Abstract
Homologous recombination hotspots increase the frequency of recombination in nearby DNA. The M26 hotspot in the ade6 gene of Schizosaccharomyces pombe is a meiotic hotspot with a discrete, cis-acting nucleotide sequence (5′-ATGACGT-3′) defined by extensive mutagenesis. A heterodimeric M26 DNA binding protein, composed of subunits Mts1 and Mts2, has been identified and purified 40,000-fold. Cloning, disruption, and genetic analyses of the mts genes demonstrate that the Mts1/Mts2 heterodimer is essential for hotspot activity. This provides direct evidence that a specific trans-acting factor, binding to a cis-acting site with a unique nucleotide sequence, is required to activate this meiotic hotspot. Intriguingly, the Mts1/Mts2 protein subunits are identical to the recently described transcription factors Atf1 (Gad7) and Pcr1, which are required for a variety of stress responses. However, we report differential dependence on the Mts proteins for hotspot activation and stress response, suggesting that these proteins are multifunctional and have distinct activities. Furthermore, ade6 mRNA levels are equivalent in hotspot and nonhotspot meioses and do not change in mts mutants, indicating that hotspot activation is not a consequence of elevated transcription levels. These findings suggest an intimate but separable link between the regulation of transcription and meiotic recombination. Other studies have recently shown that the Mts1/Mts2 protein and M26 sites are involved in meiotic recombination elsewhere in the S. pombe genome, suggesting that these factors help regulate the timing and distribution of homologous recombination.
Meiosis and homologous recombination have vital roles in the evolution of eukaryotes. Meiosis and subsequent joining of haploid gametes generates new genotypes by shuffling linkage groups (chromosomes), and recombination generates new genotypes by shuffling alleles of genes within individual chromosomes. Both processes supply genetic diversity to provide eukaryotes a mechanism for sampling different combinations of newly arising genetic variants. Meiotic recombination rates are enhanced relative to mitotic rates, presumably to maximize the amount of genetic variability in the meiotic products or to ensure homolog disjunction or both. Recombination rates are higher than average in the vicinity of DNA sites called “recombination hotspots.”
Homologous recombination hotspots are found in organisms ranging from bacteriophages to humans (1–3). Some eukaryotic hotspots function in mitosis (4), some function in meiosis (5–7), and some function in both (3, 8). Hotspots therefore influence the distribution of recombination along chromosomes and the timing of recombination during the life cycle. Recombination hotspots may contribute a significant fraction of total recombination in both prokaryotes and eukaryotes (9–11). Presumably, as for cis-acting transcriptional regulatory elements, there are proteins that interact with hotspots to mediate their biological activity. In Escherichia coli the RecBCD enzyme interacts with Chi sites to enhance recombination (12). The M26 recombination hotspot of the fission yeast Schizosaccharomyces pombe (5) is a well-characterized eukaryotic hotspot. The M26 mutation is a single base pair substitution in ade6 that increases meiotic recombination up to 20-fold relative to other ade6 alleles, such as M375 (Fig. 1) (5, 7, 13). Mutational analysis revealed that a specific 7-bp nucleotide sequence at M26 is required for hotspot activity (14) (Fig. 1), suggesting that a protein might bind to the site to increase meiotic recombination above the basal levels.
Figure 1.
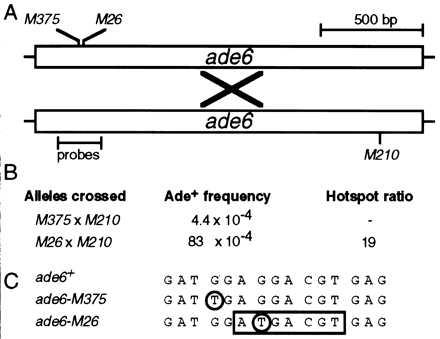
Hotspot activity of M26. (A) Schematic diagram of the ade6 gene showing positions of alleles and probes used for gel mobility shift experiments. Meiotic recombination (×) between a chromosome harboring the ade6-M26 or the ade6-M375 allele and a chromosome with the ade6-M210 allele generates a wild-type, selectable ade6+ gene. (B) Example of M26 recombination hotspot activity. Data are from Table 2. Recombination in test crosses containing M26 is increased up to 20-fold relative to recombination when using another allele, such as M375 (5). (C) DNA sequence surrounding the ade6-M26 recombination hotspot (7, 13). Both M375 and M26 are identical single base pair substitutions (circled) that generate stop codons. The M26 mutation creates a 7-bp site (box) that is bound by the Mts1/Mts2 heterodimer (15) and is required for hotspot activity in vivo (14).
To investigate the mechanism by which eukaryotic recombination hotspots function, we identified and purified 40,000-fold a heterodimeric protein that binds to the M26 DNA site (15). The polypeptides are called Mts1 and Mts2 (M-twenty-six binding proteins). Optimal binding requires the 7-bp M26 site (Fig. 1) and requires heterodimeric Mts1/Mts2 protein. Binding of Mts1/Mts2 in vitro (15) correlates almost precisely with hotspot activity in vivo (14) for single base pair substitutions in the region, suggesting that Mts1/Mts2 protein activates the M26 hotspot.
We report here that binding of Mts1/Mts2 heterodimer to the M26 site is essential for hotspot activation. While these genetic studies were underway, two laboratories described S. pombe genes identical to mts1 (called atf1 and gad7) and mts2 (called pcr1), both isolated through different lines of research (16–18). Mts1 and Mts2 proteins have roles in developmental decisions and stress responses (Fig. 2), and the proteins regulate the transcription of a variety of genes (16–20) (N.K. and W.P.W., unpublished observations). However, we show that stress response and hotspot activation are genetically distinct, and hotspot activation is not a consequence of increased transcription at ade6. Thus, hotspot activation by the Mts1/Mts2 heterodimer is independent of the known transcriptional regulatory activities of the individual Mts1 and Mts2 proteins. We speculate that the Mts1/Mts2 protein activates the M26 hotspot by altering the local chromatin structure or by directly recruiting recombination enzymes.
Figure 2.
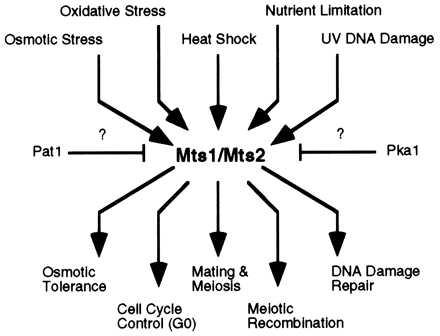
Schematic representation of biochemical pathways by using Mts1 and Mts2 proteins. Upstream signals converge via the mitogen-activated protein (MAP) kinase cascade (19, 20) and likely via Pat1 and Pka kinases (16–20). Signals diverge at Mts1 and Mts2 to activate the appropriate developmental responses. Some effector functions require Mts1, some require Mts2, and some require both Mts1 and Mts2.
MATERIALS AND METHODS
S. pombe Strains, Media, and Genetic Analyses.
S. pombe strains used for this study are listed in Table 1. A complete genealogy is available upon request. Genetic nomenclature follows the recommendations of Kohli (21). Strains were cultured in nitrogen base liquid or on nitrogen base agar minimal medium (0.67% Difco yeast nitrogen base without amino acids, 1% glucose, 2% agar for solid media) supplemented with the required amino acids, purines, and pyrimidines (100 μg/ml). Synthetic sporulation agar was prepared as described (22). Strain constructions, meiotic crosses, and analyses of recombinant frequencies were as described (23) with the two following changes: matings were for 5 days, and the spore suspensions were treated with ethanol for 15 min.
Table 1.
S. pombe strains used in this study
| Strain | Genotype | Source* |
|---|---|---|
| WSP599 | h− ura4-D18 his3-D1 leu1-32 | This study† |
| WSP547 | h− ade6-M210 ura4-D18 his3-D1 leu1-32 | This study† |
| WSP550 | h+ ade6-M210 ura4-D18 his3-D1 leu1-32 | This study† |
| WSP571 | h+ ade6-M26 ura4-D18 his3-D1 leu1-32 | This study† |
| WSP572 | h− ade6-M26 ura4-D18 his3-D1 leu1-32 | This study† |
| WSP578 | h+ ade6-M375 ura4-D18 his3-D1 leu1-32 | This study† |
| WSP579 | h− ade6-M375 ura4-D18 his3-D1 leu1-32 | This study† |
| WSP654 | h+ ade6-M210 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ | T of WSP550 |
| WSP656 | h+ ade6-M210 ura4-D18 his3-D1 leu1-32 mts2-D1∷his3+ | T of WSP550 |
| WSP639 | h− ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ | WSP654 × WSP599 |
| WSP641 | h− ura4-D18 his3-D1 leu1-32 mts2-D1∷his3+ | WSP656 × WSP599 |
| WSP642 | h+ ade6-M210 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ | WSP550 × WSP639 |
| WSP643 | h− ade6-M210 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ | WSP550 × WSP639 |
| WSP644 | h+ ade6-M26 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ | WSP571 × WSP639 |
| WSP645 | h− ade6-M26 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ | WSP571 × WSP639 |
| WSP646 | h+ ade6-M375 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ | WSP578 × WSP639 |
| WSP647 | h− ade6-M375 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ | WSP578 × WSP639 |
| WSP648 | h+ ade6-M210 ura4-D18 his3-D1 leu1-32 mts2-D1∷his3+ | WSP550 × WSP641 |
| WSP649 | h− ade6-M210 ura4-D18 his3-D1 leu1-32 mts2-D1∷his3+ | WSP550 × WSP641 |
| WSP650 | h+ ade6-M26 ura4-D18 his3-D1 leu1-32 mts2-D1∷his3+ | WSP571 × WSP641 |
| WSP651 | h− ade6-M26 ura4-D18 his3-D1 leu1-32 mts2-D1∷his3+ | WSP571 × WSP641 |
| WSP652 | h+ ade6-M375 ura4-D18 his3-D1 leu1-32 mts2-D1∷his3+ | WSP578 × WSP641 |
| WSP653 | h− ade6-M375 ura4-D18 his3-D1 leu1-32 mts2-D1∷his3+ | WSP578 × WSP641 |
| WSP671 | h+ ade6-M210 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ mts2-D1∷his3+ | WSP642 × WSP649 |
| WSP672 | h− ade6-M210 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ mts2-D1∷his3+ | WSP642 × WSP649 |
| WSP674 | h+ ade6-M26 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ mts2-D1∷his3+ | WSP644 × WSP651 |
| WSP675 | h− ade6-M26 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ mts2-D1∷his3+ | WSP644 × WSP651 |
| WSP677 | h+ ade6-M375 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ mts2-D1∷his3+ | WSP646 × WSP653 |
| WSP678 | h− ade6-M375 ura4-D18 his3-D1 leu1-32 mts1-D15∷ura4+ mts2-D1∷his3+ | WSP646 × WSP653 |
Strains were constructed by transformation (T) as in Fig. 3 or derived from standard genetic crosses (×) of the indicated strains.
Complete genealogies are available upon request.
Cloning and Sequencing mts Genes.
From 400 liters of culture we obtained about 4,000 g of cells and we conducted multiple, large-scale purifications of protein as described (15). The amino acid sequences of tryptic fragments of Mts1 (amino acids 86–112, 130–158, 213–247) and Mts2 (amino acids 37–50, 52–65) were determined by Bill Lane at the Harvard Microchemistry Facility and were used to design degenerate oligonucleotide primers for PCR amplification (24). Products of PCR amplification of genomic DNA were cloned into pBluescriptKSII+, sequenced (25) to confirm that they harbored the correct fragments, amplified, excised, and used as probes for colony hybridization (26) to identify multiple independent clones from a partial-digest, size-fractionated genomic DNA library (27). PCR analysis was used to identify one clone (pMts1-7) harboring a 4,376-bp insert with a centrally located mts1 gene and one clone (pMts2-3) harboring a 3,642-bp insert with a centrally located mts2 gene. A “primer-walk” approach was used to sequence (25) both strands of the entire genomic DNA inserts in pMts1-7 and pMts2-3 (GenBank accession numbers U87869 and U87870).
Protein Purification and Gel Mobility Shift Assay.
Proteins were generated in E. coli by expression in the pET15b vector (Novagen), purified by nickel affinity chromatography in the presence of 6 M guanidine-HCl, renatured on the column, eluted with 500 mM imidazole, and dialyzed against storage buffer. Overexpression and purification were according to the manufacturer’s instructions (Novagen), and all other conditions were as described (15). The gel mobility shift experiments were as described (15) with probes indicated in Fig. 1.
RESULTS
The mts1 and mts2 Genes Encode the Heterodimeric M26 Hotspot Binding Protein.
To conduct further biochemical and genetic studies of the M26 hotspot, we cloned and sequenced the genes encoding the Mts1/Mts2 protein (Fig. 3). The predicted polypeptides contain basic leucine zipper (bZIP) motifs characteristic of dimeric, sequence-specific DNA binding proteins. Both proteins have extensive sequence identity (≈50%), restricted to the bZIP domain, to transcription factors of the activating transcription factor/cAMP response element binding protein (ATF/CREB) family, suggesting that the Mts1/Mts2 heterodimer is a transcription factor. To confirm that the cloned genes encoded M26 recombination hotspot DNA binding proteins, we produced Mts1 and Mts2 in E. coli, purified the polypeptides, and characterized their binding affinity and specificity (Fig. 4). Heterodimeric protein bound specifically to DNA bearing the M26 site; it bound poorly to control DNA molecules that differed by a single base pair substitution within the M26 site (Fig. 4). The individual protein subunits also displayed hotspot-specific DNA binding activity, but with much lower affinity (data not shown). Thus, proteins expressed from the cloned mts genes exhibited DNA binding specificity similar to that of protein purified from S. pombe (15), confirming that the mts1 and mts2 genes encode the M26 hotspot-specific DNA binding proteins.
Figure 3.
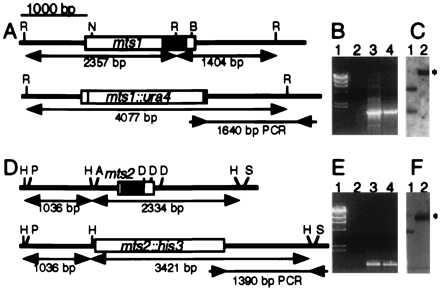
Cloning, sequence analysis, and disruption of the mts1 and mts2 genes. (A and D) The genes encode proteins with bZIP dimerization and DNA binding motifs (shaded boxes) ≈50% identical to those in ATF/CREB proteins. R, EcoRI; N, EcoNI; B, BalI; H, HincII; P, HpaI; A, AatII; D, NdeIII; S, BstEII. Standard procedures were used to disrupt the genes. (B and E) Confirmation of gene disruption by PCR. Lanes: 1, λ DNA digested with HindIII; 2, PCR product of wild-type DNA; 3, PCR product of disrupted DNA (mts1-D15::ura4+ or mts2-D1::his3+); 4, PCR product of positive control DNA (plasmids used to obtain gene targeting fragments). Primers were as shown by inward-pointing arrows in A and D. (C and F) Confirmation of gene disruption by Southern blot analysis. Lanes: 1, wild-type DNA; 2, disrupted DNA (mts1-D15::ura4+ or mts2-D1::his3+). Restriction maps are shown in A and D, and the expected positions of altered restriction fragments are indicated (∗). DNA was digested with EcoRI and probed with the 4,077-bp EcoRI–EcoRI fragment for mts1 constructs or digested with HincII and probed with the 3,421-bp HincII-HincII fragment for mts2 constructs.
Figure 4.
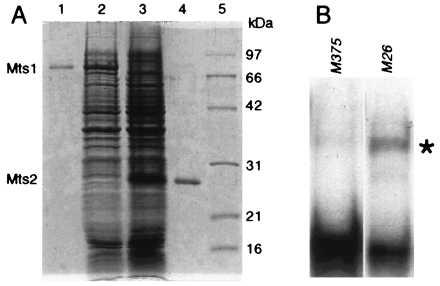
Hotspot-specific DNA binding activity of Mts1/Mts2 heterodimer purified from E. coli. (A) Coomassie-stained, 12% SDS/PAGE analysis of proteins. Lanes: 1, purified Mts1; 2, whole-cell lysate containing Mts1; 3, whole-cell lysate containing Mts2; 4, purified Mts2; 5, molecular mass standards. (B) Gel mobility shift assay by using a mixture of renatured Mts1 and Mts2 polypeptides. Position of M26-specific complex is indicated (∗). Gel shift conditions and probes were as described (15) and contained ≈5 nM probe, 25 nM Mts1, and 25 nM Mts2.
Mts1/Mts2 Heterodimer Activates the M26 Meiotic Recombination Hotspot in ade6.
To determine whether the Mts1/Mts2 heterodimer is required to activate the M26 hotspot, we used gene targeting to disrupt the genomic copies of mts1 and mts2 (Fig. 3). Although the mutants displayed a partial sterility phenotype (≈15% of wild-type mating efficiency), sufficient spores were formed to permit genetic analyses. Standard genetic techniques (5, 14, 28) were used to determine both basal and hotspot-mediated recombination levels. We measured recombination between two sets of ade6 alleles (Fig. 1) in wild-type cells and in mts mutants. Crosses between strains with ade6-M375 and ade6-M210 alleles revealed the basal recombinant frequency, whereas crosses between strains with ade6-M26 and ade6-M210 alleles revealed the sum of basal plus hotspot recombinant frequencies (5). In cells wild-type for mts1 and mts2, we observed a basal recombinant frequency of 4.4 × 10−4 and a hotspot-enhanced frequency of 83 × 10−4 (Table 2, line 1). The ratio of these two values, called the “hotspot ratio,” is a measure of the stimulation of recombination by M26. In wild-type (mts+) meioses we observed a hotspot ratio of about 19 (Table 2, line 1), similar to results reported previously (5).
Table 2.
Requirement for Mts1 and Mts2 in M26 hotspot but not basal meiotic recombination
| Line | Relevant genotypes*
|
ade6 alleles crossed and Ade+ recombinant frequency (×104)†
|
Hotspot ratio‡ | Hotspot ratio vs. wild-type basal§ | ||
|---|---|---|---|---|---|---|
| mts1/mts1 | mts2/mts2 | M375 × M210 | M26 × M210 | |||
| 1 | +/+ | +/+ | 4.4 ± 0.6 | 83 ± 7.6 | 19 | 19 |
| 2 | +/− | +/+ | 5.6 ± 1.6 | 45 ± 9.5 | 8.0 | 10 |
| 3 | −/− | +/+ | 5.3 ± 1.4 | 5.4 ± 0.0 | 1.0 | 1.2 |
| 4 | +/+ | +/− | 7.6 ± 0.1 | 27 ± 0.4 | 3.6 | 6.1 |
| 5 | +/+ | −/− | 5.3 ± 0.6 | 4.9 ± 0.8 | 0.9 | 1.1 |
| 6 | −/− | −/− | 5.7 ± 1.1 | 7.0 ± 1.1 | 1.2 | 1.6 |
The (+) indicates mts1+ or mts2+, the (−) indicates deleted mts1 (mts1-D15∷ura4+) or deleted mts2 (mts2-D1∷his3+). In addition to the listed mts1, mts2, and ade6 alleles, all strains had the genotype leu1-32 ura4-D18 his3-D1 (Table 1).
See Fig. 1 for positions of ade6 alleles. Standard genetic crosses (5, 14, 28) were conducted, and spores were plated on supplemented NBA minimal medium containing adenine (100 μg/ml) to determine the total viable spore titer (T) and on NBA medium lacking adenine to determine the ade6+ recombinant titer (R). The recombinant frequency in each experiment is R/T. Each cross was done with two pairs of strains bearing alleles in opposite configurations relative to the mating type alleles. No significant differences were observed, and the data were combined. Data are the mean ± SD of recombinant frequencies from 2 to 4 experiments.
Ratio of recombinant frequency from the M26 × M210 (hotspot) cross relative to that from the M375 × M210 (basal recombination) cross.
Ratio of recombinant frequency from the M26 × M210 (hotspot) cross relative to that from the M375 × M210 (basal recombination) cross in wild type (mts1+ mts2+).
Cells that were homozygous mutant for mts1 (Table 2, line 3) or mts2 (Table 2, line 5) exhibited basal recombinant frequencies that were statistically indistinguishable from those of wild-type (mts+) cells. We conclude that the mts1 and mts2 mutants are recombination-proficient and have an intact (wild-type) basal recombination machinery.
Although having normal basal recombination machinery, cells that were homozygous mutant for mts1 (Table 2, line 3) or mts2 (Table 2, line 5) entirely lacked recombination hotspot activity; the frequencies of recombinants in the hotspot crosses (M26 × M210) were essentially the same as the frequencies of recombinants in the basal crosses (M375 × M210). These observations, coupled with the hotspot-specific DNA binding activity of the heterodimer (Fig. 4), demonstrate that the Mts1/Mts2 heterodimer directly activates the M26 meiotic homologous recombination hotspot, but this protein has no detectable role in ade6 basal recombination. Furthermore, homodimeric Mts1 or Mts2 protein, observed to bind M26 DNA with low affinity in vitro (15), does not activate the hotspot in vivo. Double mutants (mts1− mts2−) had the same phenotype as either single mutant (Table 2, line 6), further indicating that a heterodimer of Mts1 and Mts2 is the functional unit in hotspot activation.
The mts1 and mts2 mutations were semidominant in crosses heterozygous for mts1 or mts2: the hotspot ratio was intermediate between those of wild-type and homozygous mutant crosses (Table 2, lines 2 and 4). We estimate that there are ≥200 active Mts1/Mts2 protein molecules and about 300 M26 sites per haploid genome (15), suggesting that the cis and trans components are present in approximately equal numbers. The semidominance supports those estimates and indicates that protein–DNA interaction within cells is limiting, at least when mts1 or mts2 is heterozygous.
Mts1, But Not Mts2, Is Required for Osmotic Stress Response in Mitotic Cells.
The Mts1 and Mts2 proteins are implicated in several biochemical pathways, including response to stress (Fig. 2), but the mutants exhibit no mitotic phenotype under normal laboratory culture conditions (data not shown) (16–20). We therefore tested the mutants for mitotic phenotypes under a variety of adverse conditions. Cells that were mts1 mutant were highly sensitive to osmotic stress (Fig. 5). However, the mts2 mutants were resistant to osmotic stress. We and others have observed similar differential responses to other cytotoxic and genotoxic agents (data not shown) (16, 18–20). The differential response to stress indicates that Mts1 and Mts2 have nonoverlapping functions in some pathways that sense environmental, cytotoxic, and genotoxic signals. Because hotspot activation requires a heterodimer of Mts1 and Mts2 (Table 2), we infer that the mechanism of hotspot activation is distinct from the mechanisms of developmental regulation (transcriptional transactivation of target genes) that require only the individual Mts proteins (Fig. 5).
Figure 5.

Sensitivity of mts− mutants to osmotic stress. The indicated number of cells from healthy, log-phase cultures were spotted onto supplemented NBA minimal medium containing 1 M NaCl and were incubated for 3 days at 32°C before being photographed.
Hotspot Activation Is Not a Consequence of Increased Transcription of ade6-M26.
Because Mts1/Mts2 binding to M26 activates the hotspot (Table 2) and the Mts1 and Mts2 proteins are transcription factors that induce some genes during meiotic differentiation (16–18), it is plausible that hotspot activation might result from transcriptional transactivation of ade6. However, previous studies of transcript levels of ade6-M26 (hotspot) vs. ade6-M375 (basal recombination) during meiosis (29) revealed no differential levels of ade6 expression. We have repeated those experiments to determine if ade6 message levels were altered in an M26-dependent, Mts1/Mts2-dependent, meiosis-specific fashion. Steady-state ade6 message levels were determined by Northern blot analysis of RNA from cells under a variety of conditions (±M26, ±Mts1, ±Mts2, ±meiosis). The level of ade6 mRNA was equivalent in all cases (data not shown), in agreement with the previous study (29). We conclude that increased transcription of the ade6 gene, conferred by Mts1/Mts2 binding the M26 site, is not the mechanism by which the hotspot is activated. These data, and the different Mts1 and Mts2 protein requirements for hotspot activation (Table 2) and stress response (Fig. 5), suggest that hotspot activation is a distinct function of the Mts1/Mts2 heterodimer.
DISCUSSION
We have cloned the genes, mts1 and mts2, encoding the heterodimeric protein that binds the M26 recombination hotspot (15). Disruption of either gene, or both, abolishes M26 hotspot activity (Table 2), as predicted (15). While our genetic studies were underway, two laboratories described S. pombe genes identical to mts1 (called atf1 and gad7) and mts2 (called pcr1), both isolated through different lines of research (16–18). Genetic analyses revealed that Mts1 (Atf1, Gad7) and Mts2 (Pcr1) proteins have roles in sexual development and stress response and the mutants exhibit altered induction patterns of several genes involved in those pathways (16–20). These data suggest a possible relationship between transcription and recombination. However, hotspot activity is not a consequence of increased transcription because there is no detectable difference in the level of ade6 mRNA during meiosis in strains exhibiting normal (M375) vs. hotspot (M26) recombination (29) or in cells having or lacking the Mts proteins (data not shown). Furthermore, the factors required for stress response (Fig. 5) and hotspot activation (Table 2) are genetically distinct: Mts2 is dispensible for the stress response (Fig. 5) but not for hotspot activation (Table 2). [This separability has also been inferred for hotspots in Saccharomyces cerevisiae: some promoter mutations that alter gene expression do not alter recombination hotspot activity (30, 31)]. The Mts1 and Mts2 proteins acting together or separately therefore have multiple biological roles: one in hotspot activation, one in regulating genes involved in sexual development, and one in regulating genes during stress conditions. In each case, the proteins are constitutively present (15) but apparently inactive during normal mitotic growth, permitting rapid induction of activity in times of crisis. Activation of the Mts1 protein for transcription involves signal transduction via mitogen-activated protein (MAP) kinase and cAMP-dependent kinase pathways, and perhaps others (Fig. 2) (16–20). This allows the cells to rapidly respond to environmental changes without requiring further protein synthesis. The posttranslational modifications of Mts1 and Mts2 that trigger the decision toward meiotic development (16–20) may overlap with those changes that result in meiotic hotspot activation.
Mts1/Mts2 heterodimer binding the M26 site is required for hotspot activation. Although both Mts1 and Mts2 are involved in transcriptional regulation (16, 18, 20), the relevant cis-acting DNA sites have not been defined. Nor is it known whether Mts1 and Mts2 act together, or as homodimers, or as heterodimers with other bZIP proteins when regulating transcription. We expect that different homodimers and heterodimers have different related binding sites and different biological functions (Fig. 2). The use of the same factors for two different processes during meiosis, developmental gene regulation and M26 recombination hotspot activation, provides a good example of the economy of nature.
That some transcription factors may activate recombination hotspots has also been inferred from studies with S. cerevisiae (32–34). For example, full activity of the HIS4 hotspot requires elements of the promoter region that include binding sites for Bas1, Bas2, and Rap1 transcription factors (32, 35). A deletion that includes all the binding sites for these transcription factors abolishes hotspot activity, and replacement with Rap1 binding sites restores high hotspot activity, suggesting that Rap1 protein binding can activate the hotspot (32, 35, 36). A direct demonstration that Rap1 is required for hotspot activation has not been possible however, because the Rap1 protein is essential for viability. Similarly, a direct demonstration of a discrete nucleotide sequence defining the DNA site(s) has not been reported for the natural HIS4 hotspot, but has been for the M26 hotspot (14). The Rap1 protein, like Mts1/Mts2, is a multifunctional transcription factor that is constitutively expressed in mitotic and meiotic cells. Thus, for two hotspots in two different organisms, specific transcription factors have additional roles in meiotic hotspot activation. For other hotspots no single, discrete activating factors have yet been identified, but this could be caused by redundancy of sites clustered at hotspots or limitations of the experimental approaches used (11).
A current model for hotspot activation (10, 11, 34) proposes that the DNA within “open chromatin” becomes accessible to meiotic recombination enzymes that introduce a double-strand DNA break (37, 38). Alternatively, one or a few components that are usually associated with open chromatin, rather than open chromatin itself, enhance recombination (39). Because Mts1/Mts2 protein binding to M26 is required to activate the hotspot, the protein likely remodels chromatin, or recruits recombination enzymes, or both. Meiotically induced, M26 site-dependent open chromatin is found at the M26 hotspot (40) and is entirely dependent on the presence of the Mts1/Mts2 heterodimer (K. Ohta and W.P.W., unpublished observations). [The similarity to “architectural transcription factors” (41) and chromatin remodeling enzymes involved in gene regulation (42, 43) is striking. Perhaps some factors of those processes are also components of M26 hotspot activation.] The current evidence is consistent with open chromatin being either the cause or the effect of hotspot activation: Mts1/Mts2 might create meiotically altered chromatin that serves as an assembly point for meiotic recombination enzymes, or the chromatin alteration might simply be a consequence of Mts1/Mts2 activating recombination while bound to M26. Because the Mts proteins are also transcription factors (16–20), and hotspot activation is not the result of increased transcription (29) (data not shown), we infer that the Mts1/Mts2 heterodimer has separate roles in transcriptional regulation and hotspot activation. It remains to be seen whether Mts1/Mts2 directly recruits recombination enzymes via protein–protein interactions, or whether recombination enzymes assemble at chromatin conformationally altered by Mts1/Mts2 binding to M26 DNA, or both. In either case, binding of Mts1/Mts2 to M26 sites increases recombination above basal levels, indicating that the hotspot with its binding protein regulates where and when recombination occurs within the genome.
Recombination serves a surveillance role in mitotic cells (to repair certain types of DNA damage) and recombination rates are elevated during meiosis, presumably to increase genetic diversity or to insure homolog disjunction or both. Part of the meiotic enhancement is caused by induction of meiotic recombination genes (27) and part may be because of Mts1/Mts2 protein binding to M26 sites throughout the genome (15). Intergenic recombination in mts mutants is reduced as much as 50% in four intervals tested so far (N.K. and W.P.W., unpublished observations), and each of eight M26 sites created by mutagenesis (involving one or a few base pair changes) at five sites in two widely separated loci has hotspot activity (44). These findings support the view that some natural M26 sites are active in meiosis and may contribute to elevated meiotic recombination levels.
These findings permit a speculative calculation of the fraction of meiotic recombination attributable to Mts1/Mts2 heterodimer interacting with M26 sites. There are an estimated 600 M26 sites in the diploid S. pombe genome (15). If each M26 site is, on average, as recombinogenic as ade6-M26 and each promotes recombination in 5% of meioses [the level of gene conversion at ade6-M26 (5)], then ≈30 conversion events per meiosis would be due to M26 sites. About 65% of the conversion events of ade6-M26 are accompanied by reciprocal exchange (28). Thus, ≈20 reciprocal exchange events per meiosis could be because of M26 sites. Because the S. pombe meiotic map size is ≈2,200 cM (≈44 reciprocal exchanges) (45), the predicted 600 M26 sites in the diploid genome could be responsible for ≈50% of meiotic homologous recombination. However, because no study has yet reported whether or not any naturally occurring M26 sites are recombinogenic, this hypothesis remains unproven. The availability of mts mutants that abolish ade6-M26 hotspot activity will be useful for determining what fraction of genomic M26 sites are active.
Acknowledgments
We thank Charlie Albright, Joe Farah, Mary Fox, Ramsay McFarlane, Joachim Ostermann, Philippe Szankasi, Andrew Taylor, Tony Weil and Ron Wisdom for constructive comments and Steve Lindsey and Calley Hardin for laboratory assistance. Cloning of mts1 was supported by the Damon Runyon–Walter Winchell Foundation (W.P.W.) and the National Institutes of Health (G.R.S.), the remainder was supported by the National Institutes of Health and the Vanderbilt University Department of Biochemistry (W.P.W). W.P.W. is a Leukemia Society of America Special Fellow.
ABBREVIATION
- bZIP
basic leucine zipper
Footnotes
References
- 1.Mosig G. Proc Natl Acad Sci USA. 1966;56:1177–1183. doi: 10.1073/pnas.56.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahls W P, Wallace L J, Moore P D. Cell. 1990;60:95–103. doi: 10.1016/0092-8674(90)90719-u. [DOI] [PubMed] [Google Scholar]
- 3.Wahls W P, Wallace L J, Moore P D. Mol Cell Biol. 1990;10:785–793. doi: 10.1128/mcb.10.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voelkel-Meiman K, Keil R L, Roeder G S. Cell. 1987;48:1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- 5.Gutz H. Genetics. 1971;69:331–337. doi: 10.1093/genetics/69.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuchert P, Kohli J. Genetics. 1988;119:507–515. doi: 10.1093/genetics/119.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponticelli A S, Sena E P, Smith G R. Genetics. 1988;119:491–497. doi: 10.1093/genetics/119.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treco D, Arnheim N. Mol Cell Biol. 1986;6:3934–3947. doi: 10.1128/mcb.6.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith G R. Cell. 1991;64:19–27. doi: 10.1016/0092-8674(91)90205-d. [DOI] [PubMed] [Google Scholar]
- 10.Lichten M, Goldman A S H. Annu Rev Genet. 1995;29:423–444. doi: 10.1146/annurev.ge.29.120195.002231. [DOI] [PubMed] [Google Scholar]
- 11.Wahls W P. In: Meiosis and Gametogenesis. Handel M A, editor. San Diego: Academic; 1998. pp. 37–76. [Google Scholar]
- 12.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szankasi P, Heyer W D, Schuchert P, Kohli J. J Mol Biol. 1988;204:917–925. doi: 10.1016/0022-2836(88)90051-4. [DOI] [PubMed] [Google Scholar]
- 14.Schuchert P, Langsford M, Kaslin E, Kohli J. EMBO J. 1991;10:2157–2163. doi: 10.1002/j.1460-2075.1991.tb07750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahls W P, Smith G R. Genes Dev. 1994;8:1693–1702. doi: 10.1101/gad.8.14.1693. [DOI] [PubMed] [Google Scholar]
- 16.Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanoh J, Watanabe Y, Ohsugi M, Iino Y, Yamamoto M. Genes Cells. 1996;1:391–408. doi: 10.1046/j.1365-2443.1996.d01-247.x. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe Y, Yamamoto M. Mol Cell Biol. 1996;16:704–711. doi: 10.1128/mcb.16.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiozaki K, Russell P. Genes Dev. 1996;10:2276–2228. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson M G, Samuels M, Takeda T, Toone W M, Shieh J-C, Toda T, Millar J B A, Jones N. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- 21.Kohli J. Curr Genet. 1987;11:575–589. doi: 10.1007/BF00393919. [DOI] [PubMed] [Google Scholar]
- 22.Gutz H, Heslot H, Leupold U, Loprieno N. In: Schizosaccharomyces pombe. King R C, editor. Vol. 1. New York: Plenum; 1974. pp. 395–446. [Google Scholar]
- 23.Ponticelli A S, Smith G R. Genetics. 1989;123:45–54. doi: 10.1093/genetics/123.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Compton T. In: Degenerate Primers for DNA Amplification. Innis M A, Gelfand D H, Sninsky J J, White T J, editors. San Diego: Academic; 1990. pp. 39–45. [Google Scholar]
- 25.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 27.Li Y F, Numata M, Wahls W P, Smith G R. Mol Microbiol. 1997;23:869–878. doi: 10.1046/j.1365-2958.1997.2691632.x. [DOI] [PubMed] [Google Scholar]
- 28.Grimm C, Bahler J, Kohli J. Genetics. 1994;136:41–51. doi: 10.1093/genetics/136.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimm C, Schaer P, Munz P, Kohli J. Mol Cell Biol. 1991;11:289–298. doi: 10.1128/mcb.11.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultes N P, Szostak J W. Mol Cell Biol. 1991;11:322–328. doi: 10.1128/mcb.11.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White M A, Detloff P, Strand M, Petes T D. Curr Genet. 1992;21:109–116. doi: 10.1007/BF00318468. [DOI] [PubMed] [Google Scholar]
- 32.White M A, Dominska M, Petes T D. Proc Natl Acad Sci USA. 1993;90:6621–6625. doi: 10.1073/pnas.90.14.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Massy B, Baudat F, Nicolas A. Proc Natl Acad Sci USA. 1994;91:11929–11933. doi: 10.1073/pnas.91.25.11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu T C, Lichten M. Science. 1994;263:515–518. doi: 10.1126/science.8290959. [DOI] [PubMed] [Google Scholar]
- 35.White M A, Wierdl M, Detloff P, Petes T D. Proc Natl Acad Sci USA. 1991;88:9755–9759. doi: 10.1073/pnas.88.21.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan Q, Xu F, Petes T D. Mol Cell Biol. 1995;15:1679–1688. doi: 10.1128/mcb.15.3.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keeney S, Giroux C N, Kleckner N. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 38.Bergerat A, de Massy B, Gadelle D, Varoutas P-C, Nicolas A, Forterre P. Nature (London) 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 39.Fan Q Q, Petes T D. Mol Cell Biol. 1996;16:2037–2043. doi: 10.1128/mcb.16.5.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno K, Emura Y, Baur M, Kohli J, Ohta K, Shibata T. Genes Dev. 1997;11:876–886. doi: 10.1101/gad.11.7.876. [DOI] [PubMed] [Google Scholar]
- 41.Werner M H, Burley S K. Cell. 1997;88:733–736. doi: 10.1016/s0092-8674(00)81917-0. [DOI] [PubMed] [Google Scholar]
- 42.Pazin M J, Kadonaga J T. Cell. 1997;88:737–740. doi: 10.1016/s0092-8674(00)81918-2. [DOI] [PubMed] [Google Scholar]
- 43.Pazin M J, Kadonaga J T. Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 44.Fox M E, Virgin J B, Metzger J, Smith G R. Proc Natl Acad Sci USA. 1997;94:7446–7451. doi: 10.1073/pnas.94.14.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munz P. Genetics. 1994;137:701–707. doi: 10.1093/genetics/137.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]


