Abstract
Here we describe a novel vaccine vector for expressing human immunodeficiency virus (HIV) antigens. We show that recombinant attenuated yellow fever vaccine virus 17D expressing simian immunodeficiency virus SIVmac239 Gag sequences can be used as a vector to generate SIV-specific CD8+ T-cell responses in the rhesus macaque. Priming with recombinant BCG expressing SIV antigens increased the frequency of these SIV-specific CD8+ T-cell responses after recombinant YF17D boosting. These recombinant YF17D-induced SIV-specific CD8+ T cells secreted several cytokines, were largely effector memory T cells, and suppressed viral replication in CD4+ T cells.
None of the vaccine regimens tested in human immunodeficiency virus (HIV) vaccine efficacy trials to date have either reduced the rate of HIV infection or reduced the level of HIV replication. Structural features and the enormous variability of the envelope glycoprotein have frustrated efforts to induce broadly reactive neutralizing antibodies against HIV (10). Investigators have therefore focused their attention on T-cell-based vaccines (40). Simian immunodeficiency virus (SIV) challenge of rhesus macaques vaccinated with T-cell-based vaccines has shown that it is possible to control virus replication after SIV infection (22, 41, 42). The recent STEP trial of a recombinant Ad5-vectored vaccine was widely seen as an important test of this concept (http://www.hvtn.org/media/pr/step111307.html) (9, 25). Unfortunately, vaccinees became infected at higher rates than the controls (9). While it is still not clear what caused the enhanced infection rate in the vaccinated group, future Ad5-based human vaccine trials may be difficult to justify. We therefore need to develop new vaccine vectors for delivering SIV and HIV genes. Several other viral vectors currently under consideration include nonreplicating adenovirus (Ad)-based vectors (1, 21, 22), Venezuelan equine encephalitis (VEE) virus (12, 20), adeno-associated virus (AAV) (19), modified vaccinia virus Ankara (MVA) (3, 4, 13, 15, 18, 38), NYVAC (6), cytomegalovirus (CMV) (16), and replicating Ad (30). However, only a few of these have shown promise in monkey trials using rigorous SIV challenges.
We explored whether the small (11-kb) yellow fever vaccine flavivirus 17D (YF17D) might be a suitable vector for HIV vaccines. The YF17D vaccine is inexpensive, production and quality control protocols already exist, and it disseminates widely in vivo after a single dose (27). Importantly, methods for the manipulation of the YF17D genome were recently established (7, 8, 24, 28). This effective vaccine has been safely used on >400 million people in the last 70 years (27). Additionally, the YF17D strain elicits robust CD8+ T-cell responses in humans (26). Chimeric YF17D is presently being developed as a vaccine for other flaviviruses, such as Japanese encephalitis virus (28), dengue virus (14), and West Nile virus (29). Inserts expressing a malaria B-cell epitope have been engineered into the E protein of YF17D (7). In murine models, recombinant YF17D viruses have generated robust and specific responses to engineered antigens inserted between the 2B and NS3 proteins in vivo (24, 35).
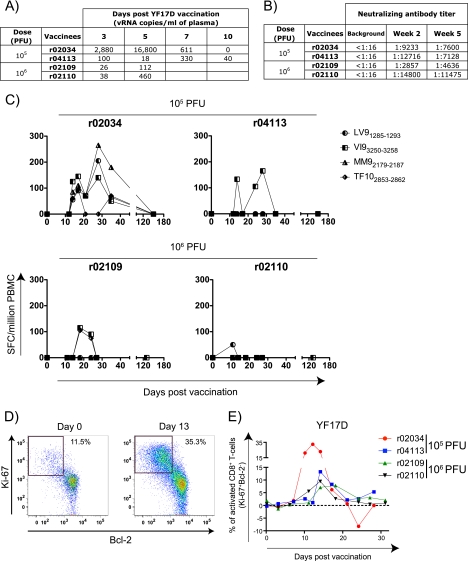
We first used the YF17D vaccine virus to infect four Mamu-A*01-positive macaques. The vaccine virus replicated in these four animals and induced neutralizing antibodies in all four macaques by 2 weeks postvaccination (Fig. 1A and B). To monitor the CD8+ T-cell immune response against YF17D, we scanned its proteome for peptides that might bind to Mamu-A*01 using the major histocompatibility complex (MHC) pathway algorithm (31). We synthesized the 52 YF17D-derived peptides most likely to bind to Mamu-A*01 based on their predicted affinity for this MHC class I molecule. We then used a gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay to screen these peptides in YF17D-immunized animals at several time points after vaccination and discovered that four Mamu-A*01-binding peptides, LTPVTMAEV (LV91285-1293), VSPGNGWMI (VI93250-3258), MSPKGISRM (MM92179-2187), and TTPFGQQRVF (TF102853-2862), were recognized in vivo (Fig. 1C). Using a previously reported protocol (26), we also observed CD8+ T-cell activation in all four animals (Fig. 1D and E). Thus, as was observed previously, the YF17D vaccine virus replicates in Indian rhesus monkeys (36) and induces neutralizing antibodies, yellow fever 17D-specific Mamu-A*01-restricted CD8+ T-cell responses, and CD8+ T-cell activation.
FIG. 1.
YF17D replicates and induces neutralizing antibodies, virus-specific CD8+ T cells, and the activation of CD8+ T cells in rhesus macaques. (A) Replication of YF17D during the first 10 days after vaccination with two different doses, as measured by quantitative PCR (Q-PCR) using the following primers: forward primer YF-17D 10188 (5′-GCGGATCACTGATTGGAATGAC-3′), reverse primer YF-17D 10264 (5′-CGTTCGGATACGATGGATGACTA-3′), and probe 6-carboxyfluorescein (6Fam)-5′-AATAGGGCCACCTGGGCCTCCC-3′-6-carboxytetramethylrhodamine (TamraQ). (B) Titer of neutralizing antibodies determined at 2 and 5 weeks after YF17D vaccination. (C) Fresh PBMC from vaccinees (100,000 cells/well) were used in IFN-γ ELISPOT assays (41) to assess T-cell responses against YF17D. We used 4 epitopes (LTPVTMAEV [LV91285-1293], VSPGNGWMI [VI93250-3258], MSPKGISRM [MM92179-2187], and TTPFGQQRVF [TF102853-2862]) predicted to bind to Mamu-A*01 as defined by the MHC pathway algorithm (31). All IFN-γ ELISPOT results were considered positive if they were ≥50 SFC/106 PBMC and ≥2 standard deviations over the background. (D) Identification of activated CD8+ T cells after vaccination with YF17D based on the expression of the proliferation and proapoptotic markers Ki-67 and Bcl-2, respectively (26). We stained whole blood cells with antibodies against CD3 and CD8. We then permeabilized and subsequently labeled these cells with Bcl-2- and Ki-67-specific antibodies. The flow graphs were gated on CD3+ CD8+ lymphocytes. (E) Expression kinetics of Ki-67 and Bcl-2 in CD8+ T cells after vaccination with YF17D.
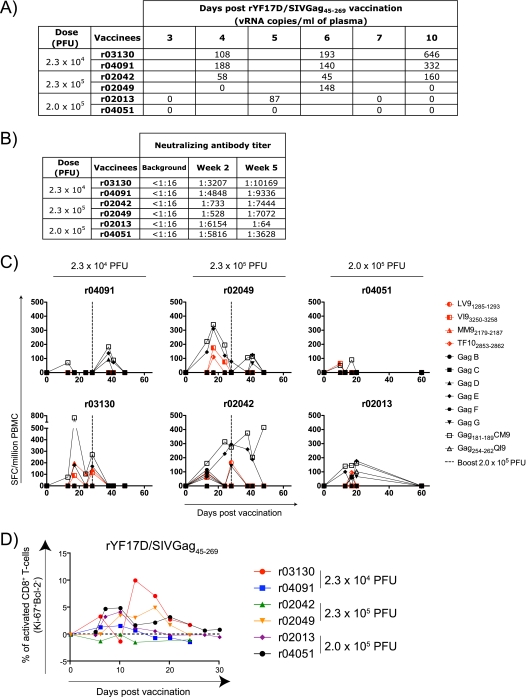
We next engineered the YF17D vaccine virus to express amino acids 45 to 269 of SIVmac239 Gag (rYF17D/SIVGag45-269) by inserting a yellow fever codon-optimized sequence between the genes encoding the viral proteins E and NS1. This recombinant virus replicated and induced neutralizing antibodies in mice (data not shown). We then tested the rYF17D/SIVGag45-269 construct in six Mamu-A*01-positive Indian rhesus macaques. We found evidence for the viral replication of rYF17D/SIVGag45-269 for five of these six macaques (Fig. 2A). However, neutralizing antibodies were evident for all six animals at 2 weeks postvaccination (Fig. 2B). Furthermore, all animals developed SIV-specific CD8+ T cells after a single immunization with rYF17D/SIVGag45-269 (Fig. 2C). To test whether a second dose of this vaccine could boost virus-specific T-cell responses, we administered rYF17D/SIVGag45-269 (2.0 × 105 PFU) to four macaques on day 28 after the first immunization and monitored cellular immune responses. With the exception of animal r04091, the rYF17D/SIVGag45-269 boost did not increase the frequency of the vaccine-induced T-cell responses. This recombinant vaccine virus also induced CD8+ T-cell activation in the majority of the vaccinated animals (Fig. 2D).
FIG. 2.
rYF17D/SIVGag45-269 replicates and induces neutralizing antibodies, virus-specific CD8+ T cells, and the activation of CD8+ T cells in rhesus macaques. (A) Replication of rYF17D/SIVGag45-269 during the first 10 days after vaccination with two different doses as measured by Q-PCR using the YF17D-specific primers described in the legend of Fig. 1. (B) Titer of neutralizing antibodies determined at 2 and 5 weeks after rYF17D/SIVGag45-269 vaccination. The low levels of neutralization for animal r02013 were observed in three separate assays. (C) Fresh PBMC from vaccinees (100,000 cells/well) were used in IFN-γ ELISPOT assays to assess T-cell responses against the YF17D vector (red) and the SIV Gag(45-269) insert (black) at several time points postvaccination. We measured YF17D-specific responses using the same epitopes described in the legend of Fig. 1. For SIV Gag-specific responses, we used 6 pools of 15-mers overlapping by 11 amino acids spanning the entire length of the SIVmac239 Gag insert. In addition, we measured Mamu-A*01-restricted responses against the dominant Gag181-189CM9 and subdominant Gag254-262QI9 epitopes. Four animals received a second dose of rYF17D/SIVGag45-269 on day 28 after the first vaccination (dashed line). (D) Expression kinetics of Ki-67 and Bcl-2 in CD8+ T cells after vaccination with rYF17D/SIVGag45-269. This assay was performed as described in the legend of Fig. 1.
We could not detect differences in vaccine-induced immune responses between the group of animals vaccinated with YF17D and the group vaccinated with rYF17D/SIVGag45-269. There was, however, considerable animal-to-animal variability. Animal r02034, which was vaccinated with YF17D, exhibited massive CD8+ T-cell activation (a peak of 35% at day 14) (Fig. 1E), which was probably induced by the high levels of viral replication (16,800 copies/ml at day 5) (Fig. 1A). It was difficult to see differences between the neutralizing antibody responses induced by YF17D and those induced by rYF17D/SIVGag45-269 (Fig. 1B and 2B). However, neutralizing antibodies in animal r02013 decreased by 5 weeks postvaccination. It was also difficult to detect differences in the YF17D-specific CD8+ T-cell responses induced by these two vaccines. Peak Mamu-A*01-restricted CD8+ T-cell responses against YF17D ranged from barely detectable (animal r02110 at day 11) (Fig. 1C) to 265 spot-forming cells (SFCs)/106 peripheral blood mononuclear cells (PBMC) (animal r02034 at day 28) (Fig. 1C). Similarly, three of the rYF17D/SIVGag45-269-vaccinated animals (animals r04091, r04051, and r02013) made low-frequency CD8+ T-cell responses against the Mamu-A*01-bound YF17D peptides, whereas the other three animals (animals r03130, r02049, and r02042) recognized these epitopes with responses ranging from 50 to 200 SFCs/106 PBMC (Fig. 2C). For almost every rYF17D/SIVGag45-269-vaccinated animal, the Gag181-189CM9-specific responses (range, 50 to 750 SFCs/106 PBMC) were higher than those generated against the Mamu-A*01-restricted YF17D epitopes (range, 0 to 175 SFCs/106 PBMC), suggesting that the recombinant virus replicated stably in vivo (Fig. 2C). Thus, the recombinant YF17D virus replicated and induced both virus-specific neutralizing antibodies and CD8+ T cells that were not demonstrably different from those induced by YF17D alone.
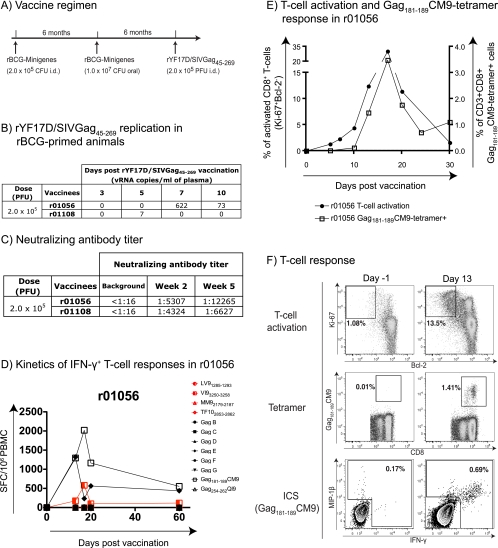
Most viral vectors are usually more efficient after a prime with DNA or recombinant BCG (rBCG) (4, 11, 15, 18). We therefore used rYF17D/SIVGag45-269 to boost two macaques that had been primed with rBCG expressing SIV proteins (Fig. 3A). We detected no SIV-specific responses after either of the two priming rBCG vaccinations. Unfortunately, while the recombinant YF17D virus replicated well in animal r01056, we found evidence for only low levels of replication of rYF17D/SIVGag45-269 on day 5 postvaccination for animal r01108 (7 copies/ml) (Fig. 3B). Both animals, however, generated neutralizing antibodies at 2 weeks postvaccination (Fig. 3C). Encouragingly, we detected high-frequency CD8+ T-cell responses in the Mamu-A*01-positive macaque (animal r01056) after boosting with rYF17D/SIVGag45-269 (Fig. 3D to F). These responses were directed mainly against the Mamu-A*01-restricted Gag181-189CM9 epitope, which is contained in the peptide pool Gag E (Fig. 3D). Furthermore, the boost induced a massive activation of animal r01056's CD8+ T cells, peaking at 35% at 17 days postvaccination (Fig. 3E). Of these activated CD8+ T cells, approximately 10% were directed against the Gag181-189CM9 epitope, with a frequency of 3.5% of CD8+ T cells (Fig. 3E). These epitope-specific CD8+ T cells made IFN-γ, tumor necrosis factor alpha (TNF-α), macrophage inflammatory protein 1β (MIP-1β), and degranulated (Fig. 3F and data not shown). Thus, an rBCG prime followed by a recombinant yellow fever 17D boost induced polyfunctional antigen-specific CD8+ T cells.
FIG. 3.
rYF17D/SIVGag45-269 vaccination induced a robust expansion of Gag-specific responses in an rBCG-primed macaque. (A) Vaccination scheme. We immunized two rhesus macaques with rBCG intradermally (i.d.) (2.0 × 105 CFU), rBCG orally (107 CFU), and rYF17D/SIVGag45-269 subcutaneously (2.0 × 105 PFU) at 6-month intervals. rBCG was engineered to express 18 minigenes containing sequences of Gag, Vif, Nef, Rev, and Tat from SIVmac239. (B) Replication of rYF17D/SIVGag45-269 during the first 10 days after vaccination as measured by Q-PCR using the YF17D-specific primers described in the legend of Fig. 1. (C) Titer of neutralizing antibodies determined at 2 and 5 weeks after rYF17D/SIVGag45-269 vaccination. (D) Fresh PBMC from animal r01056 (100,000 cells/well) were used in IFN-γ ELISPOT assays to assess T-cell responses against the YF17D vector (red) and the SIV Gag(45-269) insert (black) at several time points postvaccination. (E) Kinetics of CD8+ T-cell activation (as described in the legend of Fig. 1) and expansion of Gag181-189CM9-specific CD8+ T cells in animal r01056 after vaccination with rYF17D/SIVGag45-269. (F) Vaccination with rYF17D/SIVGag45-269 induced robust CD8+ T-cell responses against Gag181-189CM9 in r01056. CD8+ T-cell activation (Ki-67+/Bcl-2−) for baseline and day 13 are shown. Gag181-189CM9-specific responses were measured by tetramer staining and intracellular cytokine staining (ICS) with antibodies against MIP-1β and IFN-γ.
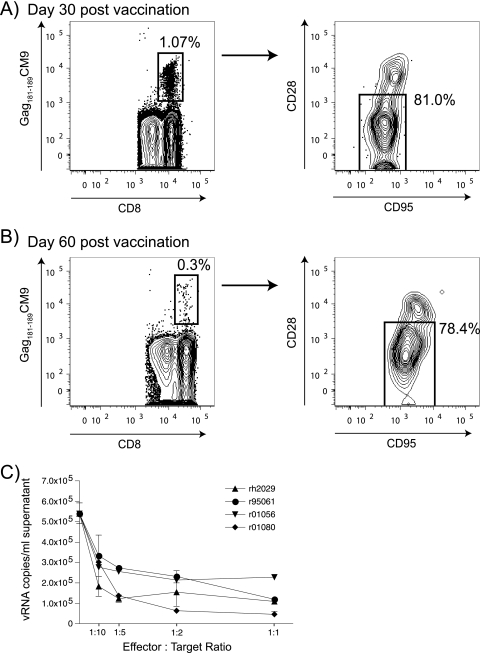
Vaccine-induced CD8+ T cells are usually central memory T cells (TCM) or effector memory T cells (TEM). These two subsets of CD8+ T cells differ in function and surface markers (23). Repeated boosting drives CD8+ T cells toward the TEM subset (23). We therefore determined whether a rBCG prime followed by a rYF17D/SIVGag45-269 boost induced TCM or TEM CD8+ T cells. Staining of PBMC obtained on day 30 postvaccination revealed that the SIV-specific CD8+ T cells were largely TEM cells since the majority of them were CD28 negative (Fig. 4A). Furthermore, these cells persisted with the same phenotype until day 60 after vaccination (Fig. 4B). It was recently suggested that TEM cells residing in the mucosae can effectively control infection after a low-dose challenge with SIVmac239 (16).
FIG. 4.
rYF17D/SIVGag45-269 vaccination of animal r01056 induced effector memory Gag181-189CM9-specific CD8+ T cells that suppressed viral replication in CD4+ targets. (A and B) Frequency and memory phenotype of tetramer-positive Gag181-189-specific CD8+ T cells in animal r01056 on day 30 (A) and day 60 (B) after rYF17D/SIVGag45-269 vaccination. CD28 and CD95 expression profiles of tetramer-positive cells show a polarized effector memory phenotype. Cells were gated on CD3+ CD8+ lymphocytes. (C) Ex vivo Gag181-189CM9-specific CD8+ T cells from animal r01056 inhibit viral replication from SIVmac239-infected CD4+ T cells. Gag181-189CM9-specific CD8+ T cells from three SIV-infected Mamu-A*01-positive animals and rYF17D/SIVGag45-269-vaccinated animal r01056 were tested for their ability to suppress viral replication from SIV-infected CD4+ T cells (39). Forty-eight hours after the incubation of various ratios of SIV-infected CD4+ T cells and Gag181-189CM9-specific CD8+ T cells, the supernatant was removed and measured for viral RNA (vRNA) copies per ml by Q-PCR. We observed no suppression when effectors were incubated with CD4+ targets from Mamu-A*01-negative animals (data not shown). Animal rh2029 was infected with SIVmac239 (viral load, ∼105 vRNA copies/ml) containing mutations in 8 Mamu-B*08-restricted epitopes as part of another study (37). Animal r01080 was vaccinated with a DNA/Ad5 regimen expressing Gag, Rev, Tat, and Nef and later infected with SIVmac239 (viral load, ∼103 vRNA copies/ml) (42). Animal r95061 was vaccinated with a DNA/MVA regimen containing Gag181-189CM9 and was later challenged with SIVmac239 (undetectable viral load) (2).
We then assessed whether rYF17D/SIVGag45-269-induced CD8+ T cells could recognize virally infected CD4+ T cells. We have shown that these vaccine-induced CD8+ T cells stain for tetramers and produce cytokines after stimulation with synthetic peptides (Fig. 3). None of these assays, however, tested whether these SIV-specific CD8+ T cells recognize SIV-infected cells and reduce viral replication. We therefore used a newly developed assay (39) to determine whether vaccine-induced CD8+ T cells can reduce viral replication in CD4+ T cells. We sorted tetramer-positive (Gag181-189CM9-specific) lymphocytes directly from fresh PBMC and incubated them for 48 h with SIVmac239-infected CD4+ T cells expressing Mamu-A*01. We assessed the percentage of CD4+ T cells that expressed SIV Gag p27 (data not shown) and the quantity of virus in the culture supernatant (Fig. 4C). Vaccine-induced CD8+ T cells reduced viral replication to the same extent as that seen with Gag181-189CM9-specific CD8+ T cells purified from three SIVmac239-infected rhesus macaques, including an elite controller rhesus macaque, animal r95061 (Fig. 4C).
The most encouraging aspect of this study is that rBCG primed a high-frequency CD8+ T-cell response after boosting with rYF17D/SIVGag45-269. These CD8+ T cells reached frequencies that were similar to those induced by an rBCG prime followed by an Ad5 boost (11). Even without the benefit of the rBCG prime, the levels of CD8+ T cells induced by a single rYF17D/SIVGag45-269 vaccination were equivalent to those induced by our best SIV vaccine, SIVmac239ΔNef. Recombinant YF17D generated an average of 195 SFCs/106 PBMC (range, 100 to 750 SFCs/106 PBMC) (n = 6), whereas SIVmac239ΔNef induced an average of 238 SFCs/106 PBMC (range, 150 to 320 SFCs/106 PBMC) (n = 3) (32). It is also possible that any YF17D/HIV recombinants would likely replicate better in humans than they have in rhesus macaques and thus induce more robust immune responses. Also, rBCG was shown previously to be effective in humans (5, 17, 33, 34) and may be more useful at priming T-cell responses in humans than it has been in our limited study with rhesus macaques. These two vectors have long-distinguished safety and efficacy histories in humans and may therefore be well suited for HIV vaccine development.
Acknowledgments
We are grateful to the staff at the Wisconsin National Primate Research Center (WNPRC) for the expert care of animals used in this study. We thank Bob DeMars for his critical comments on the manuscript. We thank Jessica Furlott, Matthew Buechler, and Laura Newman for technical help. We thank the MHC Typing Core at WNPRC for typing these animals using PCR-sequence-specific primer (PCR-SSP). We thank the AIDS Research and Reference and Reagent Program for interleukin-2 used in our assays.
This work was funded by NIH grants R01 AI076114 and R01 AI049120 to D.I.W., Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and FIOCRUZ. In addition, this work was supported by NCRR grant P51 RR000167 to the Wisconsin National Primate Research Center, University of Wisconsin—Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grants RR15459 and RR020141.
Footnotes
Published ahead of print on 20 January 2010.
REFERENCES
- 1.Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., P. Jing, B. Calore, H. Horton, D. H. O'Connor, T. Hanke, M. Piekarczyk, R. Ruddersdorf, B. R. Mothe, C. Emerson, N. Wilson, J. D. Lifson, I. M. Belyakov, J. A. Berzofsky, C. Wang, D. B. Allison, D. C. Montefiori, R. C. Desrosiers, S. Wolinsky, K. J. Kunstman, J. D. Altman, A. Sette, A. J. McMichael, and D. I. Watkins. 2002. Effects of cytotoxic T lymphocytes (CTL) directed against a single simian immunodeficiency virus (SIV) Gag CTL epitope on the course of SIVmac239 infection. J. Virol. 76:10507-10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 5.Barker, L. F., M. J. Brennan, P. K. Rosenstein, and J. C. Sadoff. 2009. Tuberculosis vaccine research: the impact of immunology. Curr. Opin. Immunol. 21:331-338. [DOI] [PubMed] [Google Scholar]
- 6.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonaldo, M. C., R. C. Garratt, P. S. Caufour, M. S. Freire, M. M. Rodrigues, R. S. Nussenzweig, and R. Galler. 2002. Surface expression of an immunodominant malaria protein B cell epitope by yellow fever virus. J. Mol. Biol. 315:873-885. [DOI] [PubMed] [Google Scholar]
- 8.Bonaldo, M. C., S. M. Mello, G. F. Trindade, A. A. Rangel, A. S. Duarte, P. J. Oliveira, M. S. Freire, C. F. Kubelka, and R. Galler. 2007. Construction and characterization of recombinant flaviviruses bearing insertions between E and NS1 genes. Virol. J. 4:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 11.Cayabyab, M. J., B. Korioth-Schmitz, Y. Sun, A. Carville, H. Balachandran, A. Miura, K. R. Carlson, A. P. Buzby, B. F. Haynes, W. R. Jacobs, and N. L. Letvin. 2009. Recombinant Mycobacterium bovis BCG prime-recombinant adenovirus boost vaccination in rhesus monkeys elicits robust polyfunctional simian immunodeficiency virus-specific T-cell responses. J. Virol. 83:5505-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engram, J. C., R. M. Dunham, G. Makedonas, T. H. Vanderford, B. Sumpter, N. R. Klatt, S. J. Ratcliffe, S. Garg, M. Paiardini, M. McQuoid, J. D. Altman, S. I. Staprans, M. R. Betts, D. A. Garber, M. B. Feinberg, and G. Silvestri. 2009. Vaccine-induced, simian immunodeficiency virus-specific CD8+ T cells reduce virus replication but do not protect from simian immunodeficiency virus disease progression. J. Immunol. 183:706-717. [DOI] [PubMed] [Google Scholar]
- 14.Guy, B., N. Nougarede, S. Begue, V. Sanchez, N. Souag, M. Carre, L. Chambonneau, D. N. Morrisson, D. Shaw, M. Qiao, R. Dumas, J. Lang, and R. Forrat. 2008. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 26:5712-5721. [DOI] [PubMed] [Google Scholar]
- 15.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen, S. G., C. Vieville, N. Whizin, L. Coyne-Johnson, D. C. Siess, D. D. Drummond, A. W. Legasse, M. K. Axthelm, K. Oswald, C. M. Trubey, M. J. Piatak, J. D. Lifson, J. A. Nelson, M. A. Jarvis, and L. J. Picker. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoft, D. F., A. Blazevic, G. Abate, W. A. Hanekom, G. Kaplan, J. H. Soler, F. Weichold, L. Geiter, J. C. Sadoff, and M. A. Horwitz. 2008. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J. Infect. Dis. 198:1491-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, P. R., B. C. Schnepp, M. J. Connell, D. Rohne, S. Robinson, G. R. Krivulka, C. I. Lord, R. Zinn, D. C. Montefiori, N. L. Letvin, and K. R. Clark. 2005. Novel adeno-associated virus vector vaccine restricts replication of simian immunodeficiency virus in macaques. J. Virol. 79:955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston, R. E., P. R. Johnson, M. J. Connell, D. C. Montefiori, A. West, M. L. Collier, C. Cecil, R. Swanstrom, J. A. Frelinger, and N. L. Davis. 2005. Vaccination of macaques with SIV immunogens delivered by Venezuelan equine encephalitis virus replicon particle vectors followed by a mucosal challenge with SIVsmE660. Vaccine 23:4969-4979. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J., B. A. Ewald, D. M. Lynch, M. Denholtz, P. Abbink, A. A. Lemckert, A. Carville, K. G. Mansfield, M. J. Havenga, J. Goudsmit, and D. H. Barouch. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82:4844-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, J., K. L. O'Brien, D. M. Lynch, N. L. Simmons, A. La Porte, A. M. Riggs, P. Abbink, R. T. Coffey, L. E. Grandpre, M. S. Seaman, G. Landucci, D. N. Forthal, D. C. Montefiori, A. Carville, K. G. Mansfield, M. J. Havenga, M. G. Pau, J. Goudsmit, and D. H. Barouch. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masopust, D., S. J. Ha, V. Vezys, and R. Ahmed. 2006. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 177:831-839. [DOI] [PubMed] [Google Scholar]
- 24.McAllister, A., A. E. Arbetman, S. Mandl, C. Pena-Rossi, and R. Andino. 2000. Recombinant yellow fever viruses are effective therapeutic vaccines for treatment of murine experimental solid tumors and pulmonary metastases. J. Virol. 74:9197-9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, and D. R. Casimiro. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, J. D., R. G. van der Most, R. S. Akondy, J. T. Glidewell, S. Albott, D. Masopust, K. Murali-Krishna, P. L. Mahar, S. Edupuganti, S. Lalor, S. Germon, C. Del Rio, M. J. Mulligan, S. I. Staprans, J. D. Altman, M. B. Feinberg, and R. Ahmed. 2008. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28:710-722. [DOI] [PubMed] [Google Scholar]
- 27.Monath, T. P. 2003. Yellow fever vaccine, p. 1095-1176. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines. Elsevier Science Health, Philadelphia, PA.
- 28.Monath, T. P., F. Guirakhoo, R. Nichols, S. Yoksan, R. Schrader, C. Murphy, P. Blum, S. Woodward, K. McCarthy, D. Mathis, C. Johnson, and P. Bedford. 2003. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J. Infect. Dis. 188:1213-1230. [DOI] [PubMed] [Google Scholar]
- 29.Monath, T. P., J. Liu, N. Kanesa-Thasan, G. A. Myers, R. Nichols, A. Deary, K. McCarthy, C. Johnson, T. Ermak, S. Shin, J. Arroyo, F. Guirakhoo, J. S. Kennedy, F. A. Ennis, S. Green, and P. Bedford. 2006. A live, attenuated recombinant West Nile virus vaccine. Proc. Natl. Acad. Sci. U. S. A. 103:6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson, L. J., and M. Robert-Guroff. 2008. Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development. Expert Opin. Biol. Ther. 8:1347-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters, B., H. H. Bui, J. Sidney, Z. Weng, J. T. Loffredo, D. I. Watkins, B. R. Mothe, and A. Sette. 2005. A computational resource for the prediction of peptide binding to Indian rhesus macaque MHC class I molecules. Vaccine 23:5212-5224. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds, M. R., A. M. Weiler, K. L. Weisgrau, S. M. Piaskowski, J. R. Furlott, J. T. Weinfurter, M. Kaizu, T. Soma, E. J. Leon, C. MacNair, D. P. Leaman, M. B. Zwick, E. Gostick, S. K. Musani, D. A. Price, T. C. Friedrich, E. G. Rakasz, N. A. Wilson, A. B. McDermott, R. Boyle, D. B. Allison, D. R. Burton, W. C. Koff, and D. I. Watkins. 2008. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J. Exp. Med. 205:2537-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skeiky, Y. A., and J. C. Sadoff. 2006. Advances in tuberculosis vaccine strategies. Nat. Rev. Microbiol. 4:469-476. [DOI] [PubMed] [Google Scholar]
- 34.Stover, C. K., G. P. Bansal, S. Langerman, and M. S. Hanson. 1994. Protective immunity elicited by rBCG vaccines. Dev. Biol. Stand. 82:163-170. [PubMed] [Google Scholar]
- 35.Tao, D., G. Barba-Spaeth, U. Rai, V. Nussenzweig, C. M. Rice, and R. S. Nussenzweig. 2005. Yellow fever 17D as a vaccine vector for microbial CTL epitopes: protection in a rodent malaria model. J. Exp. Med. 201:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trindade, G. F., R. S. Marchevsky, A. M. Fillipis, R. M. Nogueira, M. C. Bonaldo, P. C. Acero, E. Caride, M. S. Freire, and R. Galler. 2008. Limited replication of yellow fever 17DD and 17D-dengue recombinant viruses in rhesus monkeys. An. Acad. Bras. Cienc. 80:311-321. [DOI] [PubMed] [Google Scholar]
- 37.Valentine, L. E., J. T. Loffredo, A. T. Bean, E. J. Leon, C. E. Macnair, D. R. Beal, S. M. Piaskowski, Y. C. Klimentidis, S. M. Lank, R. W. Wiseman, J. T. Weinfurter, G. E. May, E. G. Rakasz, N. A. Wilson, T. C. Friedrich, D. H. O'Connor, D. B. Allison, and D. I. Watkins. 2009. Infection with “escaped” virus variants impairs control of SIVmac239 replication in Mamu-B*08+ macaques. J. Virol. 83:11514-11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel, T. U., M. R. Reynolds, D. H. Fuller, K. Vielhuber, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, M. L. Marthas, V. Erfle, S. M. Wolinsky, C. Wang, D. B. Allison, E. W. Rud, N. Wilson, D. Montefiori, J. D. Altman, and D. I. Watkins. 2003. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. J. Virol. 77:13348-13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vojnov, L., J. Reed, K. L. Weisgrau, E. Rakasz, J. T. Loffredo, S. Piaskowski, J. B. Sacha, H. L. Kolar, N. A. Wilson, R. P. Johnson, and D. I. Watkins. 2010. Effective simian immunodeficiency virus-specific CD8+ T cells lack an easily detectable, shared characteristic. J. Virol. 84:753-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watkins, D. I., D. R. Burton, E. G. Kallas, J. P. Moore, and W. C. Koff. 2008. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 14:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson, N. A., B. F. Keele, J. S. Reed, S. M. Piaskowski, C. E. MacNair, A. J. Bett, X. Liang, F. Wang, E. Thoryk, G. J. Heidecker, M. P. Citron, L. Huang, J. Lin, S. Vitelli, C. D. Ahn, M. Kaizu, N. J. Maness, M. R. Reynolds, T. C. Friedrich, J. T. Loffredo, E. G. Rakasz, S. Erickson, D. B. Allison, M. J. Piatak, J. D. Lifson, J. W. Shiver, D. R. Casimiro, G. M. Shaw, B. H. Hahn, and D. I. Watkins. 2009. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J. Virol. 83:6508-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]