Abstract
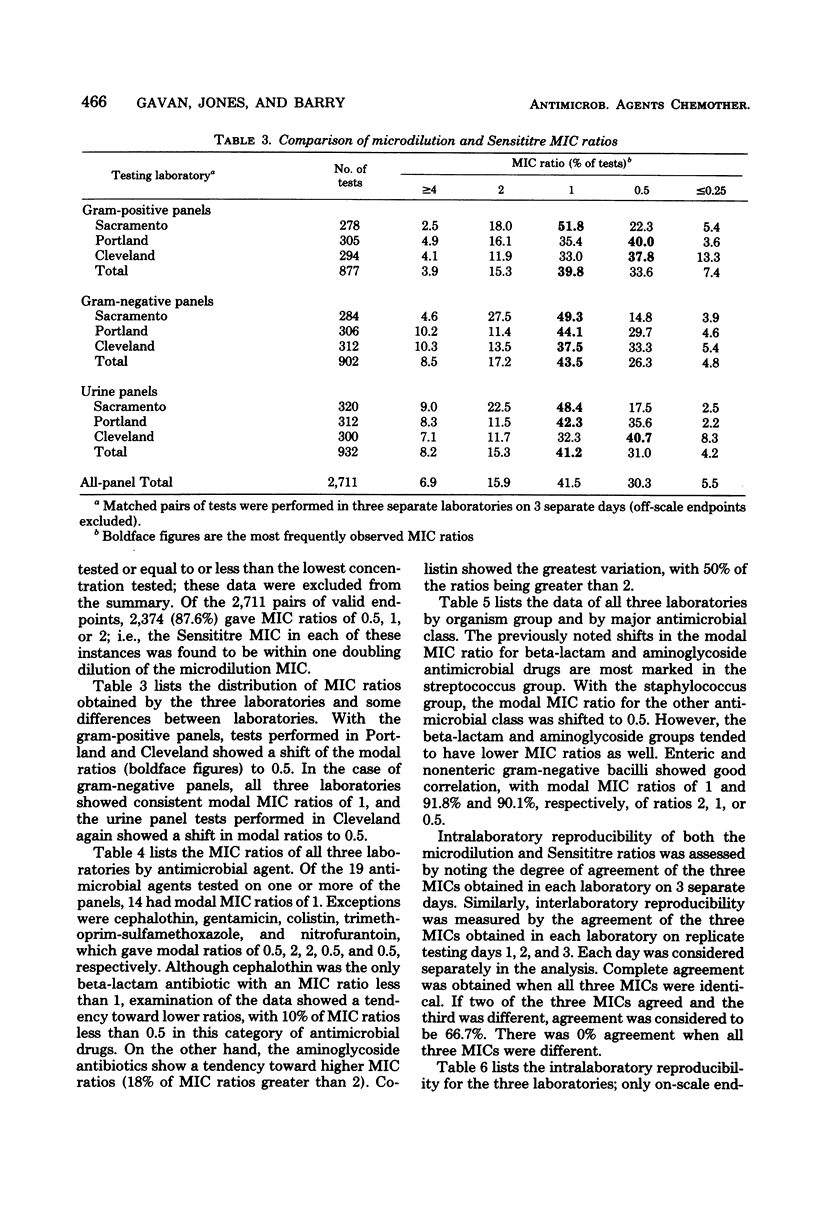
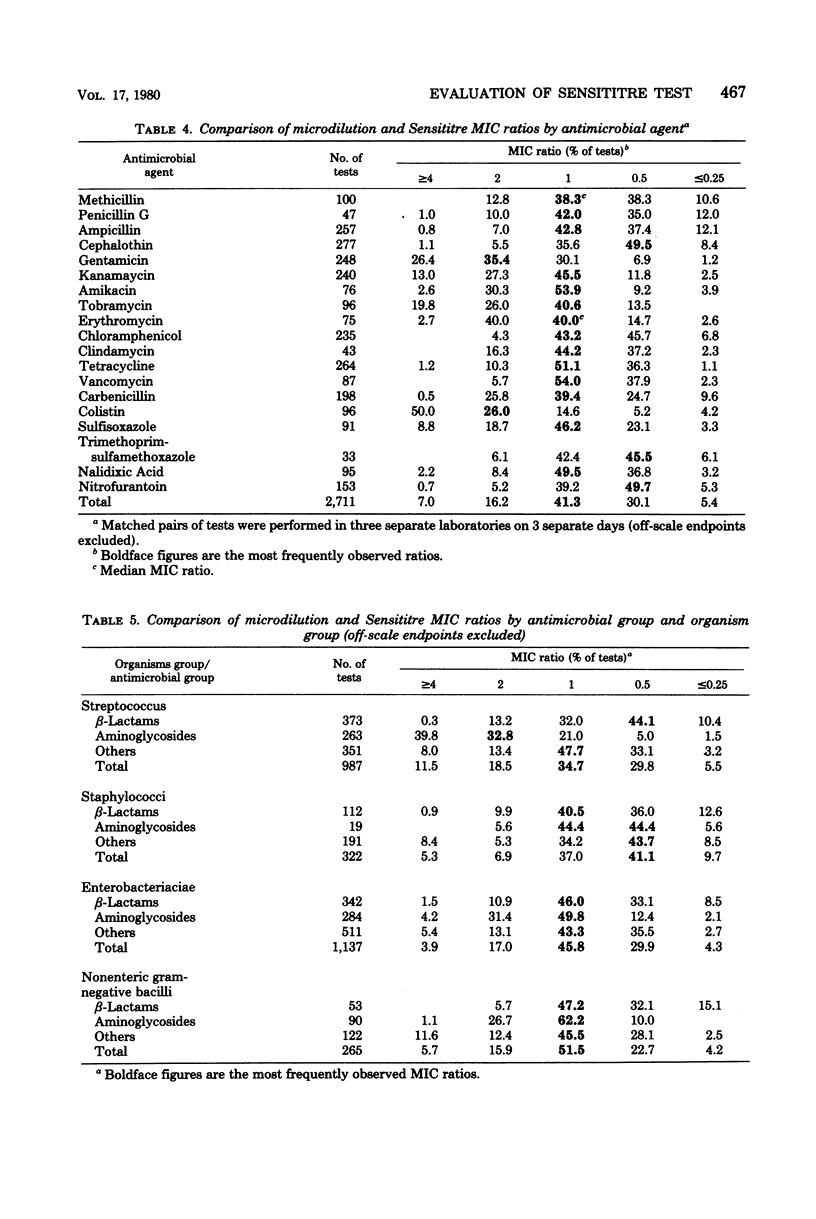
This three-center collaborative study was conducted to evaluate samples of Sensititre antimicrobial microdilution panels (GIBCO/INVENEX). Sensititre minimum inhibitory concentrations of 27 bacterial isolates were compared with those obtained by a reference microdilution method. The Sensititre and microdilution minimum inhibitory concentrations were equivalent within +/- 1 dilution in 87.6% of the comparable test results. Intralaboratory reproducibilities of the Sensititre and microdilution endpoints were equivalent with 80.4 and 82.4%, respetively, of on-scale endpoints in absolute agreement. Sensititre was more reproducible among laboratories, with nearly a 10% greater agreement of triplicate results. The Sensititre microdilution test as evaluated gave results which were essentially equivalent to those obtained with a standardized microdilution method.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N., Gavan T. L. Evaluation of the micro-media system for quantitative antimicrobial drug susceptibility testing: a collaborative study. Antimicrob Agents Chemother. 1978 Jan;13(1):61–69. doi: 10.1128/aac.13.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Gavan T. L., Town M. A. A microdilution method for antibiotic susceptibility testing: an evaluation. Am J Clin Pathol. 1970 Jun;53(6):880–885. doi: 10.1093/ajcp/53.6.880. [DOI] [PubMed] [Google Scholar]
- Phillips I., Warren C., Waterworth P. M. Determination of antibiotic sensitivities by the Sensititre system. J Clin Pathol. 1978 Jun;31(6):531–535. doi: 10.1136/jcp.31.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]


