Abstract
The native envelope (Env) spike on the surface of human immunodeficiency virus type 1 (HIV-1) is trimeric, and thus trimeric Env vaccine immunogens are currently being explored in preclinical immunogenicity studies. Key challenges have included the production and purification of biochemically homogeneous and stable trimers and the evaluation of these immunogens utilizing standardized virus panels for neutralization assays. Here we report the binding and neutralizing antibody (NAb) responses elicited by clade A (92UG037.8) and clade C (CZA97.012) Env gp140 trimer immunogens in guinea pigs. These trimers have been selected and engineered for optimal biochemical stability and have defined antigenic properties. Purified gp140 trimers with Ribi adjuvant elicited potent, cross-clade NAb responses against tier 1 viruses as well as detectable but low-titer NAb responses against select tier 2 viruses from clades A, B, and C. In particular, the clade C trimer elicited NAbs that neutralized 27%, 20%, and 47% of tier 2 viruses from clades A, B, and C, respectively. Heterologous DNA prime, protein boost as well as DNA prime, recombinant adenovirus boost regimens expressing these antigens, however, did not result in an increased magnitude or breadth of NAb responses in this system. These data demonstrate the immunogenicity of stable, homogeneous clade A and clade C gp140 trimers and exemplify the utility of standardized tier 1 and tier 2 virus panels for assessing the NAb responses of candidate HIV-1 Env immunogens.
The development and evaluation of novel HIV-1 Env immunogens are critical priorities of the HIV-1 vaccine field (2, 10, 25). The major antigenic target for neutralizing antibodies (NAbs) is the trimeric Env glycoprotein on the virion surface (4, 18, 30). Monomeric gp120 immunogens have not elicited broadly reactive NAbs in animal models (5, 13, 28, 29) or humans (16, 31), and thus several groups have focused on generating trimer immunogens that better mimic the native Env spike found on virions (3, 7, 14, 15, 20, 22, 27). It has, however, proven difficult to produce stable and conformationally homogeneous Env trimers. Strategies to modify Env immunogens have therefore been explored, including the removal of the cleavage site between gp120 and gp41 (3, 7, 23, 39, 40), the incorporation of an intramolecular disulfide bond to stabilize cleaved gp120 and gp41 moieties (6), and the addition of trimerization motifs such as the T4 bacteriophage fibritin “fold-on” (Fd) domain (8, 17, 39).
Preclinical evaluation of candidate Env immunogens is critical for concept testing and for the prioritization of vaccine candidates. Luciferase-based virus neutralization assays with TZM.bl cells (21, 24) have been developed as high-throughput assays that can be standardized (26). However, the optimal use of this assay requires the generation of standardized virus panels derived from multiple clades that reflect both easy-to-neutralize (tier 1) and primary isolate (tier 2) viruses (21, 24). A tiered approach for the evaluation of novel Env immunogens has been proposed, in which tier 1 viruses represent homologous vaccine strains and a small number of heterologous neutralization-sensitive viruses while tier 2 viruses provide a greater measure of neutralization breadth for the purpose of comparing immunogens (24).
We screened a large panel of primary HIV-1 isolates for Env stability and identified two viruses, CZA97.012 (clade C) (32) and 92UG037.8 (clade A) (17), that yielded biochemically homogeneous and stable Env trimers with well defined and uniform antigenic properties (17). The addition of the T4 bacteriophage fibritin “fold-on” (Fd) trimerization domain further increased their yield and purity (17). In the present study, we assessed the immunogenicity of these stable clade A and clade C gp140 trimers in guinea pigs. Both trimers elicited high-titer binding antibody responses and cross-clade neutralization of select tier 1 viruses as well as low-titer but detectable NAb responses against select tier 2 viruses from clades A, B, and C. These data demonstrate the immunogenicity of these stable gp140 trimers and highlight the utility of standardized virus panels in the evaluation of novel HIV-1 Env immunogens.
MATERIALS AND METHODS
HIV-1 gp140 trimers.
92UG037.8 (clade A) and CZA97.012 (clade C) gp140 trimers with C-terminal T4 bacteriophage fibritin trimerization domains (17, 39) and polyhistidine motifs were expressed in insect cells by using the Bac-to-Bac system (Invitrogen) as previously described (12, 17). Briefly, recombinant baculovirus was generated according to the manufacturer's protocol and amplified in Sf9 insect cells. For large-scale production, 12 liters of Trichoplusia ni (Hi-5) cells (2 × 106 cells/ml) were infected at the optimal multiplicity of infection. The supernatant was harvested 68 h postinfection by centrifugation and concentrated to 2 liters, followed by immediate exchange into phosphate-buffered saline (PBS) in a ProFlux M 12 tangential-flow filtration system (Millipore). After a clarifying spin and the addition of imidazole to a final concentration of 15 mM, the supernatant was loaded onto a nickel column at a flow rate of 1 ml/min, washed with 15 mM imidazole in PBS, and then washed sequentially with 40 mM and 60 mM imidazole in PBS. The protein was eluted with 300 mM imidazole in PBS. The fractions containing the purified protein were pooled, concentrated, and further purified by gel filtration chromatography on a Superose 6 column (GE Healthcare). The protein was concentrated, frozen in liquid nitrogen, and stored at −80°C.
DNA vaccines.
Human codon-optimized gene sequences for the clade C and clade A gp140 trimers with C-terminal T4 bacteriophage fibritin trimerization domains and polyhistidine motifs were synthesized commercially (Geneart) and cloned into the SalI-BamHI restriction sites of a pCMV eukaryotic expression vector. Gene inserts were verified by diagnostic restriction digests, DNA sequencing, and expression testing with 293 cells. Endotoxin-free preparations of pCMV-CZA97012-gp140 and pCMV-92UG037-gp140 (Qiagen) were utilized for all immunizations.
Recombinant adenovirus serotype 26 vectors.
Replication-incompetent, E1/E3-deleted recombinant adenovirus serotype 26 (rAd26) vectors expressing clade A and clade C gp140 trimers with C-terminal T4 bacteriophage fibritin trimerization fold-on domains (17, 39) and polyhistidine motifs were prepared as previously described (1).
Animals and immunizations.
Outbred female Hartley guinea pigs (Elm Hill) were housed at the Animal Research Facility of Beth Israel Deaconess Medical Center under approved Institutional Animal Care and Use Committee (IACUC) protocols. Guinea pigs were immunized with protein trimers (100 μg/animal) at 4- or 5-week intervals in 500 μl PBS in Ribi adjuvant (Sigma) at three sites: 2 subcutaneous (s.c.) sites (200 μl/site) and 1 intraperitoneal (i.p.) site (100 μl/site). Endotoxin-free DNA vaccines (500 μg/animal) were administered intramuscularly (i.m.) in 500 μl of PBS divided between the right and left quadriceps at 4-week intervals. Recombinant Ad26 vectors (5 × 1010 virus particles [vp]/animal) were administered intramuscularly in 500 μl saline divided between the right and left quadriceps. Serum samples were obtained from the vena cava of anesthetized animals after each immunization.
ELISA.
Serum binding antibody titers against gp140 trimers and synthetic linear peptides (92UG037.8 V1 [SYNITNNITNSITNSSVNMREEIK], 92UG037.8 V2 [SFNMTTELRDKNRKVYSLFYKLDVVQINNGNNSSNLYRLIN], 92UG037.8 V3 [TRPNNNTRKSVRIGPGQTFYATGDIIGDIRQAH], 92UG037.8 V3 scrambled [QVAHKRNTGYTPNIRDNSDIGPRTFRAQGIGTI], CZA97.012 V1 [TNATFKNNVTNDMNKEIR], CZA97.012 V2 [SFNTTTEIRDKKQQGYALFYRPDIVLLKENRNNSNNSEYILIN], and CZA97.012 V3 [RPNNNTRKSMRIGPGQTFYATGDIIGDIRQAY]) (New England Peptide) were determined by endpoint enzyme-linked immunosorbent assays (ELISAs). Ninety-six-well Maxisorp ELISA plates (Thermo Fisher Scientific) coated overnight with 100 μl/well of 1 μg/ml clade A gp140, clade C gp140, or synthetic V1-V3 peptides in PBS were blocked for 3 h with PBS containing 2% bovine serum albumin (BSA) (Sigma) and 0.05% Tween 20 (Sigma). Guinea pig sera were then added in serial dilutions and incubated for 1 h at room temperature. The plates were washed three times with PBS containing 0.05% Tween 20 and incubated for 1 h with a 1/2,000 dilution of a horseradish peroxidase (HRP)-conjugated goat anti-guinea pig secondary antibody (Jackson ImmunoResearch Laboratories). The plates were washed three times and developed with SureBlue tetramethylbenzidine (TMB) microwell peroxidase (KPL Research Products), stopped by the addition of stop solution (KPL Research products), and analyzed at 450 nm/550 nm with a Spectramax Plus ELISA plate reader (Molecular Devices) using Softmax Pro 4.7.1 software. ELISA endpoint titers were defined as the highest reciprocal serum dilution that yielded an absorbance >2-fold over background values.
TZM.bl neutralization assay.
NAb responses against tier 1 and tier 2 HIV-1 Env pseudoviruses were measured by using luciferase-based virus neutralization assays with TZM.bl cells as previously described (21, 24). These assays measure the reduction in luciferase reporter gene expression levels in TZM-bl cells following a single round of virus infection. The 50% inhibitory concentration (IC50) was calculated as the serum dilution that resulted in a 50% reduction in relative luminescence units compared with the virus control wells after the subtraction of cell control relative luminescence units. Briefly, 3-fold serial dilutions of serum samples were performed in duplicate (96-well flat-bottom plate) in 10% Dulbecco's modified Eagle's medium (DMEM) (100 μl/well). A total of 200 50% tissue culture infective doses (TCID50) of virus were added to each well in a volume of 50 μl, and the plates were incubated for 1 h at 37°C. TZM.bl cells were then added (1 × 104 cells/well in a 100-μl volume) in 10% DMEM containing DEAE-dextran (Sigma) at a final concentration of 11 μg/ml. Murine leukemia virus (MuLV) negative controls were included in all assays. Confirmatory assays were performed utilizing IgG purified from guinea pig serum by immobilized protein A columns (Pierce). To assess the reactivity to variable loop peptides, titrations of purified IgG were incubated with V3 linear peptide or a scrambled 92UG037.8 V3 negative control peptide at a final concentration of 20 μg/ml for 1 h at 37°C prior to the addition of pseudovirus. Viruses in the tier 1 panel were MW965.26 (clade C), DJ263.8 (clade A), SF162.LS (clade B), BaL.26 (clade B), 92UG037.8 (clade A), and CZA97.012 (clade C). Viruses in the tier 2 clade A panel were Q769.d22, Q168.a2, Q842.d12, 3718.v3.c11, 0330.v4.c3, and 0439.v5.c1. Viruses in the tier 2 clade B panel were previously described as the standardized clade B reference panel (21) and included WITO4160.33, AC10.0.29, REJO451.67, 6535.3, SC422661.8, and TRO.11. Viruses in the tier 2 clade C panel were also previously described as the standardized clade C reference panel (22) and included ZM109F.PB4, ZM249M.PL1, CAP45.2.00.G3, Du123.6, Du422.1, and ZM197M.PB7. All HIV-1 Env pseudoviruses were produced and titers were determined as previously described (21).
RESULTS
Production of stable, homogeneous HIV-1 gp140 trimers.
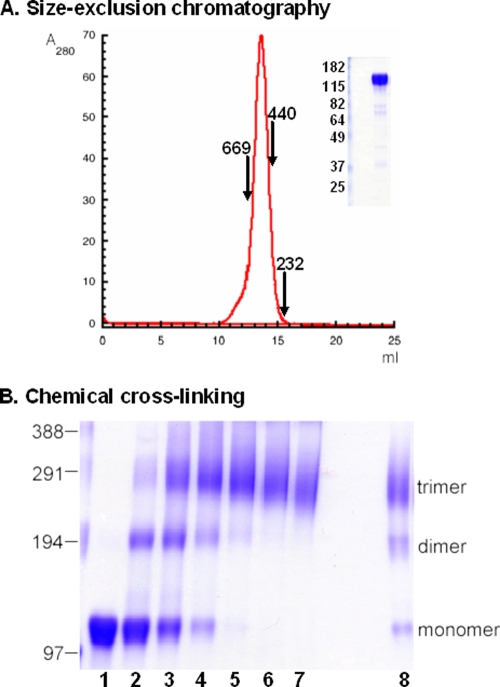
We previously reported the biochemical purity, homogeneity, and stability of the 92UG037.8 (clade A) gp140 trimer (17). The CZA97.012 (clade C) gp140 trimer showed similar purity and homogeneity. Size-exclusion chromatography and chemical cross-linking confirmed that it is both monodisperse and trimeric (Fig. 1). These immunogens contain the gp140 sequence fused to the T4 fibritin fold-on trimerization domain and a polyhistidine motif. Both trimers proved stable with no detectable dissociation for over 7 days at 4°C (data not shown).
FIG. 1.
Biochemical analysis of the clade C trimer. (A) The purified CZA97.012 (clade C) gp140 trimer was resolved by gel filtration chromatography on Superpose 6 columns. The apparent molecular mass was calculated by using the standards thyroglobulin (670 kDa), ferritin (440 kDa), and catalase (232 kDa). (Inset) Peak fractions were pooled and analyzed by SDS-PAGE. (B) The clade C trimer was treated with various concentrations (lanes 1 to 7, 0, 0.05, 0.25, 0.5, 1, 2, and 5 mM, respectively) of ethylene glycol bis(succinimidylsuccinate). Cross-linked products were analyzed by SDS-PAGE by using a 4% gel (lane 8, the gp140 trimer under nonreducing conditions). The molecular standard was cross-linked phosphorylase b (Sigma). Similar analyses were previously reported for the purified clade A 92UG037.8 gp140 trimer (17).
Binding antibody responses elicited by clade A and clade C trimers.
We next evaluated the immunogenicity of 92UG037.8 (clade A) and CZA97.012 (clade C) trimers that were selected and engineered for stability and conformational homogeneity. Guinea pigs (n = 5/group) were immunized with 100 μg of the clade A or clade C gp140 trimer in Ribi adjuvant s.c./i.p. at weeks 0, 5, and 10. Serum was obtained 4 weeks after each immunization. Env-specific binding antibodies were assessed by ELISA against both clade A and clade C gp140. High-titer binding antibody responses were observed for both clade A and clade C trimer-immunized guinea pigs (Fig. 2A and B). Responses were detected after a single immunization and increased to a mean log titer of 6.5 following the second and third immunizations. ELISA responses were comparable to the homologous and heterologous gp140 antigens. Antibody responses to the fold-on trimerization domain were also detected but were >2 logs lower in titer than those against the complete trimer immunogens (data not shown).
FIG. 2.
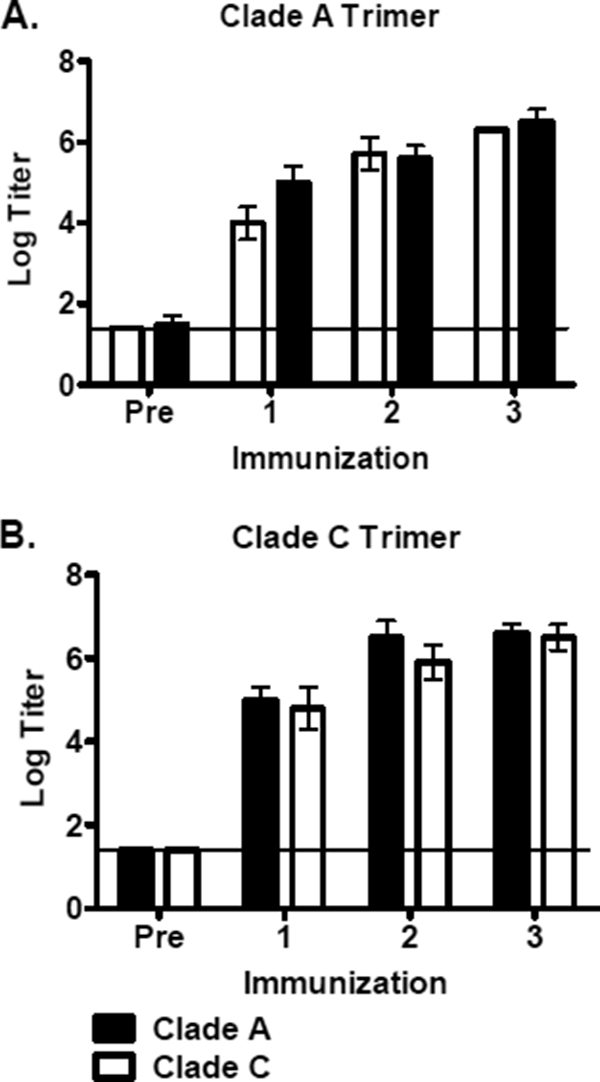
ELISA titers against gp140 in guinea pig sera. Sera obtained 4 weeks after each immunization were tested in endpoint ELISAs against the clade A and clade C trimer antigens in the clade A (A) and clade C (B) vaccinated guinea pigs. Data are presented as geometric mean titers at each time point ± standard deviations. The horizontal line indicates the background threshold.
Neutralizing antibody responses elicited by clade A and clade C trimers.
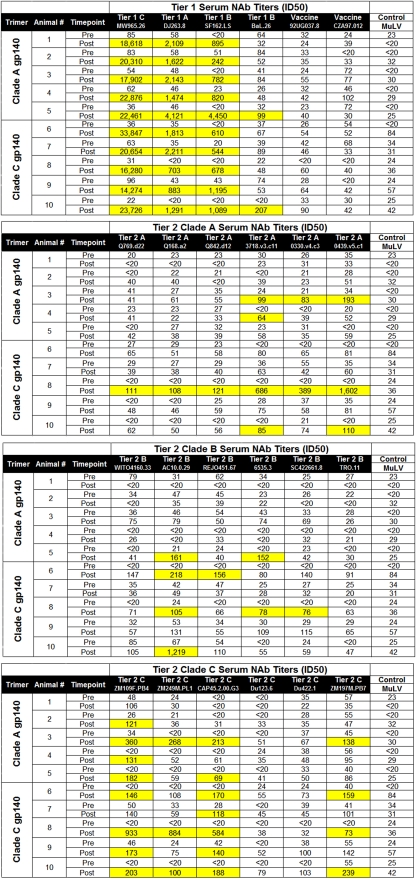
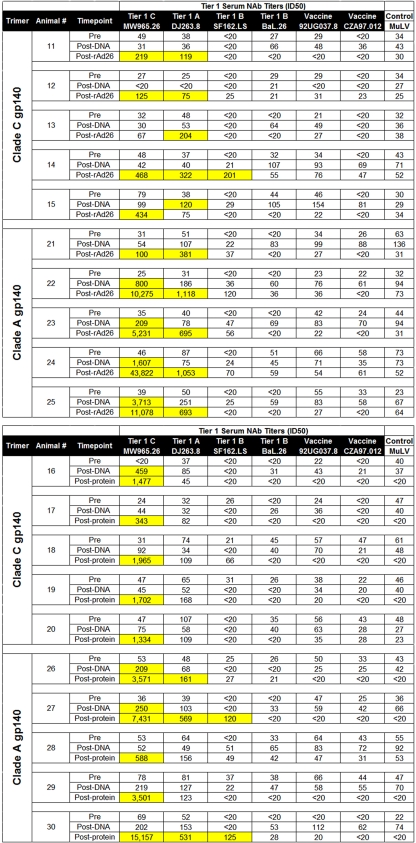
We assessed the NAb responses elicited by our stable clade A and clade C trimers using a panel of tier 1 and tier 2 viruses with a broad range of neutralization sensitivities from clades A, B, and C. We defined the criteria for positivity as 50% inhibitory dose (ID50) titers that were (i) >3-fold above preimmune background levels, (ii) >2-fold above levels of a concurrent murine leukemia virus (MuLV) control, and (iii) an absolute titer of >60. Guinea pigs immunized with either clade A or clade C trimers developed robust, cross-clade neutralizing activity against neutralization-sensitive tier 1 clade A, B, and C viruses (DJ263.8, SF162.LS, and MW965.26, respectively), with ID50 titers against MW965.26 ranging from 14,274 to 33,847 (Table 1). In contrast, no neutralization against the autologous vaccine strains was detected, presumably reflecting their inherent neutralization-resistant phenotypes.
TABLE 1.
NAb titers of guinea pig sera against panels of tier 1 and tier 2 HIV-1 isolatesa
Sera obtained preimmunization (Pre) and post-third-trimer vaccination (Post) were tested against panels of tier 1 and tier 2 clade A, B, and C pseudoviruses in TZM.bl neutralization assays. Values shown are the serum dilutions representing the ID50 titers for each animal. Values highlighted in yellow indicate positive responses defined as (i) values >3-fold above preimmune background levels, (ii) values >2-fold above those of a concurrent MuLV control, and (iii) an absolute titer of >60.
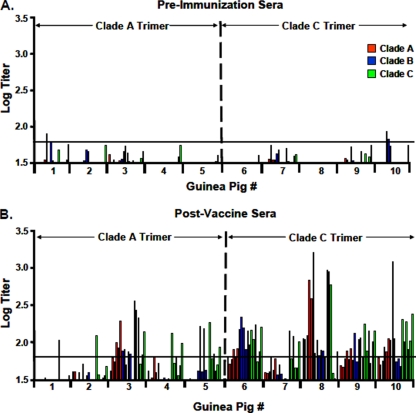
We next assessed NAb responses against more stringent tier 2 primary isolate viruses. Lower titer but reproducible NAb activity against select tier 2 clade A, B, and C viruses was detected in sera from animals following the third immunization (Table 1). The magnitude and consistency of responses against tier 2 viruses were substantially lower than those observed against tier 1 viruses. Nevertheless, a degree of tier 2 neutralization activity was consistently observed, and the clade C trimer appeared to elicit responses of higher magnitude and breadth than with the clade A trimer. A graphical summary of NAb titers against tier 2 viruses in pre- and postimmunization sera is shown in Fig. 3. Overall, the clade C trimer elicited detectable NAb responses against 27%, 20%, and 47% of tier 2 viruses tested from clades A, B, and C, respectively, whereas the clade A trimer elicited detectable NAb responses against 13%, 7%, and 27% of tier 2 viruses tested from clades A, B, and C, respectively (Table 2). There was a modest clade preference of NAbs elicited by the clade C trimer (P = 0.03 by Fisher's exact test) but not the clade A trimer (P value was not significant). These data demonstrate the immunogenicity of these stable, homogeneous clade A and clade C trimers and show the utility of this panel of Env pseudoviruses for providing a systematic tiered approach for assessing NAb responses elicited by candidate HIV-1 Env immunogens.
FIG. 3.
Summary of NAb titers against tier 2 viruses. NAb ID50 titers against six clade A (red), clade B (blue), and clade C (green) tier 2 primary isolates are summarized for each guinea pig. (A) Preimmunization. (B) Postimmunization. The horizontal line indicates a titer of 60 as the threshold for positivity.
TABLE 2.
NAb titers against tier 2 clade A, B, and C viruses
| Trimer immunogen | Tier 2 virus panel clade | No. of positive samples/total no. of samples (% positive) |
|---|---|---|
| Clade A gp140 | A | 4/30 (13) |
| B | 2/30 (7) | |
| C | 8/30 (27) | |
| Clade C gp140 | A | 8/30 (27) |
| B | 6/30 (20) | |
| C | 14/30 (47) |
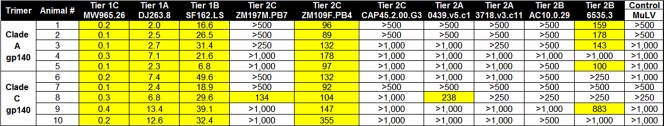
Neutralizing antibody responses of purified IgG.
To confirm the results of the serum NAb assays, we performed additional neutralization assays using IgG purified from serum of immunized animals. Purified IgG demonstrated potent neutralizing activity against the tier 1 viruses at low concentrations (0.1 to 0.4 μg/ml for MW965.26) as well as detectable neutralization activity against several tier 2 viruses, including ZM197M.PB7 (clade C), ZM109F.PB4 (clade C), 0439.v5.c1 (clade A), and 6535.3 (clade B) (Table 3). Sample NAb data for purified IgG against the tier 2 clade C virus ZM109F.PB4 are shown in Fig. 4. Control IgG from naïve sera exhibited no neutralization activity, and IgG from immunized guinea pigs did not neutralize the negative control MuLV (ID50 titers of >1,000 μg/ml) (data not shown).
TABLE 3.
IC50 NAb titers of purified guinea pig IgG against select tier 1 and 2 HIV-1 Env pseudovirusesa
IgG purified from individual guinea pigs was tested in NAb assays against a select panel of tier 1 and tier 2 viruses. Data are represented as IC50 titers in μg/ml (lower numbers reflect better neutralization). Values highlighted in yellow indicate samples with positive neutralizing activity.
FIG. 4.
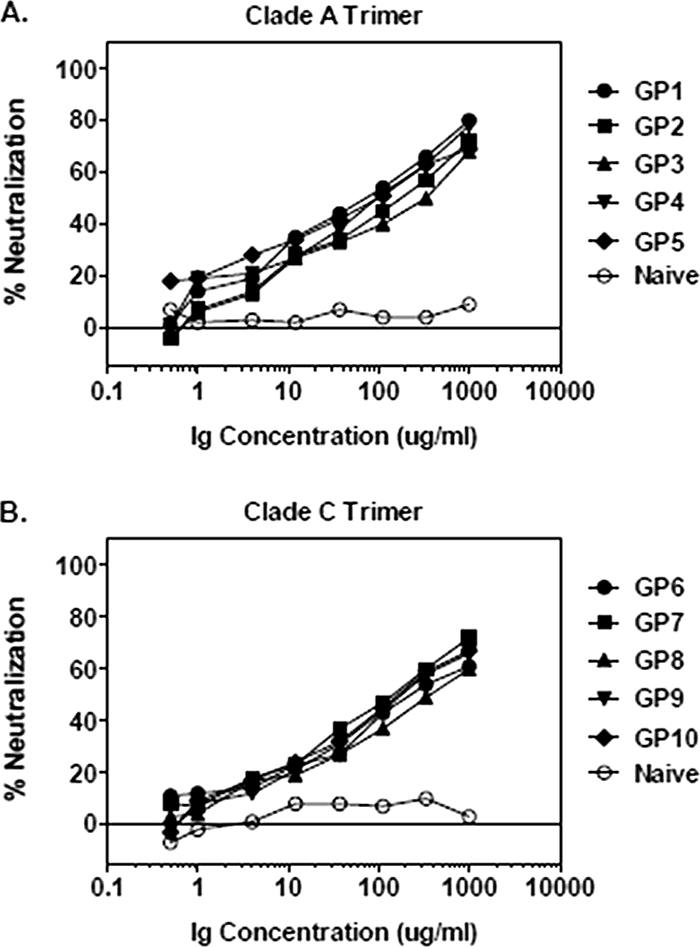
Sample NAb data for purified serum IgG against the tier 2 clade C virus ZM109F.PB4. Serial dilutions of purified IgG from clade A (A) or clade C (B) trimer-immunized guinea pigs and naïve control animals were tested for NAb activity against the tier 2 clade C virus ZM109F.PB4.
Variable loop peptide responses.
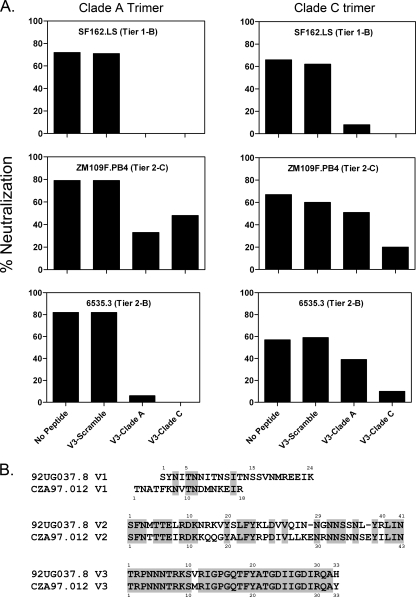
We next assessed whether antibodies elicited by our clade A and clade C trimer immunogens were directed against the Env variable loops. Antibody binding titers against the V1, V2, and V3 peptides from the clade A and clade C gp140-immunized animals were measured by ELISA. A scrambled 92UG037.8 V3 peptide was utilized as a negative control. Both clade A and clade C trimers elicited antibodies that bound to linear V3 loop peptides but not the scrambled V3 peptide (data not shown). Endpoint titers against homologous and heterologous V3 sequences were comparable, but lower ELISA titers against V1 and V2 peptides were observed (data not shown). We also performed V3 peptide competition neutralization assays with representative animals that received the clade A (guinea pig 5) and clade C (guinea pig 10) trimers. Neutralizing activity against select tier 1 and tier 2 viruses was partially blocked by both homologous and heterologous V3 loop peptides but not by the scrambled peptide (Fig. 5A), suggesting that the clade A and clade C gp140 trimers elicited NAbs that were directed in part against conserved elements in the V3 loop. A sequence alignment of the V3 loops of 92UG037.8 and CZA97.012 showed substantial sequence homology (Fig. 5B).
FIG. 5.
Variable loop peptide inhibition experiments. (A) Titrations of purified IgG obtained from the sera of representative animals immunized with the clade A (guinea pig 5) or clade C (guinea pig 10) trimer were preincubated with linear V3 loop or scrambled peptides at 20 μg/ml and then tested in NAb assays against the SF162.LS (tier 1-B), ZM109F.PB4 (tier 2-C), and 6535.3 (tier 2-B) viruses. The data shown in the graph are for a single IgG concentration of 500 μg/ml. (B) Sequence alignment of the 92UG037.8 and CZA97012 V1, V2, and V3 peptide loops. Amino acid residues highlighted in gray indicate sequence homology.
Heterologous prime/boost regimens.
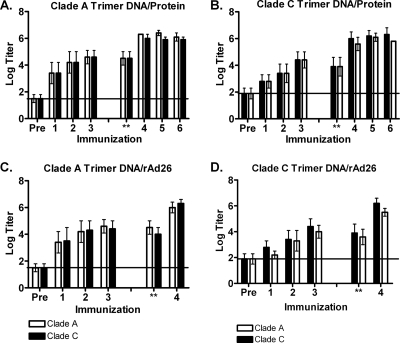
DNA priming followed by protein boosting was previously reported to elicit higher-titer antibody responses than either approach alone (35, 38). We therefore investigated DNA prime, protein boost as well as DNA prime, recombinant adenovirus serotype 26 (rAd26) boost regimens with vectors expressing the clade A and clade C trimers. Guinea pigs (n = 5/group) were primed with 0.5 mg of DNA vaccines intramuscularly (i.m.) at weeks 0, 4, and 8 and were boosted at either week 36 with a single immunization of rAd26 vectors or at weeks 36, 40, and 44 with homologous trimer proteins in Ribi adjuvant. We observed high-titer ELISA responses following immunization with both the DNA/protein (Fig. 6A and B) and the DNA/rAd26 (Fig. 6C and D) regimens. Peak titers obtained after the three DNA priming immunizations were a mean log titer of 4.8, demonstrating the efficiency of the DNA priming. Boosting with either a single rAd26 or three purified protein immunizations augmented responses to mean log titers of 6.5, which were titers that were comparable to those elicited by the protein-only regimen (Fig. 2). Low levels of tier 1 NAb responses were observed after DNA priming and increased following rAd26 or protein boosting (Table 4). However, the DNA/rAd26 and the DNA/protein regimens did not induce higher titer tier 1 NAb responses than did the protein-only regimen (Table 1).
FIG. 6.
ELISA titers against gp140 following heterologous prime/boost vaccination regimens. Sera were obtained 4 weeks after immunization with the clade A (A and C) or the clade C (B and D) trimer in the context of DNA/protein (A and B) or DNA/rAd26 (C and D) groups. Antibody titers were assessed by ELISA. Graphs show geometric mean titers for each time point ± standard deviations. Immunizations 1, 2, and 3 reflect DNA priming. 3* indicates ELISA titers after 3 immunizations but prior to boosting. 4 or 4-6 indicates rAd26 or protein boosting, respectively. The horizontal line indicates the background threshold.
TABLE 4.
NAb activity of guinea pig sera following prime/boost vaccination regimensa
Sera obtained preimmunization, after the third DNA vaccination, and postboost from guinea pigs were tested for NAb activity against tier 1 viruses. Values shown are the serum dilutions representing the ID50 titers for each animal. Highlighted values indicate positive responses, as described in Table 1.
DISCUSSION
We assessed the immunogenicity of highly purified CZA97.012 (clade C) and 92UG037.8 (clade A) Env gp140 trimer immunogens that were selected and engineered for optimal biochemical stability and conformational homogeneity. Most Env trimer immunogens reported to date are derived from clade B isolates (3, 7, 14, 15, 20, 22, 27), although recent reports have also described trimers from other clades (9, 19). However, the conformational homogeneity of trimer preparations and the capacity of trimer immunogens to neutralize standardized panels of virus isolates have often not been fully assessed. In the present study, we utilized a panel of tier 1 and tier 2 viruses from clades A, B, and C that exhibit a broad range of neutralization sensitivities to assess virus neutralization.
In a preliminary study, we assessed the immunogenicity in guinea pigs of a 92UG037.8 (clade A) gp120 monomer core immunogen, which elicited high ELISA titers but minimal to no NAb responses against tier 1 neutralization-sensitive viruses (data not shown). We therefore focused the present studies on evaluating the immunogenicity of 92UG037.8 (clade A) and CZA97.012 (clade C) trimer immunogens that were selected and engineered for maximum stability and conformational homogeneity. Guinea pigs immunized with clade A and clade C trimers demonstrated robust, cross-clade neutralizing activity against neutralization-sensitive tier 1 clade A, B, and C viruses as well as clear but low levels of neutralizing activity against select tier 2 clade A, B, and C viruses. NAb responses to tier 2 isolates were confirmed using purified IgG but were substantially lower in magnitude and less consistent than responses against tier 1 isolates.
Antibody responses elicited by the clade A and clade C trimers were directed in part against the V3 loop. V3 loop reactivity against both heterologous viruses was observed, although it is likely that a variety of other epitopes were also targeted (33). The V3 loop is a highly immunogenic region of Env, and it was previously shown that certain anti-V3 NAbs can neutralize diverse strains of HIV-1 (11, 36, 41). Moreover, a recent report described two novel monoclonal antibodies that target a broadly neutralizing epitope preferentially expressed on trimeric Env and spanning conserved regions of the V2 and V3 loops (37). Further studies will be required to determine whether NAbs elicited by our gp140 trimers target these epitopes.
Heterologous prime/boost vaccination regimens were also assessed for their potential to augment the immunogenicity of the trimer protein immunogens. DNA/protein and DNA/rAd26 regimens did not lead to an improved magnitude or breadth of antibody responses compared with the protein-only regimen in our studies. In fact, the prime/boost regimens elicited lower NAb responses despite comparable ELISA binding titers. These findings contrast with data from previous reports highlighting the improved humoral responses obtained with DNA/protein or DNA/rAd regimens compared to protein-only regimens in other systems (34, 35, 38). We hypothesize that the lower NAb activity observed for the prime/boost regimens in the present study may have been related to the fact that the DNA vaccines likely expressed a heterogeneous mixture of Env conformers or oligomers, which could have skewed the antibody responses toward nonneutralizing epitopes. Taken together, these findings suggest that the optimal regimen may be dependent on the particular antigen or system utilized.
In summary, this study demonstrates the immunogenicity of novel clade A and clade C gp140 trimers that have been selected and engineered for optimal purity and stability. Moreover, the use of standardized panels of tier 1 and tier 2 viruses from clades A, B, and C allows a rapid assessment of NAb profiles against a diversity of viruses and is useful for comparative immunogenicity studies of novel candidate HIV-1 Env immunogens.
Acknowledgments
We thank S. Harrison, Z. Ou, T. Chau, S. Chan, Y. Sun, A. Cheung, R. Dolin, B. Haynes, F. Gao, J. Mascola, and D. Burton for generous advice, assistance, and reagents. Reagents were also obtained from the NIH AIDS Research and Reference Reagent Program.
We acknowledge support from NIH grants AI084794 (D.H.B. and B.C.), AI078526 (D.H.B.), AI066924 (D.H.B.), AI066305 (D.H.B.), AI058727 (D.H.B.), and GM083680 (B.C.) and Bill and Melinda Gates Foundation CAVD grant 38619 (M.S.S.). We report no financial conflicts of interest.
Footnotes
Published ahead of print on 6 January 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barouch, D. H. 2008. Challenges in the development of an HIV-1 vaccine. Nature 455:613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beddows, S., M. Franti, A. K. Dey, M. Kirschner, S. P. Iyer, D. C. Fisch, T. Ketas, E. Yuste, R. C. Desrosiers, P. J. Klasse, P. J. Maddon, W. C. Olson, and J. P. Moore. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360:329-340. [DOI] [PubMed] [Google Scholar]
- 4.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 5.Berman, P. W., D. J. Eastman, D. M. Wilkes, G. R. Nakamura, T. J. Gregory, D. Schwartz, G. Gorse, R. Belshe, M. L. Clements, and R. A. Byrn. 1994. Comparison of the immune response to recombinant gp120 in humans and chimpanzees. AIDS 8:591-601. [DOI] [PubMed] [Google Scholar]
- 6.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bower, J. F., Y. Li, R. Wyatt, and T. M. Ross. 2006. HIV-1 Envgp140 trimers elicit neutralizing antibodies without efficient induction of conformational antibodies. Vaccine 24:5442-5451. [DOI] [PubMed] [Google Scholar]
- 8.Bower, J. F., X. Yang, J. Sodroski, and T. M. Ross. 2004. Elicitation of neutralizing antibodies with DNA vaccines expressing soluble stabilized human immunodeficiency virus type 1 envelope glycoprotein trimers conjugated to C3d. J. Virol. 78:4710-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke, B., V. R. Gomez-Roman, Y. Lian, Y. Sun, E. Kan, J. Ulmer, I. K. Srivastava, and S. W. Barnett. 2009. Neutralizing antibody responses to subtype B and C adjuvanted HIV envelope protein vaccination in rabbits. Virology 387:147-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 11.Carrow, E. W., L. K. Vujcic, W. L. Glass, K. B. Seamon, S. C. Rastogi, R. M. Hendry, R. Boulos, N. Nzila, and G. V. Quinnan, Jr. 1991. High prevalence of antibodies to the gp120 V3 region principal neutralizing determinant of HIV-1MN in sera from Africa and the Americas. AIDS Res. Hum. Retroviruses 7:831-838. [DOI] [PubMed] [Google Scholar]
- 12.Chen, B., G. Zhou, M. Kim, Y. Chishti, R. E. Hussey, B. Ely, J. J. Skehel, E. L. Reinherz, S. C. Harrison, and D. C. Wiley. 2000. Expression, purification, and characterization of gp160e, the soluble, trimeric ectodomain of the simian immunodeficiency virus envelope glycoprotein, gp160. J. Biol. Chem. 275:34946-34953. [DOI] [PubMed] [Google Scholar]
- 13.Crooks, E. T., P. L. Moore, M. Franti, C. S. Cayanan, P. Zhu, P. Jiang, R. P. de Vries, C. Wiley, I. Zharkikh, N. Schulke, K. H. Roux, D. C. Montefiori, D. R. Burton, and J. M. Binley. 2007. A comparative immunogenicity study of HIV-1 virus-like particles bearing various forms of envelope proteins, particles bearing no envelope and soluble monomeric gp120. Virology 366:245-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derby, N. R., S. Gray, E. Wayner, D. Campogan, G. Vlahogiannis, Z. Kraft, S. W. Barnett, I. K. Srivastava, and L. Stamatatos. 2007. Isolation and characterization of monoclonal antibodies elicited by trimeric HIV-1 Env gp140 protein immunogens. Virology 366:433-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey, B., M. Pancera, K. Svehla, Y. Shu, S. H. Xiang, J. Vainshtein, Y. Li, J. Sodroski, P. D. Kwong, J. R. Mascola, and R. Wyatt. 2007. Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: antigenicity, biophysics, and immunogenicity. J. Virol. 81:5579-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn, N. M., D. N. Forthal, C. D. Harro, F. N. Judson, K. H. Mayer, and M. F. Para. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654-665. [DOI] [PubMed] [Google Scholar]
- 17.Frey, G., H. Peng, S. Rits-Volloch, M. Morelli, Y. Cheng, and B. Chen. 2008. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 105:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallo, S. A., C. M. Finnegan, M. Viard, Y. Raviv, A. Dimitrov, S. S. Rawat, A. Puri, S. Durell, and R. Blumenthal. 2003. The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta 1614:36-50. [DOI] [PubMed] [Google Scholar]
- 19.Kang, Y. K., S. Andjelic, J. M. Binley, E. T. Crooks, M. Franti, S. P. Iyer, G. P. Donovan, A. K. Dey, P. Zhu, K. H. Roux, R. J. Durso, T. F. Parsons, P. J. Maddon, J. P. Moore, and W. C. Olson. 2009. Structural and immunogenicity studies of a cleaved, stabilized envelope trimer derived from subtype A HIV-1. Vaccine 27:5120-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, M., Z. S. Qiao, D. C. Montefiori, B. F. Haynes, E. L. Reinherz, and H. X. Liao. 2005. Comparison of HIV type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies. AIDS Res. Hum. Retroviruses 21:58-67. [DOI] [PubMed] [Google Scholar]
- 21.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Y., K. Svehla, N. L. Mathy, G. Voss, J. R. Mascola, and R. Wyatt. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 80:1414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao, H. X., L. L. Sutherland, S. M. Xia, M. E. Brock, R. M. Scearce, S. Vanleeuwen, S. M. Alam, M. McAdams, E. A. Weaver, Z. Camacho, B. J. Ma, Y. Li, J. M. Decker, G. J. Nabel, D. C. Montefiori, B. H. Hahn, B. T. Korber, F. Gao, and B. F. Haynes. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353:268-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascola, J. R., P. D'Souza, P. Gilbert, B. H. Hahn, N. L. Haigwood, L. Morris, C. J. Petropoulos, V. R. Polonis, M. Sarzotti, and D. C. Montefiori. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 79:10103-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montefiori, D., Q. Sattentau, J. Flores, J. Esparza, and J. Mascola. 2007. Antibody-based HIV-1 vaccines: recent developments and future directions. PLoS Med. 4:e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montefiori, D. C. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 485:395-405. [DOI] [PubMed] [Google Scholar]
- 27.Morner, A., I. Douagi, M. N. Forsell, C. Sundling, P. Dosenovic, S. O'Dell, B. Dey, P. D. Kwong, G. Voss, R. Thorstensson, J. R. Mascola, R. T. Wyatt, and G. B. K. Hedestam. 2009. Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein. J. Virol. 83:540-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nara, P. L., W. G. Robey, S. W. Pyle, W. C. Hatch, N. M. Dunlop, J. W. Bess, Jr., J. C. Kelliher, L. O. Arthur, and P. J. Fischinger. 1988. Purified envelope glycoproteins from human immunodeficiency virus type 1 variants induce individual, type-specific neutralizing antibodies. J. Virol. 62:2622-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page, M., K. H. Mills, G. C. Schild, C. Ling, V. Patel, A. McKnight, A. L. Barnard, P. Dilger, and R. Thorpe. 1991. Studies on the immunogenicity of Chinese hamster ovary cell-derived recombinant gp120 (HIV-1IIIB). Vaccine 9:47-52. [DOI] [PubMed] [Google Scholar]
- 30.Pantophlet, R., and D. R. Burton. 2006. GP120: target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 24:739-769. [DOI] [PubMed] [Google Scholar]
- 31.Pitisuttithum, P., P. Gilbert, M. Gurwith, W. Heyward, M. Martin, F. van Griensven, D. Hu, J. W. Tappero, and K. Choopanya. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661-1671. [DOI] [PubMed] [Google Scholar]
- 32.Rodenburg, C. M., Y. Li, S. A. Trask, Y. Chen, J. Decker, D. L. Robertson, M. L. Kalish, G. M. Shaw, S. Allen, B. H. Hahn, and F. Gao. 2001. Near full-length clones and reference sequences for subtype C isolates of HIV type 1 from three different continents. AIDS Res. Hum. Retroviruses 17:161-168. [DOI] [PubMed] [Google Scholar]
- 33.Scheid, J. F., H. Mouquet, N. Feldhahn, M. S. Seaman, K. Velinzon, J. Pietzsch, R. G. Ott, R. M. Anthony, H. Zebroski, A. Hurley, A. Phogat, B. Chakrabarti, Y. Li, M. Connors, F. Pereyra, B. D. Walker, H. Wardemann, D. Ho, R. T. Wyatt, J. R. Mascola, J. V. Ravetch, and M. C. Nussenzweig. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636-640. [DOI] [PubMed] [Google Scholar]
- 34.Seaman, M. S., L. Xu, K. Beaudry, K. L. Martin, M. H. Beddall, A. Miura, A. Sambor, B. K. Chakrabarti, Y. Huang, R. Bailer, R. A. Koup, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J. Virol. 79:2956-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaine, M., S. Wang, E. T. Crooks, P. Jiang, D. C. Montefiori, J. Binley, and S. Lu. 2008. Improved induction of antibodies against key neutralizing epitopes by human immunodeficiency virus type 1 gp120 DNA prime-protein boost vaccination compared to gp120 protein-only vaccination. J. Virol. 82:7369-7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel, T., R. Kurth, and S. Norley. 1994. The majority of neutralizing Abs in HIV-1-infected patients recognize linear V3 loop sequences. Studies using HIV-1MN multiple antigenic peptides. J. Immunol. 153:1895-1904. [PubMed] [Google Scholar]
- 37.Walker, L. M., S. K. Phogat, P. Y. Chan-Hui, D. Wagner, P. Phung, J. L. Goss, T. Wrin, M. D. Simek, S. Fling, J. L. Mitcham, J. K. Lehrman, F. H. Priddy, O. A. Olsen, S. M. Frey, P. W. Hammond, G. Miiro, J. Serwanga, A. Pozniak, D. McPhee, O. Manigart, L. Mwananyanda, E. Karita, A. Inwoley, W. Jaoko, J. Dehovitz, L. G. Bekker, P. Pitisuttithum, R. Paris, S. Allen, S. Kaminsky, T. Zamb, M. Moyle, W. C. Koff, P. Poignard, and D. R. Burton. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, S., R. Pal, J. R. Mascola, T. H. Chou, I. Mboudjeka, S. Shen, Q. Liu, S. Whitney, T. Keen, B. C. Nair, V. S. Kalyanaraman, P. Markham, and S. Lu. 2006. Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology 350:34-47. [DOI] [PubMed] [Google Scholar]
- 39.Yang, X., J. Lee, E. M. Mahony, P. D. Kwong, R. Wyatt, and J. Sodroski. 2002. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J. Virol. 76:4634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, C. W., Y. Chishti, R. E. Hussey, and E. L. Reinherz. 2001. Expression, purification, and characterization of recombinant HIV gp140. The gp41 ectodomain of HIV or simian immunodeficiency virus is sufficient to maintain the retroviral envelope glycoprotein as a trimer. J. Biol. Chem. 276:39577-39585. [DOI] [PubMed] [Google Scholar]
- 41.Zolla-Pazner, S., S. S. Cohen, C. Krachmarov, S. Wang, A. Pinter, and S. Lu. 2008. Focusing the immune response on the V3 loop, a neutralizing epitope of the HIV-1 gp120 envelope. Virology 372:233-246. [DOI] [PubMed] [Google Scholar]