Abstract
Recombinant vesicular stomatitis viruses (VSV) are excellent candidate vectors for vaccination against human diseases. The neurovirulence of VSV in animal models requires the attenuation of the virus for use in humans. Previous efforts have focused on attenuating virus replication. Studies presented here test an alternative approach for attenuation that uses a matrix (M) protein mutant (rM51R) VSV as a vaccine vector against respiratory infection. This mutant is attenuated for viral virulence by its inability to suppress the innate immune response. The ability of rM51R VSV vectors to protect against lethal respiratory challenge was tested using a vaccinia virus intranasal challenge model. Mice immunized intranasally with rM51R vectors expressing vaccinia virus antigens B5R and L1R were protected against lethal vaccinia virus challenge. A single immunization with the vectors provided protection against vaccinia virus-induced mortality; however, a prime-boost strategy reduced the severity of the vaccinia virus-induced disease progression. Antibody titers measured after the prime and boost were low despite complete protection against lethal challenge. However, immunized animals had higher antibody titers during the challenge, suggesting that memory B-cell responses may be important for the protection. Depletion experiments demonstrated that B cells but not CD8 T cells were involved in the protection mediated by rM51R vaccine vectors that express B5R and L1R. These results demonstrate the potential of M protein mutant VSVs as candidate vaccine vectors against human diseases.
Recombinant vesicular stomatitis virus (rVSV) vectors have been in commercial development as potential HIV vaccines and have shown promise as candidate vaccine vectors for Ebola and Marburg viruses, Lassa fever virus, influenza virus, respiratory syncytial virus, and measles virus (13, 14, 18, 19, 22-24, 36, 38, 40). The relatively low pathogenicity of VSV for humans makes it a good candidate for a vaccine vector; however, because VSV is neurovirulent in mice, VSV vectors have to be attenuated for use as vaccines. Many of the VSV-based vectors are attenuated by decreasing their ability to replicate within the host, which can also decrease the immunogenicity of the vaccine. Attenuation of VSV has been achieved by genetically altering or deleting the glycoprotein (G) (12, 13, 34-37, 41, 42) or by rearrangement of the gene order to alter protein expression (12, 46). An alternative strategy for attenuating the pathogenesis of VSV is to reduce the ability of the virus to inhibit the host innate immune response (2). The goal of the experiments presented here was to test this strategy using matrix (M) protein mutants of VSV as vaccine vectors.
Previous data from our laboratory and others has shown that the M protein of VSV is responsible for suppressing the host innate immune response by inhibiting host gene expression (3, 9, 10, 43). M protein inhibits host gene expression at multiple levels including host transcription, host mRNA transport, and translation of host mRNAs (29). A recombinant mutant virus in which the methionine at position 51 is mutated to an arginine in the M protein (rM51R) is deficient in its ability to suppress the host innate immune response but replicates in most cell types to titers that are as high or higher than the titer of an isogeneic control virus with wild-type M protein (3). The rM51R virus is able to induce interferon (IFN) production (3) and induces expression of many other genes that are important in the host antiviral response (16; also M. Ahmed and D. S. Lyles, unpublished data). The rM51R virus is attenuated for spread to the central nervous system and is attenuated for virulence in mice (1, 2, 45). The rM51R virus induces an antibody response comparable to that of the recombinant wild-type (rwt) virus in mice without causing disease (2). These results indicate that M protein mutant VSV has the ability to induce an adaptive immune response in vivo in the absence of viral virulence and support its potential as a vaccine vector. In this study we investigated the use of rM51R virus as a potential vaccine vector against respiratory infection using intranasal (i.n.) vaccinia virus challenge as a protection model.
One of the challenges in using poxviruses as a model for respiratory immunity is that poxviruses have two infectious forms of the virus, the intracellular mature virion (IMV) and the extracellular enveloped virion (EEV), which is similar to the IMV form but contains an additional envelope that is formed during budding of the viral particle from the host cell. Antibodies against surface components from both forms are important for protective immunity. Important antigens for neutralization of the IMV and EEV forms include L1R and B5R, respectively (17, 48). Another challenge is that poxvirus antigens are not highly immunogenic when delivered outside the context of a natural poxvirus infection. Immunization with purified proteins plus adjuvant or DNA vaccines requires multiple immunizations to provide protection (8, 15, 17, 20, 21, 50). Thus, the ability to induce immunity against poxviruses is a rigorous test of the effectiveness of M protein mutant VSV vectors.
We engineered the rM51R virus to express the poxvirus antigens B5R (rM51R-B5R) and L1R (rM51R-L1R). The experiments presented here demonstrate that mice immunized with the rM51R-B5R and -L1R vaccine vectors were protected from lethal intranasal vaccinia virus challenge. A single immunization provided protection, but less morbidity was observed when mice received a prime and a heterologous boost. Immunization with either B5R or L1R vectors independently also provided protection against lethal challenge. These data highlight the potential of an M protein mutant VSV that prevents suppression of the host antiviral response as a vaccine vector against respiratory infection.
MATERIALS AND METHODS
Viruses and recombinant proteins.
The recombinant M protein mutant VSV vaccine vectors, rM51R-B5R and rM51R-L1R viruses, were isolated from infectious cDNA clones as described previously (26). The rM51R-B5R and rM51R-L1R cDNAs were constructed using methods similar to those used by Whitlow et al. to construct rVSV expressing enhanced green fluorescent protein (rVSV-EGFP) described previously (47). The B5R and L1R genes used were described previously by Aldaz-Carroll et al. (4, 5). A hemagglutinin (HA) tag was added to the C terminus of both B5R and L1R at a KpnI site. A PacI site was cloned onto the 5′ and 3′ ends of B5R and L1R for its insertion into the rM51R virus using an rM51R M protein mutant VSV cDNA infectious clone, pVSV.XK4.1 (47). The primers used to add the PacI site to B5R were the forward primer 5′-CCCCTTAATTAACCTACGGAAATG-3′ and the reverse primer 5-CCCCTTAATTAACTCTAGAGGATC-3′. The primers used to add the PacI site to L1R were the forward primer 5′-CCCCTTAATTAAGCCTAACGAAATG-3′ and the same reverse primer used for B5R (5′-CCCCTTAATTAACTCTAGAGGATC-3′). Virus stocks were prepared as described previously (26).
Recombinant simian virus 5 (rSV5)-B5R and rSV5-L1R viruses were provided by Kimberly Clark and Griffith Parks (Wake Forest University Health Sciences). The B5R and L1R genes used for the rM51R virus vectors were also used in the rSV5 vectors. The recombinant B5R and L1R proteins were generated in cultures of baculovirus-infected Sf9 cells and purified using a metal affinity resin. The Salmonella enterica serovar Enteritidis FliC (flagellin) was purified as previously described (30).
Mouse strains.
Five- to 7-week-old female BALB/c mice were used in all animal studies except for the B-cell-deficient challenge study. The B-cell-deficient challenge study used 5- to 7-week-old female and male Jh knockout mice of BALB/c origin. Differences in survival and weight loss were not observed between the male Jh knockout mice and the female knockout mice.
Western blotting.
Western blot analysis of protein expression was performed as described previously (33). Briefly, BHK cells were infected with rwt (multiplicity of infection [MOI] of 5 PFU per cell), rM51R-L1R (MOI of 2.4, 7.2, or 24 PFU per cell), or rM51R-B5R (MOI of 3.5, 10.5, or 35 PFU per cell) viruses for 6 h. Anti-HA antibody (Roche) was used as a primary antibody and goat anti-rat conjugated to horseradish peroxidase (HRP) (Amersham) was used as a secondary antibody.
Single-step growth curve analysis.
BHK cells were seeded at a density of 70% and infected with rM51R, rM51R-B5R, or rM51R-L1R viruses at an MOI of 10 PFU per cell. The virus was removed after 1 h, and Dulbecco's modified Eagle's medium (DMEM)-10% fetal bovine serum (FBS) was added back to the cells. At 4, 8, 12, and 24 h postinfection, 100 μl of supernatant was collected and centrifuged at 350 × g for 5 min. The supernatant was transferred to a clean tube and stored at −80°C. Virus titers were determined by plaque assay on BHK cells.
ELISA.
Mice were immunized and boosted as described below. Serum was collected on week 3 (3 weeks postprime), week 6 (2 weeks postboost), week 8 (1 week postchallenge), and week 9 (2 weeks postchallenge). Enzyme-linked immunosorbent assays (ELISAs) were performed using a modified protocol as described previously (44). Briefly, Nunc-Immuno Maxisorp 96-well plates (Fisher) were coated with purified B5R or L1R or with purified wild-type VSV (Indiana serotype; Orsay strain) at 200 ng per well in carbonate buffer (7.5 mM Na2CO3-17.4 mM NaHCO3) overnight at 4°C. The wells were washed with wash buffer (1× phosphate-buffered saline [PBS]-0.05% Tween 20; Fisher), blocked with blocking buffer (10× Sigma blocking buffer diluted to 1×), and incubated with serum diluted in blocking buffer overnight at 4°C. The wells were washed and incubated with peroxidase-conjugated rabbit anti-mouse IgG (Jackson ImmunoResearch) at room temperature for 2 h. The wells were washed, and the antibody reactivity was detected using tetramethyl benzidine (TMB) substrate solution (Fisher) as directed by the manufacturer. The titers were reported as the reciprocal of the highest dilution of serum that gave an optical density at 450 nm (OD450) of >0.1.
Plaque reduction neutralization test (PRNT).
Serum samples were heat inactivated at 55°C for 45 min and serially diluted 2-fold in DMEM-2% FBS. Samples were incubated with 500 PFU of vaccinia virus (strain Western Reserve) diluted in DMEM-2% FBS for 1 h at 37°C. The serum and virus inoculum were absorbed onto CV1 cells for 1 h at 37°C. The inoculum was removed, and DMEM-2% FBS was added to the cells. The plates were incubated for 48 h at 37°C and visualized by crystal violet staining. The neutralization activity was determined by the reduction in number of plaques for serum samples incubated with virus compared to the virus-only control.
Immunizations and challenge.
Mice were anesthetized with isoflurane and immunized intranasally with 1 × 105 to 1 × 108 PFU of rM51R-B5R and/or rM51R-L1R in a total volume of 10 μl. On week 4, the mice were anesthetized and boosted intranasally with recombinant B5R (20 μg) and/or L1R (20 μg) proteins plus Salmonella flagellin (1 μg) or 1 × 106 PFU of rSV5-B5R and rSV5-L1R in a total volume of 10 μl. Mice that received a single primary immunization with 1 × 108 PFU of rM51R-B5R and rM51R-L1R on week 0 are indicated on figures as rM51R(B+L) 1° only. Mock-infected mice received only flagellin (1 μg) on week 4. Vector-only (indicated as rM51R+rSV5) control mice received 1 × 107 PFU of empty rM51R vector on week 0 and 1 × 106 PFU of empty rSV5 vector on week 4. Mice were challenged intranasally on week 7 postprime with 1.7 × 106 PFU of vaccinia virus (strain Western Reserve), which is equivalent to 20 times the median tolerated dose (MTD50). The challenge mice were monitored daily for weight loss and disease progression. Mice were euthanized when they lost more than 30% of their original weight, as required by IACUC guidelines of Wake Forest University Health Sciences.
CD8 T-cell depletion.
BALB/c mice were primed and boosted as described above. These mice were challenged on week 8 postprime instead of week 7 postprime as described above. On days −2, 0, and 2 postchallenge, mice were given 0.3 mg of monoclonal antibody against CD8 (clone 2.43). The efficiency of CD8 depletion was assessed by flow cytometry. In wt nondepleted mice, the percentage of CD8 T cells was 13% of total splenocytes. After three depletions the percentage of CD8 T cells was <1% of the total splenocytes.
Statistical analysis.
Statistical analysis for weight loss was performed with the SigmaStat, version 3.5, software package by repeated measures analysis of variance.
RESULTS
rM51R vaccine vectors expressing poxvirus antigens replicate to similar levels as the parental rM51R vaccine vector.
The vaccinia virus B5R and L1R genes were inserted into a separate transcription unit between the M and G genes of the rM51R virus (Fig. 1A) using reverse genetics (described in the Materials and Methods section). Both the B5R and L1R genes lack their transmembrane domains, resulting in truncated proteins that run at 35 kDa and 21 kDa, respectively (4, 5). An HA tag was added to the C terminus of each of the proteins for detection of the poxvirus antigens.
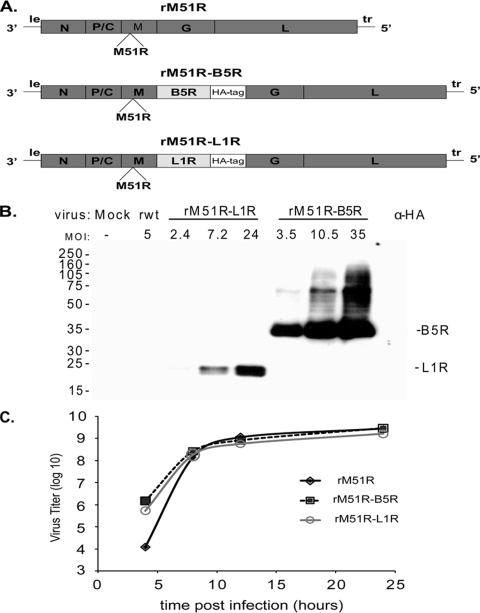
FIG. 1.
Expression of B5R and L1R and growth kinetics of recombinant M protein mutant VSV vaccine vectors expressing poxvirus antigens. (A) Schematic diagram showing the insertion of the B5R and L1R genes into the rM51R vector. (B) BHK cells were infected with rM51R-B5R or rM51R-L1R virus vectors for 6 h at the indicated multiplicity. B5R and L1R protein expression was measured by Western blot analysis using an antibody against the HA epitope tag. (C) Analysis of single-step growth comparing the vectors expressing B5R and L1R to the parental rM51R vector in BHK cells infected at an MOI of 10. The results are an average of two experiments.
Protein expression by rM51R-B5R and rM51R-L1R viruses was measured by Western blot analysis. BHK cells were infected over a range of MOIs, and the cell lysates were analyzed at 6 h postinfection. Protein expression for both B5R and L1R was detected using an antibody against the HA epitope tag. In Fig. 1B, high levels of protein expression were detected for both rM51R-B5R and rM51R-L1R viruses. The levels of B5R expression were higher than those for L1R. To measure the effect of protein expression on the replication of the vectors, a single-step growth curve was performed in BHK cells. Cells were infected at an MOI of 10, and virus titers at various times postinfection were determined by plaque assay. Similar growth kinetics were observed for each of the vectors in the single-step experiments (Fig. 1C) and also in multiple-step growth experiments (data not shown), indicating that expression of B5R and L1R proteins did not hinder the replication of the rM51R vaccine vectors.
Mice immunized with rM51R vaccine vectors expressing B5R and L1R seroconvert to L1R but not B5R.
Mice were immunized with the rM51R vectors expressing B5R and L1R to determine whether they developed detectable antibody titers after primary immunization or after boosting with B5R and L1R antigens. Mice primed with rM51R-B5R and rM51R-L1R viruses were boosted by two different methods: (i) intranasal inoculation with purified soluble B5R and L1R with Salmonella flagellin as an adjuvant (Fig. 2, +B+L+flagellin) or (ii) intranasal inoculation with recombinant SV5 vectors expressing B5R and L1R [Fig. 2, +rSV5(B+L)]. Another group of mice received a single primary immunization with rM51R-B5R and rM51R-L1R vectors [rM51R(B+L) 1° only]. As negative controls mice were mock infected and boosted with flagellin (Fig. 2B, Mock); vector-only control mice (rM51R+rSV5) were immunized with rM51R vector and boosted with rSV5 vector, in which each vector lacked the B5R and L1R antigens. As a positive control mice were immunized with a single sublethal dose of vaccinia virus.
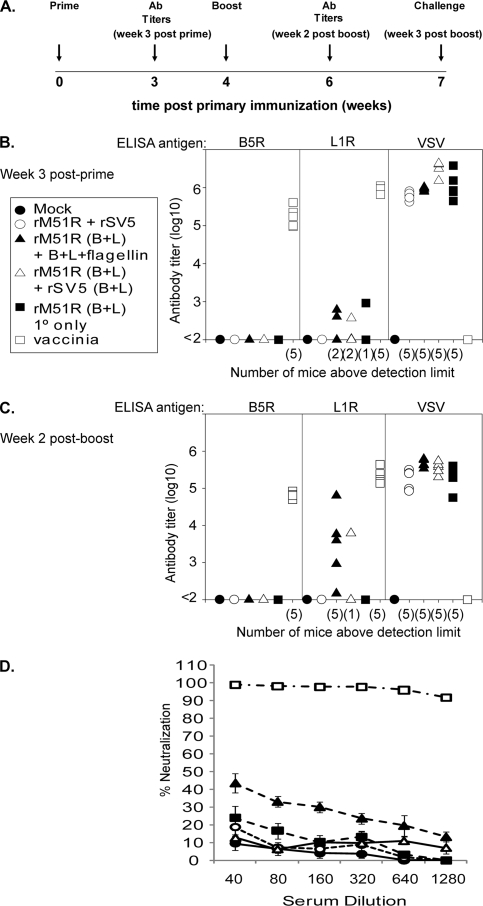
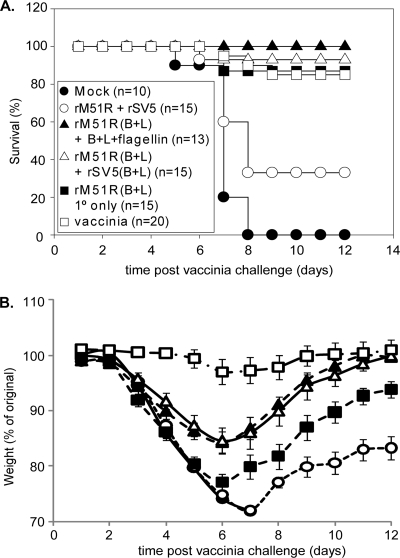
FIG. 2.
Antibody titers for mice immunized with the rM51R vaccine vectors. (A) Immunization schedule. Five- to 7-week-old BALB/c mice were immunized intranasally with 108 PFU of rM51R-B5R and rM51R-L1R viruses. At week 4 mice were boosted intranasally with purified B5R, L1R, and flagellin [rM51R(B+L) +B+L+flagellin] or 106 PFU of rSV5-B5R and rSV5-L1R [rM51R(B+L) + rSV5(B+L)] or did not receive a boost [rM51R(B+L) 1° only]. As negative controls, mice were mock infected and boosted with flagellin (Mock), immunized with empty rM51R vector and boosted with empty rSV5 vector (vector only; rM51R + rSV5). As a positive control mice were immunized with a single sublethal dose (200 PFU) of vaccinia virus (vaccinia). On week 7 immunized mice were challenged intranasally with a lethal dose (1.7 × 106 PFU/mouse) of vaccinia virus. (B and C) Antibody titers for individual mice determined by ELISA. Serum was collected at week 3 (B) and week 6 (C); the limit of detection was a serum dilution of 1:100. Data shown are from a representative experiment in which five mice were used per group. Similar results for each of these groups have been reproduced in two or more similar experiments. Number of mice with detectable titers is given in parentheses on the x axis. The reciprocal of the highest dilution of serum that gave an OD450 of >0.1 above background is shown on the y axis. (D) Virus-neutralizing activity was determined by PRNT for the serum collected on week 6 used in panel C. Data shown are the average of five individual mice per group. The error bars represent the standard error of the mean.
Figure 2A shows the timeline of the experiment. Serum was collected on week 3 (week 3 postprime), mice were boosted at week 4, and serum was collected at week 6 (week 2 postboost). Antibody titers were determined by ELISA using purified B5R, L1R, or VSV as antigen. The limit of detection for the ELISA was a serum dilution of 1:100. Figure 2B and C show titers for individual mice, with the number of individual mice with titers above the detection limit on the x axis, for week 3 postprime and week 2 postboost, respectively. Figure 2B shows that most of the mice immunized with the rM51R vaccine vectors (Fig. 2B, filled and open triangles and filled square) had antibody titers against B5R and L1R that were at or below the limit of detection although a few mice had detectable titers against L1R. All of the mice immunized with VSV vectors had high titers against the VSV antigen. The positive-control mice immunized by a sublethal dose of vaccinia virus (Fig. 2B, open squares) had high antibody titers against both B5R and L1R but not against VSV, as expected. Mice that were boosted with the purified proteins with flagellin (Fig. 2B, filled triangles) had seroconverted to L1R but not B5R; however, the titers for L1R were lower than those observed for the positive control (Fig. 2C).
Vaccinia virus neutralizing antibody activity was measured by PRNT for the serum collected at week 6 postprime (Fig. 2C, week 2 postboost shown). In mice immunized with rM51R vectors, the PRNT primarily assays antibody against L1R since most of the vaccinia virus stock used in this assay consists of IMV particles. Mice that were immunized with rM51R vaccine vectors that expressed both B5R and L1R (Fig. 2C, filled and open triangles and closed square) had detectable neutralizing activity although at a serum dilution of 1:40, the activity was less than 50% (Fig. 2D). The positive-control mice that were immunized with a sublethal dose of vaccinia virus (Fig. 2D, open squares) had neutralizing antibody activity that was >90% at a serum dilution of 1:1,280. The neutralizing antibody activity measured in Fig. 2D correlated with the ELISA titers against L1R shown in Fig. 2C, where mice that were boosted with recombinant proteins plus flagellin had higher neutralizing activity than mice that were boosted with rSV5 expressing B5R and L1R or mice that received only a single immunization. These data indicate that although vaccinia virus infection induces high serum IgG levels and neutralizing antibody activity, the purified proteins or proteins expressed from viral vectors are relatively inefficient at inducing antibody titers detected by ELISA or neutralizing activity.
Mice immunized with the rM51R vaccine vectors are protected from lethal vaccinia virus challenge.
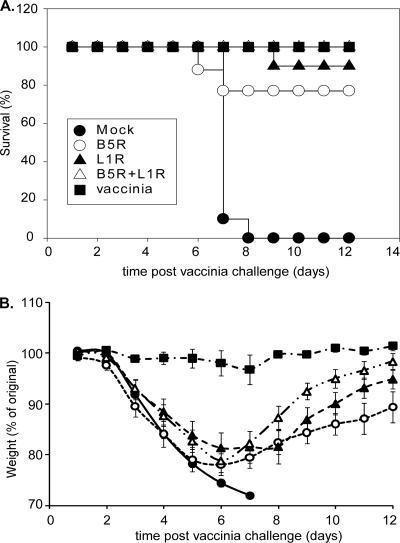
Mice immunized with the rM51R-B5R and rM51R-L1R vaccine vectors were infected with vaccinia virus intranasally with 20 MTD50 (1.7 × 106 PFU/mouse) to determine whether they were protected from lethal vaccinia virus challenge in the absence of high antibody titers. Mice that showed signs of terminal illness or lost 30% of their original weight were euthanized according to IACUC guidelines and were scored as nonsurvivors.
The results shown in Fig. 3A are represented as the percentage of mice that survived lethal challenge as a function of time postchallenge with vaccinia virus. All of the mock-immunized (Fig. 3A, filled circle) and 10 out of 15 of the mice immunized with vector only (open circle) died by day 8 postchallenge. Three out of 20 of the mice in the positive-control group immunized with a single sublethal dose of vaccinia virus (Fig. 3A, open square) died. Nearly all of the mice that were immunized with rM51R vectors survived lethal challenge. All of the mice that were immunized with rM51R vectors and boosted with the purified proteins plus flagellin (Fig. 3A, filled triangle) survived the challenge, and only 2 out of 15 mice in the immunized group that did not receive the boost (filled square) and 1 mouse from the immunized group that was boosted with rSV5-B5R and rSV5-L1R (open triangle) died on day 7 postchallenge. Single immunizations with either recombinant proteins plus flagellin (13a) or rSV5 vectors that expressed B5R and L1R (K. M. Clark and G. D. Parks, unpublished data) did not protect against lethal intranasal vaccinia virus challenge although protective immunity could be induced by multiple immunizations with these agents. These results demonstrate that mice immunized with the rM51R vectors were protected from lethal vaccinia virus challenge in the absence of high antibody titers.
FIG. 3.
Survival and weight loss for mice immunized with the rM51R vaccine vectors after lethal vaccinia virus challenge. Mice were immunized and challenged as in Fig. 2. (A) Percentage of animals that survived lethal vaccinia virus challenge. (B) Disease progression measured by weight loss after challenge. Data shown are the average of two or more similar experiments. The error bars represent the standard error of the mean. A statistically significant difference (P < 0.05) was observed in weight loss for mice that received a single inoculation or a single inoculation and a boost between days 6 and 10. Statistical analysis was performed using a repeated measures analysis of variance.
Mice that have been immunized still exhibit signs and symptoms of vaccinia virus infection after a lethal challenge by the respiratory route. In Fig. 3B, disease progression as indicated by weight loss was monitored for 12 days after the challenge for the mice described previously (Fig. 2 and 3A). The data are presented as the percentage of the original weight as a function of time. The mice that were mock infected or immunized with vector only lost the most weight, and those that survived recovered to only ∼80% of their original weight. The positive controls that were immunized with a single sublethal dose of vaccinia virus (Fig. 3B, open square) lost the least amount of weight. The mice immunized with the rM51R vaccine vectors that received a boost of purified proteins plus flagellin (Fig. 3B, filled triangle) or rSV5 vectors expressing B5R and L1R (open triangle) lost similar amounts of weight and recovered to almost their original weights. The immunized mice that did not receive a boost (Fig. 3B, filled square) lost more weight and took longer to recover than the mice that did receive a boost. The difference in weight loss between mice that were boosted and the mice that did not receive a boost was statistically significant (P < 0.05, by repeated measures analysis of variance) from days 6 to 10. Based on these results and the higher antibody titers shown in Fig. 2, mice were immunized with rM51R vaccine vectors and boosted with purified proteins using flagellin as an adjuvant in our subsequent experiments.
Immunization with low titers of rM51R vaccine vector protects against lethal challenge with vaccinia virus.
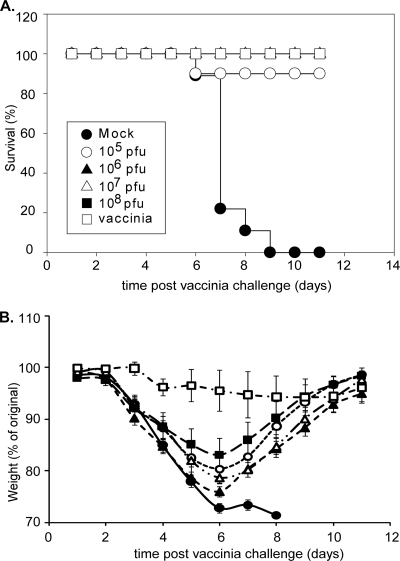
The minimal amount of rM51R vaccine vector that would protect against lethal challenge was determined by immunization with 10-fold serial dilutions of rM51R-B5R and rM51R-L1R viruses ranging from 105 to 108 PFU/mouse. As described above and outlined in Fig. 2A, the mice were boosted with purified protein plus flagellin and challenged with a lethal dose of vaccinia virus. Nearly all of the rM51R virus-immunized mice survived the challenge (Fig. 4A). One out of 10 mice from the group that received 105 PFU/mouse (Fig. 4A, open circle) died on day 6 postchallenge. All of the mock-infected mice (filled circle) died by day 9 postchallenge. These results show that complete protection can be achieved by immunization with the combined rM51R-B5R and rM51R-L1R viruses at titers as low as 106 PFU/mouse.
FIG. 4.
Survival and weight loss for mice immunized with increasing doses of the rM51R vaccine vectors. Mice were immunized and challenged as described in the legend of Fig. 2 with 10-fold serial dilutions of rM51R-B5R and rM51R-L1R viruses ranging from 105 to 108 PFU. On week 4 the mice were boosted with purified B5R, L1R, and flagellin and challenged on week 7 as described above. (A) Percentage of animals that survived lethal challenge. (B) Weight loss after challenge. Data shown are the average of two experiments (n = 10 for each experimental group). The error bars represent standard error of the mean.
Disease progression following vaccinia virus challenge was monitored by weight loss for 11 days after the challenge (Fig. 4B). These results demonstrate that mice immunized with the rM51R vectors recovered from lethal challenge when immunized with titers as low as 106 PFU, but mice that received 108 PFU showed the least signs of vaccinia virus-induced disease even though this difference was not statistically significant. Antibody titers measured at weeks 3 and 6 were similar to those described above (Fig. 2), with no statistical difference observed between the titers measured over the range of doses (data not shown).
Protection can be achieved by immunization with B5R or L1R vectors alone.
To determine whether immunization with either B5R or L1R vectors alone would protect against lethal challenge with vaccinia virus, mice were immunized intranasally with 8 × 106 PFU/mouse of rM51R-B5R or rM51R-L1R or both of the vaccine vectors. On week 4, the immunized mice were boosted with flagellin plus purified B5R, L1R, or both proteins and challenged as described above. As controls, mice were mock infected or immunized with a sublethal dose of vaccinia virus as described above. Nearly all of the mice that were immunized with a single antigen survived the challenge (Fig. 5A). Two out of 10 mice from the B5R (Fig. 5A, open circle) group and 1 out of 10 from the L1R (filled triangle) group died after the challenge. All of the mice that received both antigens (open triangle) survived. These results indicate that mice immunized with B5R or L1R alone were significantly protected from lethal challenge.
FIG. 5.
Survival and weight loss for mice immunized with either B5R or L1R alone. Mice were immunized as described in the legend of Fig. 2 with 8 × 106 PFU of either rM51R-B5R or rM51R-L1R viruses or with both viruses and then boosted with purified B5R, L1R, or both proteins plus flagellin and challenged. (A) Percentage of animals that survived lethal challenge. (B) Weight loss after challenge. Data shown are the average of two experiments (n = 10 for each experimental group). The error bars represent the standard error of the mean.
The mice that were immunized with both rM51R-B5R and rM51R-L1R (open triangle) vectors lost weight in amounts similar those of the L1R group (closed triangle) but recovered faster and closer to their original weights than mice immunized with either of the single antigens (Fig. 5B). The L1R group (filled triangle) loss less weight and recovered faster than the B5R group (open circles). These results indicated that immunization with B5R or L1R alone does protect against lethal challenge, but L1R provides protection and prevents weight loss slightly better than B5R. However, immunization with both antigens provides the best protection and reduces the severity of disease progression.
Mice immunized with rM51R vaccine vectors had earlier antibody responses upon challenge.
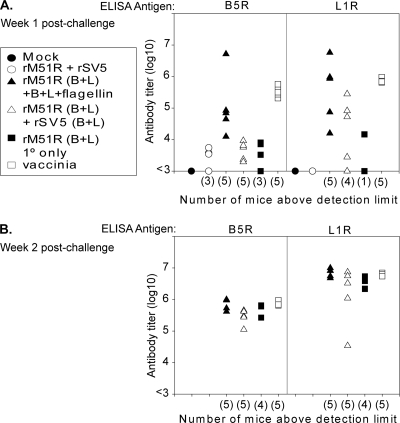
The protection of mice by immunization with rM51R vectors in the absence of high antibody titers suggests that protection could be due to an earlier recall response during the vaccinia virus challenge. To determine if mice immunized with the rM51R vaccine vectors were able to mount an earlier antibody response upon challenge, serum was collected at weeks 1 and 2 postchallenge (weeks 8 and 9 postprime) from the mice described in the legend of in Fig. 2. The IgG antibody titers against B5R and L1R were determined by ELISA as described previously. On week 1 postchallenge, mice that were immunized with the rM51R vaccine vectors and boosted had higher titers against B5R, L1R, or both than the mock-infected mice (Fig. 6A, filled circle) or mice immunized with vector only (open circle). This time frame correlates with the weight gain for mice immunized with rM51R-B5R and rM51R-L1R when all mock-infected mice had died or had been euthanized due to excessive weight loss (Fig. 3, 4, and 5). By week 2 postchallenge all the surviving mice had similar antibody titers against both B5R and L1R (Fig. 6B). These results indicate that mice immunized with the rM51R vaccine vectors and boosted had earlier antibody responses upon the challenge than mice that were naïve to poxvirus antigens. These results indicate a potential role for antibodies produced by a recall response in the protection against lethal vaccinia virus challenge in the absence of high antibody titers after the primary immunization and boost.
FIG. 6.
Antibody titers after vaccinia virus challenge. Serum was collected from the mice used in the experiment described in the legend of Fig. 2 at week 1 (A) and week 2 (B) postchallenge. Antibody titers from individual mice were measured by ELISA as described in the legend of Fig. 2. Five mice were used per group. Similar results for each of these groups have been reproduced in two or more similar experiments.
B cells but not CD8 T cells are involved in protection mediated by rM51R vaccine vectors.
The early antibody response upon challenge in mice that were immunized with rM51R vaccine vectors that expressed B5R and L1R suggests an important role for B cells in protection against lethal challenge, despite the low IgG titers and neutralizing antibody activity prior to challenge. To determine the role of B cells in protection against lethal vaccinia virus challenge, Jh knockout mice were immunized intranasally with 1 × 108 PFU of both rM51R-B5R and rM51R-L1R at week 0. At week 4, mice were boosted intranasally with recombinant B5R and L1R plus flagellin as described above. One group of mice received a single primary immunization with rM51R-B5R and rM51R-L1R vectors [rM51R(B+L) 1°only]. As controls mice were mock infected or immunized with a sublethal dose of vaccinia virus as described above. On week 7, all mice were challenged intranasally with 20 MTD50 (1.7 × 106 PFU/mouse) of vaccinia virus.
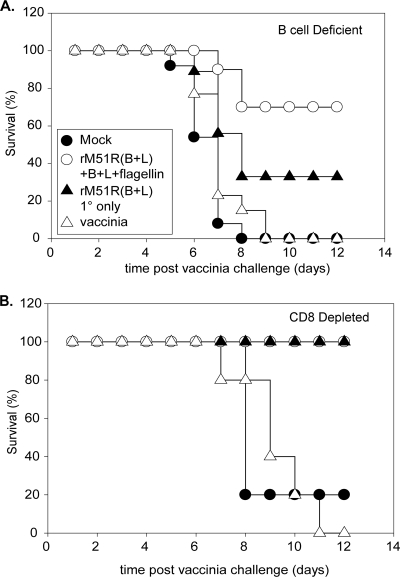
As shown in Fig. 7A, all of the mock-infected (15 out of 15) and vaccinia virus-immunized (13 out of 13) mice (filled circle and open triangle, respectively) died by day 9 postchallenge. Seven out of 10 mice that were immunized with both rM51R-B5R and rM51R-L1R and boosted with recombinant proteins B5R and L1R plus flagellin survived the lethal challenge (Fig. 7A, open circle). Only three out of nine mice that received a single primary immunization with rM51R-B5R and rM51R-L1R (Fig. 7A, filled triangle) survived the challenge. The difference in survival between mice that received a single immunization and mice that received a heterologous boost was not statistically significant. However, a comparison of the difference in survival between wt mice and Jh knockout mice revealed a statistically significant difference, regardless of whether the mice received a boost or only a primary immunization (P < 0.05). These data indicate that B cells are important in the protection that is mediated by rM51R vaccine vectors. However, the observation that some of the Jh knockout mice that were immunized with the rM51R vaccine vectors survived suggests that additional components of the immune response can provide partial protection.
FIG. 7.
Survival of Jh knockout mice and mice acutely depleted of CD8 T cells after lethal vaccinia virus challenge. (A) Jh knockout mice were immunized, boosted, and challenged as described in the legend of Fig. 2. Data shown are the results of two experiments. Mock, n = 15; rM51R(B+L) + B+L+flagellin, n = 10; rM51R(B+L) 1° only, n = 9; and vaccinia, n = 13. (B) wt BALB/c mice were immunized, boosted, and challenged on week 8 as described in the legend of Fig. 2. CD8 T cells were acutely depleted on days −2, 0, and 2 postchallenge by injection of 0.3 mg of monoclonal antibody against CD8 (clone 2.43). Data shown are from 5 mice per group.
To address the role of CD8 T cells in protecting rM51R vaccine vector-immunized mice during lethal vaccinia virus challenge, CD8 T cells were depleted prior to challenge. CD8 T cells, as well as B cells, have been shown previously to contribute to protection of mice immunized with attenuated vaccinia viruses although the relative importance of these individual components of the immune system may vary with the dose and strain of virus (6, 49). Wild-type BALB/c mice were immunized and boosted as described above. Mice that were mock infected or immunized with a sublethal dose of vaccinia virus were used as controls. T cells were depleted in immunized mice with monoclonal antibody against CD8 on days −2, 0, and 2 postchallenge. The mock-infected animals were not depleted of their T cells prior to challenge. All mice were challenged intranasally with 20 MTD50 (1.7 × 106 PFU/mouse) of vaccinia virus and monitored daily for survival.
Depletion of CD8 T cells had little, if any, effect on protection of mice immunized with rM51R-B5R and rM51R-L1R, regardless of whether they received a boost (Fig. 7B, open circle and filled triangle). In contrast, all of the vaccinia virus-immunized mice died by day 11 postchallenge. These results indicate that CD8 T cells are important for protection mediated by sublethal vaccinia virus infection but are not required for protection mediated by rM51R vaccine vectors.
DISCUSSION
The data presented here are proof of principle that M protein mutants of VSV can be effective vaccine vectors. Protection against lethal challenge with vaccinia virus was achieved with a single inoculation of rM51R vectors although the addition of a heterologous boost with recombinant proteins and flagellin provided the best protection against vaccinia virus-induced disease (Fig. 3). Neutralizing antibodies against the G protein prevent boosting with the same serotype of VSV vectors. VSV vectors with different serotypes of G protein or that lack G protein have been used to circumvent neutralizing antibodies for multiple administrations of VSV (36, 39). In principle, the rM51R vectors can be modified to contain G proteins of different serotypes. We chose instead to use the rSV5 vectors and recombinant proteins plus flagellin for boosting in these experiments. Neither the purified proteins (K. N. Delaney and S. B. Mizel, unpublished data) nor the rSV5 vectors (Clark and Parks, unpublished) elicited protection with a single immunization. However, both were effective as boosting agents in the reduction of vaccinia virus-induced morbidity.
There are two types of challenge models in mice for vaccinia virus: systemic (intraperitoneal [i.p.] or intravenous [i.v.]) and respiratory (intranasal [i.n.]) challenge. The requirements for protection can be different for these two models (25). The respiratory route reflects the natural route of transmission of smallpox in its aerosolized form. We hypothesize that a vaccine delivered via the mucosal route would be more effective against a lethal respiratory challenge. Other VSV vaccines have generated better immune responses when given intramuscularly (i.m.) or i.p. rather than by the i.n. route of inoculation (34, 35). However, rM51R-B5R and rM51R-L1R vectors provided better protection when given via the i.n. route rather than by i.m. inoculation (C. L. Braxton and D. S. Lyles, unpublished data).
There are a number of challenges to overcome to elicit a protective immune response to poxviruses. One challenge is that there are two infectious forms of the virus. The IMV form is transmitted predominately from host to host, and the EEV form is important for long-range dissemination within the host (11, 21, 32). Both the IMV and EEV forms of poxviruses give rise to neutralizing antibodies (17, 21). Antibodies that neutralize IMV do not neutralize the EEV form of the virus (27). Previous studies of the immunogenicity of subunit vaccines against smallpox have included antigens from the IMV (L1R, A27L, and D8L) and EEV (B5R, A33R, A34R, and A36R) virions (8, 15, 17, 20, 21, 25). These studies demonstrated that vaccines that contained multiple antigens provided better protection than the individual antigens alone (15, 21). In this study we chose to use L1R and B5R because they are representative antigens of the IMV and EEV forms, respectively, that have been shown to induce neutralizing antibodies (17, 48). Mice immunized with either the rM51R-L1R or rM51R-B5R vectors alone were protected from lethal intranasal challenge, with rM51R-L1R virus inducing better survival and less weight loss than rM51R-B5R virus (Fig. 5). Immunization with both rM51R-B5R and rM51R-L1R vectors provided better protection than immunization with rM51R-L1R or rM51R-B5R alone (Fig. 5). This result compares favorably with many previous studies in which antigens from both IMV and EEV were required for protection (15, 20, 21, 25).
Another challenge in establishing a protective immune response is that B5R, L1R, and other poxvirus antigens are not highly immunogenic when administered as purified proteins plus adjuvant or as DNA vaccines, as indicated by the multiple immunizations needed for protection (8, 15, 17, 20, 21, 50). The need for multiple immunizations may reflect the need for a high antigen dose since a sublethal dose of vaccinia virus induces high antibody titers (Fig. 2). In our experiments, mice that received a heterologous boost with recombinant proteins and flagellin usually had detectable antibody titers against L1R after boosting while titers against B5R often remained undetectable. These mice also had higher levels of neutralizing antibody activity than mice that received a single immunization of rM51R-B5R and rM51R-L1R or mice that were boosted with rSV5 vaccine vectors expressing B5R and L1R although the neutralizing activity was low. The difference in antibody responses against these two antigens cannot be due to differences in antigen expression since B5R was expressed at higher levels than L1R (Fig. 1).
Despite the low antibody titers and neutralizing activity following immunization with rM51R vectors, immunized mice were protected from lethal intranasal challenge with vaccinia virus (Fig. 3, 4, and 5). This protection appears to be mediated at least in part by memory B cells specific for B5R and L1R, which respond more quickly and effectively than naïve B cells during the challenge. The evidence for this comes from the high antibody titers against B5R and L1R in mice immunized with rM51R-B5R and rM51R-L1R 1 week after challenge (Fig. 6A) and from results in Jh knockout mice that lack mature B cells, in which the ability of rM51R vectors to induce protection was reduced (Fig. 7A). Jh knockout mice that were immunized with the rM51R vaccine vectors were still partially protected from lethal challenge, which indicates that additional components of the immune response are also important for protection. This partial protection does not appear to be due to CD8 T cells since their depletion had little, if any, effect. The partial protection observed in Jh knockout mice could be mediated by other T-cell subsets as well as by induced elements of the innate immune system. For example, several studies have established the importance of complement in the clearance of vaccinia virus (7, 28). Although poxvirus antigen-specific IgG may play a dominant role in complement-mediated clearance of vaccinia virus, it is quite possible that acute-phase reactants, such as C-reactive protein (CRP) and mannan-binding lectin (MBL), or elements of the alternative pathway (31) might also contribute to the activation of complement and the clearance of virus. In this regard, we have found that rabbits given more than two exposures to a flagellin fusion protein vaccine exhibit some degree of hepatic activation (S. B. Mizel, unpublished observations). It is possible that the M51R vaccine vectors sensitize the mice to flagellin-induced production of acute-phase reactants such as CRP and MBL. This might also account for the occasional survival of mice immunized with vectors that do not express B5R or L1R (Fig. 3). In future studies, we propose to evaluate this interesting possibility.
The results presented here can be generalized as an alternative strategy for the attenuation of live-virus vaccines. The usual strategy for attenuation of viral vectors, including VSV, is to decrease the ability of the vector to replicate (12, 13, 34-37, 41, 42, 46). The attenuation strategy utilized here was based on an M protein mutation that prevents suppression of the host antiviral response. This mutation attenuates the pathogenicity of the virus and promotes the adaptive immune response by increasing the innate immune response (2, 3). Ahmed et al. have shown that rM51R virus is cleared from the nasal epithelium during an intranasal infection by day 2 and is attenuated for spread to the central nervous system, where an isogenic control virus with wild-type M protein (rwt virus) spreads through the nasal mucosa and is detected in the olfactory bulb on day 7 after infection (2). The anti-VSV antibody levels induced by rM51R virus were comparable to those induced by rwt virus, indicating the potential use of rM51R as a vaccine vector (2). In this study we have shown that rM51R can be used as a vaccine vector and that it protects mice from lethal intranasal vaccinia virus challenge. Complete protection was observed when mice received a heterologous boost with recombinant proteins and flagellin; however, even with the boost, the mice lost more weight than the positive controls that were immunized with the sublethal dose of vaccinia virus. This result indicates that rM51R vectors can be further improved to achieve the level of protection afforded by a sublethal infection. The addition of immunostimulatory molecules may further increase the immunogenicity of the vector; however, future research is necessary to determine their potential enhanced effect on rM51R vectors.
Acknowledgments
This research was supported by a NIH program project grant P01-AI060642 and was performed in compliance with all relevant federal guidelines and institutional policies related to the use of animals and their care.
We thank Margie McKenzie for the production of the rM51R-B5R and rM51R-L1R viruses and James Phipps for production of the recombinant B5R and L1R. We also thank Kimberly Clark and Griffith Parks for the recombinant SV5 vectors used in this study and technical advice in performing the animal studies. In addition we thank Maryam Ahmed for advice in performing the animal studies and Kristen Delaney for the CD8 monoclonal antibody.
Footnotes
Published ahead of print on 20 January 2010.
REFERENCES
- 1.Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34-49. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, M., T. R. Marino, S. Puckett, N. D. Kock, and D. S. Lyles. 2008. Immune response in the absence of neurovirulence in mice infected with M protein mutant vesicular stomatitis virus. J. Virol. 82:9273-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 79:6260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. L. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 2005. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 341:59-71. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. U. S. A. 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benhnia, M. R., M. M. McCausland, J. Moyron, J. Laudenslager, S. Granger, S. Rickert, L. Koriazova, R. Kubo, S. Kato, and S. Crotty. 2009. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 83:1201-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berhanu, A., R. L. Wilson, D. L. Kirkwood-Watts, D. S. King, T. K. Warren, S. A. Lund, L. L. Brown, A. K. Krupkin, E. Vandermay, W. Weimers, K. M. Honeychurch, D. W. Grosenbach, K. F. Jones, and D. E. Hruby. 2008. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J. Virol. 82:3517-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black, B. L., and D. S. Lyles. 1992. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J. Virol. 66:4058-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black, B. L., R. B. Rhodes, M. McKenzie, and D. S. Lyles. 1993. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 67:4814-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blasco, R., and B. Moss. 1992. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66:4170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke, D. K., F. Nasar, M. Lee, J. E. Johnson, K. Wright, P. Calderon, M. Guo, R. Natuk, D. Cooper, R. M. Hendry, and S. A. Udem. 2007. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 81:2056-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper, D., K. J. Wright, P. C. Calderon, M. Guo, F. Nasar, J. E. Johnson, J. W. Coleman, M. Lee, C. Kotash, I. Yurgelonis, R. J. Natuk, R. M. Hendry, S. A. Udem, and D. K. Clarke. 2008. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and G gene truncation reduces neurovirulence and enhances immunogenicity in mice. J. Virol. 82:207-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Delaney, K. N., J. P. Phipps, J. B. Johnson, and S. B. Mizel. Protein vaccine elicits complement-dependent protection against respiratory challenge with vaccinia virus in mice. Viral Immunol., in press. [DOI] [PMC free article] [PubMed]
- 14.Egan, M. A., S. Y. Chong, N. F. Rose, S. Megati, K. J. Lopez, E. B. Schadeck, J. E. Johnson, A. Masood, P. Piacente, R. E. Druilhet, P. W. Barras, D. L. Hasselschwert, P. Reilly, E. M. Mishkin, D. C. Montefiori, M. G. Lewis, D. K. Clarke, R. M. Hendry, P. A. Marx, J. H. Eldridge, S. A. Udem, Z. R. Israel, and J. K. Rose. 2004. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res. Hum. Retroviruses 20:989-1004. [DOI] [PubMed] [Google Scholar]
- 15.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaddy, D. F., and D. S. Lyles. 2007. Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J. Virol. 81:2792-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71-80. [DOI] [PubMed] [Google Scholar]
- 18.Geisbert, T. W., K. M. Daddario-DiCaprio, K. J. Williams, J. B. Geisbert, A. Leung, F. Feldmann, L. E. Hensley, H. Feldmann, and S. M. Jones. 2008. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J. Virol. 82:5664-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisbert, T. W., S. Jones, E. A. Fritz, A. C. Shurtleff, J. B. Geisbert, R. Liebscher, A. Grolla, U. Stroher, L. Fernando, K. M. Daddario, M. C. Guttieri, B. R. Mothe, T. Larsen, L. E. Hensley, P. B. Jahrling, and H. Feldmann. 2005. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329-339. [DOI] [PubMed] [Google Scholar]
- 21.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, J. E., F. Nasar, J. W. Coleman, R. E. Price, A. Javadian, K. Draper, M. Lee, P. A. Reilly, D. K. Clarke, R. M. Hendry, and S. A. Udem. 2007. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 360:36-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, S. M., H. Feldmann, U. Stroher, J. B. Geisbert, L. Fernando, A. Grolla, H. D. Klenk, N. J. Sullivan, V. E. Volchkov, E. A. Fritz, K. M. Daddario, L. E. Hensley, P. B. Jahrling, and T. W. Geisbert. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11:786-790. [DOI] [PubMed] [Google Scholar]
- 24.Kahn, J. S., A. Roberts, C. Weibel, L. Buonocore, and J. K. Rose. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 75:11079-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman, D. R., J. Goudsmit, L. Holterman, B. A. Ewald, M. Denholtz, C. Devoy, A. Giri, L. E. Grandpre, J. M. Heraud, G. Franchini, M. S. Seaman, M. J. Havenga, and D. H. Barouch. 2008. Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice. J. Virol. 82:6829-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopecky, S. A., M. C. Willingham, and D. S. Lyles. 2001. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 75:12169-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustig, S., C. Fogg, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 79:13454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30-35. [DOI] [PubMed] [Google Scholar]
- 29.Lyles, D. S. 2000. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol. Mol. Biol. Rev. 64:709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott, P. F., F. Ciacci-Woolwine, J. A. Snipes, and S. B. Mizel. 2000. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect. Immun. 68:5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moulton, E. A., J. P. Atkinson, and R. M. Buller. 2008. Surviving mousepox infection requires the complement system. PLoS Pathog. 4:e1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne, L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 33.Pettit Kneller, E. L., J. H. Connor, and D. S. Lyles. 2009. hnRNPs relocalize to the cytoplasm following infection with vesicular stomatitis virus. J. Virol. 83:770-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Publicover, J., E. Ramsburg, and J. K. Rose. 2004. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J. Virol. 78:9317-9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Publicover, J., E. Ramsburg, and J. K. Rose. 2005. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J. Virol. 79:13231-13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 39.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlereth, B., J. K. Rose, L. Buonocore, V. ter Meulen, and S. Niewiesk. 2000. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J. Virol. 74:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnell, M. J., L. Buonocore, E. Boritz, H. P. Ghosh, R. Chernish, and J. K. Rose. 1998. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 17:1289-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnell, M. J., J. E. Johnson, L. Buonocore, and J. K. Rose. 1997. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell 90:849-857. [DOI] [PubMed] [Google Scholar]
- 43.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 44.Thornburg, N. J., C. A. Ray, M. L. Collier, H. X. Liao, D. J. Pickup, and R. E. Johnston. 2007. Vaccination with Venezuelan equine encephalitis replicons encoding cowpox virus structural proteins protects mice from intranasal cowpox virus challenge. Virology 362:441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trottier, M. D., D. S. Lyles, and C. S. Reiss. 2007. Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. J. Neurovirol. 13:433-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wertz, G. W., V. P. Perepelitsa, and L. A. Ball. 1998. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc. Natl. Acad. Sci. U. S. A. 95:3501-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitlow, Z. W., J. H. Connor, and D. S. Lyles. 2006. Preferential translation of vesicular stomatitis virus mRNAs is conferred by transcription from the viral genome. J. Virol. 80:11733-11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolffe, E. J., S. Vijaya, and B. Moss. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 211:53-63. [DOI] [PubMed] [Google Scholar]
- 49.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. U. S. A. 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao, Y., L. Aldaz-Carroll, A. M. Ortiz, J. C. Whitbeck, E. Alexander, H. Lou, H. L. Davis, T. J. Braciale, R. J. Eisenberg, G. H. Cohen, and S. N. Isaacs. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 25:1214-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]