Abstract
Endothelial cell (EC) migration, cell-cell adhesion, and the formation of branching point structures are considered hallmarks of angiogenesis; however, the underlying mechanisms of these processes are not well understood. Lipid phosphate phosphatase 3 (LPP3) is a recently described p120-catenin-associated integrin ligand localized in adherens junctions (AJs) of ECs. Here, we tested the hypothesis that LPP3 stimulates β-catenin/lymphoid enhancer binding factor 1 (β-catenin/LEF-1) to induce EC migration and formation of branching point structures. In subconfluent ECs, LPP3 induced expression of fibronectin via β-catenin/LEF-1 signaling in a phosphatase and tensin homologue (PTEN)-dependent manner. In confluent ECs, depletion of p120-catenin restored LPP3-mediated β-catenin/LEF-1 signaling. Depletion of LPP3 resulted in destabilization of β-catenin, which in turn reduced fibronectin synthesis and deposition, which resulted in inhibition of EC migration. Accordingly, reexpression of β-catenin but not p120-catenin in LPP3-depleted ECs restored de novo synthesis of fibronectin, which mediated EC migration and formation of branching point structures. In confluent ECs, however, a fraction of p120-catenin associated and colocalized with LPP3 at the plasma membrane, via the C-terminal cytoplasmic domain, thereby limiting the ability of LPP3 to stimulate β-catenin/LEF-1 signaling. Thus, our study identified a key role for LPP3 in orchestrating PTEN-mediated β-catenin/LEF-1 signaling in EC migration, cell-cell adhesion, and formation of branching point structures.
Angiogenesis, the formation of new blood vessels, involves several well-coordinated cellular processes, including endothelial cell (EC) migration, synthesis and deposition of extracellular matrix proteins, such as fibronectin, cell-cell adhesion, and formation of branching point structures (1-3, 19, 33); however, less is known about the underlying mechanisms of these processes (6, 8, 12, 14, 16, 17). For example, adherens junctions (AJs), which mediate cell-cell adhesion between ECs, may be involved in limiting the extent of cell migration (2, 14, 38, 40). VE-cadherin, a protein found in AJs, is a single-pass transmembrane polypeptide responsible for calcium-dependent homophilic interactions through its extracellular domains (2, 38, 40). The VE-cadherin cytoplasmic domain interacts with the Armadillo domain-containing proteins, β-catenin, γ-catenin (plakoglobin), and p120-catenin (p120ctn) (2, 15, 38, 40, 43). Genetic and biochemical evidence documents a crucial role of β-catenin in regulating cell adhesion as well as proliferation secondary to the central position of β-catenin in the Wnt signaling pathway (13, 16, 25, 31, 44). In addition, the juxtamembrane protein p120ctn regulates AJ stability via binding to VE-cadherin (2, 7, 9, 15, 21, 28, 32, 43). The absence of regulation or inappropriate regulation of β-catenin and VE-cadherin functions is linked to cardiovascular disease and tumor progression (2, 6).
We previously identified lipid phosphate phosphatase 3 (LPP3), also known as phosphatidic acid phosphatase 2b (PAP2b), in a functional assay of angiogenesis (18, 19, 41, 42). LPP3 not only exhibits lipid phosphatase activity but also functions as a cell-associated integrin ligand (18, 19, 35, 41, 42). The known LPPs (LPP1, LPP2, and LPP3) (20-23) are six transmembrane domain-containing plasma membrane-bound enzymes that dephosphorylate sphingosine-1-phosphate (S1P) and its structural homologues, and thus, these phosphatases generate lipid mediators (4, 5, 23, 35, 39). All LPPs, which contain a single N-glycosylation site and a putative lipid phosphatase motif, are situated such that their N and C termini are within the cell (4, 5, 22, 23, 35, 39). Only the LPP3 isoform contains an Arg-Gly-Asp (RGD) sequence in the second extracellular loop, and this RGD sequence enables LPP3 to bind integrins (18, 19, 22). Transfection experiments with green fluorescent protein (GFP)-tagged LPP1 and LPP3 showed that LPP1 is apically sorted, whereas LPP3 colocalized with E-cadherin at cell-cell contact sites with other Madin-Darby canine kidney (MDCK) cells (22). Mutagenesis and domain swapping experiments established that LPP1 contains an apical targeting signal sequence (FDKTRL) in its N-terminal segment. In contrast, LPP3 contains a dityrosine (109Y/110Y) basolateral sorting motif (22). Interestingly, conventional deletion of Lpp3 is embryonic lethal, since the Lpp3 gene plays a critical role in extraembryonic vasculogenesis independent of its lipid phosphatase activity (11). In addition, an LPP3-neutralizing antibody was shown to prevent cell-cell interactions (19, 42) and angiogenesis (42). Here, we addressed the hypothesis that LPP3 plays a key role in EC migration, cell-cell adhesion, and formation of branching point structures by stimulating β-catenin/lymphoid enhancer binding factor 1 (β-catenin/LEF-1) signaling.
MATERIALS AND METHODS
Antibodies and growth factors.
Preparation, purification, and characterization of rabbit anti-LPP3-RGD and anti-LPP3-C-cyto polyclonal antibodies (pAbs) have been described previously (18, 19, 41, 42). Mouse anti-VCIP (also called anti-LPP3) monoclonal antibody (MAb), anti-LPP2 pAb, and fluorescein isothiocyanate (FITC)- or Texas Red-conjugated goat or donkey IgGs were purchased from Invitrogen (Carlsbad, CA). A synthetic peptide (YRCRGDDSKVQEARKSFF conjugated to keyhole limpet hemocyanin) was used to generate mouse anti-human LPP3 monoclonal antibody by Promab (Richmond, CA), and antibodies were characterized as previously described (18, 19, 41, 42). Anti-phosphotyrosine-20 (PY20), anti-p120ctn (clone 98), anti-β-catenin (clone 14), and anti-γ-catenin (clone 15) MAbs were purchased from BD Biosciences (San Jose, CA). The anti-human VE-cadherin (clone BV6) and anti-PTEN (clone A2b1) monoclonal antibodies were purchased from Millipore/Chemicon International, Inc. (Temecula, CA). Mouse anti-human fibronectin (clone F-15), antihemagglutinin (anti-HA) (clone 12CA5), anti-FLAG, and anti-pan-cadherin (CH-19) monoclonal antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Rabbit anti-p-GSK3-β(S9) (antibody against phosphorylated glycogen synthase kinase 3β [phosphorylated at serine 9]) and anti-phospho-β-catenin (Ser33, Ser37, and Thr41) antibodies were purchased from Cell Signaling Technology, Inc. (Denver, MA). Rabbit anti-p120 (clone S-19), anti-glutathione S-transferase (anti-GST) (Z-5), anti-Grb2, and anti-LEF-1 (clone H-70) polyclonal antibodies as well as secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CD31 (CD31 also called PECAM-1 [for platelet endothelial cell adhesion molecule 1]) (clone 9G11) monoclonal antibody and recombinant human vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) were purchased from R&D Systems (Minneapolis, MN).
Recombinant cDNA and short hairpin RNA (shRNA) constructs.
The retroviral vector pLNCX2 and the amphotropic packaging cell line HEK293 (human kidney fibroblasts) were purchased from BD Biosciences. Wild-type and mutant human LPP1 (hLPP1), hLPP2, and hLPP3 cDNAs were subcloned into the pLNCX2 retroviral vector directly downstream of the human cytomegalovirus (CMV) immediate-early promoter. Two-step PCR was used to insert three copies of the hemagglutinin (HA) tag (YPYDVPDYA) at the N termini of all cDNAs as well as to generate an adhesion-defective mutant (hLPP3-RAD), a phosphatase-defective mutant (K[A]XXXXXXRP[A]; hLPP3-PD), a double mutant (hLPP3-RAD+PD), and mutants lacking the N-terminal cytoplasmic domain (pLNCX2-HA-N-Δ-Cyto-hLPP3) or C-terminal cytoplasmic domain (pLNCX2-HA-C-Δ-Cyto-hLPP3) construct (18, 19). Newly generated constructs were verified by sequencing. A set of four different shRNA constructs (in pLKO.1 lentiviral vector) targeting the human LPP3 (catalog no. SHDNA-NM_003713), phosphatase and tensin homologue (PTEN) (catalog no. SHCLNV-NM_000314), and p120ctn genes (catalog no. SHDNA-NM_001331) were purchased from Sigma-Aldrich (St. Louis, MO). The retroviral or lentiviral particles were generated according to the manufacturer's instructions. The efficiency of knockdown for each construct was determined by Western analysis. FLAG-tagged wild-type and substrate-trapping mutant (C124S) PTEN cDNA constructs were a gift of Jack E. Dixon (University of California, San Diego, CA). p120ctn (catalog no. RC222771) cDNA was obtained from Origene Technologies Inc. (Rockville, MD), and β-catenin cDNA was obtained from Addgene (Cambridge, MA). One copy of the HA epitope was added in frame to the C-terminal segments of these cDNAs by two-step PCR and then subcloned into the SfiI site of the pLNCX2 retrovirus as previously described (18, 19). The intended sequences were verified by cDNA sequencing.
Cell culture, retroviral constructs, and transfection.
Human dermal microvascular endothelial cells (hdMVECs) were purchased from Lonza (Allendale, NJ). The hdMVECs were cultured as described elsewhere (19, 41, 42). In all assays, the cells were grown in monolayers and used at either passage 3 or 4. Confluent ECs were split 1:4 or 1:1 so that, on the day of the experiments, the cells were approximately ∼50% or ∼100% confluent, respectively. Packaging and processing retroviral particles and infection of target cells were performed as previously described (18, 19). Because pooled populations of G418-resistant HEK293 cells were heterogeneous for LPP3 expression (data not shown), cell clones were isolated and expanded. For transient transfection, cell culture supernatants containing retroviral particles were added to ECs plated at ∼40% density in the presence of 8.0 μg/ml Polybrene and incubated for 12 to 16 h. The following day, complete medium was added to the cells, and the cells were then incubated for 24 to 48 h to allow maximal expression of recombinant proteins (18, 19). Protein expression was analyzed by Western blotting (WB).
Cell-cell adhesion assay.
Cell-cell adhesion (also known as cell swirling and cell aggregation) assay and quantification of cell aggregates have been previously described (18, 19). Briefly, the ECs were detached from dishes with 0.02% trypsin and 10 mM EDTA. The cells were washed with phosphate-buffered saline (PBS) and resuspended in HCMF buffer (20 mM HEPES [pH 7.3], 10 mM CaCl2, 5.0 mM KCl, 0.35 mM Na2HPO4·H2O, 137.5 mM NaCl, and 4.5 mM glucose) supplemented with 5 mM Ca2+, 1 mM Mg2+, 2.0 μg/ml of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil) (red) or 8.0 μg/ml of 3,3-dioctadecycloxacarbocyanine perchlorate (DiO) (green) (Invitrogen) at 37°C for 7 min. Labeled ECs (106 of each) were allowed to aggregate in 400 μl of HCMF buffer containing 60 U/ml DNase I in siliconized cylindrical glass vials by rotating at 90 rpm at 37°C for 12 h. The inhibitory effects of LPP3 depletion, and reexpression of either HA-tagged p120ctn (HA-p120ctn) or HA-tagged β-catenin (HA-β-catenin) on cell aggregation were quantified at the end of 12 h. A minimum of five random fields were used for each point. Experiments were carried out three times with each point analyzed in three replicate samples. Only productive cell aggregates (yellow) were counted. Unproductive (red or green) cell aggregates were ignored. Numbers were expressed as the increase (fold increase) cell aggregates.
Biochemical experiments.
For immunoprecipitation (IP) experiments, the cells were rinsed with cold PBS (pH 7.4) and solubilized with complete cell lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1 mM calcium chloride, 1 mM magnesium chloride, 1 mM sodium orthovanadate, 25 mM sodium fluoride, 1 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 μg/ml pepstatin A) or with modified radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris [pH 7.5], 0.1% SDS, 0.25% sodium deoxycholate, 1% Triton X-100, 150 mM sodium chloride, 1 mM sodium pyrophosphate, 25 mM sodium fluoride, 1 mM sodium orthovanadate, 5 mM magnesium chloride, 1 mM calcium chloride, 1 μg/ml pepstatin, 1 mM phenylmethylsulfonyl fluoride, and 1 μg/ml leupeptin) at 4°C for 30 min. Cell extracts were centrifuged at ∼21,000 × g for 30 min at 4°C, preadsorbed, and immunoprecipitated using specific antibodies. Immune complexes were washed four or five times with lysis buffer or with cold IP wash buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 0.5% NP-40, 1 mM calcium chloride, and 1 mM magnesium chloride). Sepharose-bound immune complexes were boiled in 1× Laemmli reducing sample buffer and resolved by SDS-PAGE. WB was performed as described elsewhere (19).
Far-Western analysis.
For far-Western analyses, hdMVEC monolayers were solubilized in complete cell extraction buffer as described above. IP and WB analyses were performed as described previously (18, 19). The resulting blots were saturated with 5% milk in 1× Tris-buffered saline (TBS) (25 mM Tris [pH 7.5], 150 mM NaCl) and then incubated for 1 h with 2.0 μg/ml of soluble GST-LPP3-C-cyto fusion protein (bait) in TBS containing 0.1% Tween 20 and 2 mM dithiothreitol (DTT). After the membranes were rinsed with TBS, they were analyzed by WB with an anti-GST MAb. For IP, antibodies were used at 2 to 5 μg per sample. For WB, mouse MAbs and rabbit pAbs were used at a concentration of ∼0.5 μg/ml and ∼2.0 μg/ml, respectively.
Glutathione-S-transferase (GST) pulldown assay.
The two-step PCR technique was used to generate in-frame fusions of GST and either the N-terminal cytoplasmic domain of LPP3 (GST-N-cyto or GST-NC) or the C-terminal cytoplasmic domain of LPP3 (GST-C-cyto or GST-CC). Two-step PCR, protein expression in Escherichia coli, and purification and elution of GST fusion proteins were performed as previously detailed (18, 19). SDS-PAGE and Coomassie blue staining were performed to quantify purified GST fusion protein and to verify the integrity of the protein.
Microscopy.
For fluorescence microscopy, hdMVECs were grown on coverslips, fixed with 3% paraformaldehyde (PFA), and permeabilized with 0.5% Triton X-100 in PBS, pH 7.4. Cells were subsequently incubated with the indicated primary antibodies, followed by the appropriate secondary donkey or goat anti-mouse IgGs conjugated either to FITC or Texas Red (secondary antibody [Ab] concentration, 2 μg/ml). Prolong anti-FADE (Invitrogen) containing 4′,6′-diamidino-2-phenylindole (DAPI) was used to visualize nuclei. Microscopic analyses were performed using a Zeiss Axioplan 2 microscope, and images were captured using a Zeiss digital camera equipped with AxioVision LE software.
Lymphoid enhancer binding factor 1 (LEF-1) luciferase assay.
For the luciferase assays, we used constructs containing multimeric LEF-1 binding sites fused to a luciferase reporter gene (TOPFLASH), which was a kind gift from Randall T. Moon at the University of Washington (Seattle, WA). The hdMVECs were plated at three different densities (25 to 50%) in complete medium so that after 48 h, the cells had reached 50%, 75%, and 100% confluence. These cells were then infected overnight (8 h) with supernatants containing the indicated retroviral particles and allowed to recover for 4 h in complete medium. After recovery, the cells were cotransfected with TOPFLASH (3.0 μg/106 cells) and a β-galactosidase (β-Gal) normalization plasmid (0.5 μg/106 cells) using SuperFect (Qiagen Inc., Valencia, CA). After 4 h of transfection, the dishes were replenished with complete medium. Luciferase and β-Gal activities were assayed at the indicated times using the luciferase assay system (Promega Corp., Madison, WI). Protein concentrations were adjusted for equivalent β-Gal and luciferase activities. Experiments were repeated three times, using triplicate wells in each instance. In a subset of experiments, 25% confluent hdMVECs were infected for 12 h with retroviral particles and allowed to recover in complete medium for 6 h prior to transient transfection. Transfection was performed as described above for 6 h, and ECs were maintained in serum-free medium (without growth factor) for an additional 12 h prior to assay of β-Gal and luciferase activities.
Enzyme-linked immunosorbent assay (ELISA).
ELISAs were performed using commercially available kits (R&D Systems, Biosource International, and Research Diagnostics). The limit of detection for each factor ranged from 6 pg/ml (lower limit) to 1,600 pg/ml (upper limit). The intraassay variation was 4.3 to 6.5%, while the interassay variation was approximately 6.5 to 10%.
Endothelial cell wound healing assay.
Monolayer cultures (70% confluence) of hdMVECs in 12-well plates were infected overnight (∼16 h) with either LPP3 shRNA (shLPP3) retroviral particles or retroviral particles carrying a control, nonsilencing shRNA. Fresh medium was added to the cultures, and the cultures were allowed to grow for 24 h to form confluent monolayers. The monolayers were then scratched (wounded) gently with a 200-μl micropipette tip, washed twice in sterile PBS, and allowed to recover in defined medium (DM) (EBM-2 medium supplemented with 0.1% bovine serum albumin [BSA] plus 1× ITS [insulin, transferrin, and selenium A] [Invitrogen]) at 37°C (19). At 0, 4, and 8 h after injury, the cultures were washed in PBS and fixed in 4% PFA in PBS (pH 7.5). Fixed cells were stained with eosin and hematoxylin, and images were obtained with a Zeiss phase-contrast microscope equipped with a digital camera.
Endothelial cell migration assay.
Cell migration assays were carried out using modified Transwell Boyden chambers (8-μm polycarbonate membrane). hdMVECs infected with shLPP3 or control shRNA retroviral particles were detached using 2 mM EDTA in PBS (pH 7.5). The cells were pelleted, washed once with PBS (pH 7.5), and resuspended in DM. The upper chamber was filled with 500 μl of DM containing ∼1 × 104 cells, and the lower chamber was filled with 500 μl of DM. VEGF (50 ng/ml), a chemoattractant, was added to the DM in the lower chamber. The cells were incubated for 4 h at 37°C in a CO2 incubator, and any cells that remained in the upper chamber after this incubation period were gently removed with Q-tips. Cells that migrated to the lower side of the filter were fixed with 4% PFA, stained with 0.5% crystal violet, and washed to remove excess dye. The filter inserts were then mounted on glass slides, and the cells were counted with a phase-contrast microscope. At least 10 fields at a magnification of ×200 were randomly selected for each chamber filter. Each experiment was repeated three times, with each trial performed three times.
Formation of branching point structures.
Morphogenic differentiation of ECs on type I collagen matrix was assessed by branching point structure formation as described previously with minor modifications (19, 41, 42). Briefly, ECs were detached from dishes with 3 mM EDTA, washed with PBS, suspended in defined medium (EBM-2 medium containing bFGF, VEGF, 1× insulin, transferrin, and selenium [ITS]), and plated on type I collagen matrix. Approximately 0.1 × 106 control ECs (at ∼50% density) or ECs infected with either control nonsilencing shRNA, shRNA p120ctn (shp120ctn) (a), shp120ctn (b), shRNA LPP3 (shLPP3) (a), and shLPP3 (c) retroviral constructs for 8 h, and then fresh medium was added thereafter. After ∼16 h, ECs were plated on polymerized type I collagen gel and incubated at 37°C for 12 h. The unattached ECs were then removed, and a second layer of type I collagen gel was laid over the cells. After 36 h, the medium was removed from the dishes, and the cells were washed once with PBS and photographed under a phase-contrast microscope. For quantification of branching points, at least 10 random fields were examined. Experiments were repeated three times.
Statistical analysis.
Comparisons of group means were performed using analysis of variance. Data are expressed as means ± standard errors of the means (SEMs). P values of <0.05 were considered to be statistically significant.
Digital images and software.
Digital images generated by a Zeiss microscope were saved as TIFF documents and converted to EPS format using Adobe Photoshop 7.0. Autoradiographic films (Western blots) were scanned using a UMAX flatbed scanner, and the images were saved as EPS images. Microsoft Excel bar diagrams were converted into EPS files using Adobe Photoshop 7.0. To assemble multiple images, we used QuarkXpress 7.0 software. Finally, all EPS images were converted into PDFs.
RESULTS
LPP3-induced β-catenin/LEF-1 signaling is cell density dependent.
To test the hypothesis that LPP3 may stimulate β-catenin/LEF-1 signaling, we used both subconfluent ECs (migrating and proliferating) and confluent ECs (contact inhibited). We observed high LEF-1 activity in subconfluent ECs transfected with wild-type LPP3 (see Fig. S1 in the supplemental material). Thereafter, we examined whether LPP3 induced the activation of LEF-1 using retrovirus-driven expression of wild-type LPP3 or LPP3 mutants (Fig. 1A). The LEF-1 luciferase reporter assay was performed with 50% confluent ECs to address the stimulatory effect of LPP3 in subconfluent ECs. Transfection of empty vector (construct a), hLPP1 (control construct b), and hLPP2 (control construct c) had no effect on luciferase activity; however, transfection of either mouse LPP3 (mLpp3) (construct d) or human LPP3 (hLPP3) (construct e) yielded an 8- to 9-fold increase in luciferase activity (Fig. 1B).
FIG. 1.
LPP3 activates β-catenin/LEF-1 signaling and expression of fibronectin through PTEN. (A) pLNCX2 retroviral constructs (constructs a to h). The relative positions of RGD, RGE, RAD, and phosphatase-defective (PD) mutations are shown. Murine Lpp3 (construct d) is the mouse counterpart to human LPP3. Numbers 1 to 6 represent transmembrane segments. The proposed cell binding sequence on the second extracellular loop (II nd extracellular loop) of LPP3 is shown. Three copies of influenza virus-derived hemagglutinin (HA) epitopes (3XHA) were fused N terminally and in frame to the open reading frame of the cDNAs. (B) LEF-1 luciferase activity (mean fold change) in response to expression of the indicated constructs as a function of cell density (50% cell density). (C) Phosphorylation of β-catenin (S33/S37/T41) as measured by WB. p-β-catenin, phosphorylated β-catenin. (D) Equal loading was determined by stripping and reprobing with anti-β-catenin. The position of β-catenin is indicated by a black arrowhead. (E) β-Catenin immunoprecipitates were probed for LEF-1. (F) ELISA of culture medium at (50% cell density) for fibronectin and tissue inhibitor of metalloproteinases 2 (TIMP-2). Experiments were repeated twice, using triplicate wells each time. Data are expressed as means plus SEMs (error bars) (F) or means ± SEMs (error bars) (B). Values that are significantly different are indicated as follows: *, P < 0.05; **, P < 0.01. Constructs a to h are as follows: a, vector; b, human LPP1 (hLPP1); c, hLPP2; d, mouse Lpp3 (mLpp3); e, hLPP3; f, hLPP3-RAD (cell adhesion defective); g, hLPP3-PD (phosphatase defective); h, hLPP3-RAD+PD (double mutant). (G) Anti-FLAG antibody immunoprecipitates prepared from (50% cell density) control ECs (lane 1) or ECs transfected with a FLAG-tagged wild-type (WT) PTEN (lane 2) or FLAG-tagged substrate-trapping PTEN-C124S (phosphatase defective) (lane 3) were analyzed using the indicated antibodies. (H) PTEN stimulates dephosphorylation of β-catenin and increases its stability in subconfluent ECs. ECs at 50% density were transfected with vector alone (control) or PTEN-C124S or increasing concentrations of PTEN-WT (wild-type) cDNA plasmids as indicated. After 24 h, ECs were solubilized, extracted, and centrifuged, and equal amounts of proteins were immunoprecipitated with an anti-β-catenin antibody. All data are representative of at least three separate experiments.
Next, we identified the LPP3 domain responsible for LEF-1 activity by examining the effects of an LPP3-RAD mutant, which is defective for integrin binding (construct f) and a hLPP3-PD mutant, which is defective for phosphatase activity (construct g). Transfection of construct f or g stimulated LEF-1-dependent transcription 3- or 5-fold, respectively (Fig. 1B), indicating that both domains activate LEF-1 activity. In contrast, the LPP3 mutant that lacks both adhesion and lipid phosphatase domains (hLPP3-RAD+PD or construct h) failed to stimulate luciferase activity (Fig. 1B).
To determine the mechanism of activation of LEF-1, ECs were transfected with empty vector (construct a) or hLPP1 (control construct b) or hLPP2 (control construct c) or LPP3 mutant that lacks both adhesion and lipid phosphatase domains (construct h) as described above. We observed no change in basal phosphorylation of β-catenin using any of these constructs (Fig. 1C); however, the expression of mLpp3 (construct d), hLPP3 (construct e), hLPP3-RAD (construct f), or hLPP3-PD (construct g) in ECs decreased β-catenin phosphorylation, suggesting that expression of these LPP3 constructs induced the stabilization of β-catenin (Fig. 1C). Importantly, we also observed interaction between β-catenin and LEF-1 only in ECs expressing construct d, e, f, or g (Fig. 1D and E). Thus, LPP3 functioned by preventing phosphorylation of β-catenin and, in turn, increased β-catenin stability and the β-catenin-LEF-1 binding that is required for LEF-1/T-cell factor (TCF) transcriptional activity (26, 27).
To further test the hypothesis that LPP3 stimulates LEF-1 activation, we determined the expression of the specific LEF-1 target gene, fibronectin (26, 27). Expression of fibronectin is known to mediate EC adhesion, migration, proliferation, and cellular polarity (29-34). Thus, we performed an ELISA to measure the production of fibronectin as well as a non-LEF-1-regulated protein, tissue inhibitor of metalloproteinases 2 (TIMP-2), in supernatants of 50% subconfluent EC cultures. As shown below (Fig. 1F), fibronectin production was unaffected by transfection of construct a, b, c, or h. In contrast, expression of construct d or construct e increased fibronectin concentrations by 5-fold (Fig. 1F). We noted fibronectin secretion in ECs transfected with construct f or construct g, while the TIMP-2 levels were unaltered following transfection of these constructs. Thus, LPP3 activates LEF-1-dependent transcription secondary to stabilization of β-catenin and results in fibronectin production in subconfluent ECs.
Next, we determined whether the transcriptional activity of LEF-1 is regulated by LPP3-mediated nuclear accumulation of dephosphorylated β-catenin. On the basis of the evidence that PTEN, the dual-specificity phosphatase, regulates phosphorylation of β-catenin (36, 37), we examined the possible role of this protein in LPP3-induced stabilization of β-catenin. Subconfluent ECs were transfected with FLAG-tagged wild-type PTEN or FLAG-tagged substrate-trapping PTEN mutant (catalytically inactive PTEN-C124S). Wild-type PTEN failed to coimmunoprecipitate β-catenin (Fig. 1G, lane 2); however, the phosphatase-defective PTEN mutant (PTEN-C124S) precipitated β-catenin (Fig. 1G, lane 3). To determine whether PTEN stimulates dephosphorylation of β-catenin and thereby increases its stability, we next transfected subconfluent ECs with empty vector (control), phosphatase-defective PTEN-C124S, or increasing concentrations of wild-type PTEN and examined β-catenin phosphorylation. We observed that transfection of PTEN resulted in decreased phosphorylation of β-catenin accompanied by increased nuclear accumulation of β-catenin in subconfluent ECs without a change in total cellular β-catenin levels (Fig. 1H, bottom panel). This response did not occur following expression of phosphatase-defective PTEN-C124S. Thus, in subconfluent ECs, PTEN interacted with β-catenin and stimulated its dephosphorylation. Since dephosphorylated β-catenin is stable and translocates to the nucleus to activate LEF-1, our data indicate a critical role for PTEN in the mechanism of activation of β-catenin in subconfluent ECs.
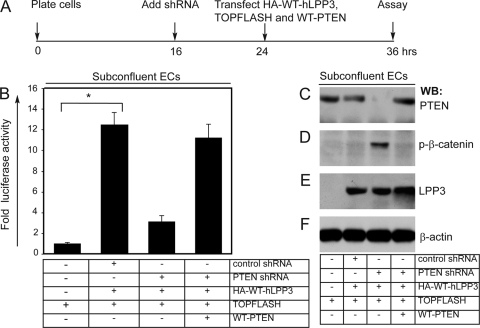
PTEN knockdown affects LPP3-mediated β-catenin/LEF-1 signaling.
Subconfluent ECs were used to examine the impact of PTEN knockdown on LPP3-mediated β-catenin/LEF-1 signaling (Fig. 2). The timeline for plating of cells, infection with shRNA particles, transfection of constructs, and LEF-1 luciferase assays are shown in Fig. 2A. In control subconfluent ECs, the LEF-1 luciferase activity was considered basal (Fig. 2B), while transfection of LPP3 in these cells increased the luciferase activity 12-fold (Fig. 2B). In contrast, PTEN knockdown reduced the LPP3-mediated LEF-1 activation (9-fold), which was also reflected in increased phosphorylation of β-catenin (Fig. 2D). The decreased LEF-1 activity after PTEN knockdown in ECs was restored, however, by ectopic expression of wild-type PTEN, and this effect was accompanied by increased β-catenin stability (Fig. 2D). The efficiency of knockdown (100%), phosphorylation of β-catenin, and expression of LPP3 and PTEN were determined by WB (Fig. 2C to F).
FIG. 2.
PTEN knockdown affects LPP3-mediated β-catenin/LEF-1 signaling. (A) Timeline of the experiment, including plating of cells, infection with lentivirus shRNA, transfection of indicated constructs, and LEF-1 (TOPFLASH) assays. Time is shown in hours. (B) Luciferase (LEF-1) assays of control subconfluent ECs, subconfluent ECs infected with control shRNA or PTEN shRNA lentivirus, and ECs expressing indicated constructs. Data are expressed as means plus SEMs (error bars). *, P < 0.05 versus control ECs. (C to F) The extent of PTEN knockdown, transfection of wild-type PTEN (to rule out off-target effects), and hLPP3 protein levels were assayed by Western blotting (WB). Experiments were carried out at least three times in triplicate.
p120ctn knockdown rescues β-catenin/LEF-1 signaling.
We sought to determine whether p120ctn knockdown restores LPP3-mediated β-catenin/LEF-1 signaling in confluent ECs (timeline of experiments shown in Fig. 3A). In control confluent ECs, the LEF-1 luciferase activity was adjusted to 1-fold (Fig. 3B); however, the knockdown of p120ctn increased LEF-1 luciferase activity by 9-fold (Fig. 3B), and this result was also reflected in an appreciable increase in total β-catenin levels (Fig. 3D). To rule out the possibility of shRNA-mediated off-target effect, HA-tagged wild-type p120ctn was transfected into p120ctn-depleted ECs. This p120-ctn ectopic expression dampened the LEF-1 luciferase activity to 2.0-fold (Fig. 3B). The 100% efficiency of the p120ctn knockdown, LPP3 transfection efficiency, and total β-catenin levels were determined by WB (Fig. 3C to F).
FIG. 3.
p120ctn regulates LPP3-mediated β-catenin/LEF-1 signaling. (A) Timeline of the experiment, including plating of cells, infection with lentivirus shRNA, transfection of indicated constructs, and LEF-1 (TOPFLASH) assays. (B) Luciferase (LEF-1) assays of control confluent ECs, confluent ECs infected with control shRNA or p120ctn shRNA lentivirus, and ECs expressing indicated constructs. Data are expressed as means plus SEMs (error bars). *, P < 0.05 versus control ECs. (C to F) The extent of p120ctn knockdown, transfection of p120ctn (to rule out off-target effects), and hLPP3 protein levels were determined by WB. Experiments were carried out at least three times in triplicate. All blots shown are representative of those obtained in at least three separate experiments.
LPP3 is required for synthesis of fibronectin and EC migration.
We next determined the effects of shRNA-mediated LPP3 gene silencing on fibronectin synthesis, which is known to be regulated by LEF-1 (26, 27). Four shRNA constructs were used for these experiments: shRNA (negative control), shLPP3(a), shLPP3(b), and shLPP3(c) (Fig. 4). Constructs shLPP3(a), shLPP3(b), and shLPP3(c) were used because each of these constructs targets a distinct region of the human LPP3 gene. The efficacy of each construct was tested in mouse NIH 3T3 fibroblasts, and the constructs showed no evidence of toxicity (data not shown). Expression of shRNA or shLPP3(a) did not affect the expression or localization of p120ctn, fibronectin, or the non-LEF-1-regulated control protein CD31 (PECAM-1) (Fig. 4A to C). In contrast, expression of shLPP3(b) and shLPP3(c) decreased the synthesis of fibronectin without affecting expression of CD31 (Fig. 4D to F and G to I, respectively). Knockdown of LPP3 was confirmed by WB analysis (Fig. 4J to Q). In addition, we did not observe any changes in morphology of the ECs (Fig. 4R to T). We observed increased β-catenin phosphorylation that was accompanied by decreased β-catenin levels (Fig. 4O and P). Moreover, we found phosphorylation of Ser-9 of GSK-3 (Fig. 4Q).
FIG. 4.
LPP3 silencing inhibits EC migration and cell-cell adhesion. (A to I) Fluorescence microscopy images of ECs showing the effect of infection with nonsilencing control shRNA (control sh) (A to C) or shLPP3(b) (D to F) or shLPP3(c) (G to I) construct on expression of p120ctn (A, D, and G), fibronectin (B, E, and H), and control protein, CD31 (C, F, and I). The cells were counterstained with DAPI alone (A, D, and G) or tetramethyl rhodamine isothiocyanate (TRITC)-phalloidin and DAPI (B, C, E, F, H, and I). Magnification, ×400. Bars, 200 μm. (J to Q) Cell extracts (total lysates) prepared from ECs infected as described above for panels A to I were analyzed by WB with the indicated antibodies. Black arrowheads indicate nonspecific signals. (R to T) Bright-field images of ECs infected with the indicated constructs. Magnification, ×200. Bar, 100 μm. All data are representative of at least three separate experiments. (U) ECs at 50 to 60% density were infected with nonsilencing control shRNA (control-sh) and shLPP3(c) constructs as described in Materials and Methods. Scratched EC monolayers were incubated in DM containing VEGF. The effect of addition of exogenous fibronectin (Fn) on EC monolayer repair is shown in the rightmost panels. (V) Quantification of chemotactic migration of ECs expressing nonsilencing control-sh and LPP3-silencing shRNAs in the absence and presence of VEGF. Data are expressed as means ± SEMs. *, P < 0.05 versus control-sh by unpaired two-tailed Student's t test.
To determine whether reduced LPP3 expression has a functional effect on EC migration, we used a wound healing assay in ECs infected with nonsilencing control or shLPP3. The repopulation of the wounded area was monitored at 0, 4, and 8 h after scratching the monolayer (see Materials and Methods). ECs expressing the nonsilencing control shRNA migrated through the wound region within 8 h (Fig. 4U, left panel). In contrast, ECs expressing shLPP3(c) showed no cell migration (Fig. 4U, middle panels). Next, we used the Transwell Boyden chamber cell migration assay following LPP3 knockdown. As shown in Fig. 4V, in the absence of VEGF, cells receiving control shRNA showed 4% migration compared with <1% migration of cells expressing shLPP3 (P < 0.05), whereas in the presence of VEGF, control cells showed 12% migration within 8 h compared with 4% migration for ECs expressing shLPP3 (P < 0.05). Thus, knockdown of LPP3 also impaired the ability of ECs to migrate.
To determine whether impaired cell migration resulted from reduced fibronectin synthesis, we added increasing concentrations of fibronectin exogenously (1.25, 2.4, 5.0, and 7.5 μg/ml). Addition of exogenous fibronectin enhanced wound closure (Fig. 4U, right panels) and cell migration in a dose-dependent manner (Fig. 4V). Maximum induction of migration was seen at 5.0 μg/ml fibronectin. Collectively, these results demonstrate that depletion of LPP3 decreases fibronectin synthesis and thereby impairs both EC migration and wound healing.
Reexpression of β-catenin reverses the effect of loss of LPP3.
To test the hypothesis that β-catenin is a key mediator of LPP3 signaling, we investigated the effects of shRNA-mediated silencing of the LPP3 gene on the formation of EC branching point structures, which are an in vitro correlate of angiogenesis (8, 17, 24, 41, 42). ECs were left untreated (control ECs) or transduced with control shRNA or shLPP3(c) constructs. To examine whether β-catenin or p120ctn reverses the loss of LPP3, LPP3-depleted ECs were either infected with retrovirus encoding full-length human β-catenin or full-length human p120ctn cDNA. Transduced cells were then subjected to the branching point structure assay using three-dimensional type I collagen as the supporting matrix protein (Fig. 5A to G). This assay was performed with defined medium containing bFGF, VEGF, and ITS, but no serum. Representative images of branching point structures are shown in Fig. 5B to G. The branching point structures in control ECs were similar to ECs transduced with nonsilencing control shRNA. We observed a significant decrease in branching points in LPP3-depleted ECs, and ECs infected with full-length p120ctn did not reverse the loss of LPP3 (Fig. 5A and E). Strikingly, the formation of branching point structures was restored in ECs infected with retrovirus encoding full-length β-catenin (Fig. 5A, F, and G). Thus, the loss of LPP3 is reversed by retroviral β-catenin expression.
FIG. 5.
Expression of β-catenin restores the effects of loss of LPP3. (A) Quantification of branching point structures was performed as described in Materials and Methods. Data are expressed as means plus SEMs. *, P < 0.05 compared with untransfected ECs. (B to G) Representative images of branching point structures. Black arrows indicate productive branching points, while white arrows indicate unproductive branching points. Results are representative of at least three separate experiments. Magnification, ×200. Bar, 200 μm. (H) Distribution of LPP3 protein in control ECs was analyzed by immunostaining with an anti-LPP3 Ab. (I) Subconfluent ECs were subjected to shRNA-mediated LPP3 knockdown. These cells were then either transfected with HA-tagged p120ctn or HA-tagged β-catenin constructs, and the effects of reexpression were analyzed by immunostaining with anti-HA (red, p120ctn) (J), anti-VE-cadherin (green) (K), anti-HA (red, β-catenin) (L), and p120ctn (green) (M), and nuclear stain DAPI (blue). Magnification, ×400. Bar, 150 μm. (N) Protein complexes were prepared from control ECs, LPP3-knockdown ECs, or LPP3-knockdown ECs transfected with indicated constructs and then analyzed by WB of whole-cell lysates with the indicated Abs. Results are representative of at least three separate experiments.
To confirm the effects of β-catenin expression on the phenotype caused by the loss of LPP3, we used ECs in which LPP3 had been knocked down (LPP3-knockdown ECs) in which p120ctn or β-catenin was ectopically reexpressed (Fig. 5H to N). On day 1 of this experiment, ECs were seeded at a low density. On day 2, the cells were either left untreated or subjected to shRNA-mediated LPP3 knockdown for 8 h. On day 3, ECs at 60% density remained untreated or were transfected with vector alone or HA-tagged p120ctn or HA-tagged β-catenin. Approximately 8 h after transfection, ECs were allowed to recover overnight in complete medium. On day 4, the cells were either plated onto coverslips for staining or solubilized in cell extraction buffer for Western analysis. As shown in Fig. 5H, LPP3 remained diffusely distributed in control ECs. Following LPP3 knockdown, expression of LPP3, β-catenin, VE-cadherin, and p120ctn proteins were reduced (Fig. 5I, K, and N). Staining of ECs with anti-HA (Fig. 5J) or anti-VE-cadherin (Fig. 5K) showed that reexpression of p120ctn failed to induce a confluent monolayer or defined AJ adhesion structures. Instead, these cells were elongated (Fig. 5J and K). Interestingly, reexpression of β-catenin induced the formation of well-defined AJs as determined by the intense p120ctn staining (Fig. 5L and M). Reexpression of β-catenin induced expression of p120ctn, VE-cadherin, and fibronectin (Fig. 5N), indicating that β-catenin reexpression reverses alterations caused by the loss of LPP3. Alternatively, these effects may be secondary to protein stabilization by junction assembly.
Depletion of LPP3 impairs EC-EC adhesion.
Next, to test the hypothesis that EC-EC adhesion mediated by VE-cadherin is regulated by LPP3 signaling, we investigated the effects of shRNA-mediated depletion of LPP3 on the formation of EC-EC adhesion by cell-cell adhesion assay as described previously (18, 19). ECs were left untreated (control ECs) or infected with control shRNA or shLPP3(c) viral particles. To examine whether p120ctn or β-catenin restores the loss of LPP3, LPP3-depleted ECs were either infected with retrovirus encoding full-length human HA-β-catenin or full-length human HA-p120ctn cDNAs as described above. These cells were labeled with red and green fluorescent chromophores and then subjected to the cell-cell adhesion assay (Fig. 6A to G). Quantification of cell aggregates is shown in Fig. 6B. Representative images of cell aggregates are shown in Fig. 6C to G. The cell aggregates in control ECs were similar to ECs infected with nonsilencing control shRNA (Fig. 6B). In LPP3-depleted ECs and transfection with HA-p120ctn did not restore the effect of loss of LPP3 (Fig. 6B, E, and F). Interestingly, the formation of cell aggregates was restored in ECs expressing β-catenin (Fig. 6B and G). The efficiency of LPP3 depletion and reexpression of constructs were determined by Western blotting (Fig. 6H). Importantly, reexpression of β-catenin induced expression of p120ctn and VE-cadherin proteins (Fig. 6H). Thus, reexpression of β-catenin restores the effect of loss of LPP3.
FIG. 6.
Depletion of LPP3 inhibits cell-cell adhesion. (A) Timeline of the experiments, including infection with lentivirus shRNA, transfection of indicated constructs, and cell-cell adhesion assays. (B) ECs (106) were labeled either with DiO (green) and Dil (red) fluorescent chromophores and subjected to cell aggregate (cell swirling) assay at 0 h. The cell aggregates (large and small) were counted at 12 h (n = 3) (*, P < 0.05 versus control ECs). (C) Photomicrograph of a mixture of control ECs labeled with DiO (green) and Dil (red) fluorescent dyes at 0 h. (D) Productive cell aggregates (yellow) are indicated by arrows. (E) Depletion of LPP3 inhibited cell-cell adhesion. (F) Reexpression of p120ctn failed to restore the loss of LPP3. (G) Reexpression of β-catenin restores the loss of LPP3. Magnification for panels C to G, ×200. Bar, 50 μm. The images of cell aggregates (cell-cell adhesion) appear out of focus. (H) Total cell extracts were subjected to Western blot analysis with the indicated antibodies (GAPDH, glyceraldehyde-3-phosphate dehydrogenase). All blots shown are representative of at least three separate experiments.
C-terminal LPP3 binding to p120ctn occurs in confluent ECs.
We sought to determine the relationship between LPP3 and p120ctn in subconfluent and confluent ECs. Thus, hdMVECs were sparsely seeded and cultured for up to 96 h in complete medium containing bFGF and VEGF. Immunoprecipitation of lysates using anti-LPP3 Ab showed that LPP3 associates with p120ctn in confluent ECs (i.e., the cells grown at high density for 72 and 96 h) and not in subconfluent cells (Fig. 7A and B). In a reciprocal experiment, p120ctn immunoprecipitates from subconfluent cells did not contain detectable amounts of LPP3 after 0, 24, or 48 h of growth (Fig. 7C and D), whereas LPP3 was detected in confluent ECs after 72 and 96 h of growth (Fig. 7D).
FIG. 7.
Endogenous LPP3 associates with p120ctn. Extracts prepared from ECs grown for 24 (25% density), 48 (50%), 72 (75%), and 96 (100%) h were subjected to immunoprecipitation (IP) and WB with the indicated antibodies (described in Materials and Methods). (A, C, and E) Anti-p120ctn antibody detected two major isoforms of p120ctn polypeptides. (B, D, and F) Anti-LPP3-C-cyto antibody detected a mature, 42-kDa species (immature form, ∼35 kDa). (G and H) Extracts from confluent ECs were immunodepleted with anti-VE-cadherin MAb and immunoprecipitated with the indicated antibodies. Immunoprecipitates were probed with anti-p120ctn MAb (G) or anti-LPP3-C-cyto pAb (H). The positions of molecular mass markers (in kilodaltons) are shown to the left of the gels. The black arrows to the right of the gels in panels B and F point to a nonspecific signal. All blots shown are representative of those obtained in at least three separate experiments. (I and J) Representative confocal microscopic assessment of LPP3 and p120ctn localization. Endothelial cells at 25% (I) and 100% (J) densities were fixed and stained with anti-p120ctn (FITC [green]) MAb, DAPI (blue), and anti-LPP3-RGD (Texas Red) pAbs. At low cell density, endogenous LPP3 (red) was diffusely distributed in the cytosol as well as plasma membrane. Increased colocalization of LPP3 with p120ctn occurred at higher cell densities. Meanwhile, at a low cell density, p120ctn (green) appeared predominantly nuclear (white arrowheads). At a higher cell density, p120ctn appeared in the nucleus, cytosol, and plasma membrane. Arrows indicate colocalization. Images shown are representative of at least three separate experiments. Magnification for panels I and J, ×400. Bar, 150 μm.
To address whether LPP3 binding to p120ctn requires VE-cadherin, cell extracts prepared from confluent ECs were subjected to immunoprecipitation with anti-VE-cadherin, anti-LPP3, and anti-p120ctn antibodies. All of these antibodies coprecipitated p120ctn (Fig. 7E and F). Anti-LPP3 and anti-p120ctn antibodies coprecipitated LPP3 in a reciprocal manner, whereas anti-VE-cadherin antibody did not (Fig. 7E and F). To further exclude the possibility that the LPP3-p120ctn interaction was mediated by VE-cadherin, cell extracts prepared from confluent ECs were subjected to immunodepletion with anti-VE-cadherin MAb prior to immunoprecipitation with anti-LPP3, anti-p120ctn, or anti-mouse IgG (control) antibody. Anti-LPP3 and anti-p120ctn antibodies coprecipitated p120ctn and LPP3 in a reciprocal manner (Fig. 7G and H); however, anti-mouse control IgG did not coprecipitate either of these proteins. These data indicate that the interaction between LPP3 and p120ctn does not require VE-cadherin.
Next, we used confocal imaging to examine the distribution of LPP3 and p120ctn proteins in ECs after 24 and 72 h of growth using an anti-LPP3-RGD polyclonal antibody (Fig. 7I and J). After the ECs were allowed to grow for 24 h, we observed intense staining of p120ctn in the nucleus; however, at 72 h, when the ECs were confluent, an appreciable level of p120ctn recruited to the inner face at levels equivalent to those of AJs (Fig. 7I and J).
We confirmed the LPP3-p120ctn interaction described above in confluent ECs using ectopically expressed LPP3 constructs (Fig. 8A). The construct encoding HA-tagged wild-type human LPP3 (pLNCX2-HA-WT-hLPP3) was stably transfected into HEK293 cells, which normally do not express LPP3. β-Catenin, γ-catenin, and p120ctn were endogenously expressed by these cells. As shown in Fig. 8B to D, IP/WB analysis of extracts prepared from HA-LPP3-expressing cells showed that HA-LPP3 coimmunoprecipitated with p120ctn; however, no HA-LPP3 was detected in β-catenin, γ-catenin, or mouse IgG immunoprecipitates (Fig. 8E to G). To determine whether HA-LPP3 was targeted to the HEK293 cell membrane where it colocalizes with p120ctn, fluorescent labeling of HA and p120ctn was performed. In accord with the IP/WB results above, HA-LPP3 colocalized with p120ctn in HEK293 cells (Fig. 8H to J).
FIG. 8.
Interaction and colocalization of LPP3 with p120ctn. (A) An illustration of the pLNCX2-driven HA-tagged pLNCX2-LPP3 wild-type retroviral construct. The regions shaded gray and numbered 1 to 6 represent the six transmembrane segments. C-term, C terminus. (B to G) Extracts from confluent HEK293 cells infected with HA-tagged pLNCX2-LPP3 were subjected to IP with the indicated antibodies and analyzed by WB with anti-p120ctn MAb (B), anti-β-catenin MAb (C), anti-γ-catenin MAb (D), and anti-HA pAb (E to G). Anti-HA pAbs detected N-glycosylated species (∼38-kDa and 40- to 42-kDa species; black arrows to the right of the gels) as well as immature LPP3 polypeptides (∼35 kDa; white asterisks on the bands on the gels). All blots shown are representative of at least three separate experiments. (H to J) Immunofluorescence labeling of HEK293 cells for HA (red)(H) and p120ctn (green) (I). Colocalized LPP3 and p120ctn appear yellow (white arrows) (J). Images shown are representative of at least three separate experiments. Magnification for panels H to J, ×200. Bar, 40 μm.
To identify the region of LPP3 (Fig. 9A) that mediates the interaction with p120ctn, we prepared recombinant proteins by fusing the N- or C-terminal region of LPP3 to GST (Fig. 9B). SDS-PAGE analysis showed that the proteins ran at the expected sizes with BSA (control) migrating at 68 kDa, GST alone at 29 kDa (Fig. 9C, lanes 2 and 3), GST-C-cyto (C-terminal fusion protein) at 32 kDa (Fig. 9C, lanes 4 and 5), and GST-N-cyto (N-terminal fusion protein) at 31 kDa (Fig. 9C, lanes 6 and 7). Far-Western analysis using GST fusion proteins as bait showed that the GST-LPP3-C-cyto antibody bound to the 120-kDa p120ctn but not to VE-cadherin, β-catenin, γ-catenin, or β1-integrin (Fig. 9D). The identity of each protein, including p120ctn, was confirmed by reprobing membranes with antibodies against VE-cadherin, p120ctn, β-catenin, γ-catenin, and β1-integrin (Fig. 9E to I). The VE-cadherin-associated p120ctn did not interact with recombinant LPP3 (Fig. 9D and E). In addition, we carried out pulldown assays with GST-LPP3 fusion proteins. GST-C-cyto (GST-CC) coprecipitated p120ctn (Fig. 9J), while no p120ctn was detected in the GST-N-cyto (GST-NC) precipitates (Fig. 9K). The membranes were also probed for the presence of the GST fusion protein (Fig. 9L and M). Taken together, these data show that the C-terminal domain of LPP3 is required for binding with p120ctn and that the specific binding of LPP3 to p120ctn occurs only in confluent ECs.
FIG. 9.
Interaction of the cytoplasmic domains of LPP3 with p120ctn. (A) Sequences of the N- and C-terminal cytoplasmic (cyto) domains of LPP3. (B) Illustrations of the GST-N-cyto (GST-NC) and GST-C-cyto (GST-CC) fusion proteins. (C) Purity of GST (∼29 kDa), GST-CC (∼32 kDa), and GST-NC (∼31 kDa) was verified by SDS-PAGE and Coomassie blue staining. Faster-migrating species represent GST alone and fusion protein degradation products. (D to I) EC extracts were subjected to IP using the indicated antibodies, and the resulting immunoprecipitates were subjected to far-Western analysis by probing the membranes with GST-LPP3-C-cyto and then anti-GST MAb. Individual lanes from the blot in panel D were excised and probed with anti-VE-cadherin MAb (E), anti-p120ctn MAb (F), anti-β-catenin MAb (G), anti-γ-catenin MAb (H), or anti-β1-integrin pAb (I). (J to M) EC extracts were subjected to GST pulldown with the indicated fusion proteins. Precipitates were analyzed by WB with anti-p120ctn (MAb) (J and K) and anti-GST MAb (L and M). All blots shown are representative of at least three separate experiments.
The C-terminal domain of LPP3 regulates the expression of p120ctn and VE-cadherin as well as formation of branching point structures.
To test the hypothesis that cell surface-associated LPP3 interacts with p120ctn, we used ECs transiently expressing pLNCX2-HA-WT-hLPP3 or mutant constructs lacking the N-terminal cytoplasmic domain (pLNCX2-HA-N-Δ-cyto-hLPP3) or C-terminal cytoplasmic domain (pLNCX2-HA-C-Δ-cyto-hLPP3) (Fig. 10A). We performed cell surface biotinylation, followed by coimmunoprecipitation (Fig. 10B and C). The anti-HA antibody coprecipitated p120ctn in cells expressing HA-WT-hLPP3 (lane 1) and HA-N-Δ-cyto-hLPP3 (lane 2) but not in cells expressing HA-C-Δ-cyto-hLPP3 (lane 3; Fig. 10B and C). Wild-type and mutant LPP3 produced smears in the blots in response to streptavidin-horseradish peroxidase (HRP) reaction (Fig. 10C). These smears, which are likely due to N glycosylation of LPP3 (41), suggest that LPP3 is exported to the cell surface. Although HA-C-Δ-cyto-hLPP3 was efficiently exported to the cell surface, this fusion protein failed to colocalize with p120ctn (data not shown). Thus, these data demonstrate that cell surface-associated LPP3 interacts with p120ctn and that the C terminus of LPP3 is required for this interaction.
FIG. 10.
The C terminus of LPP3 is required for p120ctn binding and formation of branching point structures. (A) Schematic of LPP3 retroviral constructs pLNCX2-HA-WT-hLPP3 (wild type), pLNCX2-HA-N-Δ-hLPP3 (lacking N-terminal cytoplasmic segment), and pLNCX2-HA-C-Δ-hLPP3 (lacking C-terminal cytoplasmic segment). The second extracellular loop (II nd extracellular loop) and transmembrane segments (segments 1 to 6) are shown. (B and C) ECs expressing pLNCX2-HA-WT-hLPP3(1), pLNCX2-HA-N-Δ-hLPP3(2), and pLNCX2-HA-C-Δ-hLPP3(3) were detached with 3 mM EDTA, washed, and subjected to cell surface biotinylation as described in Materials and Methods. Clarified cell extracts were immunoprecipitated with anti-HA antibody and analyzed by immunoblotting with anti-p120ctn MAb (B) and streptavidin-HRP (C). (D to J) To deplete endogenous LPP3, ECs at 50% density were infected with LPP3sh(c) (L) for 6 h and allowed to recover. After 12 h, ECs were infected with the indicated constructs (constructs 1 to 3) for 6 h, allowed to recover for 4 h, and then subjected to branching point, cell proliferation, and cell migration assays. (D and G to J) Branching point assay in three-dimensional type I collagen matrix. (D) Quantification of branching points. After 24 h, the number of branching points were scored. Data are expressed as percentages of branching points (n = 8). *, P < 0.05 versus L. (G to J) Representative images of branching point formation in response to reexpression of indicated constructs (constructs 1 to 3). Experiments were repeated three times using triplicate wells each time. The black arrows point to branching points. Magnification, ×200. Bar, 200 μm. (E) Percent bromodeoxyuridine (BrdU) incorporation of ECs in response to reexpression of indicated constructs (constructs 1 to 3). The cells were cultured on coverslips coated with 0.2% gelatin for 16 h, fixed, and stained. BrdU-positive cells were enumerated and expressed as percent cell proliferation (n = 8). *, P < 0.05 versus L. (F) Percent cell migration in response to reexpression of the indicated constructs (constructs 1 to 3). P < 0.05 versus L. (K to M) Whole-cell lysates from cells expressing constructs 1 to 3 were subjected to WB analyses with the antibodies indicated. All blots shown are representative of at least three separate experiments.
To examine the function of the interaction between LPP3 and p120ctn, we first depleted endogenous LPP3 in ECs using LPP3sh(c). ECs were then infected with HA-WT-hLPP3 (wild type), HA-N-Δ-cyto-hLPP3 (a mutant lacking the N-terminal cytoplasmic domain), or HA-C-Δ-cyto-hLPP3 (a mutant lacking the C-terminal cytoplasmic domain) construct and subjected to branching point, cell proliferation, and migration assays (Fig. 10D to F). Silencing with LPP3sh(c) decreased the number of branching points to 4% (Fig. 10D). Reexpression of HA-WT-hLPP3 and HA-N-Δ-cyto-hLPP3 restored this value to 17% and 14%, respectively. In contrast, reexpression of HA-C-Δ-cyto-hLPP3 induced a minimal increase in branching points (7%; Fig. 10D [3 branching points]). Interestingly, reexpression of HA-WT-hLPP3, HA-N-Δ-cyto-hLPP3, and HA-C-Δ-cyto-hLPP3 caused an increase in both cell proliferation (Fig. 10E) and cell migration (Fig. 10F). Representative images of branching point structures are shown in Fig. 10G to J.
Next, we prepared total cell extracts and measured p120ctn and VE-cadherin expression (Fig. 10K to M). We observed increased expression of p120ctn and VE-cadherin following reexpression of HA-WT-hLPP3 (wild type) and HA-N-Δ-cyto-hLPP3 (a mutant lacking the N-terminal cytoplasmic domain), but reexpression of HA-C-Δ-cyto-hLPP3 (a mutant lacking the C-terminal cytoplasmic domain) had no effect (Fig. 10K and L). Grb-2 protein expression was used as a control for protein loading (Fig. 10M).
DISCUSSION
Here, we demonstrated a critical role for LPP3-induced β-catenin/LEF-1 signaling in EC migration, cell-cell adhesion, and formation of branching point structures. In subconfluent ECs, LPP3 induced activation of β-catenin/LEF-1 signaling, resulting in the synthesis of the LEF-1 target fibronectin (26, 27) and VE-cadherin. In confluent ECs, however, LPP3 remained bound to p120ctn, which reduced the ability of LPP3 to stimulate β-catenin/LEF-1 signaling (Fig. 11). Thus, LPP3-induced β-catenin/LEF-1 signaling is a critical pathway involved in the angiogenic activities of ECs.
FIG. 11.
A model for LPP3-regulated β-catenin signaling in subconfluent and confluent ECs. In subconfluent ECs, LPP3 protects degradation of β-catenin in a PTEN-dependent manner. This event induces stabilization and translocation of β-catenin to the nucleus, where β-catenin interacts with LEF-1/TCF and displaces transcriptional repressors Groucho/transducin-like Enhancer of split (TLE) to activate transcription of Wnt target genes (e.g., fibronectin). In confluent ECs, excess β-catenin is phosphorylated, and the phosphorylated β-catenin is then subjected to proteosomal degradation. Consequently, p120ctn interacts with LPP3 at the plasma membrane and contributes to EC-EC adhesion and AJ formation, thereby limiting the extent of EC migration.
Since LPP3 regulates the Wnt signaling pathway (11), we posited that LPP3-induced activation of β-catenin may be important in activating the TCF/LEF-1 transcription machinery and may thus contribute to LPP3-mediated EC migration, cell-cell adhesion, and formation of branching point structures. To test this hypothesis, we examined LPP3-induced activation of β-catenin-dependent LEF-1 transcription (Fig. 1). In confluent ECs, LPP3-dependent β-catenin/LEF-1 transcription activity was not detectable (see Fig. S1 in the supplemental material); however, in subconfluent ECs, we observed marked LEF-1 activity (Fig. 1). Transfection of LPP3 in subconfluent ECs also stimulated the interaction of β-catenin with LEF-1, resulting in the production of the LEF-1 target fibronectin, a key molecule that is known to mediate EC migration and angiogenesis (10, 26, 27). In addition, both phosphatase-defective or adhesion-defective LPP3 mutants are capable of stimulating LEF-1 activity. In contrast, an LPP3 mutant carrying both mutations (PD plus RAD; acts as a dominant-negative mutant) failed to stimulate LEF-1. This result is attributable to the ability of the double mutant to titrate out endogenous LPP3. We were unable, however, to determine the precise biochemical mechanism(s) by which the phosphatase-defective LPP3 stimulated dephosphorylation of β-catenin. Nevertheless, our data show that LPP3 contains two functional domains: a lipid phosphatase domain and a cell binding domain. Moreover, these two domains function independently to stimulate LEF-1; however, both domains are required for optimal stimulation of LEF-1 (Fig. 1B). We also identified a role for the phosphatase PTEN in the stimulation of β-catenin dephosphorylation, which is a mechanism responsible for LPP3-induced β-catenin/LEF-1 signaling (Fig. 1G, H, and 2). Together, these data show that LPP3 stabilizes β-catenin in subconfluent ECs and thereby promotes LEF-1 transcriptional activity. Interestingly, in p120ctn-depleted confluent ECs, β-catenin/LEF-1 signaling was restored (Fig. 3). These data indicate that LPP3-induced β-catenin/LEF-1 signaling is cell density dependent specifically when LPP3 is not localized to the inner face of the plasma membrane at the level of AJs and not bound to p120ctn. The inability of LPP3 to stimulate β-catenin/LEF-1 signaling in confluent ECs may also be partly due to the contact inhibition phenomenon.
Angiogenic activities of ECs require growth factor stimulation, EC migration, cell-cell adhesion, and the formation of branching point structures (3, 5, 6, 8, 29, 33, 34, 37-39, 41, 42). We observed that depletion of endogenous LPP3 resulted in impairment of these events, suggesting an important role for LPP3 in angiogenesis (Fig. 4). LPP3 knockdown decreased total β-catenin, p120ctn, and VE-cadherin levels (Fig. 4, 5, and 6). Reexpression of β-catenin in LPP3-depleted cells restored the EC's ability to migrate, form AJ structures, and induce cell-cell adhesion, while p120ctn reexpression did not reverse the loss of LPP3. These results indicate the importance of β-catenin as a downstream mediator of LPP3 signaling (Fig. 5 and 6). Our current model (Fig. 11), therefore, predicts that LPP3 in the confluent EC monolayer is a negative regulator of β-catenin/LEF-1-activated transcription and that loss of EC confluence (e.g., wound) induces LPP3 activation of the β-catenin/LEF-1 pathway. This mechanism mediates de novo synthesis of LEF-1-induced protein fibronectin that contributes to negative-feedback regulation of EC migration and thus reestablishes the confluent monolayer (Fig. 4). Thus, our studies add to the increasing recognition that LPP3 plays a key role in β-catenin/LEF-1 signaling, which regulates diverse cellular responses, including cell migration, cell-cell adhesion, and the formation of branching point structures.
Since reexpression of β-catenin in LPP3-depleted ECs induced branching point structures (in rescue experiment) and cell-cell adhesion, we propose that β-catenin acts as a downstream mediator of LPP3 signaling. Whereas overabundance and degradation of β-catenin are regulated by its phosphorylation status, knockdown of LPP3 in sparse ECs decreased LEF-1 stimulation, which we attributed to reduced β-catenin stabilization. Our initial experiments indicated that silencing of either the LPP3 or p120ctn gene reduced the formation of branching point structures of ECs. We attributed this defect to the loss of p120ctn and VE-cadherin, which are two essential components of AJs. The loss of VE-cadherin from AJs is known to affect AJ stability and angiogenesis (40, 43). Subsequently, the knockdown of the LPP3 gene resulted in altered angiogenic activities of ECs, such as EC migration, cell-cell adhesion, and branching point structure formation. These data suggest that LPP3 may regulate many aspects of angiogenesis by stabilizing β-catenin function.
Interestingly, the localization of LPP3 is cell density dependent. In subconfluent ECs, LPP3 was mostly distributed in the cytosol, whereas in confluent ECs, LPP3 interacted with a fraction of p120ctn at the plasma membrane. We also identified and characterized a novel interaction between LPP3 and p120ctn proteins (Fig. 7 to 9; see Fig. S2 in the supplemental material). This interaction did not require VE-cadherin. Since LPP3-Δ-C-cyto did not colocalize with p120ctn and reexpression of LPP3-Δ-C-cyto in LPP3-knockdown ECs failed to induce branching point structures (Fig. 10) but induced cell proliferation, our data suggest that the C-terminal segment of LPP3 is required for the formation of branching point structures.
p120ctn binds the transcriptional repressor protein Kaiso (7, 30, 32). The interaction between p120ctn and Kaiso has been suggested to relieve Kaiso-mediated transcriptional repression (7, 30, 32). Whether the interaction between LPP3 and p120ctn may also release Kaiso from the cytoplasmic compartment, allowing this protein to translocate to the nucleus to repress genes, including Wnt target(s), would be of intrinsic value. Thus, investigation of possible cross talk between LPP3 and the Kaiso pathway will require further investigation. In summary, our results introduce the novel concept that LPP3 regulates PTEN-mediated β-catenin/LEF-1 transcriptional activity in subconfluent ECs, whereas LPP3 function in confluent ECs is blocked by binding to p120ctn to limit the extent of cell migration. Thus, our results demonstrate the ability of LPP3 to be activated in the subconfluent state and highlight the potentially important role of LPP3 in regulating endothelial homeostasis.
Supplementary Material
Acknowledgments
We thank Elizabetta Dejana and Tracey A. Dugan for providing helpful comments and Rodney Bowling, Jr., for generating LPP3 retroviral constructs. Excellent technical support was provided by Choun Mock and Sakina Petiwala.
This study was supported by National Institutes of Health grant (HL079356) to K.K.W.
Footnotes
Published ahead of print on 1 February 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adams, J. C., and F. M. Watt. 1993. Regulation of development and differentiation by the extracellular matrix. Development 117:1183-1198. [DOI] [PubMed] [Google Scholar]
- 2.Bazzoni, G., and E. Dejana. 2004. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 84:869-901. [DOI] [PubMed] [Google Scholar]
- 3.Bohnsack, B. L., and K. K. Hirschi. 2004. Red light, green light: signals that control endothelial cell proliferation during embryonic vascular development. Cell Cycle 3:1506-1511. [DOI] [PubMed] [Google Scholar]
- 4.Brindley, D. N., and C. Pilquil. 2009. Lipid phosphate phosphatases and signaling. J. Lipid Res. 50(Suppl.):S225-S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett, C., and K. Howard. 2003. Fly and mammalian lipid phosphate phosphatase isoforms differ in activity both in vitro and in vivo. EMBO Rep. 4:793-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet, P. 2003. Angiogenesis in health and disease. Nat. Med. 9:653-660. [DOI] [PubMed] [Google Scholar]
- 7.Daniel, J. M., and A. B. Reynolds. 1999. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol. Cell. Biol. 19:3614-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, G. E., and D. R. Senger. 2005. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97:1093-1107. [DOI] [PubMed] [Google Scholar]
- 9.Davis, M. A., R. C. Ireton, and A. B. Reynolds. 2003. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 163:525-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Langhe, S. P., F. G. Sala, P. M. Del Moral, T. J. Fairbanks, K. M. Yamada, D. Warburton, R. C. Burns, and S. Bellusci. 2005. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev. Biol. 277:316-331. [DOI] [PubMed] [Google Scholar]
- 11.Escalante-Alcalde, D., L. Hernandez, H. Le Stunff, R. Maeda, H. S. Lee, Jr-Gang-Cheng, V. A. Sciorra, I. Daar, S. Spiegel, A. J. Morris, and C. L. Stewart. 2003. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development 130:4623-4637. [DOI] [PubMed] [Google Scholar]
- 12.Folkman, J., and P. A. D'Amore. 1996. Blood vessel formation: what is its molecular basis? Cell 87:1153-1155. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin, A. M., and P. A. D'Amore. 2002. Wnt signaling in the vasculature. Angiogenesis 5:1-9. [DOI] [PubMed] [Google Scholar]
- 14.Gory-Faure, S., M. H. Prandini, H. Pointu, V. Roullot, I. Pignot-Paintrand, M. Vernet, and P. Huber. 1999. Role of vascular endothelial-cadherin in vascular morphogenesis. Development 126:2093-2102. [DOI] [PubMed] [Google Scholar]
- 15.Grosheva, I., M. Shtutman, M. Elbaum, and A. D. Bershadsky. 2001. p120catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114:695-707. [DOI] [PubMed] [Google Scholar]
- 16.Hoppler, S., and C. L. Kavanagh. 2007. Wnt signalling: variety at the core. J. Cell Sci. 120:385-393. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz, A., and M. Simons. 2008. Branching morphogenesis. Circ. Res. 103:784-795. [DOI] [PubMed] [Google Scholar]
- 18.Humtsoe, J. O., R. A. Bowling, Jr., S. Feng, and K. K. Wary. 2005. Murine lipid phosphate phosphohydrolase-3 acts as a cell-associated integrin ligand. Biochem. Biophys. Res. Commun. 335:906-919. [DOI] [PubMed] [Google Scholar]
- 19.Humtsoe, J. O., S. Feng, G. D. Thakker, J. Yang, J. Hong, and K. K. Wary. 2003. Regulation of cell-cell interactions by phosphatidic acid phosphatase 2b/VCIP. EMBO J. 22:1539-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes, R. O. 2007. Cell-matrix adhesion in vascular development. J. Thromb. Haemost. 5:32-40. [DOI] [PubMed] [Google Scholar]
- 21.Ichii, T., and M. Takeichi. 2007. p120-catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes Cells 12:827-839. [DOI] [PubMed] [Google Scholar]
- 22.Jia, Y. J., M. Kai, I. Wada, F. Sakane, and H. Kanoh. 2003. Differential localization of lipid phosphate phosphatases 1 and 3 to cell surface subdomains in polarized MDCK cells. FEBS Lett. 552:240-246. [DOI] [PubMed] [Google Scholar]
- 23.Kai, M., I. Wada, S. Imai, F. Sakane, and H. Kanoh. 1996. Identification and cDNA cloning of 35-kD phosphatidic acid phosphatase (type 2) bound to plasma membranes. Polymerase chain reaction amplification of mouse H2O2-inducible hic53 clone yielded the cDNA encoding phosphatidic acid phosphatase. J. Biol. Chem. 271:18931-18938. [DOI] [PubMed] [Google Scholar]
- 24.Kamei, M., M. Kamei, W. B. Saunders, K. J. Bayless, L. Dye, G. E. Davis, and B. M. Weinstein. 2006. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442:453-456. [DOI] [PubMed] [Google Scholar]
- 25.Korswagen, H. C., and H. C. Clevers. 1999. Activation and repression of wingless/Wnt target genes by the TCF/LEF-1 family of transcription factors. Cold Spring Harb. Symp. Quant. Biol. 64:141-147. [DOI] [PubMed] [Google Scholar]
- 26.Loscertales, M., A. J. Mikels, J. K. Hu, P. K. Donahoe, and D. J. Roberts. 2008. Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development 135:1365-1376. [DOI] [PubMed] [Google Scholar]
- 27.Marsden, M., and D. W. DeSimone. 2001. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development 128:3635-3647. [DOI] [PubMed] [Google Scholar]
- 28.Noren, N. K., P. Liu, K. Burridge, and B. Kreft. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150:567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsson, A. K., A. Dimberg, J. Kreuger, and L. Claesson-Welsh. 2006. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 7:359-371. [DOI] [PubMed] [Google Scholar]
- 30.Park, J. I., H. Ji, S. Jun, D. Gu, H. Hikasa, L. Li, S. Y. Sokol, and P. D. McCrea. 2006. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: distinct catenin subfamilies promote Wnt signals. Dev. Cell 11:683-695. [DOI] [PubMed] [Google Scholar]
- 31.Parmalee, N. L., and J. Kitajewski. 2008. Wnt signaling in angiogenesis. Curr. Drug Targets 9:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prokhortchouk, A., B. Hendrich, H. Jørgensen, A. Ruzov, M. Wilm, G. Georgiev, A. Bird, and E. Prokhortchouk. 2001. The p120catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 15:1613-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risau, W. 1997. Mechanisms of angiogenesis. Nature 386:671-674. [DOI] [PubMed] [Google Scholar]
- 34.Ruoslahti, E. 1992. Control of cell motility and tumour invasion by extracellular matrix interactions. Br. J. Cancer 66:239-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sciorra, V. A., and A. J. Morris. 2002. Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim. Biophys. Acta 1582:45-51. [DOI] [PubMed] [Google Scholar]
- 36.Sharma, M., W. W. Chuang, and Z. Sun. 2002. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J. Biol. Chem. 277:30935-30941. [DOI] [PubMed] [Google Scholar]
- 37.Uegaki, K., Y. Kanamori, J. Kigawa, W. Kawaguchi, R. Kaneko, J. Naniwa, M. Takahashi, M. Shimada, T. Oishi, H. Itamochi, and N. Terakawa. 2006. PTEN is involved in the signal transduction pathway of contact inhibition in endometrial cells. Cell Tissue Res. 323:523-528. [DOI] [PubMed] [Google Scholar]
- 38.Vestweber, D. 2008. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 28:223-232. [DOI] [PubMed] [Google Scholar]
- 39.Waggoner, D. W., J. Xu, I. Singh, R. Jasinska, Q. X. Zhang, and D. N. Brindley. 1999. Structural organization of mammalian lipid phosphate phosphatases: implications for signal transduction. Biochim. Biophys. Acta 439:299-316. [DOI] [PubMed] [Google Scholar]
- 40.Wallez, Y., and P. Huber. 2008. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta 1778:794-809. [DOI] [PubMed] [Google Scholar]
- 41.Wary, K. K., G. D. Thakker, J. O. Humtsoe, and J. Yang. 2003. Analysis of VEGF-responsive genes involved in the activation of endothelial cells. Mol. Cancer 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wary, K. K., and J. O. Humtsoe. 2005. Anti-lipid phosphate phosphohydrolase-3 (LPP3) antibody inhibits bFGF- and VEGF-induced capillary morphogenesis of endothelial cells. Cell Commun. Signal. 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wildenberg, G. A., M. R. Dohn, R. H. Carnahan, M. A. Davis, N. A. Lobdell, J. Settleman, and A. B. Reynolds. 2006. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127:1027-1039. [DOI] [PubMed] [Google Scholar]
- 44.Zerlin, M., M. A. Julius, and J. Kitajewski. 2008. Wnt/Frizzled signaling in angiogenesis. Angiogenesis 11:63-69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.