Abstract
The mammalian SWI/SNF chromatin-remodeling complex facilitates DNA access by transcription factors and the transcription machinery. The characteristic member of human SWI/SNF-A is BAF250/ARID1, of which there are two isoforms, BAF250a/ARID1a and BAF250b/ARID1b. Here we report that BAF250b complexes purified from mammalian cells contain elongin C (Elo C), a BC box binding component of an E3 ubiquitin ligase. BAF250b was found to have a BC box motif, associate with Elo C in a BC box-dependent manner, and, together with cullin 2 and Roc1, assemble into an E3 ubiquitin ligase. The BAF250b BC box mutant protein was unstable in vivo and was autoubiquitinated in a manner similar to that for the VHL BC box mutants. The discovery that BAF250 is part of an E3 ubiquitin ligase adds an enzymatic function to the chromatin-remodeling complex SWI/SNF-A. The immunopurified BAF250b E3 ubiquitin ligase was found to target histone H2B at lysine 120 for monoubiquitination in vitro. To date, all H2B monoubiquitination was attributed to the human homolog of yeast Bre1 (RNF20/40). Mutation of Drosophila osa, the homolog of BAF250, or depletion of BAF250 by RNA interference (RNAi) in cultured human cells resulted in global decreases in monoubiquitinated H2B, implicating BAF250 in the cross talk of histone modifications.
Accessibility to DNA in eukaryotic cells is regulated by chromatin structure, which consists of DNA wrapped around histone octamers (two each of H2A, H2B, H3, and H4) to form nucleosomes. Chromatin structure is modulated by chromatin-remodeling complexes and/or chromatin-modifying complexes, which posttranslationally modify histones. Chromatin-remodeling complexes can slide, restructure, or eject nucleosomes. SWI/SNF is a chromatin-remodeling complex with a superfamily 2 ATPase catalytic subunit (BRG1 or BRM) that functions like a DNA translocase (4). Mammalian SWI/SNF complexes are made up of approximately 10 subunits depending on cell type (39). Subunits such as BAF53 and BAF60 are present in different isoforms, increasing the number of possible complexes. Recently, it has been shown that subunit isoform switching is important in selecting different neuronal developmental programs (18). The mammalian SWI/SNF catalytic core includes BRG1 or BRM, BAF155, BAF170, and INI1, which is necessary and sufficient for remodeling in vitro (22, 27). Two structurally and functionally distinct types of SWI/SNF complexes can be differentiated using cation- and anion-exchange chromatography. SWI/SNF-A and SWI/SNF-B differ in their largest subunits, which are BAF250/ARID1 for SWI/SNF-A and BAF180 plus BAF200/ARID2 for SWI/SNF-B (17). BAF250 has a DNA binding domain known as ARID (AT-rich interaction domain), and two isoforms, BAF250a/ARID1a and BAF250b/ARID1b, are present in mammalian cells.
BAF250a and BAF250b are homologous to Drosophila Osa, a component of the Drosophila Brahma (Brm) complex. Osa, along with Brahma and Moira, which make up the catalytic core, was originally identified as a Trithorax group protein (trxG) in a screen for suppressors of Polycomb mutations (15). Trithorax and Polycomb group proteins (PcG) regulate the expression of Homeobox (HOX) genes early in development. PcG proteins such as Ring1a/b and E(z)/EZH2 are repressors of HOX gene transcription, while trxG proteins such as Trx/MLL and Ash1 are activators (29). Recently, BAF250a- or BAF250b-deficient mouse embryonic stem cells have been characterized and found to exhibit defects in self-renewal capacity and increased differentiation (10, 41). Collectively, these properties indicate that BAF250 plays an important role in early development.
The two human isoforms of BAF250/ARID1 are highly conserved. The BAF250a and BAF250b N-terminal ARID and C-terminal homology regions are 62% and 76% identical, respectively (12). While the SWI/SNF-A complex is an activator of HOX gene expression (15), in other cellular or chromatin contexts SWI/SNF may either activate or repress transcription. The two BAF250 isoforms are found in separate SWI/SNF complexes and are thought to target SWI/SNF to specific genes (36). In vivo studies have shown that BAF250a is a coactivator for the glucocorticoid receptor (34) and an essential gene for FAS-mediated apoptosis (19), while BAF250b interacts with Smad2/3 in response to the cytokine transforming growth factor β (TGF-β) (40). Both BAF250a and BAF250b associate with E2F transcription factors and play important roles in cell cycle control (21). Although the BAF250 ARID may contribute to targeting of SWI/SNF-A to specific genes, ARID binds DNA in a sequence-independent manner and is not required for BRG1 localization (6, 38).
Here we describe a newly discovered association between BAF250b and components of an E3 ubiquitin ligase. E3 ubiquitin ligases are responsible for target selection by binding both substrate and the corresponding ubiquitin-conjugating enzyme (E2). E2, in turn, receives its ubiquitin from ubiquitin-activating enzymes (E1). E3 ubiquitin ligases such as the Skp1, cullin 1, F box protein (SCF), and Von Hippel-Lindau (VHL) complexes serve as scaffolds, which link the substrate recognition module with the catalytically active RING domain in Roc1/Rbx1/Hrt1. VHL or F box proteins act as the substrate recognition module by binding the substrate and adapters, elongin B/C (Elo B/C) or Skp1, respectively, and the N-terminal domain of a cullin protein (35). Based on the crystal structure of cullin 1 and sequence homology, the cullins share similar N-terminal domain structures, which resemble elongated stalks and contain three copies of the five-helix cullin repeat motif. The Roc1 RING domain is embedded within the globular C terminus of cullins and does not make direct contact with the substrate binding protein (42). Elo B/C binding to VHL and other substrate recognition proteins, such as the SOCS box proteins, is mediated by a 10-amino-acid motif known as the BC box whose consensus sequence is XLXXX(C,S)XXX(A,I,L,V) (where X stands for any amino acid) (14). Mutations in the BC box of VHL lead to VHL autoubiquitination, which results in degradation by the proteasome (13, 28).
In the present work, we show that BAF250 associates with elongin C (Elo C), cullin 2 (Cul2), and Roc1 to form an E3 ubiquitin ligase. BAF250b immunoprecipitates with Elo C through a BC box, which when mutated results in BAF250b autoubiquitination and degradation in a proteasome-dependent manner. We find immunopurified BAF250b to associate with Elo C, Cul2, Roc1, and SWI/SNF components to monoubiquitinate histone H2B on lysine-120 (H2BK120) in a nucleosomal context. RNA interference (RNAi) knockdown of BAF250 results in a global decrease in monoubiquitinated H2B and HoxA9 mRNA levels in cultured human cells. The Drosophila osa mutants exhibit lower levels of monoubiquitinated H2B and genetically interact with Cul2. To date, the only known H2BK120 E3 ubiquitin ligase is human Bre1 (RNF 20/40), which is conserved in Saccharomyces cerevisiae (43). The discovery that the trxG protein BAF250/ARID1 functions as an E3 ubiquitin ligase adapter for histone H2BK120 has important implications for chromatin remodeling by SWI/SNF and H2B monoubiquitination, an upstream event of H3K4 trimethylation associated with gene activation.
MATERIALS AND METHODS
Plasmids and cell culture.
The T7-tagged BAF250 derivatives were constructed in the pCGN vector as described previously (12). Elongin C (a gift of M. Pagano) was subcloned into the p3XFlag-CMV-10 expression vector (Sigma). Myc-Cul2 and Myc-Cul5 (in pcDNA3) were generous gifts of M. Pagano.
HeLa and HEK293 cells were maintained in Dulbecco's modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone), l-glutamine, and penicillin-streptomycin (Invitrogen) and cultured in a humidified incubator with 5% CO2. The HeLa cell line 16e (12) was maintained in the same medium containing 10 to 30 ng/ml doxycycline (Dox; Sigma). For induction of Flag-BAF250b, the concentration of doxycycline was reduced to 1 ng/ml for 24 h and doxycycline was then removed entirely from the medium on the following day.
Immunoprecipitation.
For immunoprecipitation assays, 1 μg each of empty vector or plasmids encoding T7-tagged BAF250b or mutants were transiently transfected (Lipofectamine 2000; Invitrogen) with Flag-Elo C or Myc-Cul2 into HeLa cells. For comparisons of T7-BAF250b mutants in Fig. 2E, 8 μg of empty vector or 4 μg of wild-type (WT), 4 μg of ΔA, 20 μg of BC*, or 8 μg of ΔA/BC* plasmid DNA was transiently transfected. A total of 0.5 to 1 mg of nuclear extract was incubated with anti-T7 antibody (Novagen), followed by the addition of 30 μl 50% protein A- and/or protein G-Sepharose bead slurry (GE Healthcare) or 30 μl 50% Flag antibody-conjugated agarose bead slurry (M2; Sigma). The beads were washed four times with 0.5 ml of HEMG buffer (25 mM HEPES [pH 7.6], 0.1 mM EDTA, 12.5 mM MgCl2, 20% glycerol) containing 0.1% NP-40 and the indicated KCl concentration (0.3 M or 0.5 M) before analysis by SDS-PAGE. Immunoprecipitated proteins were detected by immunoblotting with antibodies to T7 (Novagen), Flag (M2; Sigma), or Myc (9E10 or PRB-150C; Covance).
FIG. 2.
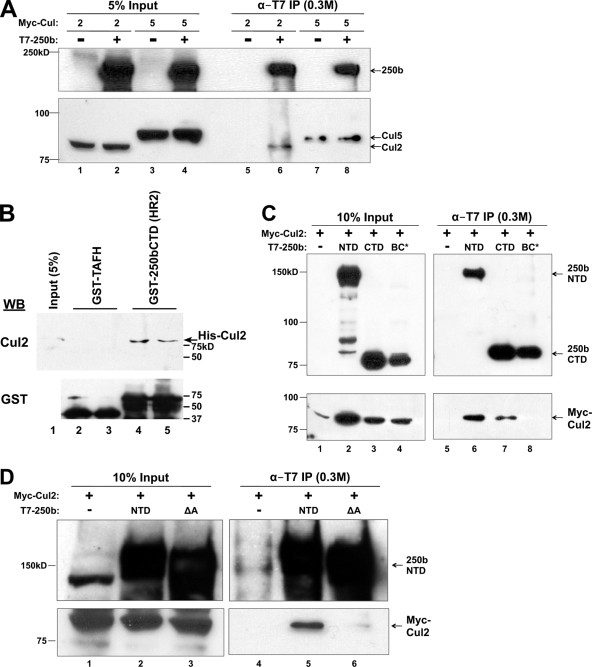
Association of Cul2 with BAF250b. (A) BAF250b associates specifically with Cul2 and not Cul5. T7 immunoprecipitates of transiently transfected T7-BAF250b with Myc-Cul2 (lanes 1, 2, 5, and 6) or Myc-Cul5 (lanes 3, 4, 7, and 8) were washed in a buffer containing 0.3 M KCl and analyzed by immunoblotting with an antibody to T7 (α-T7) or Myc. (B) Bacterially expressed Cul2 interacts with BAF250bCTD. GST pulldown assays were performed as for Fig. 1C with His-Cul2 expressed in E. coli. (C) Cul2 association with the BAF250b C terminus is BC box dependent. Lysates from cells transiently transfected with T7-BAF250bNTD, T7-BAF250bCTD, or T7-BAF250bCTDBC* and with Myc-Cul2 were immunoprecipitated with T7 antibody, washed in a buffer containing 0.3 M KCl, and analyzed by immunoblotting with an antibody to T7 or Myc. (D) Cul2 association with BAF250b N terminus is ARID dependent. Lysates from cells transiently transfected with T7-BAF250bNTD or the T7-BAF250b N terminus with the ARID deletion (ΔA) and Myc-Cul2 were immunoprecipitated with T7 antibody, washed in a buffer containing 0.3 M KCl, and analyzed by immunoblotting with an antibody to T7 or Myc. (E) Endogenous Cul2 association with BAF250b is BC box dependent. T7 immunoprecipitates of transiently transfected T7-BAF250b, T7-BAF250b with ARID deleted (ΔA), the T7-BAF250b BC box mutant (BC*), or T7-BAF250b with ARID deleted and the BC box mutated (ΔA and BC*) were washed in a buffer containing 0.3 M KCl and analyzed by immunoblotting with an antibody to T7 or endogenous Cul2. (F) A schematic summarizing the interactions between BAF250b constructs and Elo C and Cul2. n.d., not determined. (G) Association of endogenous BAF250a/b and Cul2. BAF250a and BAF250b were immunoprecipitated from HEK293 cells and probed for Cul2 and BAF250. The bands corresponding to BAF250a and BAF250b are indicated by ▪ and ▪▪, respectively. (H) Endogenous Roc1 is enriched in immunopurified Flag-BAF250b complex. Flag-BAF250b immunoprecipitated from induced (+) or uninduced (−) 16e cell nuclear extracts was washed in a buffer containing 0.3 M (lanes 3 and 4) or 0.5 M (lanes 5 and 6) KCl and eluted with a Flag peptide. Eluted material was analyzed by immunoblotting for endogenous Roc1 or Flag-BAF250b.
FIG. 8.
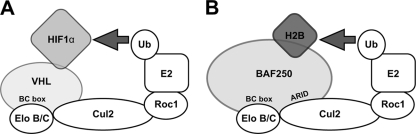
A model of the BAF250-containing E3 ubiquitin ligase. (A) VHL is a substrate adapter associated with the Elo B/C-Cul2-Roc1 complex, which ubiquitinates HIF1α. (B) BAF250 forms an E3 ubiquitin ligase with Elo B/C, Cul2, and Roc1 that targets histone H2B.
Endogenous BAF250 was immunoprecipitated from HEK293 nuclear extract by incubation with antibodies specific for BAF250a (PSG3; Santa Cruz Biotechnology) or BAF250b (KMN1; Santa Cruz Biotechnology) cross-linked to protein A/G beads as follows. A 30-μl portion of the beads was incubated with 15 μg each of PSG3 or KMN1 antibody in phosphate-buffered saline (PBS) with 0.1% Triton X-100 before addition of 30 μl of 11.7 mM BS3 cross-linker (Thermo Scientific). The BS3 activity was quenched by addition of 100 μl of 50 mM Tris (pH 7.5), and the beads were washed three times with 1 ml of HEMG buffer containing 0.1 M KCl before incubation with 1 mg of HEK293 nuclear extract for 2 h. The beads were then washed three times in 500 μl of HEMG buffer containing 0.3 M KCl and 0.1% NP-40 and bound proteins analyzed by SDS-PAGE and immunoblotting.
Protein stability assay.
HeLa cells (2 × 106 per 10-cm plate) were seeded and transiently transfected with 1 μg each of T7-BAF250b or BAF250b mutants using Lipofectamine 2000 (Invitrogen). At 24 h posttransfection, cells were treated with 100 μg/ml cycloheximide (CHX) or 5 μM MG132 for the times indicated. Whole-cell extracts were prepared and separated by 8% SDS-PAGE. Immunoblots were probed with antibody to T7 or BRG1 (H-88; Santa Cruz Biotechnology). For Cul2 cotransfection assays, 1 μg Myc-Cul2 was transiently cotransfected into HeLa cells with 1 μg of vector, T7-BAF250b, or BAF250b mutants. To compare the effects of Cul2 and Cul5 on the stability of BAF250b and mutants, 1 μg Myc-Cul2 or Myc-Cul5 was transiently cotransfected into HeLa cells with 1 μg of vector, T7-BAF250b, or BAF250b mutants.
To determine if the BAF250b C-terminal domain (CTD) is polyubiquitinated, HeLa cells were transiently transfected with T7-BAF250b CTD and Myc-Cul2 constructs and treated with 5 μM MG132 in culture. Nuclear extracts were prepared with 2 mM N-ethylmaleimide (NEM), a deubiquitinase inhibitor. Proteins immunoprecipitated with anti-T7 antibody were washed four times in HEMG buffer (with 0.3 M KCl and 0.5% NP-40) before immunoblotting with ubiquitin, T7, and Myc antibodies.
In vitro ubiquitination assay.
Flag-BAF250b complexes were immunopurified from 35 to 40 mg of nuclear extracts from stable cell line 16e (either induced or uninduced for BAF250b overexpression) by incubation with a 150-μl packed-bead volume of Flag M2 agarose beads (Sigma). Bound beads were washed with 5 ml of HEMG buffer containing 0.5 M KCl and 0.1% NP-40. The salt concentration was then adjusted by washing with 0.5 ml of 0.1 M KCl-HEMG. Flag-BAF250b complexes were eluted by incubation in 200 μl of 0.3 μg/μl Flag peptide (Sigma) in 0.1 M KCl HEMG at 25°C for 30 min.
In vitro ubiquitination assays were conducted essentially as described previously (5). Thirty microliters of Flag-BAF250b complexes (purified from induced 16e cells [16e+]) or the negative-control eluant (purified from uninduced 16e cells [16e−]) was dialyzed into ubiquitination buffer (50 mM Tris-HCl [pH 7.9], 5 mM MgCl2, 2 mM NaF, 0.6 mM dithiothreitol [DTT]) at 4°C for 1 h. After adjusting for changes in volume during dialysis, equivalent amounts of 16e−, Flag-BAF250b complexes, or recombinant Flag-tagged Ring1b were incubated with 2 mM ATP, 0.1 μg E1 (Boston Biochem), 0.5 μg E2 (UbcH5c; Boston Biochem), 1 μg hemagglutinin antigen-tagged ubiquitin (HA-Ub) (Boston Biochem), and 5 to 10 μg of in vitro-reconstituted oligonucleosomes (wild type or H2BK120A) in 30- to 40-μl reaction volumes for 30 min at 37°C. Ubiquitination assay reactions were resolved by 15% SDS-PAGE, and HA antibody was used to determine the levels of HA-Ub modified proteins by immunoblotting. Immunoblots were then reprobed with H2B antibody.
siRNA knockdown.
HEK293 cells were seeded in 10-cm plates at 70 to 80% confluence. A 3.6-μg amount of small interfering RNA (siRNA) oligonucleotides (Dharmacon) targeting luciferase, BAF250a, BAF250b, or BAF250a and -b was transiently transfected into the cells at 24 h after seeding and incubated for an additional 24 h before each 10-cm plate was split into six 35-mm plates. At 48 h after transfection, two 35-mm plates each were treated with TriReagent (Sigma) for RNA extraction or harvested for protein analysis as either acid extracts or whole-cell extracts. To examine histone posttranslational modifications in vivo, acid extracts were prepared by incubation of HEK293 cells in 1 cell pellet volume of PBS with 0.2 N HCl overnight at 4°C. The siRNAs used were as follows: luciferase (sense sequence, FlCUUACGCUGAGUACUUCGA, where Fl is fluorescein), BAF250a (sense sequence, GCAGGCACCACUAACUUAU, GAUGAGACCUCAGCCAUAU), and BAF250b (sense sequence, FlAACCGCUCGGAAUUCCUUU, where Fl is fluorescein).
Drosophila experiments.
The osa308 and osa4H alleles have been described previously (7). osa308/osa4H larvae were homogenized in whole-cell extract buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1 mM EDTA, 4% NP-40, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM NaF, 1 mM NaVO4) at 4°C overnight and spun down at 14,000 rpm for 15 min. Histone extracts were prepared by addition of 1 pellet volume of 0.2 N HCl-1 mM PMSF plus NEM. ap-GAL4 was obtained from the Bloomington Drosophila Stock Center, and UAS-cul-2RNAi was obtained from the Vienna Drosophila RNAi center. Wing imaginal discs were stained as previously described (7). Mouse anti-Nub (23) was used at a 1:10 dilution, and guinea pig anti-Sens (25) was used at a 1:1,000 dilution. All discs were stained and imaged in parallel on a Zeiss LSM 510 confocal microscope.
GST pulldown assay.
The GST-BAF250bCTD(HR2) plasmid was designed to express the C-terminal portion (HR2) of BAF250bCTD (residues 1786 to 2165). Glutathione S-transferase (GST) fusion proteins were purified from 90 and 35 μg of total Escherichia coli cell lysate expressing GST-BAF250bCTD(HR2) or GST-TAFH (37), respectively. They were mixed with 35 μg of histones extracted from HeLa cells, and bound proteins were washed in HEMG buffer containing 0.3 M KCl and analyzed by SDS-PAGE and immunoblotting. Human Elo C and Cul2 cDNAs were subcloned into pET15b to generate polyhistidine-tagged Elo C and Cul2 in E. coli. The bacterial cell lysate was mixed with the GST fusion proteins described above, and bound proteins were washed in HEMG buffer containing 0.1 M KCl and analyzed by SDS-PAGE and immunoblotting.
RESULTS
BAF250 interacts with elongin C.
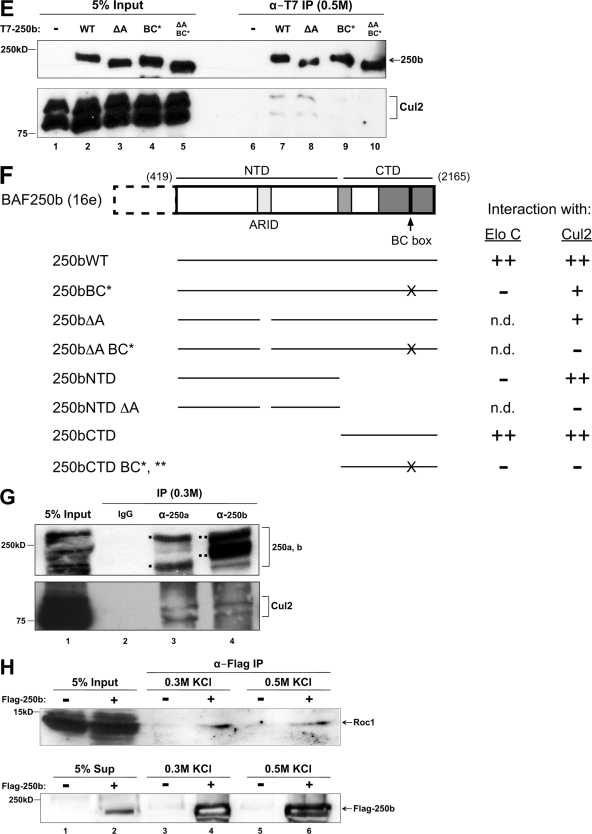
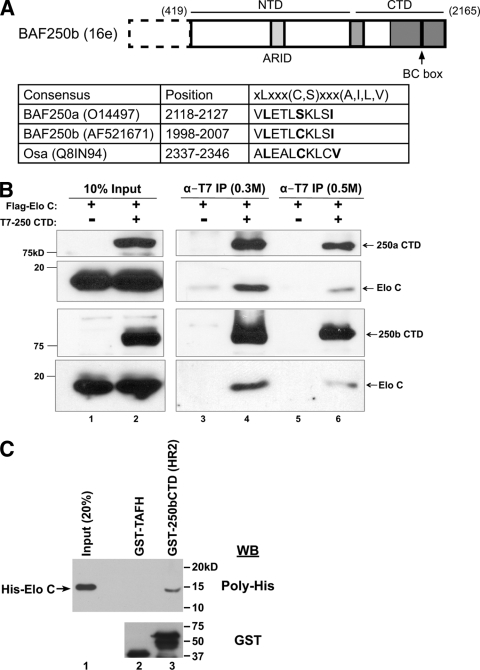
To find novel BAF250-associated proteins, an expression plasmid encoding Flag-tagged BAF250b was stably transfected into HeLa cells to create an inducible cell line named 16e (12). After glycerol gradient sedimentation of 16e nuclear extract, fractions containing BAF250b were pooled and subjected to immunoaffinity purification with anti-Flag antibody. The immunoprecipitate was separated by SDS-PAGE and stained with silver before individual bands were cut out and analyzed by mass spectrometry. A unique peptide (AMLSGPGQFAENETNEVNFR) that mapped to elongin C (Elo C) was recovered. Because Elo C is known to bind the BC box motif, defined as XLXXX(C,S)XXX(A,I,L,V) (2), we searched for an equivalent sequence in a subunit of the SWI/SNF complex. Using known BC boxes (14), a training set was applied to the Multiple EM for Motif Elicitation (MEME) program (1). The MEME motif output file was then submitted to the Motif Alignment and Search Tool (MAST) to scan the sequences of human SWI/SNF subunits BAF250, BAF200/ARID2, BAF180, BRG1, BRM, BAF170, BAF155, BAF60a/b/c, BAF57, BAF53a/b, INI1, BAF45a/b/c/d and Drosophila Osa, BAP170, and Polybromo. MAST identified candidate BC box motifs in the C-terminal domains (CTD) of BAF250a, BAF250b, and the Drosophila ortholog Osa (Fig. 1A). Matches were not found in any other SWI/SNF subunits.
FIG. 1.
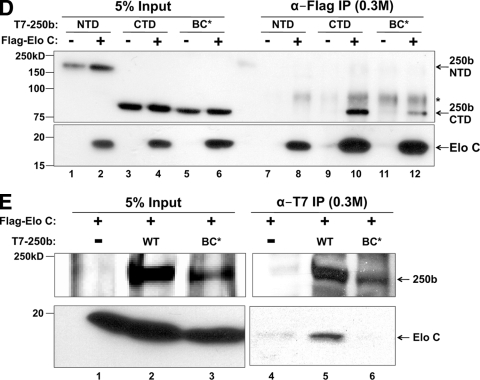
BAF250 contains a BC box and associates with Elo C. (A) Schematic diagram of BAF250b (GenBank accession no. AF521671). 16e, amino acids 419 to 2165; NTD, 419 to 1547; CTD, 1518 to 2165. Putative BC boxes identified by MAST scanning of human BAF250a/b and Drosophila Osa are shown. Amino acid numbering in the second column corresponds to the GenBank accession numbers listed in parenthesis. Sequences of consensus and putative BC boxes are listed in the third column, with key residues highlighted in bold. (B) The BAF250a/b C-terminal domain associates with Elo C. Upper two panels, cells were transiently transfected with Flag-Elo C alone (lanes 1, 3, and 5) or together with T7-BAF250aCTD (amino acids 1635 to 2285, GenBank accession no. O14497) (lanes 2, 4, and 6). Lysates were immunoprecipitated (IP) with α-T7 antibody, washed in a buffer containing 0.3 M KCl (lanes 3 and 4) or 0.5 M KCl (lanes 5 and 6), and analyzed by immunoblotting with an antibody to T7 or Flag. Lower two panels, the same experiment was performed with T7-BAF250bCTD. (C) Bacterially expressed Elo C interacts with BAF250b CTD. Polyhistidine (His)-tagged Elo C was expressed in E. coli, and the lysate was mixed with purified GST-BAF250bCTD (HR2) (lane 3) or control GST-TAFH (lane 2). Immunoblotting (WB) with an antibody to the poly-His tag shows specific binding of Elo C to BAF250bCTD. HR2 represents the second conserved region in BAF250bCTD, which contains the BC box (residues 1786 to 2165, boxed in gray in Fig. 1A). (D) BAF250bCTD interaction with Elo C is BC box dependent. Flag antibody immunoprecipitates of transiently transfected Flag-Elo C with T7-BAF250bNTD, T7-BAF250bCTD, or T7-BAF250bCTD with the BC box mutation (BC*) were washed in a buffer containing 0.3 M KCl and immunoblotted with an antibody to T7 or Flag. *, nonspecific band. (E) BAF250b interacts with Elo C in a BC box-dependent manner. T7 antibody immunoprecipitates of transiently transfected vector, T7-BAF250b, or T7-BAF250bBC* with Flag-Elo C were washed in a buffer containing 0.3 M KCl and immunoblotted with an antibody to T7 or Flag.
To experimentally verify the bioinformatics data, HeLa cells were transiently transfected with T7-BAF250aCTD, T7-BAF250bCTD, or vector, along with Flag-Elo C (Fig. 1B). T7-BAF250CTD was immunoprecipitated, washed in a buffer containing either 0.3 M (lanes 3 and 4) or 0.5 M (lanes 5 and 6) KCl, and subjected to SDS-PAGE followed by immunoblotting. Probing with anti-T7 antibody demonstrated efficient immunoprecipitation of BAF250aCTD and BAF250bCTD under both salt wash conditions. Further, Flag-Elo C was found to coprecipitate with BAF250CTD in the presence of 0.3 M and 0.5 M KCl. We also carried out GST pulldown assays using bacterially expressed polyhistidine-tagged Elo C and GST fused to BAF250bCTD (HR2) (Fig. 1C). His-Elo C bound specifically to GST-BAF250bCTD and not to the control GST-TAFH protein (37), indicating that the observed interaction between Elo C and BAF250bCTD is not mediated by another protein(s) in mammalian cells. Thus, the bioinformatics, immunoprecipitation, and GST pulldown data support the mass spectrometry result and indicate that Elo C interacts with the CTD of BAF250.
Full-length BAF250a is difficult to express irrespective of the vector or cell line (data not shown); thus, we focused on BAF250b (16e) (Fig. 1A). To determine whether BAF250b and Elo C interact in a BC box-dependent manner, a single amino acid substitution was made in BAF250bCTD, changing the conserved leucine at position 2 of the BC box to a proline. Based on the crystal structure of the VHL protein bound to Elo B/C, the 10 amino acids of the BC box motif form a small alpha helix, which directly contacts Elo C (32). The L-to-P mutation in the BAF250b BC box was designed to disrupt the potentially related alpha helix structure. This mutant is referred to as BC* and was compared with the wild-type BC box in the following immunoprecipitation assays. Flag-Elo C or vector was transiently cotransfected in HeLa cells with the BAF250b N terminus (BAF250bNTD), BAF250bCTD, or BAF250bCTD with the BC box mutation (BC*). Immunoprecipitation with anti-Flag antibody from nuclear extract revealed that the BAF250b CTD, and not the NTD, was associated with Elo C, and this was greatly diminished when the BC box was mutated (Fig. 1D). To examine the association of near-full-length BAF250bWT with Elo C, a Flag-Elo C plasmid was transiently cotransfected with empty vector, T7-BAF250bWT, or T7-BAF250bBC*. Immunoprecipitation of the nuclear extract with anti-T7 antibody followed by blotting with anti-Flag antibody demonstrated that Flag-Elo C interacts with BAF250bWT and not BAF250bBC* (Fig. 1E). Thus, immunoprecipitation experiments with the BC* mutant demonstrate that Elo C interacts with BAF250bCTD in a BC-box dependent manner.
BAF250b interacts with cullin 2.
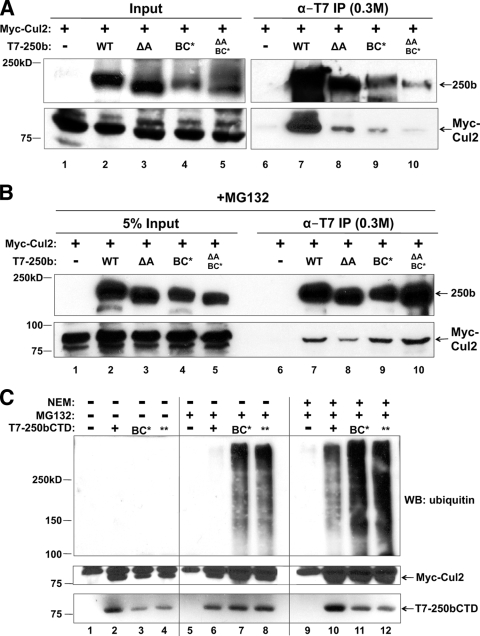
Because Elo B/C E3 ubiquitin ligases contain either a cullin 2 (Cul2) or a cullin 5 (Cul5) subunit, we wanted to determine which cullin associates with BAF250b. To that end, we transiently transfected HeLa cells with Myc-Cul2 or Myc-Cul5 and T7-BAF250b or empty vector (Fig. 2A). Immunoprecipitation with anti-T7 antibody from nuclear extract showed that Myc-Cul2 preferentially coprecipitated with T7-BAF250b, while Myc-Cul5 was not enriched in the presence of T7-BAF250b. Furthermore, GST pulldown assays with bacterially expressed Cul2 and BAF250bCTD demonstrated a specific interaction between the two proteins (Fig. 2B). To determine which domain(s) in BAF250b associates with Cul2, T7-BAF250bNTD, T7-BAF250bCTD, or T7-BAF250bCTDBC* was transiently cotransfected with Myc-Cul2. Nuclear extract was prepared for immunoprecipitation with anti-T7 antibody, and we found that both BAF250bNTD and BAF250bCTD pulled down Cul2 (Fig. 2C). The coprecipitation of BAF250bCTD with Cul2 was greatly diminished in the presence of the BC* mutation, indicating that the interaction might be mediated primarily by Elo B/C. The BAF250bNTD interaction with Cul2 was unexpected because most Cul2 or Cul5 boxes are adjacent to the BC box. By analogy to the crystal structures of VHL and Cul1 (32, 42), we would expect the BAF250 CTD and Elo B/C to bind the N terminus of the Cul2 stalk region. However, BAF250a and BAF250b are large proteins, and the BAF250 NTD could also interact with Cul2 at a secondary contact site along the Cul2 NTD stalk. We predicted that the BAF250b NTD interaction with Cul2 would be mediated by a sequence in the NTD that is conserved among multicellular organisms. Within the BAF250 and Osa NTDs, there is a conserved ARID. To determine if BAF250bNTD binds Cul2 via the ARID, we repeated the coimmunoprecipitation assay using Myc-Cul2 transiently cotransfected with a version of BAF250bNTD lacking the ARID (ΔA). Immunoprecipitation with α-T7 antibody from nuclear extract showed that the Myc-Cul2 interaction with BAF250bNTD was greatly diminished when the ARID was deleted (Fig. 2D).
To examine how BAF250b associates with endogenous Cul2, empty vector, T7-BAF250bWT, T7-BAF250b without the ARID (ΔA), T7-BAF250bBC*, or a T7-BAF250b double mutant lacking the ARID and with BC* (ΔA and BC*) was transiently transfected in HeLa cells. Immunoprecipitation with α-T7 antibody from nuclear extract demonstrated that wild-type BAF250b associated with endogenous Cul2 (Fig. 2E, lane 7). Deletion of the ARID did not significantly perturb the Cul2 association under these conditions (lane 8). Mutation of the BC box significantly reduced the levels of coprecipitated Cul2 (lanes 9 and 10). Together, the immunoprecipitation data suggest that BAF250b recruits Elo B/C and Cul2 through the BC box in the CTD. There may be a secondary Cul2 contact site within the ARID. These results are summarized in Fig. 2F.
To examine endogenous BAF250 interaction with Cul2, mouse IgG or antibodies specific for BAF250a or BAF250b were cross-linked to protein A/G-Sepharose beads. The beads were then incubated with HEK293 nuclear extract and analyzed by immunoblotting for Cul2. Both endogenous BAF250a and BAF250b were found to be associated with endogenous Cul2 (Fig. 2G). The Roc1 protein, a component of an E3 ligase, is embedded within the globular cullin C terminus and does not directly contact the substrate recognition module (42). We examined association between BAF250b and endogenous Roc1 through Cul2 (Fig. 2H). Roc1 coprecipitated with Flag-250b in a buffer containing 0.5 M KCl. These results support the idea that BAF250b serves as a substrate recognition module for an Elo B/C-, Cul2-, and Roc1-based E3 ubiquitin ligase.
BAF250bBC* is autoubiquitinated and degraded by the proteasome.
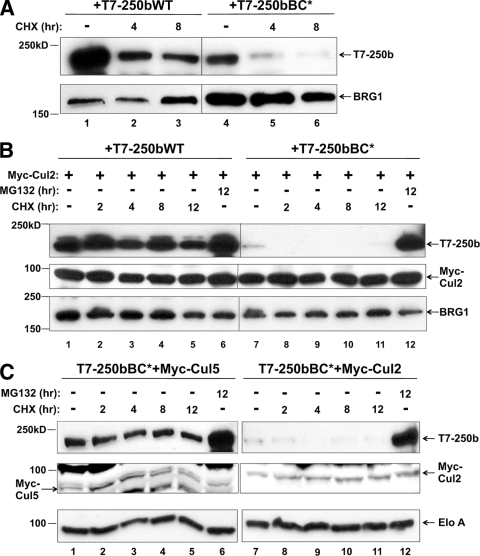
For substrate recognition modules like VHL, mutations in or around the BC box result in increased autoubiquitination and degradation by the proteasome (13). BAF250b may display similar properties when the BC box is mutated, as seen by the lower protein level of BC* in Fig. 1E. To determine if BAF250bWT and/or BAF250bBC* was degraded by the proteasome, we transiently transfected HeLa cells with T7-BAF250b and treated with the protein synthesis inhibitor cycloheximide (CHX) (Fig. 3A). Inhibiting protein synthesis revealed differences in BAF250bWT and BC* protein levels. Another SWI/SNF subunit, BRG1, was not affected, as seen in the immunoblot probed with anti-BRG1 antibody. If BAF250bBC* degradation is due to autoubiquitination, then BC* degradation is predicted to be dependent upon Cul2 interaction and exacerbated by higher levels of Cul2. BAF250bWT or BAF250bBC* was transiently cotransfected with Myc-Cul2 and treated with CHX or MG132 (Fig. 3B). Cotransfection of Cul2 further destabilized BC*, suggesting that BAF250bBC* degradation is stimulated by overexpressed Cul2. To demonstrate specificity, we also compared the destabilizing activities of Cul2 and Cul5 by transiently cotransfecting T7-BAF250bBC* with either Myc-Cul2 or Myc-Cul5 (Fig. 3C). Because we did not see preferential enrichment of Cul5 in coimmunoprecipitations with BAF250b (Fig. 2A), cotransfection of Myc-Cul5 was predicted not to promote BAF250bBC* degradation. Indeed, Myc-Cul5 had no effect on the stability of BC*.
FIG. 3.
BC* degradation by the proteasome is Cul2 dependent. (A) Stability of BAF250b and BC* protein. HeLa cells transiently transfected with T7-BAF250bWT (lanes 1 to 3) or T7-BAF250bBC* (lanes 4 to 6) were treated with vehicle (−) or cycloheximide (CHX) for the times indicated. Whole-cell extracts were immunoblotted for T7-250b and endogenous BRG1. (B) BC* degradation is exacerbated by overexpression of Cul2. HeLa cells were transiently transfected with T7-BAF250b (lanes 1 to 6) or T7-BAF250bBC* (lanes 7 to 12) and Myc-Cul2 (lanes 1 to 12). Cells were treated with MG132 or CHX for the times indicated. Whole-cell extracts were immunoblotted for T7, Myc, or endogenous BRG1. (C) The degradation of BAF250bBC* is exacerbated by Cul2 but not Cul5. HeLa cells were transiently transfected with T7-BAF250bBC* (lanes 1 to 12) and Myc-Cul5 (lanes 1 to 6) or Myc-Cul2 (lanes 7 to 12). Cells were treated with MG132 or CHX for the times indicated. Whole-cell extracts were immunoblotted for T7, Myc, or endogenous Elo A (loading control).
Consistent with the notion that BC* is preferentially autoubiquitinated, comparisons of BAF250b and BC* mutants proved to be difficult with transient cotransfection of Myc-Cul2 because BC* mutants were preferentially degraded during the immunoprecipitation step (Fig. 4A). Inhibiting the proteasome changed the association between BAF250b mutants and Cul2: Myc-Cul2 was transiently cotransfected with an empty vector or a plasmid encoding T7-BAF250bWT, T7-BAF250b without the ARID (ΔA), T7-BAF250bBC*, or a T7-BAF250b double mutant lacking the ARID and with BC* (ΔA and BC*) (Fig. 4B). The cells were treated with MG132 and a deubiquitinase inhibitor, N-ethylmaleimide. Immunoprecipitation with anti-T7 antibody from the nuclear extract showed that WT and mutant BAF250b proteins recruited equivalent amounts of Myc-Cul2 (lanes 7 to 10). This suggests that proteasomal and deubiquitinase inhibition stabilized the association between BAF250b mutants and Cul2 by trapping the BAF250b E3 ligase complex in the process of being autoubiquitinated before being degraded by the proteasome.
FIG. 4.
BAF250b is ubiquitinated and degraded by the proteasome. (A) BAF250bCTD BC* mutants are degraded during immunoprecipitation. T7 antibody immunoprecipitates of transiently transfected T7-BAF250b, T7-BAF250b with ARID deleted (ΔA), the T7-BAF250b BC box mutant (BC*), or T7-BAF250b with ARID deleted and the BC box mutated (ΔA and BC*), with Myc-Cul2, were washed in a buffer containing 0.5 M KCl and 0.1% NP-40 and analyzed by immunoblotting with an antibody to T7 or Myc. The film exposure for the input panels was shorter than that for the IP panels. (B) MG132 and NEM stabilize BAF250b mutant proteins in the presence of Myc-Cul2. HeLa cells were transiently transfected with T7-BAF250b, T7-BAF250b with ARID deleted (ΔA), the T7-BAF250b BC box mutant (BC*), or T7-BAF250b with ARID deleted and the BC box mutated (ΔA, BC*) and with Myc-Cul2, treated with 5 μM MG132, and harvested in the presence of NEM. BAF250b proteins were immunoprecipitated with an antibody to T7 and probed for Myc-Cul2. (C) BAF250bCTDBC* is autoubiquitinated. T7 antibody immunoprecipitates of cells transiently transfected with vector (−) (lanes 1, 5, and 9), T7-BAF250bCTDWT (+) (lanes 2, 6, and 10), T7-BAF250bCTDBC* (lanes 3, 7, and 11), or T7-BAF250bCTDBC** (lanes 4, 8, and 12) and Myc-Cul2 (lanes 1 to 12) were washed in a buffer containing 0.3 M KCl and 0.5% NP-40 and immunoblotted for ubiquitin (endogenous), Myc, and T7.
To directly examine ubiquitination of BAF250b, we chose BAF250bCTD because it is expressed to higher levels than near-full-length BAF250bWT due to its small size (75 kDa compared with 200 kDa). The CTD binds to both Elo C and Cul2 (Fig. 1B, C, and D and 2B and C), and we reasoned that the addition of polyubiquitin chains would be easier to visualize in this context. The BAF250bCTD BC* was degraded in a manner similar to that for BAF250bWT BC* in cells without inhibitor treatment (data not shown). Myc-Cul2 expression plasmids were cotransfected with an empty vector (−), T7-BAF250bCTD (+), T7-BAF250bCTD BC* (BC*), or a T7-BAF250bCTD BC box double mutant where, in addition to the leucine-to-proline substitution used so far (BC*), the conserved cysteine was mutated to phenylalanine (BC**) (Fig. 4C). Nuclear extract from cells treated with vehicle (lanes 1 to 4), MG132 (lanes 5 to 8), or MG132 plus NEM (lanes 9 to 12) was immunoprecipitated with α-T7 antibody and probed for endogenous ubiquitin (Fig. 4C, upper panel). Lanes 1 to 4 demonstrated that without MG132 or NEM, polyubiquitination of BAF250bCTD, or of any other protein, could not be detected. Upon MG132 addition, the immunoblot probed for ubiquitin revealed that BC* and BC**, but not BAF250bCTD, were heavily polyubiquitinated (lanes 5 to 8). In lanes 9 to 12, which received MG132 in culture and NEM during nuclear extract preparation, the polyubiquitination was visible on BC*, BC**, and BAF250bCTD. Thus, upon proteasome inhibition, polyubiquitination of the BC* mutants was detected, while that of BAF250bCTD was detected only upon inhibition of both the proteasome and deubiquitinating enzymes. Thus, like VHL, a mutation in the BC box of BAF250b appears to destabilize the protein through autoubiquitination and proteasomal degradation.
The BAF250b (SWI/SNF)-Elo B/C-Cul2-Roc1 complex is an E3 ubiquitin ligase for monoubiquitination of histone H2B.
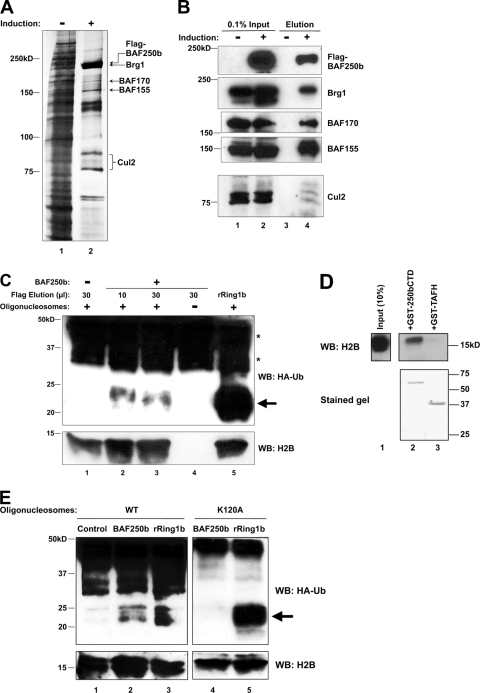
Because BAF250b is part of the SWI/SNF-A chromatin-remodeling complex with demonstrated activity on nucleosomes, we considered the core histones as potential substrates for the BAF250b-containing E3 ubiquitin ligase complex. To address this, an in vitro ubiquitination assay was carried out with reconstituted nucleosomes. 16e cells were induced to overexpress Flag-BAF250b, and anti-Flag antibody-conjugated M2 agarose beads were used to immunopurify the BAF250b (and SWI/SNF)-Elo B/C-Cul2-Roc1 complex under stringent salt conditions (0.5 M KCl). Uninduced 16e nuclear extract was also subjected to Flag antibody immunopurification as a negative control. A silver-stained protein gel of the Flag peptide-eluted proteins demonstrated the purification of the BAF250b complex containing components of the SWI/SNF complex (BRG1, BAF170, and BAF155) as well as Cul2, all of which was verified by immunoblotting (Fig. 5A and B). Although the Flag antibody purification of uninduced nuclear extract usually contained a small number of nonspecific protein bands on a silver-stained gel, we show here that even an eluted material containing many background proteins (e.g., Fig. 5A, lane 1) did not show any ubiquitin-conjugating activity (see below).
FIG. 5.
The BAF250b complex contains an E3 ligase activity that ubiquitinates histone H2B at K120. (A) Immunopurification of Flag-BAF250b complex. A silver-stained gel of SWI/SNF purified from a HeLa cell line, 16e, induced to express Flag-BAF250b is shown (lane 2). Purification from uninduced (−) 16e cells is shown as a negative control (lane 1). The SWI/SNF subunits identified by mass spectrometry are indicated. (B) Identification of SWI/SNF subunits by immunoblotting. Endogenous BRG1, BAF170, BAF155, and E3 ligase subunit Cul2 were detected in Flag-BAF250b-immunopurified material eluted with Flag peptide from induced cells (lane 4). An antibody to Flag was used to detect Flag-BAF250b. (C) The BAF250b complex monoubiquitinates histone(s) in a ubiquitination assay. An in vitro nucleosome ubiquitination assay mixture was reconstituted with the Flag-BAF250b complex (lanes 2 to 4), control Flag-eluted material (lane 1), or recombinant Ring1b (lane 5) and HA-ubiquitin (lanes 1 to 5). The products were immunoblotted (WB) with an antibody to HA and H2B. Ten microliters of eluted material correlates roughly with each lane in the silver-stained gel shown in panel A. Oligonucleosomes were omitted from the reaction in lane 4. Arrow, ubiquitinated H2A/H2B; *, HA-Ub-conjugated E1 and E2. (D) Bacterially expressed BAF250bCTD interacts with histone H2B. GST-BAF250bCTD (HR2) (lane 2) and control GST-TAFH (lane 3) proteins were expressed and purified from E. coli and incubated with histones extracted from HeLa cells. Immunoblotting (WB) with an antibody to H2B shows specific binding of H2B to BAF250bCTD. The lower panel shows the upper portion of the same gel stained with Coomassie blue. (E) The BAF250b complex monoubiquitinates histone H2B on K120. An in vitro nucleosome ubiquitination assay was performed as for panel C with the Flag-BAF250b complex (lanes 2 and 4), control material (lane 1), or recombinant Ring1b (lanes 3 and 5). The products were immunoblotted with an antibody to HA and H2B. The nucleosomes used were reconstituted in vitro with wild-type H2B (lanes 1 to 3) or H2B with lysine-120 mutated to alanine (H2BK120A) (lanes 4 and 5). Arrow, ubiquitinated H2A/H2B; *, HA-Ub-conjugated E1 and E2.
For the in vitro ubiquitination assay, E1, E2, HA-tagged ubiquitin (HA-Ub), in vitro-reconstituted oligonucleosomes, and Mg-ATP were added to the purified Flag-BAF250b complex. The E2 ubiquitin-conjugating enzyme used in this experiment was UbcH5c, the E2 favored by VHL, our reasoning being that VHL and BAF250 both utilize Elo B/C and Cul2. UbcH5c is also the E2 for Ring1b (used as a positive control in this experiment), whereas RNF20/40, the known E3 ubiquitin ligase for monoubiquitination of histone H2B, utilizes the E2 UbcH6. The products of the in vitro ubiquitination assay were separated by SDS-PAGE and immunoblotted for HA-Ub (Fig. 5C). Monoubiquitinated histones H2A and H2B, when tagged with HA, are expected to migrate at approximately 25 kDa on an HA immunoblot. The immunopurified material from uninduced 16e nuclear extract did not produce a band of ∼25 kDa (Fig. 5C, lane 1). The addition of purified Flag-BAF250b complex to the in vitro ubiquitination assay mixture produced a band corresponding in size to monoubiquitinated histone H2A or H2B (indicated with arrow), suggesting that the immunopurified complex contained ubiquitination activity (lanes 2 and 3). This product was not detected when nucleosomes were omitted from the reaction (lane 4). Recombinant Ring1b (rRing1b), a known E3 ubiquitin ligase for histone H2A, was included as a positive control for the assay and nucleosome integrity (lane 5). Background bands above 30 kDa (*) in all lanes resulted from E1 and E2 enzymes conjugated to HA-Ub. The result suggests that the immunopurified BAF250b complex contains an E3 ubiquitin ligase activity targeted to nucleosomes and modifying either H2A or H2B.
Because Drosophila osa is a member of the Trithorax group (see Discussion), we hypothesized that the BAF250b complex might target histone H2B for monoubiquitination, a mark associated with HOX gene activation. Furthermore, the GST-tagged BAF250b C terminus was found to interact with histone H2B extracted from HeLa cells in a GST pulldown assay (Fig. 5D). To test whether BAF250b monoubiquitinates H2B, we reconstituted nucleosomes with histone H2B in which lysine-120, the lysine that accepts the ubiquitin moiety, had been mutated to an alanine (H2BK120A) and repeated the in vitro ubiquitination assay (Fig. 5E). Again, the immunopurified material from uninduced 16e nuclear extract did not produce any band corresponding in size to ubiquitinated histones (Fig. 5E, lane 1), and the addition of the BAF250b complex produced a band of ∼25 kDa in the presence of WT nucleosomes (lane 2), as did the addition of rRing1b (lane 3). Remarkably, no band of ∼25 kDa was detected when the nucleosomes were reconstituted with H2BK120A (lane 4). The reactions carried out with the mutant nucleosomes are shown in a separate panel because their substrates differed from the reactions carried out with WT nucleosomes. The recombinant Ring1b served as an ideal positive control because its target H2A was present in both WT and H2BK120A nucleosomes (lanes 3 and 5). Thus, we conclude that the BAF250b (SWI/SNF)-Elo B/C-Cul2-Roc1 E3 ligase complex specifically ubiquitinates histone H2B on lysine-120. Contamination by the known H2B E3 RNF20/40 was unlikely, as RNF20/40 utilizes the E2 human Rad6 (16) or UbcH6 and not UbcH5 (26), which was used in our experiment. RNF20/40 was also undetectable in immunoblots of Flag-16e purified material probed with an antibody to RNF20/40 (data not shown). Furthermore, purified material from uninduced cells, which contained many more proteins than induced cells (Fig. 5A, compare lanes 1 and 2), showed no ubiquitinating activity on histones.
Knockdown of BAF250a and BAF250b by siRNA results in decreased levels of global H2B-Ub and H3K4me3.
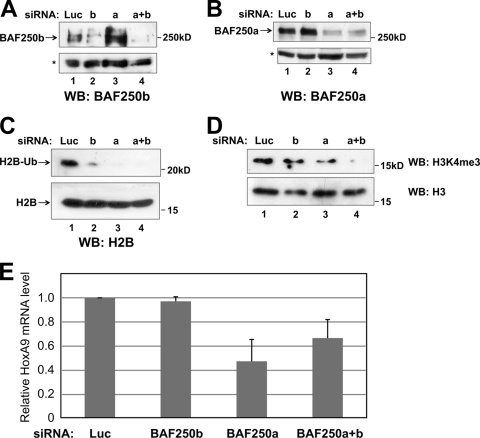
If BAF250 contributes to the global pool of H2B-Ub, then knockdown of BAF250 by siRNA is predicted to reduce global levels of H2B-Ub. To test this, siRNA oligonucleotides against luciferase (control), BAF250b, and BAF250a were transiently transfected into HEK293 cells. After 48 h, the cells were used to prepare a whole-cell extract, an acid extract, and RNA. Immunoblotting of the whole-cell extract demonstrated that the siRNAs reduced the levels of endogenous BAF250a and BAF250b (Fig. 6A and B). Immunoblotting of the acid extract with an antibody against H2B demonstrated that global levels of H2B-Ub were decreased in the cells depleted of BAF250a and/or BAF250b by siRNA compared with the control cells that received luciferase siRNA (Fig. 6C). Because BAF250a is more abundant than BAF250b, knockdown of BAF250a alone had an effect similar to the knockdown of both. The levels of monoubiquitinated H2A were not affected by BAF250a and/or BAF250b knockdown (data not shown). Furthermore, immunoblotting with an antibody to H3 lysine-4 trimethylation, a mark that is dependent on H2B-Ub, revealed a similar decrease in the global levels of H3K4me3 (Fig. 6D). Consistent with the classification of Osa as a trxG protein, siRNA knockdown of BAF250a resulted in a global decrease in HoxA9 mRNA levels detected by quantitative reverse transcription-PCR (qRT-PCR) (Fig. 6E). Collectively, these results suggest that BAF250 functions in vivo as a TrxG protein by acting as a positive regulator of HOX genes, possibly through monoubiquitination of histone H2BK120.
FIG. 6.
Global levels of H2B-Ub and H3K4me3 are decreased following knockdown of BAF250a and BAF250b by RNAi. (A) Knockdown of BAF250 by RNAi. Whole-cell extracts from HEK293 cells transiently transfected with siRNAs against luciferase (control), BAF250a, BAF250b, or both BAF250a and BAF250b (a+b) were immunoblotted with an antibody to BAF250b. *, nonspecific band used as a loading control. (B) The same extracts as for panel A were immunoblotted with an antibody to BAF250a. (C) Decreased global H2B-Ub levels upon BAF250 siRNA knockdown. Acid extracts from HEK293 cells depleted of BAF250a (lane 3), BAF250b (lane 2), or BAF250a+b (lane 4) were immunoblotted with an antibody to H2B. The band migrating above 20 kDa in the upper panel corresponds to the size of H2B-Ub. The upper and lower panels are from the same gel. The blot was separated for probing because of the difference in signal intensity between H2B-Ub and H2B. (D) The same extracts as for panel C were immunoblotted with an antibody specific to H3K4me3 (upper panel) and H3 (lower panel). (E) BAF250a is a positive regulator of the HoxA9 gene. Quantitative RT-PCR of HoxA9 mRNA levels in HEK293 cells transiently transfected with siRNAs targeting luciferase (control), BAF250a, BAF250b, or both BAF250a and BAF250b (a+b) is shown. Error bars indicate standard deviations.
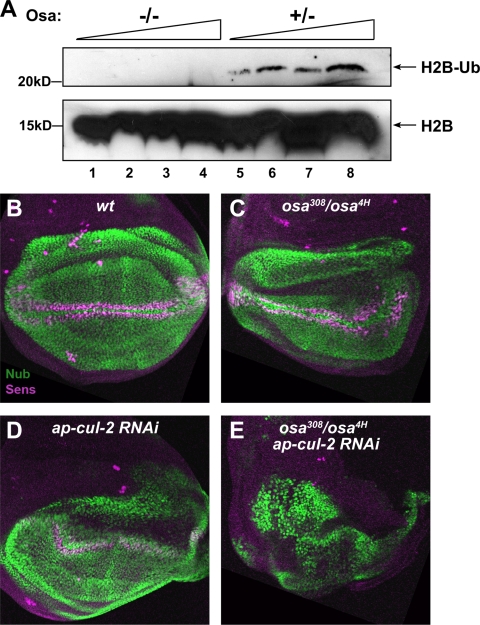
A Drosophila Osa mutant has reduced levels of monoubiquitinated H2B and functions synergistically with Cul2 in vivo.
To test whether the activity of BAF250 on H2B is evolutionarily conserved, we next examined the levels of monoubiquitinated H2B in mutants with mutations in the Drosophila BAF250 homolog osa. Acid extracts were made from the hypomorphic allelic combination osa308/osa4H (−/−), osa308/+, or osa4H/+ (+/−) larvae and probed for H2B (Fig. 7A). We found homozygous osa mutant larvae to exhibit significantly lower levels of H2B-Ub than heterozygous control larvae. To test whether the association with Cul2 might contribute to the function of Osa during development, we looked for genetic interactions in the wing imaginal disc, a tissue that has been shown to require osa (7) (Fig. 7B to E). In osa308/osa4H mutant wing discs, ectopic expression of the Wingless target gene nubbin (nub) (23) was observed in the notum, as previously described (7), but the endogenous domain of Nub expression in the wing pouch was only mildly affected (Fig. 7C). Expression of Senseless (Sens), a marker for sensory organ precursors at the wing margin (25) was weak but still present in these osa mutants (Fig. 7C). Since no cul-2 mutants have been reported, we used RNA interference to reduce the function of the gene. Expression of a transgenic UAS-cul-2RNAi line in the dorsal compartment of the wing disc with the apterous (ap)-GAL4 driver produced a mild distortion of Nub expression and a slight reduction in Sens expression (Fig. 7D). However, expressing cul-2RNAi in the osa mutant background completely abolished Sens expression and dramatically disrupted Nub expression (Fig. 7E). The synergistic effects of reducing osa and cul2 function suggest that the two proteins act together in vivo.
FIG. 7.
Analysis of Drosophila Osa mutants. (A) Osa mutant larvae have decreased levels of H2B-Ub. Acid extracts made from the hypomorphic allelic combination osa308/osa4H (−/−), osa308/+, or osa4H/+ (+/−) larvae were immunoblotted with an antibody to H2B. Increasing amounts (1× to 4×) of acid extracts from (−/−) or (+/−) larvae were loaded in lanes 1 to 4 and 5 to 8, respectively. The upper and lower panels are from the same gel. The blot was separated for probing because of the difference in signal intensity between H2B-Ub and H2B. (B to E) Third-instar wing imaginal discs oriented with dorsal up and posterior to the right were stained with anti-Nub (green) and anti-Sens (magenta) antibodies. (B) Wild type; (C) osa308/osa4H; (D) ap-GAL4/+, UAS-cul-2RNAi/+; (E) ap-GAL4/+; osa308, UAS-cul-2RNAi/osa4H. Reduction of either osa or cul-2 function causes slight abnormalities in Nub expression throughout the wing pouch and Sens expression at the wing margin, but when both are reduced simultaneously, Sens expression is completely absent and the Nub expression domain is distorted.
DISCUSSION
In this study, we have shown that BAF250b interacts with Elo C and Cul2. The interaction with Elo C and Cul2 is dependent on a BC box in the CTD of BAF250b, whereas the Cul2 interaction requires both ARID and the BC box. In vivo, the single amino acid substitution in the BC box (BC*) resulted in the degradation of the BC box mutant BAF250b through autoubiquitination. The Cul2-dependent regulation of BC* supports the idea that BAF250b serves as an E3 ubiquitin ligase substrate recognition module in vivo. We have determined histone H2B to be a substrate for the BAF250 complex in a nucleosomal context. We propose that this complex is assembled in a manner similar to that for the well-characterized VHL complex, which targets HIF1α (Fig. 8). We previously reported that the ARID and the CTD of BAF250 are important for transcriptional activation (12). BAF250CTD was also shown to interact with the glucocorticoid receptor to activate transcription (12, 24, 34). It is possible that the coactivator function of BAF250 is in part mediated through its association with Elo C and Cul2.
Until now, all H2B-Ub was thought to arise from the action of the heterodimeric RNF20/40 E3 ubiquitin ligase, although it has been noted that depletion of RNF20 by RNAi affected transcription of only a subset of genes (31). H2B-Ub has been shown to be required for transcriptional activation in vitro (26) and associates with transcriptionally active genes in vivo (8, 20). RNF20 is sufficient for ubiquitin ligase activity in vitro and shares approximately 30% homology with S. cerevisiae Bre1, which performs the analogous function in yeast (43). Interestingly, the yeast BAF250 ortholog Swi1 lacks an identifiable BC box necessary for interaction with Elo B/C. The Elo B/C interaction domain and the Swi1 ARID are also not highly conserved (38). Swi1 has not been identified in genetic screens for factors affecting H2B monoubiquitination (11), and multiple groups have reported on the elimination of H2B-Ub upon deletion of bre1 in yeast. Thus, the probability of Swi1 possessing E3 ubiquitin ligase activity seems low. Because yeast is a unicellular organism, the layers of epigenetic regulation necessary in mammals would not be required. The BAF250 E3 ubiquitin ligase activity therefore may reflect an evolutionary adaptation unique to the demands of developmental regulation in multicellular organisms.
The BAF250-Elo B/C-Cul2-Roc1 complex would also be regulated by assembly and neddylation of the cullin subunit, whereas RNF20/RNF40 is presumably constitutively active (43). Dynamic regulation of the BAF250 E3 ubiquitin ligase assembly and activity by neddylation, the COP9 signalosome, and TIP120A/CAND1 complexes would allow the type of temporal regulation necessary in early development. Recent studies of developing cells have demonstrated that temporal regulation of different transcription factor complexes is important to cell fate decisions (9).
Both BAF250a and BAF250b are expressed in mammalian embryonic stem cells. Chromatin immunoprecipitation studies suggest that Nanog, Oct4, and Sox2 occupy the BAF250b promoter, while E2F4 occupies the BAF250a promoter (3). The dependence of mammalian embryonic stem cells on BAF250a and -b argues that these are genuine trxG members. Although trxG and PcG proteins have antagonistic roles in controlling HOX gene expression, many of the trxG and PcG proteins studied have chromatin-remodeling and chromatin-modifying capabilities. Posttranslational modifications of histones, such as H3 lysine-4 trimethylation (H3K4me3) and H2B monoubiquitination (H2B-Ub), show a positive correlation with transcription activation. H2B-Ub is required for transcription from a chromatinized template in vitro (26) and regulates H3K4me3 levels in vivo (30, 33). In contrast, H2A monoubiquitination is considered a transcriptionally repressive mark set by Ring1a/b, part of Polycomb repressor complex 1 (5). In accordance with Osa's antagonistic role in regard to Ring1a/b in HOX gene regulation, we show that BAF250a is a positive regulator of HoxA9 and that the BAF250b complex is specific for histone H2B in a nucleosomal context. Indeed, in vitro ubiquitination assays and in vivo knockdown experiments indicate that H2B is a target of the BAF250 E3 ubiquitin ligase. BAF250 is not essential to SWI/SNF targeting in vivo or to chromatin remodeling in vitro (6, 27). Thus, we do not think that BAF250 siRNA knockdown has disrupted SWI/SNF remodeling activity in general. Furthermore, Osa and Cul2 synergistically interact in genetic analysis carried out with the Drosophila wing discs, confirming the importance of association with Cul2 to the function of Osa in vivo. The discovery that BAF250 associates with Elo B/C, Cul2, and Roc1 to form an E3 ubiquitin ligase that specifically monoubiquitinates histone H2B in a nucleosomal context provides a mechanistic explanation for Osa's classification as a trxG member.
The identification of a novel ubiquitination pathway mediated by the chromatin-remodeling complex SWI/SNF-A raises several important questions. Is the H2B-Ub mark set before or after remodeling? Does H2B-Ub affect chromatin remodeling? Is the H2B ubiquitination mediated by BAF250 gene specific? If so, what distinguishes such genes from those targeted by the E3 ligase RNF20/40? Genome-wide analysis of the occupancy of BAF250, RNF20/40, and H2B-Ub will help to identify genes targeted by the ubiquitin ligase activity of BAF250, which would facilitate future biochemical studies.
Acknowledgments
This work was supported in part by an American Cancer Society grant (RSG-01-248-01-CCE to N.T.), a grant from the Mathers Charitable Foundation (to N.T.), a Public Health Service training grant (GM007238 to X.S.L.), and a grant by the National Institutes of Health (GM56131 to J.E.T.). We are grateful to Danny Reinberg for his generous support of the project.
We thank Takako Furukawa for performing the initial purification of the BAF250b complex; the NYU Protein Analysis Facility for mass spectrometry service; Ryo Koyama-Nasu, Michele Pagano, Michael Garabedian, Steve Cohen, and Hugo Bellen for reagents, help, and valuable advice; and Angus Wilson for critical reading of the manuscript. We thank Evelyn Kono, Laurent Pascual Le Tallec, and Yan Li for their help with this project.
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Bailey, T. L., and M. Gribskov. 1998. Combining evidence using p-values: application to sequence homology searches. Bioinformatics 14:48-54. [DOI] [PubMed] [Google Scholar]
- 2.Blanchette, P., C. Y. Cheng, Q. Yan, G. Ketner, D. A. Ornelles, T. Dobner, R. C. Conaway, J. W. Conaway, and P. E. Branton. 2004. Both BC-box motifs of adenovirus protein E4orf6 are required to efficiently assemble an E3 ligase complex that degrades p53. Mol. Cell. Biol. 24:9619-9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, L. A., T. I. Lee, M. F. Cole, S. E. Johnstone, S. S. Levine, J. P. Zucker, M. G. Guenther, R. M. Kumar, H. L. Murray, R. G. Jenner, D. K. Gifford, D. A. Melton, R. Jaenisch, and R. A. Young. 2005. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122:947-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairns, B. R. 2007. Chromatin remodeling: insights and intrigue from single-molecule studies. Nat. Struct. Mol. Biol. 14:989-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, R., Y. Tsukada, and Y. Zhang. 2005. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 20:845-854. [DOI] [PubMed] [Google Scholar]
- 6.Collins, R. T., T. Furukawa, N. Tanese, and J. E. Treisman. 1999. Osa associates with the Brahma chromatin remodeling complex and promotes the activation of some target genes. EMBO J. 18:7029-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, R. T., and J. E. Treisman. 2000. Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev. 14:3140-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davie, J. R., and L. C. Murphy. 1994. Inhibition of transcription selectively reduces the level of ubiquitinated histone H2B in chromatin. Biochem. Biophys. Res. Commun. 203:344-350. [DOI] [PubMed] [Google Scholar]
- 9.Deato, M. D., and R. Tjian. 2007. Switching of the core transcription machinery during myogenesis. Genes Dev. 21:2137-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao, X., P. Tate, P. Hu, R. Tjian, W. C. Skarnes, and Z. Wang. 2008. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc. Natl. Acad. Sci. U. S. A. 105:6656-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang, W. W., S. Venkatasubrahmanyam, A. G. Ianculescu, A. Tong, C. Boone, and H. D. Madhani. 2003. A conserved RING finger protein required for histone H2B monoubiquitination and cell size control. Mol. Cell 11:261-266. [DOI] [PubMed] [Google Scholar]
- 12.Inoue, H., T. Furukawa, S. Giannakopoulos, S. Zhou, D. S. King, and N. Tanese. 2002. Largest subunits of the human SWI/SNF chromatin-remodeling complex promote transcriptional activation by steroid hormone receptors. J. Biol. Chem. 277:41674-41685. [DOI] [PubMed] [Google Scholar]
- 13.Kamura, T., C. S. Brower, R. C. Conaway, and J. W. Conaway. 2002. A molecular basis for stabilization of the von Hippel-Lindau (VHL) tumor suppressor protein by components of the VHL ubiquitin ligase. J. Biol. Chem. 277:30388-30393. [DOI] [PubMed] [Google Scholar]
- 14.Kamura, T., K. Maenaka, S. Kotoshiba, M. Matsumoto, D. Kohda, R. C. Conaway, J. W. Conaway, and K. I. Nakayama. 2004. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 18:3055-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennison, J. A., and J. W. Tamkun. 1988. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 85:8136-8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, J., M. Guermah, R. K. McGinty, J. S. Lee, Z. Tang, T. A. Milne, A. Shilatifard, T. W. Muir, and R. G. Roeder. 2009. RAD6-mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell 137:459-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemon, B., C. Inouye, D. S. King, and R. Tjian. 2001. Selectivity of chromatin-remodelling cofactors for ligand-activated transcription. Nature 414:924-928. [DOI] [PubMed] [Google Scholar]
- 18.Lessard, J., J. I. Wu, J. A. Ranish, M. Wan, M. M. Winslow, B. T. Staahl, H. Wu, R. Aebersold, I. A. Graef, and G. R. Crabtree. 2007. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron 55:201-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo, B., H. W. Cheung, A. Subramanian, T. Sharifnia, M. Okamoto, X. Yang, G. Hinkle, J. S. Boehm, R. Beroukhim, B. A. Weir, C. Mermel, D. A. Barbie, T. Awad, X. Zhou, T. Nguyen, B. Piqani, C. Li, T. R. Golub, M. Meyerson, N. Hacohen, W. C. Hahn, E. S. Lander, D. M. Sabatini, and D. E. Root. 2008. Highly parallel identification of essential genes in cancer cells. Proc. Natl. Acad. Sci. U. S. A. 105:20380-20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minsky, N., E. Shema, Y. Field, M. Schuster, E. Segal, and M. Oren. 2008. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat. Cell Biol. 10:483-488. [DOI] [PubMed] [Google Scholar]
- 21.Nagl, N. G., Jr., X. Wang, A. Patsialou, M. Van Scoy, and E. Moran. 2007. Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. EMBO J. 26:752-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narlikar, G. J., M. L. Phelan, and R. E. Kingston. 2001. Generation and interconversion of multiple distinct nucleosomal states as a mechanism for catalyzing chromatin fluidity. Mol. Cell 8:1219-1230. [DOI] [PubMed] [Google Scholar]
- 23.Ng, M., F. J. Diaz-Benjumea, J. P. Vincent, J. Wu, and S. M. Cohen. 1996. Specification of the wing by localized expression of wingless protein. Nature 381:316-318. [DOI] [PubMed] [Google Scholar]
- 24.Nie, Z., Y. Xue, D. Yang, S. Zhou, B. J. Deroo, T. K. Archer, and W. Wang. 2000. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol. Cell. Biol. 20:8879-8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolo, R., L. A. Abbott, and H. J. Bellen. 2000. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102:349-362. [DOI] [PubMed] [Google Scholar]
- 26.Pavri, R., B. Zhu, G. Li, P. Trojer, S. Mandal, A. Shilatifard, and D. Reinberg. 2006. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell 125:703-717. [DOI] [PubMed] [Google Scholar]
- 27.Phelan, M. L., S. Sif, G. J. Narlikar, and R. E. Kingston. 1999. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol. Cell 3:247-253. [DOI] [PubMed] [Google Scholar]
- 28.Schoenfeld, A. R., E. J. Davidowitz, and R. D. Burk. 2000. Elongin BC complex prevents degradation of von Hippel-Lindau tumor suppressor gene products. Proc. Natl. Acad. Sci. U. S. A. 97:8507-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuettengruber, B., D. Chourrout, M. Vervoort, B. Leblanc, and G. Cavalli. 2007. Genome regulation by polycomb and trithorax proteins. Cell 128:735-745. [DOI] [PubMed] [Google Scholar]
- 30.Shahbazian, M. D., K. Zhang, and M. Grunstein. 2005. Histone H2B ubiquitylation controls processive methylation but not monomethylation by Dot1 and Set1. Mol. Cell 19:271-277. [DOI] [PubMed] [Google Scholar]
- 31.Shema, E., I. Tirosh, Y. Aylon, J. Huang, C. Ye, N. Moskovits, N. Raver-Shapira, N. Minsky, J. Pirngruber, G. Tarcic, P. Hublarova, L. Moyal, M. Gana-Weisz, Y. Shiloh, Y. Yarden, S. A. Johnsen, B. Vojtesek, S. L. Berger, and M. Oren. 2008. The histone H2B-specific ubiquitin ligase RNF20/hBRE1 acts as a putative tumor suppressor through selective regulation of gene expression. Genes Dev. 22:2664-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stebbins, C. E., W. G. Kaelin, Jr., and N. P. Pavletich. 1999. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 284:455-461. [DOI] [PubMed] [Google Scholar]
- 33.Sun, Z. W., and C. D. Allis. 2002. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418:104-108. [DOI] [PubMed] [Google Scholar]
- 34.Trotter, K. W., H. Y. Fan, M. L. Ivey, R. E. Kingston, and T. K. Archer. 2008. The HSA domain of BRG1 mediates critical interactions required for glucocorticoid receptor-dependent transcriptional activation in vivo. Mol. Cell. Biol. 28:1413-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vodermaier, H. C. 2004. APC/C and SCF: controlling each other and the cell cycle. Curr. Biol. 14:R787-R796. [DOI] [PubMed] [Google Scholar]
- 36.Wang, X., N. G. Nagl, D. Wilsker, M. Van Scoy, S. Pacchione, P. Yaciuk, P. B. Dallas, and E. Moran. 2004. Two related ARID family proteins are alternative subunits of human SWI/SNF complexes. Biochem. J. 383:319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X., D. M. Truckses, S. Takada, T. Matsumura, N. Tanese, and R. H. Jacobson. 2007. Conserved region I of human coactivator TAF4 binds to a short hydrophobic motif present in transcriptional regulators. Proc. Natl. Acad. Sci. U. S. A. 104:7839-7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilsker, D., A. Patsialou, S. D. Zumbrun, S. Kim, Y. Chen, P. B. Dallas, and E. Moran. 2004. The DNA-binding properties of the ARID-containing subunits of yeast and mammalian SWI/SNF complexes. Nucleic Acids Res. 32:1345-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, J. I., J. Lessard, and G. R. Crabtree. 2009. Understanding the words of chromatin regulation. Cell 136:200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xi, Q., W. He, X. Zhang, H. Le, and J. Massague. 2008. Genome-wide impact of the BRG1 SWI/SNF chromatin remodeler on the transforming growth factor transcriptional program. J. Biol. Chem. 283:1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan, Z., Z. Wang, L. Sharova, A. Sharov, C. Ling, Y. Piao, K. Aiba, R. Matoba, W. Wang, and M. Ko. 2008. BAF250B-associated SWI/SNF chromatin-remodeling complex is required to maintain undifferentiated mouse embryonic stem cells. Stem Cells 26:1155-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng, N., B. A. Schulman, L. Song, J. J. Miller, P. D. Jeffrey, P. Wang, C. Chu, D. M. Koepp, S. J. Elledge, M. Pagano, R. C. Conaway, J. W. Conaway, J. W. Harper, and N. P. Pavletich. 2002. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416:703-709. [DOI] [PubMed] [Google Scholar]
- 43.Zhu, B., Y. Zheng, A. D. Pham, S. S. Mandal, H. Erdjument-Bromage, P. Tempst, and D. Reinberg. 2005. Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol. Cell 20:601-611. [DOI] [PubMed] [Google Scholar]