Abstract
A substantial proportion of human immunodeficiency virus type 1 (HIV-1)-infected individuals has cross-reactive neutralizing activity in serum, with a similar prevalence in progressors and long-term nonprogressors (LTNP). We studied whether disease progression in the face of cross-reactive neutralizing serum activity is due to fading neutralizing humoral immunity over time or to viral escape. In three LTNP and three progressors, high-titer cross-reactive HIV-1-specific neutralizing activity in serum against a multiclade pseudovirus panel was preserved during the entire clinical course of infection, even after AIDS diagnosis in progressors. However, while early HIV-1 variants from all six individuals could be neutralized by autologous serum, the autologous neutralizing activity declined during chronic infection. This could be attributed to viral escape and the apparent inability of the host to elicit neutralizing antibodies to the newly emerging viral escape variants. Escape from autologous neutralizing activity was not associated with a reduction in the viral replication rate in vitro. Escape from autologous serum with cross-reactive neutralizing activity coincided with an increase in the length of the variable loops and in the number of potential N-linked glycosylation sites in the viral envelope. Positive selection pressure was observed in the variable regions in envelope, suggesting that, at least in these individuals, these regions are targeted by humoral immunity with cross-reactive potential. Our results may imply that the ability of HIV-1 to rapidly escape cross-reactive autologous neutralizing antibody responses without the loss of viral fitness is the underlying explanation for the absent effect of potent cross-reactive neutralizing humoral immunity on the clinical course of infection.
The need for an effective vaccine to prevent the global spread of human immunodeficiency virus type 1 (HIV-1) is well recognized. The ability to elicit broadly neutralizing antibodies (BrNAbs) is believed to be crucial to developing a successful vaccine, ideally to acquire protective immunity or, alternatively, to achieve a nonprogressive infection with viral loads sufficiently low to limit HIV-1 transmission (1, 39).
During natural infection, antibodies that are able to neutralize autologous virus variants are elicited in the majority of HIV-1-infected individuals. Early in infection, these neutralizing antibodies (NAbs) are mainly type specific, due to the fact that they are primarily directed against the variable domains in the viral envelope, and allow for the rapid escape of HIV-1 from antibody neutralization (8, 9, 14, 15, 20, 28, 41). Escape from type-specific neutralizing humoral immunity has been associated with enormous sequence variation, particularly in variable loops 1 and 2 (V1V2) of the envelope protein where large insertions and deletions are observed, as well as with changes in the number of potential N-linked glycosylation sites (PNGS) in the envelope protein (8, 15, 19, 22, 25, 27-31, 41). The rapid escape of HIV-1 from autologous type-specific NAbs seems to be the underlying explanation for the absent correlation between autologous humoral immunity and HIV-1 disease course. Furthermore, we recently observed that the changes in envelope that are associated with escape from autologous neutralizing humoral immunity do not coincide with a loss of viral fitness (7), providing an additional explanation for the lack of protection from disease progression by the autologous type-specific NAb response.
In the last couple of years, the focus of research has shifted toward neutralizing humoral immunity with cross-reactive activity, defined as the ability to neutralize a range of heterologous HIV-1 variants from different subtypes. It has become apparent that about one-third of HIV-1-infected individuals develop cross-reactive neutralizing activity in serum. However, the prevalence of cross-reactive neutralizing activity in serum was similar for HIV-infected individuals with a progressive disease course and long-term nonprogressors (LTNP) (11, 12, 34, 37).
We studied the underlying explanation for this observation in three LTNP and three progressors who all had high-titer cross-reactive neutralizing activity in serum within 2 to 4 years after seroconversion (SC). In all individuals, we observed that the potent and cross-reactive neutralizing immunity was preserved during the entire course of infection. However, the presence of cross-reactive neutralizing activity in serum did not prevent rapid viral escape from humoral immunity, which coincided with changes in envelope similar to those described for escape from type-specific autologous humoral immunity. Although broadly neutralizing antibodies are assumed to target the more conserved epitopes that may lie in crucial parts of the viral envelope, escape from cross-reactive neutralizing activity did not coincide with a loss in viral fitness. Our findings underscore that vaccine-elicited cross-reactive neutralizing immunity should protect against HIV-1 acquisition, since protection from disease progression, even by humoral immunity with strong cross-reactivity, may be an unachievable goal.
MATERIALS AND METHODS
Participants and viruses.
The six individuals studied here were selected from the Amsterdam Cohort Studies on HIV and AIDS in homosexual men. LTNP were defined as HIV-1-infected individuals who have ≥10 years of asymptomatic follow-up with stable CD4 counts that are still above 400 cells/μl in the ninth year of follow-up. Typical progressors were defined as HIV-1-infected individuals who progressed to AIDS within 7 years post-SC. All individuals were infected with HIV-1 subtype B. Five individuals were seropositive at entry into the cohort studies (seroprevalent cases with an imputed SC date on average 18 months before entry into the cohort [21, 38]), whereas participant H18969 seroconverted during active follow-up (8). None of the individuals received combination antiretroviral therapy during the follow-up period for the present study.
Clonal virus variants were obtained as previously described (32, 36). For further study, we selected a maximum of five virus variants per individual per time point to be tested for autologous neutralization sensitivity. Viruses were selected on the basis of their replication capacities to get a mix of different virus variants that had coexisted in vivo. To prevent a change in the neutralization sensitivity of the virus variants during in vitro culture, the number of virus passages in peripheral blood mononuclear cells (PBMC) was kept to a minimum (2).
The Amsterdam Cohort Studies were conducted in accordance with the ethical principles set out in the declaration of Helsinki, and written consent was obtained prior to data collection. The study was approved by the Academic Medical Center Institutional Medical Ethics Committee.
U87/pseudovirus assay for testing of HIV-1 cross-reactive neutralizing activity in serum.
Sera from these six individuals were tested for neutralizing activity in a pseudovirus assay developed by Monogram Biosciences. The tier 2-3 virus panel that we used for determining cross-neutralizing activity in serum consisted of HIV-1 pseudoviruses from subtypes A (n = 5), B (n = 6), C (n = 7), and D (n = 5). Viruses were obtained recently after transmission or during the chronic phase of infection and included both moderately neutralization sensitive and neutralization resistant primary HIV-1 variants, based on previously determined neutralization sensitivities to subtype B sera and monoclonal antibodies b12, 2G12, and 4E10 (4, 33, 34). Not all sera were tested against all viruses of the panel. Pseudotyped viral particles were produced by cotransfecting HEK293 cells with an expression vector carrying the HIV-1-derived gp160 gene (eETV) and an HIV-1 genomic vector carrying a luciferase reporter gene (pRTV1.F-lucP.CNDO-ΔU3). At 48 h after transfection, pseudovirus stocks were harvested, and small aliquots were tested for infectivity using U87 target cells expressing CD4, CCR5, and CXCR4. Pseudovirus stocks were tested and normalized for infectivity prior to testing in the neutralization assay.
A recombinant virus assay involving a single round of virus infection was used to measure cross-neutralization activity of the sera (23, 28). Diluted pseudoviruses were incubated for 1 h at 37°C with serial dilutions of serum, after which the U87 target cells were added. The ability of participant sera to neutralize viral infection was assessed by measuring luciferase activity 72 h after viral inoculation in comparison to a control infection with a virus pseudotyped with amphotropic murine leukemia virus envelope proteins gp70SU and p15TM (aMLV). Neutralization titers are expressed as the reciprocal of the plasma dilution that inhibited virus infection by 50% (IC50). Neutralization titers were considered positive if they were three times greater than the negative aMLV control and were ≥100. The lowest serum dilution used in the assay was 1:40.
PBMC-based assay for testing HIV-1 autologous neutralizing activity in serum.
Clonal virus variants of participants were tested for their relative neutralization sensitivities against autologous serum and pooled sera from healthy, uninfected individuals. PBMC were obtained from buffy coats from 10 healthy seronegative blood donors and pooled prior to use. Cells were isolated by Ficoll-Isopaque density gradient centrifugation and then stimulated for 3 days in Iscove modified Dulbecco medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 U/ml), ciproxin (5 μg/ml), and phytohemagglutinin (PHA; 5 μg/ml) at a cell concentration of 5 × 106/ml. After inoculation, the cells (106/ml) were grown in the absence of PHA in medium supplemented with recombinant interleukin-2 (20 U/ml; Chiron Benelux, Amsterdam, The Netherlands) and Polybrene (5 μg/ml; hexadimethrine bromide; Sigma, Zwijndrecht, The Netherlands). To prevent possible complement-mediated antibody inhibition of virus infection, complement in human sera and fetal bovine serum was inactivated by a 30-min incubation at 56°C.
From each virus isolate, an inoculum of 20 50% tissue culture infective doses in a total volume of 50 μl was incubated for 1 h at 37°C with decreasing concentrations of the serum (starting concentration, 1:50) in 96-well microtiter plates. Subsequently, 105 PHA-stimulated PBMC were added to the mixtures of virus with serum. After 4 h of incubation, PBMC were washed once in 100 μl of phosphate-buffered saline, after which fresh medium was added. On day 11, virus production in culture supernatants was analyzed in an in-house p24 antigen capture enzyme-linked immunosorbent assay (35). Background measurements were performed with pooled sera from uninfected individuals, and neutralization titers were expressed as the reciprocal serum dilution that established the 50% inhibitory concentrations (IC50s) of virus infection. Experiments were performed in triplicate. When possible, the IC50s were determined by linear regression. To calculate IC50s for viruses that were not inhibited by the 1:50 serum dilution, we assumed that 50% inhibition would have occurred at a 1:25 serum dilution.
Preparation of chimeric viruses.
To exclude an effect of additional mutations in other genes than Env on the viral replication rate, we generated a panel of chimeric NL4-3 viruses, in which the original envelope was replaced with the envelopes of virus variants that were isolated from our participants. For each time point, envelopes from a minimum of two and a maximum of eight viruses were analyzed.
env fragments from HXB2 nucleotides (nt) 5658 to 9171 were amplified by PCR using Expand High-Fidelity PCR System (Roche Applied Science). Chimeric NL4-3/Env viruses were produced by homologous recombination of the Env PCR products with a pNL4-3 vector (kindly provided by J. Alcami). In short, pNL4-3 was restricted with XbaI (HXB2 nt 6114) and XhoI (HXB2 nt 8898) and was subsequently cotransfected with an env PCR product into 293T cells in a 24-well plate by using the calcium phosphate method. After 2 days, PHA-stimulated PBMC from healthy seronegative blood donors were added to the culture, and the next day the PBMC were transferred to a culture flask. Supernatants were harvested when positive for p24, as determined by using an in-house p24 antigen capture enzyme-linked immunosorbent assay (35). The presence of the correct env in NL4-3 was confirmed by sequencing.
Sequence analysis.
The HIV envelope gp160 gene was PCR amplified from DNA isolated from PBMC that were infected in vitro with a single clonal HIV-1 variant and subsequently sequenced as described previously (3, 6, 26). The nucleotide sequences of all virus clones from an individual were aligned by using CLUSTAL W in the software package BioEdit (16) and edited manually. The reference sequence HXB2 was included in the alignment to number each aligned residue according to the corresponding position in this reference sequence. Genetic analyses were performed on gp160 sequences starting at nucleotide position 91, which excludes the Env signal peptide. PNGS were identified using N-glycosite (43) at the HIV database website (https://www.hiv.lanl.gov/content/sequence/GLYCOSITE/glycosite.html) (42). Net charges of gp160 were calculated by counting all charged amino acid residues per sequence, where residues R and K counted as +1, H as +0.293, and D and E as −1. Nonsynonymous substitution (dN) and synonymous substitution (dS) rates for the different regions of env were calculated by using the Synonymous Nonsynonymous analysis program (https://hiv.lanl.gov/content/sequence/SNAP/SNAP.html). dN/dS ratios were calculated between successive time points by averaging the dN/dS ratios between all individual pairs of env sequences from the two time points. Positively selected codons were identified by using DataMonkey (http://www.datamonkey.org) with the REL, FEL, and SLAC method and were assumed to be truly positively selected if two methods were significant (P < 0.05). To ensure a correct calculation of dN/dS ratios and positive selection in the variable loops, the codon alignments of these regions were corrected manually. Codons containing indels were excluded in this method.
Characterization of HIV-1 replication kinetics.
Replication kinetics of the clonal virus variants were determined by using chimeric NL4-3/Env viruses on pooled PBMC which were obtained and stimulated as described above. A total of 2 × 106 PHA-stimulated PBMC were inoculated with 500 50% tissue culture infective doses of a given chimeric NL4-3/Env HIV-1 variant in a volume of 2 ml at 37°C for 2 h in a shaking water bath. Subsequently, cells were washed with 10 ml of Iscove modified Dulbecco medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 U/ml) and resuspended at a concentration of 106 cells/ml for culture. Fresh PHA-stimulated PMBC (106) in a volume of 1 ml were added at days 5 and 8. Cultures were maintained for 11 days. Then, 75 μl of supernatant for the determination of p24 antigen production was harvested each day. The concentration of p24 in all samples was determined at the same time using an in-house p24 antigen capture enzyme-linked immunosorbent assay (29). p24 production per ml of supernatant was determined and corrected for the differences in volume of culture supernatant. Per individual, the period of logarithmic expansion of viral p24 production was determined, and only this timeline was used for further analyses.
Statistical analysis.
Statistical analyses were performed by using the SPSS 16 software package. Changes in replication kinetics were compared by using an unpaired two-sample t test. Changes in the length and the number of PNGS in Env were assessed by using the Kruskal-Wallis analysis of variance.
Nucleotide sequence accession numbers.
The sequences used in the present study have been deposited in GenBank under accession numbers EU744055 to EU744096 and GU455425 to GU455525.
RESULTS
Longitudinally preserved cross-reactive neutralizing serum activity in three LTNP and three progressors.
We previously demonstrated a similar prevalence of cross-reactive neutralizing activity in sera of LTNP and progressors at time points relatively early in infection (37). In the present study, we first wanted to study whether a progressive disease course was associated with a more rapid loss of cross-reactive neutralizing serum activity at the later stages of disease. To this end, we selected three LTNP and three progressors from the Amsterdam Cohort Studies on HIV infection and AIDS, for whom we previously established cross-reactive neutralizing activity in serum samples that were obtained at around years 2 and 4 post-SC (37). For these patients, we analyzed cross-reactive neutralizing activity in sera that were obtained at multiple time points during the course of infection, up to the moment of clinical AIDS diagnosis or initiation of HAART (highly active antiretroviral therapy) in the three progressors and in one LTNP who ultimately progressed to AIDS or until end of follow-up in the other two LTNP (Fig. 1). HIV-1-specific neutralizing activity was measured in a cell-based infectivity assay using a panel of 23 recombinant viruses pseudotyped with envelope proteins from HIV-1 subtype A, B, C, and D. Due to the limited availability of serum, some sera were only tested against a subset of this virus panel.
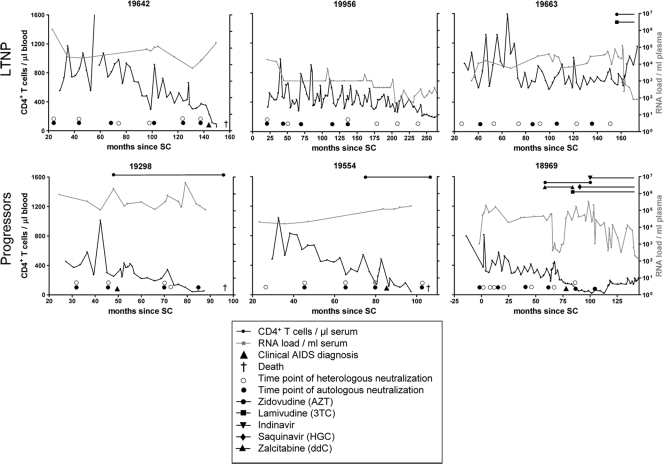
FIG. 1.
CD4+ T-cell count, viral RNA load, and antiretroviral treatment during the course of infection of three LTNP (top) and three progressors (bottom). The CD4+ T-cell count are shown in black with the legend on the left y axis, while the viral RNA load are indicated in gray with the legend on the right y axis. The detection limit for the measurement of RNA load was 1,000 copies/ml of plasma, which decreased to 400 copies/ml plasma later in time (for participant H19956 from 200 months onward). The length and type of antiretroviral therapy are indicated at the top of each diagram.
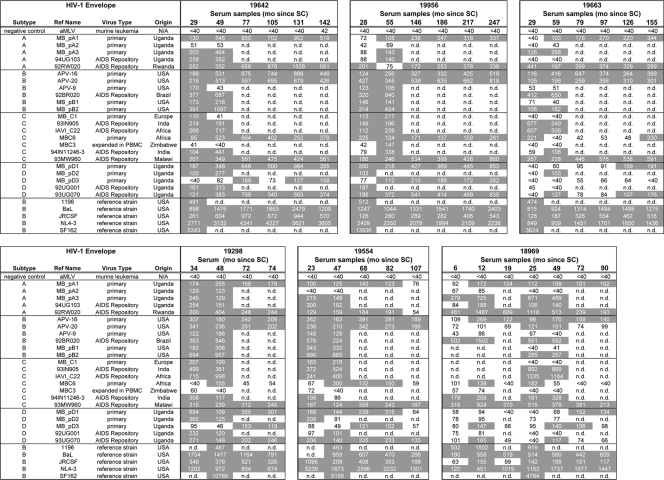
HIV-specific cross-reactive neutralizing activity, defined as an IC50 of ≥100 against at least 50% of the viruses from three or more subtypes, was observed in sera from all six individuals. For participant H18969, cross-reactive neutralizing serum activity developed as early as 12 months post-SC (Fig. 2). In contrast, serum from participant H19663 did not show cross-reactive neutralizing activity until 59 months post-SC, although serum obtained 29 months post-SC from this participant was already able to neutralized virus variants from different subtypes (Fig. 2). In the remaining four participants, cross-reactive neutralizing serum activity was observed at ∼30 months post-SC (Fig. 2). However, serum samples from earlier time points were not available for these four participants, indicating that cross-reactive neutralizing activity could have been present earlier in infection. Without exception, cross-reactive neutralizing serum activity was conserved longitudinally in both LTNP and progressors. Neutralizing serum titers increased over the course of infection until the end of follow-up in two LTNP or until around the moment of clinical AIDS diagnosis for the four participants who developed AIDS. After clinical AIDS diagnosis, cross-reactive neutralizing serum activity declined, although the breadth of neutralization was preserved (Fig. 1 and 2).
FIG. 2.
Breadth and potency of HIV-1-specific neutralizing activity in sera obtained during the course of infection from three LTNP (top) and three progressors (bottom). IC50s, given as the reciprocal serum dilution determined using a U87-based neutralizing assay, are shown for serum samples obtained during the course of infection against a panel of 23 heterologous virus variants. Due to limiting amounts of serum, some sera were only tested against a subset of this virus panel. In the left columns, a description of the virus panel is given; the tier 2-3 virus panel consisted of HIV-1 pseudoviruses from subtype A, B, C, and D. The references panel (bottom part) included strains 1196, BaL, JRCSF, NL4-3, and SF162. As a negative control (NC), the amphotropic murine leukemia virus was used. IC50 titers ≥ 1:100 and exceeding three times the background reading for that sample are indicated in gray. n.d., not done.
Decreasing neutralizing humoral immunity against autologous HIV-1 during the course of infection.
The observation that cross-reactive neutralizing serum activity was preserved during the course of infection in both LTNP and progressors excludes the possibility that loss of humoral immunity precedes disease progression. To investigate whether viruses from LTNP and progressors showed a difference in their ability to escape from autologous humoral immunity, we analyzed the efficacy of neutralizing serum activity against autologous virus variants.
Clonal HIV-1 variants were isolated from PMBC that were obtained at approximately the same time points at which the sera were collected. Although one to five clones per time point were isolated from earlier time points in participant H19956, attempts to isolate clonal HIV-1 variants from PBMC that were obtained at time points after 150 months post-SC were not successful. From participant H19642, both R5 and X4 HIV-1 variants were isolated at the time point just before clinical AIDS diagnosis, while from earlier time points only R5 variants were obtained. From participant H19554, both R5 and X4 HIV-1 variants were isolated at around 5.5 and 7 years after SC, but only R5 HIV-1 variants were isolated after clinical AIDS diagnosis.
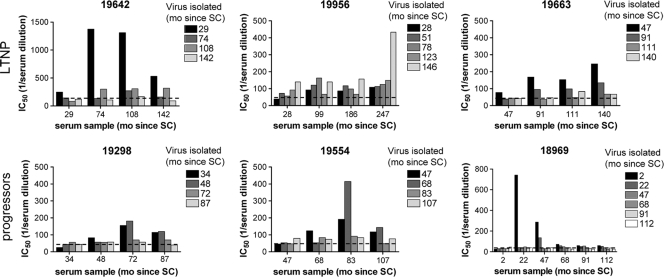
For each individual, autologous neutralizing activity in sera obtained at or close to the time points of virus isolation were measured against a maximum of five randomly selected clonal HIV-1 variants per time point, both R5 and X4 HIV-1 variants when applicable. The number of HIV-1 variants that could be tested was limited by the amount of participant serum that was available. Neutralization of autologous virus variants was observed in all six individuals, although the level of neutralization was diverse (Fig. 3). In agreement with findings by others (8, 9, 14, 15, 20, 22, 28, 30, 41), virus variants were poorly neutralized by contemporaneous serum and sera from earlier time points, a finding suggestive of viral escape. In general, the neutralizing titer in serum was highest against the earliest virus variants and was much less potent against virus variants from subsequent time points. Moreover, this limited autologous neutralizing activity against early viruses was lost after AIDS diagnosis in those individuals who ultimately progressed to AIDS. For participant H19956, we observed a different pattern of neutralization, although it should be mentioned that the viruses from this participant were isolated from much earlier time points than the sera that were available for testing. For this reason, titers against all viruses were somewhat higher than what was observed in the other patients, and the highest titer was observed for the last serum sample tested against an earlier virus variant. However, only a single virus variant was obtained from the 123 and 146 months post-SC time points, respectively, not allowing firm conclusions on the effect of humoral immunity in this individual.
FIG. 3.
Development of autologous humoral immune responses during the course of infection in three LTNP (top) and three progressors (bottom). The average IC50s, determined by linear regression, of ≤5 virus variants per time point are indicated. The time points of virus isolation are indicated in the top right corner of each panel. Bars with identical shading represent inhibition of virus isolates from one time point by sera of different time points (as indicated on the x axis). The dashed lines represent background measurements using pooled sera from healthy uninfected individuals. Note that the maximum value on the y axis in the graph of participant H19642 and H18969 are higher than in the other graphs. IC50, 50% inhibitory concentration; mo, months; SC, seroconversion.
Overall, for viruses from the same time point, neutralizing titers in sera varied only minimally. In all six individuals, autologous neutralizing activity was lost already in the asymptomatic phase of infection, before clinical AIDS was diagnosed. Moreover, we did not observe any difference between LTNP and progressors in autologous neutralizing activity. Escape from autologous neutralization did not coincide with changes in plasma viral RNA load and/or CD4+ T-cell counts (Fig. 1 and 3). We also did not observe a difference in neutralization sensitivity between R5 and X4 HIV-1 variants.
Our data show that autologous neutralizing antibody responses could no longer be mounted later in infection and that the autologous neutralizing activity that was elicited early in infection diminished over time, while at the same time heterologous responses were preserved.
Evolution of the envelope protein during the course of infection in individuals with cross-reactive neutralizing activity in serum.
Next, we analyzed the molecular changes in the viral envelope during the clinical course of HIV-1 infection that coincided with escape from neutralizing humoral immunity with cross-reactive neutralizing activity. To this end, full-length gp160 sequences were generated from a median of five virus variants (range, 1 to 10) per time point (Table 1). Phylogenetic analysis of all sequences using the neighbor-joining method revealed clustering of sequences per individual and excluded superinfection and contamination of samples (data not shown).
TABLE 1.
Envelope characteristics of the isolated virus variants per individual per time point
| LNTP or progressor | Time post-SC (mo) | No. of virus samples | Avg sequence characteristic (SD)a |
|||||
|---|---|---|---|---|---|---|---|---|
| gp160 length (aa) | No. of PNGS |
dN/dS |
||||||
| gp160 | C region | V region | C region (gp120) | V region (gp120) | ||||
| LTNP | ||||||||
| H19642 | P = 0.008 | P = 0.003 | P = 0.009 | P = 0.026 | ||||
| 29 | 4 | 828.4 (2.61) | 29.6 (2.30) | 12.8 (0.84) | 12.0 (1.23) | |||
| 49 | 5 | 834.8 (2.23) | 34.2 (1.84) | 15.0 (1.55) | 14.2 (0.75) | 0.573 (0.195) | 1.074 (0.447) | |
| 74 | 5 | 833.7 (3.56) | 34.2 (0.75) | 15.2 (0.41) | 14.5 (1.05) | 0.512 (0.206) | 0.996 (0.305) | |
| 108 | 4 | 836.4 (6.80) | 34.4 (1.52) | 15.2 (0.45) | 13.8 (1.30) | 0.377 (0.114) | 0.984 (0.292) | |
| 131 | 4 | 840.0 (1.63) | 31.8 (1.89) | 14.3 (0.50) | 13.0 (1.41) | 0.422 (0.175) | 1.316 (0.365) | |
| 142 | 5 | 839.2 (3.56) | 31.8 (0.84) | 14.4 (0.55) | 13.4 (0.55) | 0.321 (0.132) | 0.773 (0.405) | |
| H19956 | P = 0.174 | P = 0.100 | P = 0.166 | P = 0.076 | ||||
| 28 | 5 | 829.8 (3.49) | 30.6 (1.52) | 13.0 (0.71) | 13.6 (0.89) | |||
| 51 | 3 | 826.3 (1.16) | 28.3 (0.58) | 12.3 (0.58) | 12.0 (0.00) | 0.952 (0.246) | 1.186 (0.446) | |
| 78 | 2 | 828.5 (0.71) | 29.5 (0.71) | 12.5 (0.71) | 13.0 (0.00) | 0.602 (0.135) | 1.042 (0.297) | |
| 123 | 1 | 832.0 | 28.0 | 11.0 | 13.0 | 0.617 (0.048) | 1.249 (0.721) | |
| 146 | 1 | 841.0 | 29.0 | 11.0 | 14.0 | |||
| H19663 | P = 0.003 | P = 0.500 | P = 0.021 | P = 0.061 | ||||
| 47 | 5 | 831.6 (3.21) | 29.6 (1.520) | 13.2 (0.84) | 12.2 (1.10) | |||
| 91 | 6 | 844.3 (8.62) | 30.2 (0.98) | 11.7 (0.52) | 14.2 (1.47) | 0.852 (0.333) | 2.483 (1.722) | |
| 111 | 6 | 850.0 (8.37) | 31.0 (1.55) | 12.5 (0.84) | 14.5 (1.38) | 0.890 (0.338) | 1.371 (0.527) | |
| 140 | 4 | 857.5 (0.58) | 29.8 (0.50) | 12.0 (0.00) | 13.8 (0.50) | 1.012 (0.460) | 1.284 (0.355) | |
| Progressors | ||||||||
| H19298 | P = 0.005 | P = 0.044 | P = 0.006 | P = 0.011 | ||||
| 34 | 2 | 836.0 (2.83) | 31.0 (0.00) | 13.0 (0.00) | 14.0 (0.00) | |||
| 48 | 5 | 846.7 (4.46) | 32.7 (1.03) | 13.2 (0.41) | 15.2 (1.17) | 0.788 (0.202) | 1.085 (0.390) | |
| 72 | 5 | 843.6 (1.52) | 32.8 (0.84) | 14.2 (0.45) | 14.6 (0.55) | 0.557 (0.211) | 0.641 (0.184) | |
| 87 | 5 | 837.4 (2.07) | 31.6 (0.55) | 14.2 (0.45) | 12.8 (0.45) | 0.487 (0.171) | 0.412 (0.139) | |
| H19554 | P = 0.205 | P = 0.002 | P = 0.081 | P = 0.004 | ||||
| 47 | 5 | 835.8 (1.60) | 33.8 (0.41) | 13.0 (0.00) | 14.8 (0.41) | |||
| 68 | 4 | 840.8 (8.90) | 33.0 (1.00) | 12.4 (0.55) | 14.8 (0.84) | 0.361 (0.092) | 1.234 (0.481) | |
| 83 | 6 | 833.3 (6.74) | 31.5 (0.84) | 12.7 (0.52) | 13.5 (0.55) | 0.339 (0.156) | 1.364 (0.482) | |
| 107 | 3 | 832.0 (3.46) | 30.3 (1.26) | 12.3 (0.50) | 13.0 (0.82) | 0.228 (0.095) | 1.073 (0.466) | |
| H18969 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||||
| 2 | 8 | 813.4 (2.20) | 27.4 (1.06) | 10.9 (0.35) | 11.5 (0.93) | |||
| 22 | 8 | 826.1 (3.87) | 31.6 (1.06) | 12.1 (0.64) | 14.3 (1.17) | 1.592 (0.529) | 2.741 (1.928) | |
| 47 | 10 | 829.5 (5.19) | 31.5 (1.72) | 13.7 (0.67) | 13.4 (0.97) | 1.411 (0.512) | 1.964 (1.158) | |
| 68 | 6 | 832.2 (8.47) | 33.0 (2.61) | 13.2 (0.75) | 15.5 (2.59) | 0.757 (0.297) | 1.573 (0.482) | |
| 91 | 8 | 836.5 (3.12) | 33.0 (1.60) | 12.9 (0.64) | 16.1 (1.25) | 0.611 (0.301) | 1.488 (0.414) | |
| 112 | 3 | 838.0 (1.41) | 33.5 (2.12) | 12.5 (0.71) | 17.0 (1.41) | 0.435 (0.154) | 1.284 (0.374) | |
The average sequence characteristic for all viruses from one time point is presented, with the standard deviations given in parentheses. Changes in sequence characteristics over the course of infection within each individual were calculated by using the Kruskal-Wallis test. P values are as indicated. The dN/dS ratios are a comparison between viruses of that time point and viruses of the previous time point.
Escape of HIV-1 from type-specific NAbs has been associated with increases in the length of the viral envelope and the number of potential N-linked glycosylation sites in Env (8, 30, 31, 41). For the virus variants that were isolated from the six individuals in the present study, we observed an increase in length of gp160 during the course of infection. For viruses of participants H19642 and H18969, this extension of the length of the envelope protein reached a plateau, while the envelope length of viruses from participants H19289 and H19544 decreased at later time points (Table 1). The plateau or decrease in the length of the envelope protein coincided with fading autologous neutralizing activity in these participants (Fig. 3). The changes in gp160 length could be completely attributed to the variable regions, except for viruses from participant H19663, in which minor insertions in C3 were observed. Insertions and deletions were observed in V1 and V4 for viruses from all participants, while additional changes in the other variable regions of gp120 were observed for viruses from some participants (data not shown).
A similar pattern of change over the course of infection was observed for the number of PNGS. The changes in PNGS in gp160 of all individuals over time were caused by the acquisition and/or loss of PNGS in both the constant and variable regions of gp160 (Table 1). For all individuals, the number and/or location of the PNGS in the C3 and V1V2 region of gp120 changed over time. Moreover, additional changes in other regions of the envelope protein were observed in viruses from participants H19642, H19956, H19663, and H19554 (data not shown). Changes in gp160 length and PNGS did not always occur simultaneously in time. For example, in viruses from participant H19642, the number of PNGS decreased already from 4 years post-SC onward, while the average length of gp160 still increased (Table 1).
Changes in the net charge of the V1V2 loop during infection have previously been reported to be correlated with higher neutralizing titers (5). Apart from an increase in the net charge of V2 over time in viruses from all individuals, we did not observe any uniform changes in the envelope net charge over the course of infection (data not shown).
To characterize regions in the envelope protein that were positively selected over the course of infection, we calculated the selection pressure per codon using virus variants from all different time points for each individual, as well as the dN/dS ratio for the variable and constant regions between virus variants from successive time points. Positively selected codons were observed in all regions of gp160 and did not reveal specific mutations that correlated with neutralization sensitivity. However, dN/dS ratios were highest for the variable regions, suggesting that the selection pressure was strongest in these regions. Moreover, evidence for positive selection of the constant regions was absent in viruses from all participants except for viruses obtained from participant H18969 between 0 and 4 years post-SC (Table 1). dN/dS ratios decreased over time and were similar for viruses from LTNP and progressors.
Overall, we did not observe any differences in the length, number of PNGS, or net charge between gp160 of viruses from LTNP and progressors. In addition, similar regions of the viral envelope showed evidence of positive selection. These results indicate that the evolution of HIV-1 over the course of infection is similar in both LTNP and progressors with cross-reactive neutralizing serum activity.
Escape from cross-neutralizing activity does not coincide with a loss of viral replication capacity in vitro.
Cross-reactive neutralizing activity is assumed to be directed against more conserved regions in the viral envelope. Escape mutations in these regions may therefore have an impact on the viral replication fitness. We studied here whether escape from autologous humoral immunity with cross-reactive neutralizing activity was associated with a reduction in viral replication fitness. Although the molecular changes that we observed were similar for viruses from LTNP and progressors, this does not exclude that specific amino acid changes in LTNP viruses or molecular changes in the background of these viruses have a higher impact on viral replication rate than similar changes in the background of the HIV-1 variants from the progressors we studied here. Therefore, by affecting the viral replication rate, humoral immunity could still, although indirectly, contribute to the differential clinical course in LTNP and progressors. Since some individuals in our study received antiretroviral monotherapy for certain periods of time, HIV-1 variants with drug resistance mutations may have been selected. To exclude an effect of these and any other mutations outside Env on the viral replication rate, we generated a panel of chimeric NL4-3 viruses in which the original envelope gene was replaced with the envelope genes of the virus variants that were isolated from our participants during the clinical course of infection. Replication kinetics were determined by the logarithmic expansion of equal viral inocula in PHA-stimulated PBMC and analyzed as p24 production during the period of logarithmic expansion. From participant H19956, too few clonal virus variants were available for analysis of the replication rate.
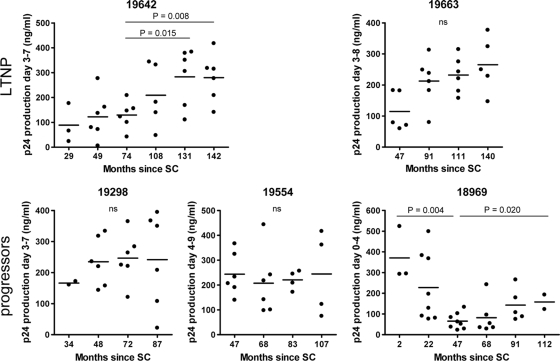
Replication kinetics varied between viruses from a single individual, and even between viruses obtained from the same time point. Over the course of infection, we generally observed either stable or increasing replication rates (Fig. 4), suggesting that escape from cross-neutralizing activity did not coincide with a reduction of the viral replicative capacity. However, the replication rates of HIV-1 variants from participant H18969 decreased during the first 47 months of infection, which coincided with the presence of autologous neutralizing activity in serum (Fig. 4). This might imply that for HIV-1 variants from this individual, an effect of NAb escape mutations on viral replication fitness cannot be excluded. We observed that an increase or decrease in replication rate did not correlate with changes in plasma viral RNA load (Fig. 1 and 4) and that there was no difference in replication kinetics between R5 and X4 HIV-1 variants of these individuals.
FIG. 4.
Replication kinetics of clonal virus variants obtained during the course of infection from three LTNP (top) and three progressors (bottom). Replication rates of individual chimeric NL4-3/Env variants are expressed as the p24 production during the logarithmic expansion after infection of PHA-stimulated PBMC. The horizontal lines represent the means. Note that the maximum value on the y axis in the graphs is different for each individual. SC, seroconversion.
DISCUSSION
HIV-1-specific cross-reactive humoral immunity is assumed to be directed against relatively conserved regions on the viral envelope. As a consequence, HIV-1 may be unable to rapidly escape from cross-reactive NAb pressure, suggesting that a broad and potent humoral immune response may influence the clinical course of infection. However, we have recently demonstrated that the prevalence of cross-reactive neutralizing activity in serum is similar among HIV-infected individuals with a progressive disease course and LTNP (37). This absent correlation between disease course and cross-reactive neutralizing activity in serum could either point to fading humoral immunity in the progressive course of infection or to viral escape from antibody pressure, as has been shown to occur in response to type-specific neutralizing humoral immunity (8, 9, 14, 15, 20, 22, 28, 30, 41).
In the longitudinal analysis performed in our present study, cross-reactive neutralizing humoral immunity was preserved in both LTNP and progressors, even after the moment of AIDS diagnosis in those individuals who ultimately progressed to AIDS. In contrast, autologous neutralizing activity was only observed against viruses that were isolated early in infection. Moreover, this limited autologous neutralizing activity against early viruses was lost after AIDS diagnosis. These findings not only point toward a rapid selection of HIV-1 variants that resisted the neutralizing activity in serum, they also demonstrate the inability of the infected host to generate novel neutralizing antibody specificities against these escape variants.
One could argue that the apparent discrepancy between preserved cross-reactive neutralizing activity but fading autologous neutralizing activity could relate to differences in sensitivities of the assays used for their detection (13). Cross-reactive neutralizing activity was tested against a panel of pseudoviruses in a U87-based assay, whereas autologous neutralizing activity was tested in a PBMC-based assay with replicating viruses. However, we have previously shown that the relative potency of neutralizing serum activity as detected by these two assays is comparable (37). The different profiles of autologous versus heterologous neutralizing activity over the course of infection as observed in the present study are thus likely to reflect true differences in the development and persistence of these components of neutralizing serum activity.
We recently demonstrated that escape from type-specific autologous neutralizing activity in serum did not influence the in vitro replication fitness of HIV-1 (7). However, our observation that rapid escape of HIV-1 from autologous humoral immunity with cross-reactive neutralizing activity also had no impact on the viral replicative fitness was somewhat unexpected since BrNAbs are considered to target conserved epitopes which, by definition, carry crucial functions for the virus. It is tempting to speculate that replication fitness is restored by compensatory mutations that may rapidly be selected. This is currently under investigation.
Overall, the similar potency of humoral immunity, the similar dynamics of viral escape, and the absent impact of escape on the replication kinetics of viruses from both LTNP and progressors argue against a role for NAb in the clinical course of infection. In agreement, we and others have shown in comprehensive cohort analyses that the presence of cross-reactive neutralizing activity was not associated with prolonged AIDS free survival (12, 24). Indeed, HIV-1 cellular immunity and host genetic background seem to have a more pronounced effect on disease progression (17, 18).
Escape from autologous neutralizing humoral immunity with cross-reactive activity coincided with an increase in the length and number of PNGS of gp160 and an increase in the net charge of the V2 region. Similar changes were observed in HIV-1 variants that escaped from autologous neutralizing humoral immunity with only type-specific activity (8, 22, 30). This may either suggest that the same mechanisms apply for escape from different antibody specificities or that the relevant changes for escape from cross-reactive neutralizing antibodies are masked by changes that are selected by type-specific antibodies. Positive selection pressure was mainly observed in the variable regions of the envelope protein. Interestingly, two novel highly potent cross-reactive neutralizing antibodies directed against a conformational epitope in the V2V3 region have recently been described (40), suggesting that the variable regions can indeed be targeted by cross-reactive neutralizing antibodies.
We are currently studying the exact nature of the humoral immune response in the individuals in our study which will reveal whether the cross-reactive neutralizing activity is determined by a single high-affinity antibody or by a combination of multiple coexisting neutralizing antibodies directed at multiple distinct regions of the envelope. The results from these analyses will help to define which changes in the viral envelope are relevant for escape from cross-reactive neutralizing activity.
Taken together, our findings seem to underscore the absent role for cross-reactive neutralizing humoral immunity in the protection from disease progression due to the ability of HIV-1 to rapidly escape from this immune pressure without a loss of viral fitness. Whereas vaccine-elicited cellular immunity may be able to control viremia and thereby contribute to protection from disease progression (10), our results support the notion that vaccine-elicited BrNAbs may only be relevant for protection from the acquisition of infection.
Acknowledgments
We thank the participants of the Amsterdam Cohort Studies for their continuous participation in the study. We are grateful to Diana Edo Matas for her help with the sequence analysis. We thank José Alcami for kindly providing the pNL4-3 vector.
The Amsterdam Cohort Studies on HIV infection and AIDS, a collaboration between the Amsterdam Health Service, the Academic Medical Center of the University of Amsterdam, the Sanquin Blood Supply Foundation, the University Medical Center Utrecht, and the Jan van Goyen Clinic, are part of The Netherlands HIV Monitoring Foundation and are financially supported by the Center for Infectious Disease Control of The Netherlands National Institute for Public Health and the Environment. This study was financially supported by The Netherlands Organization for Scientific Research (grant 918.66.628), the Dutch AIDS fund (grant 2004064), the European Community's Six Framework Programme Europrise (FP6/2007-2012) under grant number 037611, and the European Community's Seventh Framework Programme NGIN (FP7/2007-2013) under grant agreement 201433. It was also partially funded by an NIH Small Business Innovation Research grant (5R44AI062522) awarded to Monogram Biosciences. The funding organizations had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print on 13 January 2010.
REFERENCES
- 1.Barouch, D. H. 2008. Challenges in the development of an HIV-1 vaccine. Nature 455:613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaumont, T., E. Quakkelaar, A. van Nuenen, R. Pantophlet, and H. Schuitemaker. 2004. Increased sensitivity to CD4 binding site-directed neutralization following in vitro propagation on primary lymphocytes of a neutralization-resistant human immunodeficiency virus IIIB strain isolated from an accidentally infected laboratory worker. J. Virol. 78:5651-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaumont, T., A. van Nuenen, S. Broersen, W. A. Blattner, V. V. Lukashov, and H. Schuitemaker. 2001. Reversal of HIV-1 IIIB toward a neutralization resistant phenotype in an accidentally infected laboratory worker with a progressive clinical course. J. Virol. 75:2246-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blay, W. M., S. Gnanakaran, B. Foley, N. A. Doria-Rose, B. T. Korber, and N. L. Haigwood. 2006. Consistent patterns of change during the divergence of human immunodeficiency virus type 1 envelope from that of the inoculated virus in simian/human immunodeficiency virus-infected macaques. J. Virol. 80:999-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. Van der Noordaa. 1991. A rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunnik, E. M., M. S. D. Lobbrecht, A. van Nuenen, and H. Schuitemaker. 2010. Escape from autologous humoral immunity of HIV-1 is not associated with a decrease in replicative capacity. Virology 397:224-230. [DOI] [PubMed] [Google Scholar]
- 8.Bunnik, E. M., L. Pisas, A. C. van Nuenen, and H. Schuitemaker. 2008. Autologous neutralizing humoral immunity and evolution of the viral envelope in the course of subtype B human immunodeficiency virus type 1 infection. J. Virol. 82:7932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks, S. G., B. Schweighardt, T. Wrin, J. Galovich, R. Hoh, E. Sinclair, P. Hunt, J. M. McCune, J. N. Martin, C. J. Petropoulos, and F. M. Hecht. 2006. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J. Virol. 80:6155-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks, S. G., and B. D. Walker. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406-416. [DOI] [PubMed] [Google Scholar]
- 11.Doria-Rose, N. A., R. M. Klein, M. M. Manion, S. O'Dell, A. Phogat, B. Chakrabarti, C. W. Hallahan, S. A. Migueles, J. Wrammert, R. Ahmed, M. Nason, R. T. Wyatt, J. R. Mascola, and M. Connors. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 83:188-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Euler, Z., M. J. van Gils, E. M. Bunnik, P. Phung, B. Schweighardt, T. Wrin, and H. Schuitemaker. Cross-reactive neutralizing humoral immunity does not protect from HIV-1 disease progression. J. Infect. Dis., in press. [DOI] [PubMed]
- 13.Fenyo, E. M., A. Heath, S. Dispinseri, H. Holmes, P. Lusso, S. Zolla-Pazner, H. Donners, L. Heyndrickx, J. Alcami, V. Bongertz, C. Jassoy, M. Malnati, D. Montefiori, C. Moog, L. Morris, S. Osmanov, V. Polonis, Q. Sattentau, H. Schuitemaker, R. Sutthent, T. Wrin, and G. Scarlatti. 2009. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One 4:e4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost, S. D., T. Wrin, D. M. Smith, S. L. Kosakovsky Pond, Y. Liu, E. Paxinos, C. Chappey, J. Galovich, J. Beauchaine, C. J. Petropoulos, S. J. Little, and D. D. Richman. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. U. S. A. 102:18514-18519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray, E. S., P. L. Moore, I. A. Choge, J. M. Decker, F. Bibollet-Ruche, H. Li, N. Leseka, F. Treurnicht, K. Mlisana, G. M. Shaw, S. S. Karim, C. Williamson, and L. Morris. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 81:6187-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 17.Hogan, C. M., and S. M. Hammer. 2001. Host determinants in HIV infection and disease. Part 2: genetic factors and implications for antiretroviral therapeutics. Ann. Intern. Med. 134:978-996. [DOI] [PubMed] [Google Scholar]
- 18.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni, S. S., A. Lapedes, H. Tang, S. Gnanakaran, M. G. Daniels, M. Zhang, T. Bhattacharya, M. Li, V. R. Polonis, F. E. McCutchan, L. Morris, D. Ellenberger, S. T. Butera, R. C. Bollinger, B. T. Korber, R. S. Paranjape, and D. C. Montefiori. 2009. Highly complex neutralization determinants on a monophyletic lineage of newly transmitted subtype C HIV-1 Env clones from India. Virology 385:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahalanabis, M., P. Jayaraman, T. Miura, F. Pereyra, E. M. Chester, B. Richardson, B. Walker, and N. L. Haigwood. 2009. Continuous viral escape and selection by autologous neutralizing antibodies in drug-naive human immunodeficiency virus controllers. J. Virol. 83:662-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascola, J. R., P. D'Souza, P. Gilbert, B. H. Hahn, N. L. Haigwood, L. Morris, C. J. Petropoulos, V. R. Polonis, M. Sarzotti, and D. C. Montefiori. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 79:10103-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore, P. L., N. Ranchobe, B. E. Lambson, E. S. Gray, E. Cave, M. R. Abrahams, G. Bandawe, K. Mlisana, S. S. Bdool Karim, C. Williamson, and L. Morris. 2009. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog. 5:e1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piantadosi, A., D. Panteleeff, C. A. Blish, J. M. Baeten, W. Jaoko, R. S. McClelland, and J. Overbaugh. 2009. HIV-1 neutralizing antibody breadth is affected by factors early in infection, but does not influence disease progression. J. Virol. 83:10269-10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinter, A. 2007. Roles of HIV-1 Env variable regions in viral neutralization and vaccine development. Curr. HIV Res. 5:542-553. [DOI] [PubMed] [Google Scholar]
- 26.Quakkelaar, E. D., F. P. van Alphen, B. D. Boeser-Nunnink, A. C. van Nuenen, R. Pantophlet, and H. Schuitemaker. 2007. Susceptibility of recently transmitted subtype B human immunodeficiency virus type 1 variants to broadly neutralizing antibodies. J. Virol. 81:8533-8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rademeyer, C., P. L. Moore, N. Taylor, D. P. Martin, I. A. Choge, E. S. Gray, H. W. Sheppard, C. Gray, L. Morris, and C. Williamson. 2007. Genetic characteristics of HIV-1 subtype C envelopes inducing cross-neutralizing antibodies. Virology 368:172-181. [DOI] [PubMed] [Google Scholar]
- 28.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong, R., F. Bibollet-Ruche, J. Mulenga, S. Allen, J. L. Blackwell, and C. A. Derdeyn. 2007. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J. Virol. 81:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rong, R., B. Li, R. M. Lynch, R. E. Haaland, M. K. Murphy, J. Mulenga, S. A. Allen, A. Pinter, G. M. Shaw, E. Hunter, J. E. Robinson, S. Gnanakaran, and C. A. Derdeyn. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 5:e1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagar, M., X. Wu, S. Lee, and J. Overbaugh. 2006. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J. Virol. 80:9586-9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. Y. De Goede, R. P. Van Steenwijk, J. M. A. Lange, J. K. M. Eeftink Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweighardt, B., Y. Liu, W. Huang, C. Chappey, Y. S. Lie, C. J. Petropoulos, and T. Wrin. 2007. Development of an HIV-1 reference panel of subtype B envelope clones isolated from the plasma of recently infected individuals. J. Acquir. Immune. Defic. Syndr. 46:1-11. [DOI] [PubMed] [Google Scholar]
- 34.Simek, M. D., W. Rida, F. H. Priddy, P. Pung, E. Carrow, D. S. Laufer, J. K. Lehrman, M. Boaz, T. Tarragona-Fiol, G. Miiro, J. Birungi, A. Pozniak, D. McPhee, O. Manigart, E. Karita, A. Inwoley, W. Jaoko, J. Dehovitz, L. G. Bekker, P. Pitisuttithum, R. Paris, L. M. Walker, P. Poignard, T. Wrin, P. E. Fast, D. R. Burton, and W. C. Koff. 2009. HIV-1 elite neutralizers: individuals with broad and potent neutralizing activity identified using a high throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83:7337-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tersmette, M., I. N. Winkel, M. Groenink, R. A. Gruters, P. Spence, E. Saman, G. van der Groen, F. Miedema, and J. G. Huisman. 1989. Detection and subtyping of HIV-1 isolates with a panel of characterized monoclonal antibodies to HIV-p24 gag. Virology 171:149-155. [DOI] [PubMed] [Google Scholar]
- 36.Van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrecht-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Coutinho, F. Miedema, and H. Schuitemaker. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Invest. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Gils, M. J., Z. Euler, B. Schweighardt, T. Wrin, and H. Schuitemaker. 2009. Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS 23:2405-2414. [DOI] [PubMed] [Google Scholar]
- 38.van Griensven, G. J., E. M. de Vroome, J. Goudsmit, and R. A. Coutinho. 1989. Changes in sexual behavior and the fall in incidence of HIV infection among homosexual men. BMJ 298:218-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker, B. D., and D. R. Burton. 2008. Toward an AIDS vaccine. Science 320:760-764. [DOI] [PubMed] [Google Scholar]
- 40.Walker, L. M., S. K. Phogat, P. Y. Chan-Hui, D. Wagner, P. Phung, J. L. Goss, T. Wrin, M. D. Simek, S. Fling, J. L. Mitcham, J. K. Lehrman, F. H. Priddy, O. A. Olsen, S. M. Frey, P. W. Hammond, G. Miiro, J. Serwanga, A. Pozniak, D. McPhee, O. Manigart, L. Mwananyanda, E. Karita, A. Inwoley, W. Jaoko, J. Dehovitz, L. G. Bekker, P. Pitisuttithum, R. Paris, S. Allen, S. Kaminsky, T. Zamb, M. Moyle, W. C. Koff, P. Poignard, and D. R. Burton. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, M., B. Gaschen, W. Blay, B. Foley, N. Haigwood, C. Kuiken, and B. Korber. 2004. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology 14:1229-1246. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, Y. J., C. Fracasso, J. R. Fiore, A. Björndal, G. Angarano, A. Gringeri, and E. M. Fenyö. 1997. Augmented serum neutralizing activity against primary human immunodeficiency virus type 1 (HIV-1) isolates in two groups of HIV-1-infected long-term nonprogressors. J. Infect. Dis. 176:1180-1187. [DOI] [PubMed] [Google Scholar]