was used to reverse transcribe 2 μg of total RNA, using a SuperScript Preamplification System (Gibco-BRL). The first cDNA strand was then amplified by PCR using both the 3′ end primer, used for the RT reaction, and a specific 5′ end primer (cDNAR, 5′-CCATGGCTTTAAACGAAGCC-3′). The cDNAR primer contained an NcoI restriction site that overlaps the ATG start codon of H. capsulatum Ole1 cDNA. The amplified product was gel purified with a Geneclean II Kit (Bio 101 Inc.) after 30 cycles consisting of 45 s at 94°C, 60 s at 53°C, and 120 s at 72°C. It was then subcloned into TA vector (Invitrogen) to generate plasmid pTOleH. The amplified fragment was sequenced to ensure that no misincorporation had occurred during amplification. Subsequently, pTOleH was digested with NcoI-XhoI to release an Ole1 cDNA fragment that subsequently was ligated into NcoI/XhoI-digested pLD to generate the pDH plasmid. Plasmid pWU55 (50) containing the Podospora anserina URA5 gene and a cassette with telomeres separated by a kanamycin resistance gene was digested with NheI in which the XbaI-NheI fragment derived from the pDH plasmid was cloned, generating pD3 plasmid. The pD3 plasmid contained the Ole1 cDNA of the G217B strain under the transcriptional control of the upregulated Ole1 promoter of H. capsulatum Downs strain (9).
Electrotransformation of H. capsulatum.
Plasmid pD3 was linearized with PacI and used to transform the G217B ura5-23 strain as previously described (50). Insertion of pD3 into the Histoplasma genome was checked by enzymatic restrictions and Southern blot analysis. The modified strain containing the pD3 vector inserted into the Histoplasma chromosome was named HcD3.
Heat shock gene expression in H. capsulatum.
Expression of the H. capsulatum Hsp70 gene was determined in the highly virulent G217B strain and in the modified HcD3 strain as described previously (4).
Murine macrophage infection with H. capsulatum strains.
MΦ were obtained from hematopoietic stem cells aseptically harvested from tibial bone marrow of 42-day-old male CD-1 mice and selected in Iscove's modified Dulbecco's medium (IMDM; Gibco-BRL) supplemented with 10% fetal calf serum (FCS) and 30% of the conditioned LB6 cell supernatant, as a source of macrophage colony-stimulating factor (M-CSF). Aliquots of bone marrow-derived cells (approximately 2.0 × 107 cells) were dispensed into 750-ml Falcon tissue culture flasks (growth surface, 175 cm2) and placed at 37°C in a humidified atmosphere containing 5% CO2 to allow MΦ to adhere. After 24 h, IMDM containing nonadhered cells was removed and replaced with fresh medium. Aliquots of MΦ from 7-day cultures were transferred to IMDM supplemented with 10% FCS and then plated on 13-mm glass coverslips (thickness 1) in 24-well tissue culture plates overnight (o.n.). Yeast phase cells were grown at 34°C prior to the infection. Logarithmically growing cells of H. capsulatum G217B wild-type and HcD3 strains were collected by centrifugation at 3,200 × g for 10 min, added to the 7-day culture MΦ monolayer (90% confluent growth with approximately 7 × 106 MΦ per flask) at a multiplicity of infection (MOI) of 10:1, and incubated at 37°C in 5% CO2. Adherence to and ingestion by MΦ of yeast cells were evaluated using phase-contrast and Nomarski optics in a Zeiss Axioskop microscope with high-numerical-aperture objective lenses (Zeiss Corp) and by serial dilution plating of fungi recovered after MΦ lysis.
Mouse model of infection of H. capsulatum.
BALB/c × CD-1 mice were injected with the yeast form of the highly virulent G217B strain or the genetically modified HcD3 H. capsulatum strain at a concentration between 1 × 106 and 5 × 107 cells/mouse as described previously (20). Mouse survival was monitored up to 2 months. Mice that survived the infection with the highest concentration of H. capsulatum HcD3 strain were challenged with a lethal dose (5 × 107 cells/mouse) of the virulent H. capsulatum G217B strain.
RESULTS
Effect of constitutive expression of Δ9-desaturase on Histoplasma growth.
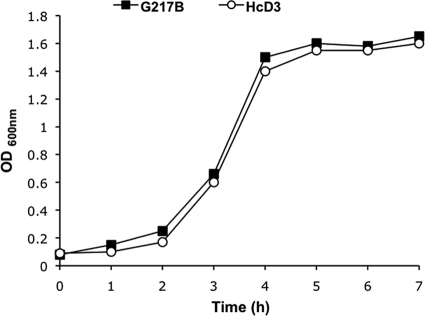
The G217B and HcD3 H. capsulatum strains (the latter is a G217B mutant strain carrying an extra copy of its own Δ9-desaturase gene under the transcriptional control of the upregulated Downs Δ9-desaturase promoter) were grown at 34 and 37°C, and their growth rates were monitored up to 30 h. Growth rates of both strains were similar at 30° and 34°C, with a doubling time of about 7.0 h (data not shown). Growth rates of the G217B and HcD3 strains of H. capsulatum were comparable also at 37°C (Fig. 1).
FIG. 1.
Growth curve of yeast form of H. capsulatum G217B and HcD3 strains at 37°C. The values of the graph represent one of four independent growth curves.
Hsp70 gene expression in the H. capsulatum HcD3 strain.
The yeast forms of the highly virulent H. capsulatum G217B and of HcD3 strains were grown at 34° and 37°C to mid-log phase and then heat shocked for 30 min at different temperatures. Total RNA was purified and immediately processed for Northern blot analysis. The Hsp70 gene was highly expressed in H. capsulatum G217B (from 34° to 37°C, from 34° to 42°C, and from 37° to 42°C), whereas in strain HcD3 Hsp70 transcription was detectable only from 34° to 42°C (Fig. 2). Previously, we have shown that Hsp70 and Hsp82 transcription was altered in the H. capsulatum G217B strain when cultures were treated by the addition of oleic acid (UFA) or BA, which increased membrane fluidity (4).
FIG. 2.
Hsp70 gene expression in the H. capsulatum HcD3 strain. Yeast cells of H. capsulatum G217B and of HcD3 strains were grown at 34° and 37°C and heat shocked for 30 min at different temperatures. The Hsp70 gene was highly expressed in strain G217B (from 34° to 37°C, from 34° to 42°C, and from 37° to 42°C), whereas in strain HcD3 Hsp70 transcription was detectable only from 34° to 42°C.
H. capsulatum MΦ infection.
H. capsulatum G217B and HcD3 strains grown at 34°C were used to infect MΦ. Yeast phase cells were added to a MΦ monolayer at 37°C, and infection proceeded for different lengths of time at an MOI of 10:1. Internalization and quantification of yeast cells inside MΦ were evaluated using phase-contrast and Nomarski optics with a Zeiss Axioskop microscope (Fig. 3A). We incubated the fungal cells at 34°C prior to infection and then transferred them to 37°C so that cells could experience a heat shock. Though H. capsulatum HcD3 was internalized inside MΦ, no fungal growth was detectable at 8.0 h after infection (Fig. 3B), very likely because of an altered MPS and impaired heat shock response (Fig. 2).
FIG. 3.
Murine MΦ infection with H. capsulatum. Yeast phase cells were grown at 34°C before infection. (A) Yeast phase cells of strain HcD3 as well as the control G217B strain were internalized inside MΦ. (B) Yeast cells recovered from MΦ lysed at different time points of infection were counted. G217B grew inside MΦ while no growth was detectable for the HcD3 strain. Statistical differences were evaluated by a two-tailed Student's t test. P values less than 0.05 were considered significant. Each experiment in duplicate was performed at least three times. The figure shows one of such experiments. The pictures were taken after the macrophages were washed. Arrows point to internalized yeasts cells.
Mouse infection.
In Histoplasma the standard concentration of yeast cells injected to study the effects of drugs or vaccine particles is 1 × 107, which kills all mice in about 3 weeks. Increasingly high concentrations of yeast phase cells of the virulent G217B or of the genetically modified HcD3 H. capsulatum strain (106, 107, and 5 × 107) were injected intravenously into CD-1 mice (10/group). Survival of infected mice was monitored daily. Mice infected with 106 yeast cells of the virulent H. capsulatum strain died within 40 days, whereas all mice infected with the same dose of the HcD3 strain survived (Fig. 4A). At a dose of 107 yeast cells all mice infected with the virulent strain of H. capsulatum died after 23 days, whereas all mice infected with the modified HcD3 strain survived up to 30 days after infection (Fig. 4B). Mice infected with the killing dose of yeast cells of the virulent G217B strain (5 × 107) died within 6 days, while 7 out of 10 mice infected with the same inoculum of the modified HcD3 strain survived up to 45 days after infection (Fig. 4C). After 40 days from the initial infection with the HcD3 strain, the surviving mice were challenged with a lethal dose (5 × 107 yeast cells) of the virulent G217B strain. Figure 4D shows that 20 days (60 days from the initial infection) after infection six mice of the initial group still survived.
FIG. 4.
Mouse infection with H. capsulatum. (A) Mice infected with 106 yeast cells of strain G217B died within 40 days after infection, whereas all mice infected with strain HcD3 survived. (B) About 107 G217B yeast cells killed all mice after 23 days, whereas all mice infected with 107 HcD3 yeast cells survived up to 30 days after infection. (C) All mice infected with 5 × 107 G217B yeast cells died within 6 days, while 7 out of 10 mice infected with the same inoculum of HcD3 survived up to 45 days after infection. (D) Surviving mice were challenged with a lethal dose (5 × 107 yeast cells) of the virulent strain G217B. After 20 days (60 days from the initial infection with attenuated HcD3 strain) six mice still survived.
DISCUSSION
H. capsulatum is the most common cause of invasive fungal pulmonary disease worldwide (27). Fungal infections have increased tremendously in the past 20 years because of the AIDS epidemic (12). Available antifungal drugs are only fungistatic, and vaccines against fungal pathogens are badly needed but are not yet available.
Historically, vaccines against microbial pathogens have been based on the use of attenuated strains or on the injection of purified antigenic microbial proteins. One strategy to develop new vaccines is the use of live attenuated strains (5). These procedures have accomplished a striking reduction of infection and diseases. Purification of microbial components and molecular biology techniques improved our knowledge of the immune system and of the mechanisms of virulence of pathogens, allowing the production of attenuated mutants, the expression of vaccine proteins in live vectors, the purification and synthesis of microbial antigens, and the induction of immune responses through nucleic acids, proteins, and polysaccharides (8, 22, 25, 30, 37).
Some of the classical methods to obtain vaccines are based on the use of attenuated strains or on the injection of one or a few antigens that stimulate an immune response. The first vaccines using purified antigens were developed against pathogens with little antigenic variability that resulted in life immunity to further exposure to the same pathogen. Eventually, problems emerged in trying to develop vaccines against bacteria with a high level of antigenic variability. For example, vaccines were developed by inducing antibodies that recognize the capsular polysaccharide (CPS). In spite of the problem of antigenic variability, successful vaccines were developed, e.g., for Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis. However, these pathogens have highly variable capsular serotypes, and there is rarely cross-protection between serotypes. Further, strains can shift in serotype, and novel serotypes can develop. Thus, for CPS-based vaccines, capsule diversity still needs to be tested continuously (41). In general, this results in the low efficacy of these vaccines since the natural mechanisms of protection against infective agents consist of a complex response against the entire microorganism and combined antigens. Further, vaccines comprised of infective attenuated particles, obtained by a thermal or chemical treatment, may contain denatured antigens that do not induce the appropriate immunological response. New strategies using multivalent vaccines are also being employed and are beginning to be successful against these more difficult pathogens. However, novel approaches to the design of vaccines need to be based on antigens that are not under immune pressure during infection. A successful acellular vaccine against Bordetella pertussis based on the identification of major virulence factors has been produced (35). Further, a vaccine containing three important virulence factors that confer protection against intragastric Helicobacter pylori is being tested in humans (41). More recently, the possibility of sequencing entire genomes and the use of bioinformatics have led to the opportunity to identify genes coding for specific antigens (24). However, though these very powerful techniques are providing important data on potential antigens to be used in vaccines, they still rely on data to be generated on each single microorganism, and results cannot be generalized to other pathogens. With respect to fungal pathogens, sequences of entire genomes are available only for Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus, and information on virulence factors is far from being exhaustive.
There is ample evidence that HSPs have been implicated in the stimulation and generation of both innate and adaptive immunity in a wide variety of microorganisms, eliciting humoral responses during natural infection with various pathogens. Anti-HSP70 antibodies have been detected in a significant number of patients with chronic parasitosis such as schistosomiasis, malaria, and leishmaniasis. In addition HSP70 of Plasmodium falciparum, Schistosoma mansoni, H. capsulatum, and Mycobacterium spp. have also been shown to induce a cell-mediated immune response (2, 20). A putative role for HSP90 in immunity has also been proposed (20). However, vaccination with a recombinant protein does not protect mice against a sublethal challenge with virulent strains. Thus, while HSPs do have antigenic properties, they do not mediate protection. Many questions therefore remain unanswered about the role of HSPs in adaptive immunity.
Our approach, in contrast, is based on the manipulation of the heat shock response to reduce the synthesis of HSPs, rather than stimulate an increase of HSP accumulation, to produce a strain hampered in the process of stress adaptation. We have modified H. capsulatum with a gene involved in the regulation of the MPS, causing impairment of the pathogen's stress response that resulted in a parallel failure of the pathogen to survive within murine MΦ (as shown in Salmonella in the accompanying paper [34]) and to cause disease in a mouse model of infection. Further, the genetically modified attenuated H. capsulatum strain induced protection in mice after subsequent challenge with a highly virulent strain.
We along with other authors have shown that synthesis of HSPs is controlled by abrupt local variations of a number of factors that include membrane lipid composition, membrane lipid-protein interactions, membrane lipid dynamics (MPS modification), and physical reorganization of lipids (3, 38, 39, 42, 45-47). Stubbs and Slater (40) have proposed that perturbation of membrane lipid-protein interactions (coupled with curvature stress, causing unfavorable packing of nonlamella-forming lipids) induces nonlamellar HII lipid phases and altered signal transduction pathways. We have shown that the specificity of gene expression is obtained by the uneven distribution of these membrane domains that precisely sense biological and physical environmental regulating signals and different forms of stresses (44).
It is well known that the lack of expression of HSPs has profound effects on all organismal growth and during the embryogenesis of higher organisms (a stage in the life cycle of Metazoa or plants when specific genes are highly coregulated and transcribed) (31). Though in all organisms heat shock genes are highly transcribed under stress conditions, there are specific stages in the embryogenesis of Metazoa (e.g., the Drosophila pupae) during which, under stress conditions, heat shock genes are poorly transcribed, and accumulation of HSPs does not occur, generating phenocopies (morphologically altered individuals but with identical genotypes). Goldschmidt (10) originally described the emergence of phenocopies in Drosophila. Later, Mitchell and Lipps (23) associated this phenomenon with the lack of HSP synthesis. Petersen and Mitchell demonstrated that induction of thermotolerance under nonlethal heat shock conditions prevents the appearance of phenocopies (32). Thermotolerance is a well-known phenomenon associated with synthesis of HSPs at moderate temperatures that allow the cell and the whole organism to tolerate subsequent, higher stress conditions (15, 16, 28, 29, 33). Different types of embryological defects have been described in other organisms depending on the exact timing of the stress (6, 10, 31). All of these defects induced by heat can be prevented by inducing thermotolerance (31). Furthermore, Waddington (48) obtained phenocopies of Drosophila when embryos were exposed to ether (a membrane fluidizer like BA), which is now known to interfere with the MPS, an effect that has been shown to be strictly involved in the induction of the heat shock response (44, 45).
The regularity with which these defects emerge under stressful conditions during embryogenesis of Metazoa and plants across different taxa suggests that HSPs may play a rather general role in the proper expression of developmentally regulated genes (21). These findings suggest that, since these genes and the transcription factors involved are different and evolutionarily distant, the effect of HSPs (which are highly conserved in sequence and functions) is stochastic and not protein specific. It is likely that among other effects, an insufficient amount of HSPs also provokes the improper folding of regulatory proteins that control, during embryogenesis, the correct expression of highly transcribed developmentally regulated genes.
We have assumed that developmentally regulated genes of Metazoa and the virulence genes of pathogens may represent groups of sequences that may be dependent for their expression on the proper levels of HSPs under stress conditions. At the onset of infection, the pathogen's genome undergoes a stage of simultaneous transcriptional regulation of heat shock and virulence genes. We postulated that since the appearance of phenocopies in higher organisms is due to a stochastic effect of HSPs on highly transcribed gene products, a similar situation could occur in pathogens when they invade a host. Thus, the possibility to manipulate the heat shock response could be used as a tool to obtain strains altered in the expression of genes involved in adaptation and virulence, thus generating a strain altered in its phenotype (a temperature-sensitive nonvirulent strain), similar to the phenocopies described in higher organisms.
Intracellular pathogens at the onset of infection induce a genetic response that includes in addition to the transcriptional activation of stress genes other species-specific genes, broadly defined as virulence genes. The latter class of gene products, which are directly involved in the mechanisms of invasion/adaptation, operate in a coordinate fashion and are responsible for the capacity of the pathogen to invade, replicate, and induce disease in the host (11, 43). Thus, expression of stress genes and those involved in virulence is temporarily associated and genetically coordinated in their tempo of transcription (11). However, the details of the regulation of virulence genes and how they coordinately act have not been elucidated yet.
We suggest that inhibition of HSP accumulation (through changes in the MPS of the pathogen) might interfere with the process of adaptation, invasion, and disease in microbial pathogens, thus generating an attenuated form of a pathogen. In Histoplasma the standard concentration of yeast cells injected to study the effects of drugs or vaccine particles is between 1 × 106 and 1 × 107 that kills mice in about 3 weeks. We also used a concentration five times higher (5 × 107) that kills all mice in less than a week. This concentration of yeast cells is higher than a mouse would receive in natural environment. CD-1 mice were injected with increasing lethal concentrations of yeast cells (106 and 107) or with the same concentration of the modified HcD3 strain. Mice that were given the highly virulent strain died between 23 and 35 days, while those receiving the HcD3 strain survived up to 40 days. Further, when virulent H. capsulatum yeast cells at the extremely high concentration of 5 × 107 yeast cells were injected, all mice died within 6 days, while after 45 days 70% of the mice injected with the HcD3 strain still survived. The latter group of surviving mice was then challenged with a lethal dose of 5 × 107 virulent G217B yeast cells. After 20 days, more than 60% of mice were still alive, suggesting that the initial inoculation with the attenuated HcD3 strain conferred protection from the subsequent challenge with the virulent strain. It should also be pointed out that when mice were injected with a standard number of virulent yeast cells (106 or 107), all mice died between 35 and 22 days, respectively, while all mice injected with the modified strain survived after 40 and 30 days, respectively.
We have shown that it is possible to obtain with a single genetic modification a new procedure to produce an attenuated strain from an otherwise highly virulent pathogen by repressing the heat shock response. Though the mechanism by which a broad reduction in the levels of HSPs is linked to pathogenicity is not clear yet, it is intriguing to suggest that this might be due to the reduced capacity of the genetically modified bacteria to fold proteins properly under stress conditions (at the onset of infection). Among other proteins, regulatory proteins involved in the appropriate timing of expression of virulence genes synthesized during early stages of infection may be strongly and stochastically affected by the reduced amount of chaperones (HSPs). This, in turn, may alter the proper pattern of expression of virulence genes. This might explain why evolutionarily distant pathogens such as Salmonella and H. capsulatum are both attenuated in their capacity to infect macrophage cells or mice (in Histoplasma) though they belong to two different biological domains possessing major differences in the mechanism of virulence and in the DNA sequences involved. To address this key question, we have initiated a separate microarray study to determine potential changes in the pattern of expression of virulence (and other) genes in wild-type and genetically modified pathogens.
Since the modified H. capsulatum strain induces protection, it is reasonable to suggest that the overall antigenic profile of H. capsulatum may be unaffected or only slightly affected, thus presenting a full antigenic repertoire to the host immune system, while the capacity to express virulence genes might be severely hampered under stress conditions. This methodology has a major advantage over other techniques of attenuation since it does not require the identification of species-specific genes and development of a particular genetic procedure for each pathogen. Our method is, in contrast, based on a phenomenon shared by all organisms, the control of the heat shock response via modification of the MPS, that we and other authors have extensively shown to be analogous in both prokaryotic and eukaryotic cells. Thus, it may be possible that this procedure to obtain attenuation of virulence might be applied to other very important intracellular pathogens, such as mycobacteria, staphylococci, streptococci, and parasites. While further studies are needed, this technique provides a new opportunity for the development of a new, safer, and more effective class of attenuated strains for human and animal use.
Acknowledgments
This work was supported in part by grants from MIUR/2009, Italy, and from the Hungarian National Scientific Research Foundation (OTKA NK 68379).
We do not have any commercial interest or conflict of interest relevant to this study.
We thank Sergio Colonna-Romano for his technical help.
This paper is dedicated to the memory of George S. Kobayashi, Washington University, School of Medicine, Division of Infectious Diseases, St. Louis, MO.
Footnotes
Published ahead of print on 5 February 2010.
REFERENCES
- 1.Acharya, P., R. Kumar, and U. Tatu. 2007. Chaperoning a cellular upheaval in malaria: heat shock proteins in Plasmodium falciparum. Mol. Biochem. Parasitol. 153:85-94. [DOI] [PubMed] [Google Scholar]
- 2.Allendoerfer, R., B. Maresca, and G. S. Deepe, Jr. 1996. Cellular immune responses to recombinant heat shock protein 70 from Histoplasma capsulatum. Infect. Immun. 64:4123-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balogh, G., I. Horvath, E. Nagy, Z. Hoyk, O. Bensaude, and L. Vigh. 2005. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 272:6077-6086. [DOI] [PubMed] [Google Scholar]
- 4.Carratù, L., S. Franceschelli, C. L. Pardini, G. Kobayashi, I. Horvath, L. Vigh, and B. Maresca. 1996. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc. Natl. Acad. Sci. U. S. A. 93:3870-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheminaya, C., and M. Hensel. 2008. Rational design of Salmonella recombinant vaccines. Int. J. Med. Microbiol. 298:87-98. [DOI] [PubMed] [Google Scholar]
- 6.Chow, K. L., and K. W. Chan. 1999. Stress-induced phenocopy of C. elegans defines functional steps of sensory organ differentiation. Dev. Growth Differ. 41:629-637. [DOI] [PubMed] [Google Scholar]
- 7.Feige, U., and W. van Eden. 1996. Infection, autoimmunity and autoimmune disease. EXS 77:359-373. [DOI] [PubMed] [Google Scholar]
- 8.Fox, K. L., H. H. Yildirim, M. E. Deadman, E. K. H. Schweda, E. R. Moxon, and D. W. Hood. 2005. Novel lipopolysaccharide biosynthetic genes containing tetranucleotide repeats in Haemophilus influenzae, identification of a gene for adding O-acetyl groups. Mol. Microbiol. 58:207-216. [DOI] [PubMed] [Google Scholar]
- 9.Gargano, S., G. Di Lallo, G. S. Kobayashi, and B. Maresca. 1995. A temperature-sensitive strain of Histoplasma capsulatum has an altered delta 9-fatty acid desaturase gene. Lipids 30:899-906. [DOI] [PubMed] [Google Scholar]
- 10.Goldschmidt, R. 1935. Gen und Ausseneigenschaft (Untersuchungen an Drosophila) I. Z. Indukt. Abstamm. Vererbungsl. 69:70-131. [Google Scholar]
- 11.Groisman, E. A., and H. Ochman. 1997. How Salmonella became a pathogen. Trends Microbiol. 5:343-349. [DOI] [PubMed] [Google Scholar]
- 12.Haddad, N. E., and W. G. Powderly. 2001. The changing face of mycoses in patients with HIV/AIDS. AIDS Read. 11:365-368, 375-378. [PubMed] [Google Scholar]
- 13.Henderson, B., E. Allan, and A. R. Coates. 2006. Stress wars: the direct role of host and bacterial molecular chaperones in bacterial infection. Infect. Immun. 74:3693-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocabiyik, S. 2009. Essential structural and functional features of small heat shock proteins in molecular chaperoning process. Protein Pept. Lett. 16:613-622. [DOI] [PubMed] [Google Scholar]
- 15.Kotak, S., J. Larkindale, U. Lee, P. von Koskull-Döring, E. Vierling, and K. D. Scharf. 2007. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 10:310-316. [DOI] [PubMed] [Google Scholar]
- 16.Kregel, K. C. 2002. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 92:2177-2186. [DOI] [PubMed] [Google Scholar]
- 17.Kur, J., L. Holec-Gasior, and E. Hiszczyńska-Sawicka. 2009. Current status of toxoplasmosis vaccine development. Expert Rev. Vaccines 8:791-808. [DOI] [PubMed] [Google Scholar]
- 18.Launois, P., F. Tacchini-Cottier, and M. P. Kieny. 2008. Cutaneous leishmaniasis: progress towards a vaccine. Expert Rev. Vaccines 7:1277-1287. [DOI] [PubMed] [Google Scholar]
- 19.Liberek, K., A. Lewandowska, and S. Zietkiewicz,. 2008. Chaperones in control of protein disaggregation. EMBO J. 27:328-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maresca, B., and G. Kobayashi. 1994. Hsp70 in parasites: as an inducible protective protein and as an antigen. Experientia 50:1067-1074. [DOI] [PubMed] [Google Scholar]
- 21.Maresca, B., and J. H. Schwartz. 2006. Sudden origins: a general mechanism of evolution based on stress protein concentration and rapid environmental change. Anat. Rec. B New Anat. 289:38-46. [DOI] [PubMed] [Google Scholar]
- 22.Masoud, H., I. Sadovskaya, T. De Kievit, E. Altman, J. C. Richards, and J. S. Lam. 1995. Structural elucidation of the lipopolysaccharide core region of the O-chain-deficient mutant strain A28 from Pseudomonas aeruginosa serotype 06 (International Antigenic Typing Scheme). J. Bacteriol. 177:6718-6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell, H. K., and L. S. Lipps. 1978. Heat shock and phenocopy induction in Drosophila. Cell 15:907-918. [DOI] [PubMed] [Google Scholar]
- 24.Mora, M., and J. L. Telford. Genome-based approaches to vaccine development. J. Mol. Med., in press. [DOI] [PubMed]
- 25.Moxon, E. R., and C. Tang. 2000. Challenge of investigating biologically relevant functions of virulence factors in bacterial pathogens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:643-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy, E., Z. Balogi, I. Gombos, M. Akerfelt, A. Björkbom, G. Balogh, Z. Török, A. Maslyanko, A. Fiszer-Kierzkowska, K. Lisowska, P. J. Slotte, L. Sistonen, I. Horváth, and L. Vígh. 2007. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc. Natl. Acad. Sci. U. S. A. 104:7945-7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nosanchuk, J. D., and A. Gacser. 2008. Histoplasma capsulatum at the host-pathogen interface. Microbes Infect. 10:973-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patriarca, E. J., G. S. Kobayashi, and B. Maresca. 1992. Mitochondrial activity and heat-shock response during morphogenesis in the pathogenic fungus Histoplasma capsulatum. Biochem. Cell Biol. 70:207-214. [DOI] [PubMed] [Google Scholar]
- 29.Patriarca, E. J., and B. Maresca. 1990. Acquired thermotolerance following heat shock protein synthesis prevents impairment of mitochondrial ATPase activity at elevated temperatures in Saccharomyces cerevisiae. Exp. Cell Res. 190:57-64. [DOI] [PubMed] [Google Scholar]
- 30.Peiser, L., K. Makepeace, A. Plüddemann, S. Savino, J. C. Wright, M. Pizza, R. Rappuoli, E. R. Moxon, and S. Gordon. 2006. Identification of Neisseria meningitidis nonlipopolysaccharide ligands for class A macrophage scavenger receptor by using a novel assay. Infect. Immun. 74:5191-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen, N. S. 1990. Effects of heat and chemical stress on development. Adv. Genet. 28:275-296. [DOI] [PubMed] [Google Scholar]
- 32.Petersen, N. S., and H. K. Mitchell. 1987. The induction of a multiple wing hair phenocopy by heat shock in mutant heterozygotes. Dev. Biol. 121:335-341. [DOI] [PubMed] [Google Scholar]
- 33.Piper, P. 1996. Induction of heat shock proteins and thermotolerance. Methods Mol. Biol. 53:313-317. [DOI] [PubMed] [Google Scholar]
- 34.Porta, A., Z. Török, I. Horvath, S. Franceschelli, L. Vígh, and B. Maresca. 2010. Genetic modification of the Salmonella membrane physical state alters the pattern of heat shock response. J. Bacteriol. 192:1988-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rappuoli, R. 1996. Acellular pertussis vaccines: a turning point in infant and adolescent vaccination. Infect. Agents Dis. 5:21-28. [PubMed] [Google Scholar]
- 36.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Schweda, E. K. H., J. C. Richards, D. W. Hood, and E. R. Moxon. 2007. Expression and structural diversity of the lipopolysaccharide of Haemophilus influenzae: implication in virulence. Int. J. Med. Microbiol. 297:297-306. [DOI] [PubMed] [Google Scholar]
- 38.Shigapova, N., Z. Torok, G. Balogh, P. Goloubinoff, L. Vigh, and I. Horvath. 2005. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 328:1216-1223. [DOI] [PubMed] [Google Scholar]
- 39.Slater, S. J., M. B. Kelly, F. J. Taddeo, C. Ho, E. Rubin, and C. D. Stubbs. 1994. The modulation of protein kinase C activity by membrane lipid bilayer structure. J. Biol. Chem. 269:4866-4871. [PubMed] [Google Scholar]
- 40.Stubbs, C. D., and S. J. Slater. 1996. The effects of non-lamellar forming lipids on membrane protein-lipid interactions. Chem. Phys. Lipids 81:185-195. [DOI] [PubMed] [Google Scholar]
- 41.Telford, J. L. 2008. Bacterial genome variability and its impact on vaccine design. Cell Host Microbe 3:408-416. [DOI] [PubMed] [Google Scholar]
- 42.Török, Z., P. Goloubinoff, I. Horvath, N. M. Tsvetkova, A. Glatz, G. Balogh, V. Varvasovszki, D. A. Los, E. Vierling, J. H. Crowe, and L. Vigh. 2001. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. U. S. A. 98:3098-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu, X., T. Latifi, A. Bougdour, S. Gottesman, and E. A. Groisman. 2006. The PhoP/PhoQ two-component system stabilizes the alternative sigma factor RpoS in Salmonella enterica. Proc. Natl. Acad. Sci. U. S. A. 103:13503-13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vigh, L., and B. Maresca. 2002. Dual role of membranes in heat stress: as thermosensors modulate the expression of stress genes and, by interacting with stress proteins, re-organize their own lipid order and functionality, p. 173-178. In K. B. Storey and J. M. Storey (ed.), Cell and molecular responses to stresses. Elsevier, Amsterdam, The Netherlands.
- 45.Vigh, L., B. Maresca, and J. L. Harwood. 1998. Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem. Sci. 23:369-374. [DOI] [PubMed] [Google Scholar]
- 46.Vigh, L., P. V. Escriba, A. Sonnleitner, M. Sonnleitner, S. Piotto, B. Maresca, and I. Horvath. 2005. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog. Lipid Res. 44:303-344. [DOI] [PubMed] [Google Scholar]
- 47.Vigh, L., H. Nakamoto, J. Landry, A. Gomez-Munoz, J. L. Harwood, and I. Horvath. 2007. Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann. N. Y. Acad. Sci. 1113:40-51. [DOI] [PubMed] [Google Scholar]
- 48.Waddington, C. H. 1957. The strategy of the genes. A discussion of some aspects of theoretical biology. Allen and Unwin, London, United Kingdom.
- 49.Woods, J. P., and W. E. Goldman. 1992. In vivo generation of linear plasmids with addition of telomeric sequences by Histoplasma capsulatum. Mol. Microbiol. 6:3603-3610. [DOI] [PubMed] [Google Scholar]
- 50.Woods, J. P., E. L. Heinecke, and W. E. Goldman. 1998. Electrotransformation and expression of bacterial genes encoding hygromycin phosphotransferase and beta-galactosidase in the pathogenic fungus Histoplasma capsulatum. Infect. Immun. 66:1697-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]