Abstract
The clinicopathological phenotypes of sporadic Creutzfeldt-Jakob disease (sCJD) correlate with the allelotypes (M or V) of the polymorphic codon 129 of the human prion protein (PrP) gene and the electrophoretic mobility patterns of abnormal prion protein (PrPSc). Transmission of sCJD prions to mice expressing human PrP with a heterologous genotype (referred to as cross-sequence transmission) results in prolonged incubation periods. We previously reported that cross-sequence transmission can generate a new prion strain with unique transmissibility, designated a traceback phenomenon. To verify experimentally the traceback of sCJD-VV2 prions, we inoculated sCJD-VV2 prions into mice expressing human PrP with the 129M/M genotype. These 129M/M mice showed altered neuropathology and a novel PrPSc type after a long incubation period. We then passaged the brain homogenate from the 129M/M mouse inoculated with sCJD-VV2 prions into other 129M/M or 129V/V mice. Despite cross-sequence transmission, 129V/V mice were highly susceptible to these prions compared to the 129M/M mice. The neuropathology and PrPSc type of the 129V/V mice inoculated with the 129M/M mouse-passaged sCJD-VV2 prions were identical to those of the 129V/V mice inoculated with sCJD-VV2 prions. Moreover, we generated for the first time a type 2 PrPSc-specific antibody in addition to type 1 PrPSc-specific antibody and discovered that drastic changes in the PrPSc subpopulation underlie the traceback phenomenon. Here, we report the first direct evidence of the traceback in prion infection.
Creutzfeldt-Jakob disease (CJD) is a lethal transmissible neurodegenerative disease caused by an abnormal isoform of prion protein (PrPSc), which is converted from the normal cellular isoform (PrPC) (1, 23). The genotype (M/M, M/V, or V/V, where M and V are allelotypes) at polymorphic codon 129 of the human prion protein (PrP) gene and the type (type 1 or type 2) of PrPSc in the brain are major determinants of the clinicopathological phenotypes of sporadic CJD (sCJD) (15-18). Type 1 and type 2 PrPSc are distinguishable according to the size of the proteinase K-resistant core of PrPSc (PrPres) (21 and 19 kDa, respectively), reflecting differences in the proteinase K cleavage site (at residues 82 and 97, respectively) (15, 18). According to this molecular typing system, sCJD can be classified into six subgroups (MM1, MM2, MV1, MV2, VV1, or VV2).
The homology of the PrP genes between inoculated animals and the inoculum determines the susceptibility to prion infection. Transmission of sCJD prions to mice expressing human PrP with a nonhomologous genotype (referred to as cross-sequence transmission) results in a relatively long incubation period (10, 12). Meanwhile, the cross-sequence transmission can generate a new prion strain. Transmission of sCJD-VV2 prions to mice expressing human PrP with the 129M/M genotype generates unusual PrPres intermediate in size between type 1 and type 2 (10). We have designated this unusual PrPres with an upward size shift (Sh+) from the inoculated type 2 template MM[VV2]2Sh+ PrPres, where the notation is of the following form: host genotype [type of inoculated prion] type of generated PrPres.
Similar to the MM[VV2]2Sh+ PrPres, the intermediate-sized PrPres has been observed in the plaque-type of dura mater graft-associated CJD (p-dCJD) (10, 13). Furthermore, a transmission study using p-dCJD prions revealed that PrP-humanized mice with the 129V/V genotype were highly susceptible to p-dCJD prions despite cross-sequence transmission (10). In addition, these 129V/V mice inoculated with p-dCJD prions produced type 2 PrPres (10). These findings suggest that p-dCJD could be caused by cross-sequence transmission of sCJD-VV2 prions to individuals with the 129M/M genotype. We have designated this phenomenon “traceback.” The traceback phenomenon was discovered for the first time by a transmission study using variant CJD (vCJD) prions (2). Mice expressing bovine PrP were highly susceptible to vCJD prions because vCJD was caused by cross-sequence transmission of bovine spongiform encephalopathy prions to human. These findings suggest that a traceback study can be a powerful tool to identify the origin of prions (2, 10, 11). However, the traceback phenomenon has not been verified experimentally despite the abundant circumstantial evidence described above.
To verify the traceback of sCJD-VV2 prions, we inoculated sCJD-VV2 prions into PrP-humanized mice with the 129M/M genotype as an experimental model of p-dCJD. Thereafter, we inoculated these MM[VV2]2Sh+ prions into PrP-humanized mice with the 129M/M or 129V/V genotype and compared the incubation period, neuropathology, and the type of PrPres in the brain. Here, we report the first direct evidence of the traceback in prion infection.
MATERIALS AND METHODS
Production of PrPres type-specific polyclonal antibodies.
A synthetic peptide corresponding to human PrP residues 82 to 98 was used as the immunogen for type 1 PrPres-specific antibody Tohoku 1 because residues 82 to 96 were retained in type 1 PrPres but not in type 2 PrPres after proteinase K digestion (18). For type 2 PrPres-specific antibody Tohoku 2, a short synthetic peptide corresponding human PrP residues 97 to 103 was used as the immunogen because the length of the immunogen peptide is critical for the production of proteolytic cleavage site-specific polyclonal antibodies (25, 26). Cysteine residues were added to the C terminus of each peptide, which was utilized for conjugation to bovine thyroglobulin via EMCS [N-(6-maleimidocaproyloxy)succinimide] (Dojin). For the initial injection, 100 μg of conjugate was emulsified in complete Freund's adjuvant and subcutaneously injected into rabbits. For the boosting injections, 100 μg of conjugate was emulsified in incomplete Freund's adjuvant and was subcutaneously injected on days 7, 21, 35, 49, 63, 84, and 91. At day 98, the rabbits were sacrificed, and serum was collected. Antibodies were purified by affinity chromatography using the immunogen peptides. Another type 1 PrPres-specific monoclonal antibody, POM2, reacts with repeated octapeptide epitopes 59 to 65, 67 to 73, 75 to 81, and 83 to 89 of human and murine PrP (21, 22).
Production of knock-in mice and transgenic mice.
The production of knock-in mice expressing human PrP with 129M/M (Ki-Hu129M/M mice) and Ki-Hu129V/V mice has been reported previously (2). Ki-Hu129M/M mice and knock-in mice expressing human PrP with 129M/M and four octapeptide repeats (Ki-Hu129M4R/M4R) were crossed with transgenic mice expressing human PrP with 129M (Tg-Hu129M) and four octapeptide repeats (Tg-Hu129M4R), respectively (10). The expression levels of human PrP in the brains from Tg+Ki-Hu129M/M and Tg+Ki-Hu129M4R/M4R mice were 1.2-fold and 9.8-fold, respectively, the levels observed in Ki-Hu129M/M mice.
Human brain inocula.
Brain tissues were obtained at autopsy from CJD patients after informed consent for research use was received. The diagnosis of CJD and the type of PrPSc were confirmed by neuropathological examination, PrPSc immunohistochemistry, and Western blotting as described previously (7, 27). The genotype and the absence of mutations in the open reading frame of the PrP gene were determined by sequence analysis (8). The CJD cases selected for the transmission studies were typical of the sCJD-MM1 and sCJD-VV2 subgroups. In the sCJD-VV2 (AK) isolate, the plaque-type PrP deposition in the brain and the absence of periodic synchronous discharges on electroencephalogram were confirmed. More detailed information of the patient was reported previously (4). Isolates sCJD-MM1 (H3) and sCJD-VV2 (AK) showed the same levels of transmissibility to PrP-humanized mice as other sCJD-MM1 and sCJD-VV2 isolates, respectively (27).
Transmission experiments.
Human brain homogenates (10%) and mouse brain homogenates (10%) were prepared as described previously (9). Intracerebral transmission was performed using 20 μl of the homogenates (27). The inoculated mice were sacrificed after the onset of disease, and their brains were immediately frozen or fixed in 10% buffered formalin.
Immunohistochemistry.
Formalin-fixed mouse brains were treated with 60% formic acid for 1 h to inactivate the infectivity and then were embedded in paraffin. Tissue sections were pretreated by hydrolytic autoclaving before PrP immunohistochemistry (7). The N-terminal PrP (PrP-N) antiserum was used as the primary antibody (6). Goat anti-rabbit immunoglobulin polyclonal antibody labeled with the peroxidase-conjugated dextran polymer EnVision+ (DakoCytomation) was used as the secondary antibody.
Expression of a GST-recombinant PrP fusion protein.
The open reading frame of the human PrP gene was amplified by PCR with human DNA. The amplified fragment was cloned into pBluescript plasmid (Stratagene). With the plasmid construct, N-terminally or C-terminally truncated human PrP gene fragments were amplified by PCR. The primers used for the amplification are shown in Table 1. These primers introduced BamHI sites at the 5′ end of the fragments and XhoI sites at the 3′ end of the fragments. The amplified fragments were cloned into pGEM-T Easy plasmid (Promega). After digestion with BamHI and XhoI, the fragments were inserted into the BamHI/XhoI sites of the expression vector pGEX-4T-1 (GE Healthcare). Escherichia coli BL21(DE3) cells were transformed with the pGEX-4T-1 plasmid constructs, and recombinant PrP fragments fused to glutathione S-transferase (GST) were purified using glutathione Sepharose 4B beads (GE Healthcare) according to the manufacturer's instructions. GST-tagged recombinant PrP fragments were subjected to 13% SDS-PAGE and Western blotting.
TABLE 1.
Primers used for the amplification of truncated human PrP gene fragments
| Primer function and name | Sequence |
|---|---|
| Amplification of C-terminal truncation fragment | |
| BamHI-23 | 5′-GGATCCAAGAAGCGCCCGAAGCCTGGAGGA-3′ |
| 89-XhoI | 5′-CTCGAGTCACCAGCCACCACCATGAGGCTG-3′ |
| 90-XhoI | 5′-CTCGAGTCAACCCCAGCCACCACCATGAGG-3′ |
| 91-XhoI | 5′-CTCGAGTCATTGACCCCAGCCACCACCATG-3′ |
| 92-XhoI | 5′-CTCGAGTCATCCTTGACCCCAGCCACCACC-3′ |
| 93-XhoI | 5′-CTCGAGTCAACCTCCTTGACCCCAGCCACC-3′ |
| 94-XhoI | 5′-CTCGAGTCAGCCACCTCCTTGACCCCAGCC-3′ |
| 95-XhoI | 5′-CTCGAGTCAGGTGCCACCTCCTTGACCCCA-3′ |
| 96-XhoI | 5′-CTCGAGTCAGTGGGTGCCACCTCCTTGACC-3′ |
| Amplification of N-terminal truncation fragment | |
| BamHI-82 | 5′-CGTGGATCCGGACAGCCTCATGGTGGTGGCTGG-3′ |
| BamHI-84 | 5′-CGTGGATCCCCTCATGGTGGTGGCTGGGGTCAA-3′ |
| BamHI-86 | 5′-CGTGGATCCGGTGGTGGCTGGGGTCAAGGAGGT-3′ |
| BamHI-88 | 5′-CGTGGATCCGGCTGGGGTCAAGGAGGTGGCACC-3′ |
| BamHI-90 | 5′-CGTGGATCCGGTCAAGGAGGTGGCACCCACAGT-3′ |
| BamHI-92 | 5′-CGTGGATCCGGAGGTGGCACCCACAGTCAGTGG-3′ |
| BamHI-94 | 5′-CGTGGATCCGGCACCCACAGTCAGTGGAACAAG-3′ |
| BamHI-97 | 5′-CGTGGATCCAGTCAGTGGAACAAGCCGAGTAAG-3′ |
| 230-XhoI | 5′-CCGCTCGAGTCACGATCCTCTCTGGTAATAGGCCTG-3′ |
Enzyme-linked immunosorbent assay (ELISA).
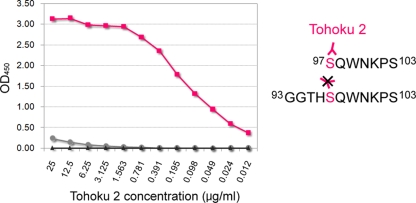
Synthetic peptides corresponding to human PrP residues 97 to 103 and 93 to 103 were used as the antigens. Plates were individually coated with 50 ng/50 μl/well antigen or with 0.1% bovine serum albumin (BSA). The polyclonal antibody Tohoku 2 was serially diluted and added to each well as the primary antibody. A goat anti-rabbit IgG Fab′ fraction labeled with horseradish peroxidase was used as the secondary antibody. The color was developed with ο-phenylenediamine.
Western blotting.
PrPSc was extracted from mouse brains with collagenase treatment as described previously (5) with some modifications. Samples were subjected to 13% SDS-PAGE and Western blotting as described previously (2). The monoclonal antibody 3F4 (Signet Laboratories), PrP-N (Immuno-Biological Laboratories), C-terminal PrP ([PrP-C] Immuno-Biological Laboratories), POM2, Tohoku 1, and Tohoku 2 were used as the primary antibodies. Anti-mouse EnVision+ and anti-rabbit EnVision+ were used as the secondary antibodies. The signal intensities of the Western blots were quantified with Quantity One software using a VersaDoc 5000 (Bio-Rad Laboratories) imaging device.
Statistical analysis.
Incubation times and signal intensities of PrPres bands are expressed as mean ± standard error of the mean (SEM).
RESULTS
Transmission of MM[VV2]2Sh+ prions to PrP-humanized mice with the 129M/M or 129V/V genotype.
To verify the traceback of sCJD-VV2 prions, we performed intracerebral inoculation of a brain homogenate from as sCJD-VV2 patient into Tg+Ki-Hu129M/M mice. Thereafter, we performed the second passage of the brain homogenate from a Tg+Ki-Hu129M/M mouse inoculated with sCJD-VV2 prions (MM[VV2]2Sh+ prions). Since Tg+Ki-Hu129M/M mice were established before the Ki-Hu129M/M mice were produced, we used them in the primary transmission of sCJD-VV2 prions. The data of the primary transmission have been reported previously (10). The mean incubation time of Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions was 723 ± 79 days (number of diseased animals/number of inoculated animals, 4/4) (Table 2). In the second passage, we inoculated MM[VV2]2Sh+ prions intracerebrally into Ki-Hu129M/M mice or Ki-Hu129V/V mice. Ki-Hu129M/M mice inoculated with MM[VV2]2Sh+ prions showed long incubation times of 685 ± 17 days (6/6). In contrast, the mean incubation time of Ki-Hu129V/V mice inoculated with MM[VV2]2Sh+ prions was shortened to 309 ± 3 days (7/7). In spite of cross-sequence transmission, the mean incubation time of Ki-Hu129V/V mice was much shorter than that of Ki-Hu129M/M mice. Immunohistochemical analysis of the brains from Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions showed large plaque-type PrP deposits spread throughout the cerebral gray matter and thalamus (Fig. 1). Ki-Hu129M/M mice inoculated with MM[VV2]2Sh+ prions showed similar patterns of PrP deposition to those of Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions. In contrast, Ki-Hu129V/V mice inoculated with MM[VV2]2Sh+ prions showed diffuse synaptic-type PrP deposits in the gray matter and small plaque-type deposits restricted to the cerebral white matter. These patterns of PrP deposition were identical to those of Ki-Hu129V/V mice inoculated with sCJD-VV2 prions. Thus, we confirmed that Ki-Hu129V/V mice were highly susceptible to MM[VV2]2Sh+ prions that originated from sCJD-VV2 prions and that the neuropathology of Ki-Hu129V/V mice inoculated with MM[VV2]2Sh+ prions was identical to that of the Ki-Hu129V/V mice inoculated with the parental sCJD-VV2 prions.
TABLE 2.
Transmission of sCJD prions to humanized mice with the 129M/M or 129V/V genotype
| Inoculum | Incubation period (days [mean ± SEM]) in the indicated mouse straina |
||
|---|---|---|---|
| Tg+Ki-Hu129M/M (1.2×)b | Ki-Hu129M/M (1×)b | Ki-Hu129V/V (1×)b | |
| sCJD-MM1 (H3) | 429 ± 6 (6/6) | 467 ± 24 (8/8) | 774 ± 32 (6/6) |
| sCJD-VV2 (AK) | 723 ± 79 (4/4) | 633 ± 49 (6/6) | 312 ± 7 (4/4) |
| MM[VV2]2sh+ | ND | 685 ± 17 (6/6) | 309 ± 3 (7/7) |
Values in parentheses are the number of diseased animals/number of inoculated animals. ND, not done.
The expression level of human PrP in the brain.
FIG. 1.
The changes in neuropathology through cross-sequence transmission and traceback transmission. Immunohistochemical analysis of PrPSc in the brains from PrP-humanized mice inoculated with sCJD-VV2 prions or Tg+Ki-Hu129M/M mouse-passaged sCJD-VV2 prions. Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions or Ki-Hu129M/M mice inoculated with MM[VV2]2Sh+ prions showed prominent plaque-type PrP deposits throughout the cerebral gray matter. In contrast, plaque-type PrP deposits were restricted to within the white matter in the brains from Ki-Hu129V/V mice inoculated with sCJD-VV2 prions or MM[VV2]2Sh+ prions. G, gray matter; W, white matter.
Characterization of PrPres in the mouse brains using PrPres type-specific antibodies.
Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions produced unusual PrPres with an upward size shift from the inoculated type 2 template (10). To characterize these type 2Sh+ PrPres, we produced PrPres type-specific antibodies. Type 1 PrPres-specific polyclonal antibody Tohoku 1 reacted with epitopes located between residues 82 and 96 of human PrP (Fig. 2). Type 2 PrPres-specific polyclonal antibody Tohoku 2 reacted with a synthetic peptide corresponding to human PrP residues 97 to 103 (the immunogen peptide) but not with the peptide at residues 93 to 103 (Fig. 3). The amino group at the N terminus of the immunogen peptide might constitute an essential part of the epitopes for Tohoku 2, as reported in other proteolytic cleavage site-specific antibodies (25, 26). Therefore, Tohoku 1 should specifically detect type 1 PrPres, and Tohoku 2 should specifically detect the N-terminal cleavage site of type 2 PrPres after proteinase K digestion (Fig. 4A).
FIG. 2.
Epitope mapping of polyclonal antibody Tohoku 1. (A) GST-tagged C-terminally truncated human PrP fragments were probed with PrP-N, POM2, or Tohoku 1. POM2 (21) reacted with all PrP fragments. In contrast, Tohoku 1 reacted with PrP fragments comprised of residues 23 to 94, 23 to 95, and 23 to 96. In addition, weak reactivity to PrP fragments 23 to 89, 23 to 90, 23 to 91, 23 to 92, and 23 to 93 was also observed. (B) GST-tagged N-terminally truncated human PrP fragments were probed with 3F4, PrP-C, POM2, or Tohoku 1. POM2 reacted with only the PrP fragment of residues 82 to 230. Tohoku 1 reacted with PrP fragments comprised of residues 82 to 230, 84 to 230, 86 to 230, 88 to 230, and 90 to 230. PrP residues 97 to 230, corresponding to proteinase K-digested type 2 PrPres fragment, was not detected by Tohoku 1 or POM2. Low-molecular-weight bands lacking the PrP-C epitope should be degradation products. The reactivity of Tohoku 1 antibody (light blue), POM antibody (dark blue), and the other antibodies is summarized in a schematic diagram on the right.
FIG. 3.
Characterization of polyclonal antibody Tohoku 2 by peptide ELISA. Tohoku 2 specifically reacted with a synthetic peptide corresponding to human PrP residues 97 to 103 (▪) but not with peptide at residues 93 to 103 (•). Control wells were coated with 0.1% BSA (▴). OD450, optical density at 450 nm. The reactivity of Tohoku 2 antibody (pink) is summarized in a schematic diagram on the right. Tohoku 2 should recognize the N terminus of the human PrP fragment comprised of residues 97 to 103.
FIG. 4.
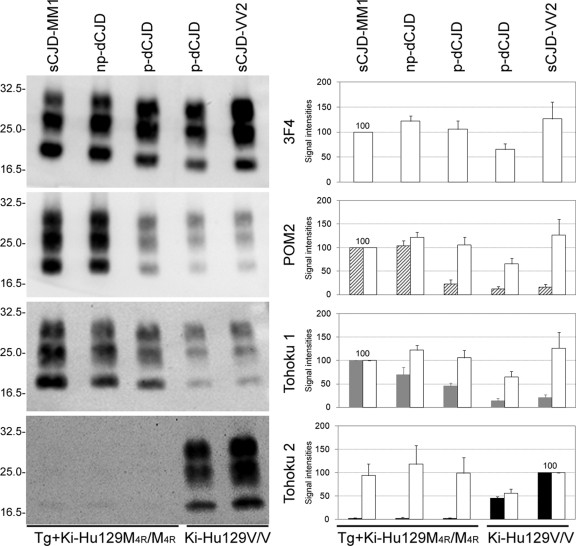
Characterization of PrPres using PrPres type-specific antibodies. (A) The epitopes for type 1 PrPres-specific polyclonal antibody Tohoku 1 (grey) or type 2 PrPres-specific polyclonal antibody Tohoku 2 (black) are summarized in a schematic diagram. POM2 also specifically detects type 1 PrPres (21). 3F4 detects all types of PrPres. (B) Characterization of the human brain inocula used for the transmission studies. Western blot analysis using POM2 and Tohoku 1 revealed that the sCJD-VV2 brain contained minority subpopulations that could be detected by type 1 PrPres-specific antibodies, as reported previously (21, 28). Meanwhile, using type 2 PrPres-specific antibody Tohoku 2, the minority type 2 PrPres subpopulation could be detected even in the sCJD-MM1 brain. The mean signal intensity of PrPres in the sCJD-MM1 brain was assigned as 100/mm2 in each experiment using 3F4 (white bars), POM2 (hatched bars), or Tohoku 1 (gray bars). The mean signal intensity of PrPres in the sCJD-VV2 brain was assigned as 100/mm2 in each experiment using Tohoku 2. The signal intensities of PrPres are expressed as mean ± SEM (n = 3). (C) Western blot analysis using PrPres type-specific antibodies revealed that drastic changes in the PrPres subpopulations underlie the traceback phenomenon. In the cross-sequence transmission of sCJD-VV2 prions to Tg+Ki-Hu129M/M mice, POM2/Tohoku 1-reactive subpopulations were increased, whereas the Tohoku 2 reactive-subpopulation was decreased. Conversely, in the traceback transmission of MM[VV2]2Sh+ prions to Ki-Hu129V/V mice, POM2/Tohoku 1-reactive subpopulations were decreased, whereas the Tohoku 2 reactive-subpopulation predominated. The signal intensity of PrPres from Ki-Hu129M/M mice inoculated with sCJD-MM1 was assigned as 100/mm2 in each experiment using 3F4 (white bars), POM2 (hatched bars), or Tohoku 1 (gray bars). The signal intensity of PrPres from Ki-Hu129V/V mice inoculated with sCJD-VV2 was assigned as 100/mm2 in each experiment using Tohoku 2 (black bars). The signal intensity of PrPres is expressed as the mean ± SEM (n = 4).
First, with these PrPres type-specific antibodies, we performed Western blot analysis of the PrPres in the human brain inocula used in this transmission study (Fig. 4B). In addition to the newly generated type-specific antibodies, we used the monoclonal antibody POM2, which also specifically detects type 1 PrPres (Fig. 2) (21), as a reference antibody. Conventional typing of PrPres using monoclonal antibody 3F4, which detects all PrPres types, showed only a single PrPres type in the brain of an sCJD-MM1 patient or in that of an sCJD-VV2 patient. With the PrPres type-specific antibodies, however, small amounts of POM2/Tohoku 1-reactive subpopulations were observed in the sCJD-VV2 brain (Fig. 4B). The mean signal intensity of PrPres in the sCJD-MM1 brain was assigned as 100/mm2 in each experiment using 3F4, POM2, or Tohoku 1 (n = 3). In the Western blot analysis using 3F4, the mean signal intensity of PrPres in the sCJD-VV2 brain was 224/mm2 (Fig. 4B, white bars). In contrast, the signal intensities of POM2/Tohoku 1-reactive PrPres bands in the sCJD-VV2 brain were 12/mm2 (Fig. 4B, hatched bars) and 48/mm2 (gray bars), respectively. Thus, using type 1 PrPres-specific antibody, the sCJD-VV2 brain contained minority subpopulations that could be detected by type 1 PrPres-specific antibodies, as reported previously (21, 28). The sizes of POM2/Tohoku 1-reactive bands were smaller than those of type 1 PrPres in the sCJD-MM1 brain. Thus, POM2 and Tohoku 1 could detect the intermediate-sized PrPres in addition to type 1 PrPres. Furthermore, trace amounts of the Tohoku 2-reactive subpopulation were observed in the sCJD-MM1 brain (Fig. 4B, black bars). The mean signal intensity of PrPres in the sCJD-VV2 brain was assigned as 100/mm2 in each experiment using Tohoku 2 (n = 3). The mean signal intensity of Tohoku 2-reactive PrPres bands in the sCJD-MM1 brain was 2/mm2. Thus, using type 2 PrPres-specific antibody, the minority type 2 PrPres subpopulation could be detected even in the sCJD-MM1 brain.
Second, we performed Western blot analysis of PrPres in the mouse brains using the PrPres type-specific antibodies. Western blot analysis using 3F4 showed that Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions produced type 2Sh+ PrPres that was located between type 1 PrPres from Ki-Hu129M/M mice inoculated with sCJD-MM1 prions and type 2 PrPres from Ki-Hu129V/V mice inoculated with sCJD-VV2 prions (Fig. 4C) (10). These PrPres were probed with type-specific antibodies Tohoku 1, Tohoku 2, or POM2. The brains from Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions contained POM2/Tohoku 1-reactive PrPres subpopulations in which the PrPres were smaller than those of type 1 PrPres from Ki-Hu129M/M inoculated with sCJD-MM1 prions. However, the signal intensities of these POM2/Tohoku 1-reactive bands were apparently decreased compared to those detected by 3F4 (Fig. 4C, bar graphs). The mean signal intensity of PrPres from Ki-Hu129M/M mice inoculated with sCJD-MM1 prions was assigned as 100/mm2 in each experiment using 3F4, POM2, or Tohoku 1 (n = 4). In the brains from Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions, the mean signal intensity of 3F4-reactive PrPres bands was 154/mm2 (white bars). However, the mean signal intensities of POM2/Tohoku 1-reactive PrPres bands were 34/mm2 (hatched bar) and 113/mm2 (gray bar), respectively. Since more of the epitopes for Tohoku 1 were located at the C terminus than for POM2 (Fig. 2), the signal intensities of Tohoku 1-reactive bands might be higher than those of POM2-reactive bands. Thus, the brains from Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions contained the intermediate-sized PrPres, but certain subpopulations that could not be detected by POM2 or Tohoku 1 must also have been present. The Tohoku 2-reactive subpopulation was not observed in Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions or Ki-Hu129M/M mice inoculated with sCJD-MM1 prions (Fig. 4C, black bars). The mean signal intensity of PrPres from Ki-Hu129V/V mice inoculated with sCJD-VV2 prions was assigned as 100/mm2 in each experiment using Tohoku 2 (n = 4). The brains from Ki-Hu129V/V mice inoculated with sCJD-VV2 prions contained small amounts of POM2/Tohoku 1-reactive subpopulations in addition to the Tohoku 2-reactive majority subpopulation. Thus, in the cross-sequence transmission of sCJD-VV2 prions to Tg+Ki-Hu129M/M mice, POM2/Tohoku 1-reactive subpopulations were increased, whereas the Tohoku 2 reactive-subpopulation was decreased. Therefore, the upward size shift from type 2 to type 2Sh+ in the Western blot analysis using 3F4 reflected the shift of the majority PrPres subpopulation from the Tohoku 2-reactive subpopulation to the POM2/Tohoku 1-reactive subpopulation.
In the second passage of the brain homogenate from Tg+Ki-Hu129M/M mouse inoculated with sCJD-VV2 prions (MM[VV2]2Sh+ prions: host genotype [type of inoculated prions] type of generated PrPres), Ki-Hu129M/M mice inoculated with MM[VV2]2Sh+ prions produced the intermediate-sized PrPres that were identical in size to parental MM[VV2]2Sh+ prions when probed with 3F4 (Fig. 4C). Similar to the brains from Tg+Ki-Hu129M/M mice inoculated with sCJD-VV2 prions, the brains from Ki-Hu129M/M mice inoculated with MM[VV2]2Sh+ prions contained POM2/Tohoku 1-reactive subpopulations but not the Tohoku 2-reactive subpopulation. In the brains from Ki-Hu129M/M mice inoculated with MM[VV2]2Sh+ prions, the mean signal intensities of 3F4, POM2, and Tohoku 1-reactive PrPres bands were 176/mm2 (Fig. 4C, white bars), 85/mm2 (hatched bars), and 139/mm2 (gray bars), respectively. Meanwhile, Western blot analysis using 3F4 showed that Ki-Hu129V/V mice inoculated with MM[VV2]2Sh+ prions produced type 2 PrPres; i.e., the intermediate-sized PrPres reverted to type 2 when MM[VV2]2Sh+ prions were transmitted to Ki-Hu129V/V mice. Moreover, in the traceback transmission of MM[VV2]2Sh+ prions to Ki-Hu129V/V mice, POM2/Tohoku 1-reactive subpopulations were decreased, whereas the Tohoku 2 reactive-subpopulation predominated. In the brains from Ki-Hu129V/V mice inoculated with MM[VV2]2Sh+ prions, the mean signal intensities of 3F4, POM2, and Tohoku 1-reactive PrPres bands were 179/mm2 (Fig. 4C, white bars), 13/mm2 (hatched bars), and 43/mm2 (gray bars), respectively, whereas the mean signal intensity of the Tohoku 2-reactive PrPres bands was 159/mm2 (black bars). Thus, PrPres type-specific antibodies revealed that cross-sequence transmission of sCJD-VV2 prions generated a new prion strain (MM[VV2]2Sh+ prions) with an altered proportion of PrPres subpopulations and that the altered proportion reverted to the original proportion through the traceback transmission to Ki-Hu129V/V mice.
Traceback study of p-dCJD prions reevaluated with the PrPres type-specific antibodies.
We reported previously that p-dCJD prions showed the intermediate-sized PrPres, and that Ki-Hu129V/V mice inoculated with p-dCJD prions showed accumulation of type 2 PrPres (10). To characterize these PrPres in the brains from PrP-humanized mice inoculated with p-dCJD prions, we performed Western blot analysis using PrPres type-specific antibodies (Fig. 5). Since Tg+Ki-Hu129M4R/M4R mice were already established before the Ki-Hu129M/M mice were produced, we used them in the traceback study of p-dCJD or nonplaque-type dCJD (np-dCJD) prions. Subsequently, we confirmed that Tg+Ki-Hu129M4R/M4R, Ki-Hu129M/M, and Tg+Ki-Hu129M/M mice produced PrPres identical in size in the transmission studies using various CJD prions (10). POM2/Tohoku 1-reactive subpopulations existed in the brains from Tg+Ki-Hu129M4R/M4R mice inoculated with p-dCJD prions, but the signal intensities were apparently decreased compared to those detected by 3F4 (Fig. 5). The mean signal intensity of PrPres from Tg+Ki-Hu129M4R/M4R mice inoculated with sCJD-MM1 prions was assigned as 100/mm2 in each experiment using 3F4, POM2, or Tohoku 1 (n = 3). In the brains from Tg+Ki-Hu129M4R/M4R mice inoculated with p-dCJD prions, the mean signal intensity of 3F4-reactive PrPres bands was 105/mm2 (Fig. 5, white bars). However, the mean signal intensities of POM2/Tohoku 1-reactive PrPres bands were 23/mm2 (hatched bars) and 45/mm2 (gray bars), respectively. The POM2/Tohoku 1-reactive bands were smaller than those of type 1 PrPres from Tg+Ki-Hu129M4R/M4R mice inoculated with np-dCJD prions or sCJD-MM1 prions. In addition, trace amounts of the Tohoku 2-reactive subpopulation were observed in the brains from Tg+Ki-Hu129M4R/M4R mice inoculated with p-dCJD prions, np-dCJD prions, or sCJD-MM1 prions (Fig. 5, black bars). The mean signal intensity of PrPres from Ki-Hu129V/V mice inoculated with sCJD-VV2 prions was assigned as 100/mm2 in each experiment using Tohoku 2 (n = 3). Since the sizes of these Tohoku 2-reactive bands were identical to those of type 2 PrPres from Ki-Hu129V/V mice inoculated with sCJD-VV2 prions, Tohoku 2 could specifically detect type 2 PrPres. Meanwhile, in the transmission of p-dCJD prions to Ki-Hu129V/V mice, POM2/Tohoku 1-reactive subpopulations were decreased, whereas the Tohoku 2-reactive subpopulation predominated. In the brains from Ki-Hu129V/V mice inoculated with p-dCJD prions, the mean signal intensities of 3F4, POM2, and Tohoku 1-reactive PrPres bands were 65/mm2 (Fig. 5, white bars), 12/mm2 (hatched bars), and 13/mm2 (gray bars), respectively, whereas the mean signal intensity of Tohoku 2-reactive PrPres bands was 45/mm2 (black bars). Thus, the changes in PrPres subpopulation observed in this traceback study of p-dCJD prions were identical to those observed in the traceback study of MM[VV2]2Sh+ prions.
FIG. 5.
Traceback study of p-dCJD prions reevaluated with PrPres type-specific antibodies. In the traceback transmission of p-dCJD prions to Ki-Hu129V/V mice, POM2/Tohoku 1-reactive subpopulations were decreased, whereas the Tohoku 2 reactive-subpopulation predominated. In addition, trace amounts of the Tohoku 2-reactive subpopulation were observed in the brains from Tg+Ki-Hu129M4R/M4R mice inoculated with p-dCJD prions, np-dCJD prions, or sCJD-MM1 prions. The signal intensity of PrPres from Tg+Ki-Hu129M4R/M4R mice inoculated with sCJD-MM1 was assigned as 100/mm2 in each experiment using 3F4 (white bars), POM2 (hatched bars), or Tohoku 1 (gray bars). The signal intensity of PrPres from Ki-Hu129V/V mice inoculated with sCJD-VV2 was assigned as 100/mm2 in each experiment using Tohoku 2 (black bars). The signal intensity of PrPres is expressed as the mean ± SEM (n = 3).
DISCUSSION
In order to protect humans and animals from infectious diseases, it is often crucial to determine the origin of the isolates that may lie at the origin of epidemics. In the case of conventional pathogens, this is relatively simple and primarily involves the sequencing of pathogen-associated nucleic acids. Because prions lack informational nucleic acids, however, the unambiguous assignment of a given infection to a specific source is very often impossible. Therefore, methods aimed at characterizing stable prion properties after passaging through hosts would be extremely valuable.
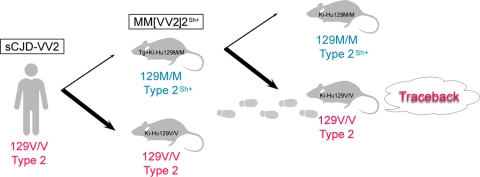
Here, we demonstrate the first direct evidence of traceback in prion infection. Ki-Hu129V/V mice were highly susceptible to the Tg+Ki-Hu129M/M mouse-passaged sCJD-VV2 prions (MM[VV2]2Sh+ prions) despite cross-sequence transmission (Fig. 6). In addition, MM[VV2]2Sh+ prions and sCJD-VV2 prions exhibited similar neuropathologies and the identical PrPres types when inoculated into Ki-Hu129V/V mice; i.e., the altered disease phenotypes and unusual PrPres type of MM[VV2]2Sh+ prions reverted to those of the parental sCJD-VV2 prions. Furthermore, we generated for the first time type 2 PrPres-specific polyclonal antibody Tohoku 2 in addition to type 1 PrPres-specific polyclonal antibody Tohoku 1. These PrPres type-specific antibodies revealed that drastic changes in the PrPres subpopulations underlie the traceback phenomenon.
FIG. 6.
Diagram of the traceback studies. The cross-sequence transmission of sCJD-VV2 prions to Tg+Ki-Hu129M/M mice generated a new prion strain (MM[VV2]2Sh+ prions) with altered conformational properties and disease phenotypes after a long incubation period. In the secondary transmission, Ki-Hu129V/V mice were highly susceptible to these MM[VV2]2Sh+ prions despite cross-sequence transmission. Furthermore, the altered conformational properties and disease phenotypes reverted to the original ones. If atypical prion strains emerge through cross-sequence transmission, traceback studies can be a reliable tool to identify the origin of prions.
The present study clearly shows that traceback studies can be a reliable tool to identify the origin of prions if atypical prion strains emerge through cross-sequence transmission. Although the numbers of animals and human brain inocula used for the transmission were limited in the present study, we demonstrated experimentally the traceback phenomenon: Ki-Hu129V/V mice were highly susceptible to MM[VV2]2Sh+ prions that originated from sCJD-VV2 prions. In the cross-sequence transmission of sCJD-VV2 prions to Tg+Ki-Hu129M/M mice, POM2/Tohoku 1-reactive subpopulations were increased, whereas the Tohoku 2-reactive subpopulation was decreased. In contrast, the altered proportion of PrPres subpopulations reverted to the original proportion through the traceback transmission to Ki-Hu129V/V mice. Similar changes in the PrPres subpopulations were observed in the traceback transmission of p-dCJD prions to Ki-Hu129V/V mice. Therefore, the present study shows again that p-dCJD could be caused by cross-sequence transmission of sCJD-VV2 prions to individuals with the 129M/M genotype.
The drastic changes in the PrPres subpopulations can be the molecular basis of the traceback phenomena. We suppose that the subpopulation change observed in the cross-sequence transmission is due to adaptation and/or a selection process (3, 20), which requires a relatively long incubation period. In contrast, the subpopulation change observed in the traceback transmission might be due to reemergence of the parental prions. Since the emerging prion strain generated by the cross-sequence transmission retains the memory of the parental prions within its conformational properties or repertoire of PrPSc subpopulations, the parental PrPSc subpopulation reemerges and becomes predominant if the emerging prion strain is transmitted to the original host. Therefore, the incubation period can be shortened, and the altered disease phenotypes revert to the original ones in traceback transmission.
Unexpectedly, type 2 PrPres-specific antibody Tohoku 2 revealed that trace amounts of type 2 PrPres coexisted with type 1 PrPres in the brain of an sCJD-MM1 patient. In addition, PrP-humanized mice with the 129M/M genotype inoculated with sCJD-MM1 prions could produce trace amounts of type 2 PrPres in addition to type 1 PrPres. The additional type 2 PrPres was detected in Tg+Ki-Hu129M4R/M4R but not in Ki-Hu129M/M mice. Since Tg+Ki-Hu129M4R/M4R mice express human PrP with four octapeptide repeats at 9.8-fold the level observed in Ki-Hu129M/M mice, these differences might account for the subtle change. Further large-scale studies are needed to determine whether an additional type 2 PrPres can be detected by Tohoku 2 in other human CJD cases formerly classified as type 1.
The present study raises the possibility that cooccurrence of multiple PrPres subpopulations in the same brain might be a general phenomenon. Both type 1 and type 2 PrPres can be detected in the same brain in 35% of sCJD patients examined (19, 24). By using type 1 PrPres-specific antibodies, the minority type 1 subpopulation can be detected with type 2 in all sCJD patients or variant CJD patients formerly classified as type 2 (21, 28). In accord with these reports, small amounts of type 1 (and the intermediate-sized) PrPres were detected by type 1 PrPres-specific antibodies in Ki-Hu129V/V mice inoculated with sCJD-VV2 prions in the present study. In addition, trace amounts of type 2 PrPres were detected by type 2 PrPres-specific antibody in the sCJD-MM1 patient or Tg+Ki-Hu129M4R/M4R mice inoculated with sCJD-MM1 prions. These findings are in line with a report that diverse PrPres fragments can be detected by N-terminal amino acid sequencing in the same brain even though only a single PrPres type is detected by conventional Western blot analysis (18). Since the conventional Western blot analysis using antibodies that react with all PrPres types failed to detect type 1 PrPres unless type 1 PrPres represented more than 30 to 40% of total PrPres in experimentally mixed type 1 and type 2 brain samples, the cooccurrence of multiple PrPres subpopulations might be underestimated (21, 28). Therefore, the “type” of PrPSc determined by the conventional typing system might merely represent the predominant PrPres subpopulation among multiple subpopulations.
However, the biological importance of the minority PrPres subpopulation detected by the PrPres type-specific antibodies or by N-terminal amino acid sequencing remains to be determined. Insufficient proteinase K digestion can generate type 1-specific antibody-reactive PrP bands in brain samples from sCJD or vCJD patients classified as type 2 (14). Otherwise, the size of the PrPres fragment might not always reflect the conformation of PrPSc; e.g., the minority MM1 PrPres subpopulation detected in sCJD-MM2 patients might differ from the genuine MM1 PrPres of sCJD-MM1 patients. It remains unknown whether the minority PrPres subpopulation has infectivity and pathogenicity to cause prion disease. A concise and attractive explanation would be that the proportion of PrPSc subpopulations in the brain determines the disease phenotype, transmissibility, and the type of PrPSc determined by the conventional typing system, but further studies are needed to elucidate why multiple PrPres subpopulations can be detected in the same brain. Therefore, the significance of the conventional molecular typing system using antibodies that react with all PrPres types is likely to continue to be used in the classification of sCJD.
In conclusion, we verified experimentally that traceback studies can be a reliable tool to identify the origin of prions. The present study shows that the changes in PrPres subpopulations correlate with the changes in prion strain-specific properties, e.g., transmissibility and disease phenotypes, in the traceback transmission. Hereafter, the proportion of PrPres subpopulations in human CJD cases should be analyzed quantitatively using the PrPres type-specific antibodies Tohoku 1 and Tohoku 2.
Acknowledgments
We thank Y. Ishikawa, H. Kudo and K. Abe for excellent technical assistance and B. Bell for critical review of the manuscript.
This study was supported by the Program for Promotion of Fundamental Studies in Health Sciences of National Institute of Biomedical Innovation (S.M. and T.K.), a Grant-in-Aid from the Ministry of Health, Labor and Welfare (A.K., S.M., and T.K.), and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (A.K. and T.K.).
Footnotes
Published ahead of print on 20 January 2010.
REFERENCES
- 1.Aguzzi, A., and A. M. Calella. 2009. Prions: protein aggregation and infectious diseases. Physiol. Rev. 89:1105-1152. [DOI] [PubMed] [Google Scholar]
- 2.Asano, M., S. Mohri, J. W. Ironside, M. Ito, N. Tamaoki, and T. Kitamoto. 2006. vCJD prion acquires altered virulence through trans-species infection. Biochem. Biophys. Res. Commun. 342:293-299. [DOI] [PubMed] [Google Scholar]
- 3.Collinge, J., and M. R. Scott. 2007. A general model of prion strains and their pathogenicity. Science 318:930-936. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima, R., Y. Shiga, M. Nakamura, J. Fujimori, T. Kitamoto, and Y. Yoshida. 2004. MRI characteristics of sporadic CJD with valine homozygosity at codon 129 of the prion protein gene and PrPSc type 2 in Japan. J. Neurol. Neurosurg. Psychiatry 75:485-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grathwohl, K. U. D., M. Horiuchi, N. Ishiguro, and M. Shinagawa. 1996. Improvement of PrPSc-detection in mouse spleen early at the preclinical stage of scrapie with collagenase-completed tissue homogenization and Sarkosyl-NaCl extraction of PrPSc. Arch. Virol. 141:1863-1874. [DOI] [PubMed] [Google Scholar]
- 6.Kitamoto, T., T. Muramoto, C. Hilbich, K. Beyreuther, and J. Tateishi. 1991. N-terminal sequence of prion protein is also integrated into kuru plaques in patients with Gerstmann-Sträussler syndrome. Brain Res. 545:319-321. [DOI] [PubMed] [Google Scholar]
- 7.Kitamoto, T., R. W. Shin, K. Doh-ura, N. Tomokane, M. Miyazono, T. Muramoto, and J. Tateishi. 1992. Abnormal isoform of prion proteins accumulates in the synaptic structures of the central nervous system in patients with Creutzfeldt-Jakob disease. Am. J. Pathol. 140:1285-1294. [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamoto, T., M. Ohta, K. Doh-ura, S. Hitoshi, Y. Terao, and J. Tateishi. 1993. Novel missense variants of prion protein in Creutzfeldt-Jakob disease or Gerstmann-Sträussler syndrome. Biochem. Biophys. Res. Commun. 191:709-714. [DOI] [PubMed] [Google Scholar]
- 9.Kitamoto, T., S. Mohri, J. W. Ironside, I. Miyoshi, T. Tanaka, N. Kitamoto, S. Itohara, N. Kasai, M. Katsuki, J. Higuchi, T. Muramoto, and R. W. Shin. 2002. Follicular dendritic cell of the knock-in mouse provides a new bioassay for human prions. Biochem. Biophys. Res. Commun. 294:280-286. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi, A., M. Asano, S. Mohri, and T. Kitamoto. 2007. Cross-sequence transmission of sporadic Creutzfeldt-Jakob disease creates a new prion strain. J. Biol. Chem. 282:30022-30028. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi, A., M. Asano, S. Mohri, and T. Kitamoto. 2009. A traceback phenomenon can reveal the origin of prion infection. Neuropathology 29:619-624. [DOI] [PubMed] [Google Scholar]
- 12.Korth, C., K. Kaneko, D. Groth, N. Heye, G. Telling, J. Mastrianni, P. Parchi, P. Gambetti, R. Will, J. Ironside, C. Heinrich, P. Tremblay, S. J. DeArmond, and S. B. Prusiner. 2003. Abbreviated incubation times for human prions in mice expressing a chimeric mouse-human prion protein transgene. Proc. Natl. Acad. Sci. U. S. A. 100:4784-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kretzschmar, H. A., S. Sethi, Z. Földvári, O. Windl, V. Querner, I. Zerr, and S. Poser. 2003. Iatrogenic Creutzfeldt-Jakob disease with florid plaques. Brain Pathol. 13:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notari, S., S. Capellari, J. Langeveld, A. Giese, R. Strammiello, P. Gambetti, H. A. Kretzschmar, and P. Parchi. 2007. A refined method for molecular typing reveals that co-occurrence of PrPSc types in Creutzfeldt-Jakob disease is not the rule. Lab. Invest. 87:1103-1112. [DOI] [PubMed] [Google Scholar]
- 15.Parchi, P., R. Castellani, S. Capellari, B. Ghetti, K. Young, S. G. Chen, M. Farlow, D. W. Dickson, A. A. F. Sima, J. Q. Trojanowski, R. B. Petersen, and P. Gambetti. 1996. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann. Neurol. 39:767-778. [DOI] [PubMed] [Google Scholar]
- 16.Parchi, P., S. Capellari, S. G. Chen, R. B. Petersen, P. Gambetti, N. Kopp, P. Brown, T. Kitamoto, J. Tateishi, A. Giese, and H. Kretzschmar. 1997. Typing prion isoforms. Nature 386:232-234. [DOI] [PubMed] [Google Scholar]
- 17.Parchi, P., A. Giese, S. Capellari, P. Brown, W. Schulz-Schaeffer, O. Windl, I. Zerr, H. Budka, N. Kopp, P. Piccardo, S. Poser, A. Rojiani, N. Streichemberger, J. Julien, C. Vital, B. Ghetti, P. Gambetti, and H. Kretzschmar. 1999. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 46:224-233. [PubMed] [Google Scholar]
- 18.Parchi, P., W. Zou, W. Wang, P. Brown, S. Capellari, B. Ghetti, N. Kopp, W. J. Schulz-Schaeffer, H. A. Kretzschmar, M. W. Head, J. W. Ironside, P. Gambetti, and S. G. Chen. 2000. Genetic influence on the structural variations of the abnormal prion protein. Proc. Natl. Acad. Sci. U. S. A. 97:10168-10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parchi, P., R. Strammiello, S. Notari, A. Giese, J. P. M. Langeveld, A. Ladogana, I. Zerr, F. Roncaroli, P. Cras, B. Ghetti, M. Pocchiari, H. Kretzschmar, and S. Capellari. 29 August 2009. Incidence and spectrum of sporadic Creutzfeldt-Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neuropathol. doi: 10.1007/s00401-009-0585-1. [DOI] [PMC free article] [PubMed]
- 20.Peretz, D., R. A. Williamson, G. Legname, Y. Matsunaga, J. Vergara, D. R. Burton, S. J. DeArmond, S. B. Prusiner, and M. R. Scott. 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921-932. [DOI] [PubMed] [Google Scholar]
- 21.Polymenidou, M., K. Stoeck, M. Glatzel, M. Vey, A. Bellon, and A. Aguzzi. 2005. Coexistence of multiple PrPSc types in individuals with Creutzfeldt-Jakob disease. Lancet Neurol. 4:805-814. [DOI] [PubMed] [Google Scholar]
- 22.Polymenidou, M., R. Moos, M. Scott, C. Sigurdson, Y. Z. Shi, B. Yajima, I. Hafner-Bratkovic, R. Jerala, S. Hornemann, K. Wuthrich, A. Bellon, M. Vey, G. Garen, M. N. James, N. Kav, and A. Aguzzi. 2008. The POM monoclonals: a comprehensive set of antibodies to non-overlapping prion protein epitopes. PLoS One 3:e3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prusiner, S. B., M. R. Scott, J. P. DeArmond, and F. E. Cohen. 1998. Prion protein biology. Cell 93:337-348. [DOI] [PubMed] [Google Scholar]
- 24.Puoti, G., G. Giaccone, G. Rossi, B. Canciani, O. Bugiani, and F. Tagliavini. 1999. Sporadic Creutzfeldt-Jakob disease: co-occurrence of different types of PrPSc in the same brain. Neurology 53:2173-2176. [DOI] [PubMed] [Google Scholar]
- 25.Saido, T. C., S. Nagao, M. Shiramine, M. Tsukaguchi, H. Sorimachi, H. Murofushi, T. Tsuchiya, H. Ito, and K. Suzuki. 1992. Autolytic transition of μ-calpain upon activation as resolved by antibodies distinguishing between the pre- and post-autolysis forms. J. Biochem. 111:81-86. [DOI] [PubMed] [Google Scholar]
- 26.Saido, T. C., T. Iwatsubo, D. M. A. Mann, H. Shimada, Y. Ihara, and S. Kawashima. 1995. Dominant and differential deposition of distinct β-amyloid peptide species, AβN3(pE), in senile plaques. Neuron 14:457-466. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi, Y., S. Mohri, J. W. Ironside, T. Muramoto, and T. Kitamoto. 2003. Humanized knock-in mice expressing chimeric prion protein showed varied susceptibility to different human prions. Am. J. Pathol. 163:2585-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yull, H. M., D. L. Ritchie, J. P. M. Langeveld, F. G. van Zijderveld, M. E. Bruce, J. W. Ironside, and M. W. Head. 2006. Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am. J. Pathol. 168:151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]