Abstract
CD8+ T cells (TCD8+) play a crucial role in immunity to viruses. Antiviral TCD8+ are initially activated by recognition of major histocompatibility complex (MHC) class I-peptide complexes on the surface of professional antigen-presenting cells (pAPC). Migration of pAPC from the site of infection to secondary lymphoid organs is likely required during a natural infection. Migrating pAPC can be directly infected with virus or may internalize antigen derived from virus-infected cells. The use of experimental virus infections to assess the requirement for pAPC migration in initiation of TCD8+ responses has proven difficult to interpret because injected virus can readily drain to secondary lymphoid organs without the need for cell-mediated transport. To overcome this ambiguity, we examined the generation of antigen-specific TCD8+ after immunization with recombinant adenoviruses that express antigen driven by skin-specific or ubiquitous promoters. We show that the induction of TCD8+ in response to tissue-targeted antigen is less efficient than the response to ubiquitously expressed antigen and that the resulting TCD8+ fail to clear all target cells pulsed with the antigenic peptide. This failure to prime a fully functional TCD8+ response results from a reduced period of priming to peripherally expressed antigen versus ubiquitously expressed antigen and correlated with a brief burst of pAPC migration from the skin, a requirement for induction of the response to peripheral antigen. These results indicate that a reduced duration of pAPC migration after virus infection likely reduces the amplitude of the TCD8+ response, allowing persistence of the peripheral virus.
The induction of effector CD8+ T cells (TCD8+) is a vital step in the eradication or control of many viral infections. The induction of antiviral TCD8+ requires the presentation of virally derived peptides in complex with major histocompatibility complex (MHC) class I on the surface of specialized professional antigen-presenting cells (pAPC), most commonly a subset of dendritic cells (DC) that bear the CD8α chain (1, 29). The CD8α+ DC reside only in secondary lymphoid organs and not in the tissues, implying that cell-mediated transport or drainage of virus particles to a lymph node is required for initiation of a TCD8+ response. Partial inhibition of DC migration from the skin can impair the initiation of a TCD8+ response (2). After influenza infection in the lungs, there is a burst of DC migration, followed by a refractory period in which no DC migration occurs (19). The functional consequences of this refractory period of DC migration have not been explored.
A number of viruses, particularly human papillomaviruses, infect the skin and are ignored by the immune response for extended periods of time (31). We sought to explore the possibility that, after a low-level peripheral virus infection of the skin, changes in DC migration may limit the availability of antigen in the draining lymph node and thus the induction of a TCD8+ response. There are a number of confounding factors that make the study of DC migration in the initiation of an antiviral TCD8+ response difficult. Virus particles may directly drain to the lymph node within seconds (11, 13, 25). In addition, many viruses will alter DC functions, including migration, after infection of the DC itself. This may occur via specific viral modulation of DC function (16) or via nonspecific shut down of host protein synthesis (26), both of which will affect migration. Thus, it is often not possible to distinguish between the effects of virus infection upon DC migration, drainage of virus directly to the lymph node, and the natural response that follows migration of DC responding to a peripheral virus infection.
There is currently no mouse model of a peripheral virus infection that is confined to the skin, as no natural mouse papillomavirus has ever been isolated. Therefore, to address these issues, we have made use of another small DNA virus, namely, an adenovirus vector that is replication deficient (rAd). These vectors express influenza virus nucleoprotein (NP) under the control of a ubiquitous (cytomegalovirus [CMV] immediate-early) or tissue-targeted promoter (K14, targeted to keratinocytes, the site of papillomavirus replication). Antigen driven by the K14 promoter is expressed only in skin cells, so only uninfected DC can present antigen in this system, removing the need to account for modulation of the function of virus-infected DC.
We demonstrate that when antigen is expressed in only keratinocytes in the skin, the efficiency of TCD8+ induction is reduced and the time period for which antigen is available to prime effector cells is reduced dramatically. DC-mediated transport is required for antigen to reach the lymph node where a TCD8+ response is initiated. The reduced time period of antigen presentation is the result of a transient blockade in DC migration from the site of infection. The blockade in DC migration reduced the delivery of viral antigen to the lymph node needed to induce a TCD8+ response. The resulting TCD8+ response to peripheral viral antigen is not capable of clearing all target cells presenting a viral peptide, thus allowing the persistence of peripheral virus-infected cells. These results provide a potential mechanism for the long-term evasion of the immune response by papillomaviruses following natural infection and also have important implications for tissue targeted gene therapy vectors.
MATERIALS AND METHODS
Mice.
All mice were housed in specific-pathogen-free conditions in the animal facility at the Pennsylvania State M. S. Hershey College of Medicine. Experiments and breeding were conducted under the guidelines of the institutional animal care and use committee. F5 mice (21) were originally obtained from the NIAID contract facility at Taconic Farms (Germantown, NY) and, where indicated, were bred to B6.SJL congenic mice (Taconic). C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA).
Recombinant viruses.
The manufacture of replication-deficient (E1, E3 deleted) rAd vectors encoding influenza virus nucleoprotein derived from influenza virus A/PR8 and driven by CMV, K14, or surfactant protein C (SPC) promoters has been previously described (28). Viruses used here were made similarly, but the amino acid sequence of the nucleoprotein was altered at the Db-restricted determinant (NP366-374) from ASNENMETM to ASNENMDAM as previously described (25) in order to facilitate recognition by the F5 T-cell receptor (TCR) transgenic TCD8+. Recombinant vaccinia virus (rVACV) expressing influenza virus nucleoprotein from A/NT60/68 was kindly provided by Tim Elliott (Cancer Research UK Wessex Regional Medical Oncology Unit, University of Southampton, Southampton, United Kingdom).
Immunizations.
Female C57BL/6 mice (6 to 10 weeks old) were injected intravenously (i.v.) with 107 PFU of rVACV or rAd in the dosages indicated. To generate TCD8+ after i.v. injection, splenocytes from mice immunized with viruses were stimulated in vitro for 6 days with splenocytes pulsed with antigenic peptides at a concentration of 1 ng/ml.
For intradermal (i.d.) ear injections, mice were anesthetized, and rAd was injected into each ear pinna (∼20 μl/ear). At the times indicated postinfection, the spleens and draining cervical lymph nodes were removed and homogenized, and T-cell expansion and/or effector activity was assessed as described below. For all TCD8+ assays, whether directly ex vivo or after in vitro culture, live cells were recovered via centrifugation over a lymphocyte separation medium gradient (LSM; density = 1.077 g/ml; Mediatech, Herndon, VA), and viable cells were harvested from the LSM-medium interface. Cells were then tested for effector activity or proliferation.
Cell lines and cultures.
All media were purchased from Invitrogen (Grand Island, NY). Cytotoxic T-cell culture was in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS), 5 × 10−5 M β-mercaptoethanol, antibiotics (penicillin and streptomycin), nonessential amino acids, sodium pyruvate (1 mM), and 2 mM glutamine (RP-10). Iscove modified Dulbecco medium supplemented with 10% FBS, antibiotics (penicillin and streptomycin), and 2 mM glutamine was used during effector assays and for adoptive transfers where shown.
Adoptive transfer of TCR Tg T cells.
T-cell enriched populations were obtained from TCR transgenic (TCR Tg) mice as follows. Lymph nodes (popliteal, inguinal, brachial, axillary, and superficial cervical) and spleens were harvested, and a single-cell suspension was generated. Live cells were isolated by centrifugation over LSM. Cells were washed and resuspended, and 1 × 106 to 5 × 106 cells were injected into each recipient via the lateral tail vein. Unless the experimental protocol required otherwise, mice were immunized 24 h after the adoptive transfer of T cells.
Analysis of cell division in vivo.
To analyze cell division in vivo, adoptively transferred TCR Tg T cells were labeled with the dye 5- (and 6-) carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE; Molecular Probes, Eugene, OR). Mononuclear cells isolated over an LSM gradient were washed and then labeled with 5 mM CFDA-SE for 10 min at 37°C, washed, and injected i.v. into recipient mice. Primary lymphocytes were harvested at the times indicated after immunization and analyzed for cell division as indicated by dilution of the CFDA-SE dye.
Spleens were harvested from two mice per group and homogenized separately. Mononuclear cells were isolated by centrifugation over LSM. Cells were incubated in 2.4G2 (9) supernatant-20% normal mouse serum for 20 min on ice to block Fc receptor-mediated uptake of antibody and then stained with anti-CD8-phycoerythrin (PE) Cy5 (clone 53-6.7) and either anti-Vb11-PE (clone RR3-15) or anti-CD45.1-PE (clone A20) for 40 min on ice.
Flow cytometric analysis.
Cells were washed thoroughly prior to analysis (typically a minimum of three washes), and then data were acquired by using either a FACScan, a FACSCalibur, or a FACSCanto flow cytometer (Becton Dickinson, San Jose, CA) and analyzed by using FlowJo software (Treestar, San Carlos, CA). Only cells expressing CD8 and either Vβ11 or CD45.1 were analyzed for CFDA-SE staining. All antibodies were from BD Pharmingen (San Diego, CA).
Intracellular cytokine production analysis.
Splenocytes were harvested, and mononuclear cells were isolated over an LSM gradient. The resulting cells were restimulated in the presence of APC bearing the NP366-374 peptide. At 6 days after restimulation, live cells were harvested by centrifugation over LSM. The resulting cells were incubated with or without 1 μM NP366-374 peptide for 2 h, at which time 5 μg/ml of brefeldin A was added, and the cells were incubated for an additional 4 h. Cells were then stained with anti-CD8-PE-Cy5 antibody and fixed with 1% paraformaldehyde for 20 min. After a washing step, cells were permeabilized with 0.5% saponin and stained with anti-gamma interferon (IFN-γ)-fluorescein isothiocyanate (FITC; clone XMG 1.2) for 40 min on ice and analyzed as indicated above. The results shown are the percentages of CD8+ cells that produced IFN-γ.
In vivo killing assay.
Red blood cell-depleted splenocytes were purified from B6.SJL mice by suspension in ACK lysis buffer (Cambrex, Walkersville, MD). The resulting cells were washed once and incubated with either the concentrations of NP366-374 shown or 1 μM control peptide from chicken egg ovalbumin (OVA) (OVA257-264, SIINFEKL) for 40 min at room temperature. All peptides were made in the Macromolecular Core Facility at Penn State College of Medicine. Cells were then washed twice to remove unbound peptide and labeled with 5 μM (NP peptide-pulsed) or 0.5 μM (OVA peptide-pulsed) CFDA-SE. Cells were counted and mixed in equal ratios, and 2 × 106 cells/peptide were injected into previously immunized mice. At 18 h after injection, the spleens were harvested from recipient mice and homogenized, and the red blood cell-depleted splenocyte population was analyzed for the presence or absence of CFDA-SE-labeled cells by flow cytometric analysis. Similar results were obtained when the spleens were removed from recipients 4 h after the injection of CFDA-SE-labeled cells.
DC migration assay.
FITC (8 mg/ml, Sigma) was dissolved in equal amounts of dibutyl phthalate (Sigma) and acetone and then vortexed vigorously. C57BL/6 mice were immunized with approximately 4 × 106 PFU rAdLacZ i.d. in each ear. Immunized or nonimmunized mice received 25-μl aliquots of FITC solution applied to each ear with a pipette tip. After 18 h, the draining lymph nodes were harvested, microdissected, and incubated in 2 mg/ml of collagenase D (Roche Diagnostics, Indianapolis, IN) at 37°C for 30 min. The resulting single cell suspensions were analyzed by flow cytometry.
Ex vivo proliferation.
DC obtained from lymph nodes were incubated with CD11c microbeads (Miltenyi Biotec, Auburn, CA) and positively sorted by using an AutoMACS system. A total of 5 × 104 CD8-enriched CFDA-SE-labeled TCR-Tg cells were added to 105 sorted DCs in 200 μl of RPMI 1640 containing 10% FBS, 1% nonessential amino acids, penicillin-streptomycin, sodium pyruvate, and 2 mM l-glutamine in 96-well U-bottom plates (Costar; Corning, Corning, NY). Each culture was performed in duplicate. Cultures were analyzed for proliferation after 60 h.
Removal of the infection site.
At times indicated after i.d. ear infection, the mice were anesthetized, and the site of infection was surgically removed. At 3 days postimmunization, the draining lymph nodes were removed and analyzed for cell division in vivo.
Statistical analysis.
Statistical analyses were determined where indicated using a two-tailed unpaired Student t test. In each case, tests were used to distinguish between values obtained after immunization with either AdK14NP versus AdCMVNP, AdK14NP versus AdSPCNP, or AdK14NP versus no immunization. Where analysis of in vivo killing assays was performed, percentages of cells remaining in the CFDA-SE high peak were compared. Where statistics are shown superimposed on representative histograms, they represent statistics from at least three repeat experiments. All error bars shown on plots represent the standard error of the mean, and the statistical significance is indicated (*, P < 0.05; **, P < 0.01).
RESULTS
Characterization of rAd.
In order to study the initiation of a TCD8+ response to a peripheral virus infection in the skin, we designed a system in which a nonreplicating adenovirus vector expresses a model antigen, influenza virus nucleoprotein (NP) from the A/NT60 strain, driven by a tissue-targeted promoter. The use of NP as an antigen allows the use of F5 transgenic mice bearing a TCR specific for an antigenic peptide (NP366-374)) in complex with Db, facilitating both the study of early events after infection and the study of the duration of antigen presentation. The production of NP was driven either by the ubiquitous CMV promoter (AdCMVNP), by a promoter that drives the expression primarily in keratinocytes (K14, AdK14NP) or, as a negative control, by a promoter that drives expression in type II pneumocytes in the lung (SPC, AdSPCNP).
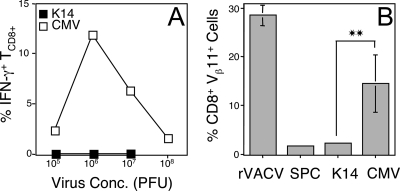
Although many promoters have been described as “tissue specific,” these promoters may also drive expression in other tissues, which would be a concern in the interpretation of our results. Therefore, we infected mice i.v. with each of our rAd to allow access of the injected virus to the maximal number of tissues, including pAPC populations. We then examined the functional relevance of the TCD8+ response to antigen expressed in tissues other than our target tissues. As shown (Fig. 1A), cells from mice primed with AdCMVNP generated a TCD8+ response, as measured by production of IFN-γ by in vitro restimulated splenocytes after exposure to the NP366-374 peptide. We used an assay where cells were restimulated with peptide, since it is the most sensitive assay that can detect a response when no response can be detected directly ex vivo. In contrast to the response observed with AdCMVNP, the response to AdK14NP was not significantly different (P < 0.01) from that observed with no immunization in five separate experiments. These results demonstrate that cells that are infected after i.v. immunization, including pAPC, do not express K14-driven antigen at a level sufficient to generate effector TCD8+. Similar results were obtained when primary ex vivo activation of TCD8+ was examined (not shown).
FIG. 1.
TCD8+ responses after i.v. immunization with AdCMVNP or AdK14NP. Mice were immunized i.v. with AdCMVNP (□) or AdK14NP (▪) (A) or additionally with AdSPCNP or rVACV-NT60 NP (B). (A) Production of IFN-γ in response to NP366-374 peptide by in vitro restimulated splenocytes removed 30 days after immunization. Error bars are too small to observe. There was no statistically significant difference between the response observed in mice infected with either AdK14NP or AdSPCNP compared to uninfected mice. (B) Expansion of adoptively transferred F5 TCD8+ (CD8+ Vβ11+) 4 days after immunization. **, P < 0.01.
Throughout our studies we used activation and expansion of TCR transgenic TCD8+ as a measure for TCD8+ priming. To ensure that the higher precursor frequency of F5 TCD8+ after adoptive transfer did not result in a differential ability of tissue-targeted antigen to expand TCD8+, we immunized mice i.v. with AdK14NP, AdCMVNP, or AdSPCNP. As shown in Fig. 1B, neither AdK14NP nor AdSPCNP expanded the numbers of adoptively transferred TCR transgenic TCD8+ above background levels. In contrast, specific TCD8+ were dramatically expanded in mice immunized with either AdCMVNP or rVACV encoding nucleoprotein from A/NT60.
Induction of TCD8+ specific for viral antigen expressed in the skin.
In order to allow expression of rAd-encoded antigen driven by the K14 promoter, we infected mice in the skin, where rAd can infect keratinocytes. To examine whether rAd infection could drive proliferation of specific naive TCD8+ after infection of keratinocytes, we immunized mice i.d. in the ear pinnae with AdCMVNP, AdSPCNP, or AdK14NP. At 24 h prior to infection, we transferred CFDA-SE-labeled F5 splenocytes into mice to allow specific TCD8+ proliferation to be measured. At 3 days postimmunization, the superficial cervical lymph nodes draining the ear were removed and a single-cell suspension analyzed for dilution of the CFDA-SE stain in adoptively transferred F5 TCD8+. As expected, AdSPCNP failed to trigger proliferation of F5 TCD8+, since the virus did not drain to areas containing cells that are able to produce protein driven by the SPC promoter (Fig. 2A). In contrast, significant proliferation was observed after i.d. immunization with either AdK14NP (Fig. 2B) or AdCMVNP (Fig. 2C). There was a significant decrease (P < 0.01) in the number of F5 TCD8+ proliferating in response to AdK14NP (45.7%) compared to AdCMVNP (94%), which likely reflects both an enhanced expression of antigen driven by the CMV promoter within keratinocytes, as well as expression of antigen within other cell types, including pAPC.
FIG. 2.
Proliferation and effector activity of TCD8+ after i.d. immunization with AdCMVNP or Adk14NP. Mice were immunized i.d. in the ear pinnae with AdSPCNP (A and D), AdK14NP (B and E), or AdCMVNP (C and F). Proliferation of adoptively transferred F5 TCD8+ in the draining cervical lymph node was assessed 3 days postinfection by monitoring CFDA-SE dilution (A to C). The data shown are representative, but the statistics shown are derived from four repeat experiments. (D to K) Cytotoxic activity was assessed 3 days (D to G) or 30 days (H to K) postinfection by examining clearance of cells pulsed with either the OVA257-264 peptide (labeled with 0.5 μM CFDA-SE, lower peak) or with the NT60 NP366-374 peptide (labeled with 5 μM CFDA-SE, upper peak) from the spleen 18 h after injecting target cells. Similar results were obtained when splenocytes were taken 4 h after the injection of labeled cells. *, P < 0.05; **, P < 0.01. The data in panels D to F and H to J are representative, and cumulative data from three independent experiments are shown with statistical analyses in panels G and K.
Cells often proliferate prior to deletion or entry into an unresponsive state during the induction of peripheral tolerance (12), and expression of constitutively expressed antigen within keratinocytes may induce tolerance of TCD8+ (6, 35). Antigen-specific proliferation of TCR transgenic TCD8+, therefore, does not necessarily imply maturation to effector and memory status. To examine whether cells responding to tissue-targeted rAd-encoded antigen acquired lytic function, we adoptively transferred F5 splenocytes and immunized the recipient mice with rAd. We then examined the clearance of CFDA-SE-labeled splenocytes pulsed with either the NP366-374 peptide or a control peptide (OVA257-264). As shown in Fig. 2D to F, we found that the adenoviruses did not stimulate clearance of OVA peptide-pulsed cells. AdCMVNP-immunized mice completely cleared NP366-374 pulsed cells (Fig. 2F), with the number of target cells remaining not being statistically significant from zero. AdSPCNP-immunized mice failed to clear any cells pulsed with the NP366-374 peptide (Fig. 2D), but AdK14NP-immunized mice showed significant clearance of cells pulsed with the NP366-374 peptide (Fig. 2E), demonstrating induction of specific cytolytic activity by the AdK14NP virus. However, in seven experiments examining cytolytic activity in a primary response, the number of residual NP peptide-pulsed cells in mice immunized with AdK14NP consistently remained statistically different (P < 0.05) from those in mice immunized with AdCMVNP (Fig. 2G). The reduced clearance of NP peptide-pulsed cells indicates a reduction in the efficiency of antigen-specific cytolytic activity compared to AdCMVNP, similar to that seen during initiation of the proliferative response. The reduced efficacy of cytolytic activity of TCD8+ primed with AdK14NP is preserved in the memory response (Fig. 2K, P < 0.01), since 30 days postinfection the clearance of NP366-374 pulsed cells was incomplete after immunization with AdK14NP (Fig. 2I) but not after immunization with AdCMVNP (Fig. 2J).
Localization of effector responses.
After immunization with AdK14NP, we detected proliferation of F5 TCD8+ in the lymph node draining the site of infection but only minimal proliferation in the spleen (Fig. 3A). In contrast, proliferation was readily detectable in both the draining node and spleen after immunization with AdCMVNP (Fig. 3A). Lower numbers of F5 TCD8+ were present in the spleen 5 days after immunization with AdK14NP (Fig. 3B, P < 0.01), even when the amount of AdK14NP used to immunize was 100-fold higher than that of AdCMVNP (data not shown). This deficit in localization of responding TCD8+ to the spleen appeared more pronounced in the memory response where, 30 days after immunization, very few adoptively transferred F5 TCD8+ could be detected after immunization with AdK14NP (Fig. 3C, P < 0.01). The decrease in detectable TCD8+ was not due to clearance of the adoptively transferred F5 TCD8+ since these cells were easily detectable after immunization with AdCMVNP (Fig. 3C).
FIG. 3.
Localization of TCD8+ after i.d. immunization with AdCMVNP or AdK14NP. CFDA-SE-labeled (A) or unlabeled (B and C) F5 TCD8+ were adoptively transferred into mice that were immunized 24 h later with rAd as shown. Spleens and lymph nodes were removed 3 days (A), 4 days (B) or 30 days (C) after immunization, and F5 TCD8+ (CD8+, CD45.1+) proliferation (A), quantified by dilution of CFDA-SE, or the numbers of cells (B and C) were measured by flow cytometry. (A) Representative plots are shown, but the statistics from three independent experiments are included. The numbers of proliferating cells in both spleen and node were different in AdCMVNP- and AdK14NP-infected mice (P < 0.01). Panels B and C: **, P < 0.01; *, P < 0.05.
Timing of the TCD8+ response.
In our system it is possible that AdCMVNP virus particles drain to the lymph node and infect pAPC that can directly present NP peptide to TCD8+, a scenario we have previously observed after infection with VACV (25). In contrast, antigen expressed from AdK14NP must be translated within keratinocytes, transferred to pAPC (likely DC), and transported via afferent lymphatics to the draining lymph node. To examine the differences in the requirement for pAPC-mediated transport from the site of infection, we removed the site of infection at various times postinfection. By removing the site of infection, we could remove the contribution of cell-mediated transport, which requires infection of cells at the site, antigen expression, transfer of antigen to pAPC, and then migration of these pAPC to the lymph node. However, we would not remove the contribution of drainage of particles directly to the lymph node and infection of cells in situ. We found that immediate removal of the site of infection reduced the proliferation of adoptively transferred F5 TCD8+ in response to AdK14NP infection to almost background levels but did not impact the response after infection with AdCMVNP (Fig. 4A). In contrast, removal of the site of infection 24 h postinfection did not alter the response to AdK14NP. These results are consistent with a differential requirement for cell-mediated trafficking of antigen from the site of infection, with AdK14NP requiring cell-mediated trafficking and AdCMVNP generating a response in the absence of this process.
FIG. 4.
Time course and efficacy of TCD8+ activation after i.d. infection with AdCMVNP or AdK14NP. Mice were immunized i.d. and tested for either the ability to stimulate proliferation of adoptively transferred F5 TCD8+ via CFDA-SE dilution (A and B) or the effector activity via clearance of specific peptide-pulsed CFDA-SEHI labeled targets (C) on day 3 (A) or days 2, 3, and 4 (B and C) postinfection. (A) The site of infection was removed at the times shown postinfection. (D) The sensitivity of effector F5 TCD8+ was assessed by measuring the clearance of cells pulsed with various amounts of NP366-374 peptide 6 days after i.d. immunization. The data in panel C are expressed as a percentage of the maximal killing of NP366-374 peptide-pulsed CFDA-SEHI cells compared to levels after immunization with AdSPCNP. *, P < 0.05; **, P < 0.01.
Drainage of solute, and presumably of virus particles, to the lymph node can occur within seconds of injection (11, 14), but the migration of DC from the skin to draining lymph nodes is thought to take at least 10 to 16 h (14, 20). Thus, it might be expected that a response to a peripheral viral antigen may be delayed compared to the response to a comparable antigen that can be expressed in secondary lymphoid organs. To address whether TCD8+ respond to peripheral viral antigen with different kinetics of proliferation and acquisition of effector function, we examined the proliferation of adoptively transferred F5 TCD8+ at various times after i.d. immunization with AdK14NP or AdCMVNP. As shown in Fig. 4B, we observed that the number of proliferating cells after immunization with AdK14NP was lower than after immunization of AdCMVNP (P < 0.01). However, there were no gross differences in the kinetics of proliferation of F5 TCD8+ after immunization with AdCMVNP or AdK14NP, with proliferation beginning at 3 days postinfection for both. Similarly, mice immunized with either virus acquired the ability to clear injected splenocytes pulsed with the NP366-374 peptide at 3 days postinfection (Fig. 4C). These results demonstrate a clear correlation between the initiation of proliferation and the acquisition of effector activity that has previously been demonstrated after immunization with herpes simplex virus type 1 (HSV-1) (24). Our results show, somewhat surprisingly, that there is no major difference in the time course of priming of TCD8+ to peripheral antigen or antigen that can be expressed within secondary lymphoid organs.
We had observed that the ability of TCD8+ primed by peripheral virus antigen to clear peptide-pulsed cells was incomplete (Fig. 2E, G, I, and K). This observation could be due to the lower number of cells primed by peripheral antigen (Fig. 3) or to a difference in the ability of individual TCD8+ to lyse target cells. To examine whether there was a deficiency in the ability of TCD8+ primed by peripheral viral antigen to lyse target cells, we adoptively transferred F5 splenocytes and immunized recipient mice with AdSPCNP, AdK14NP, or AdCMVNP. CFDA-SE-labeled splenocytes loaded with either the OVA peptide as a control or with titrated amounts of the NP366-374 peptide were injected, and clearance of these cells was measured 18 h after injection. We found that in mice immunized with AdCMVNP, complete clearance of cells pulsed with NP peptide concentrations down to 10−7 M was observed (Fig. 4D). Progressively less clearance was observed at peptide concentrations down to 10−10 M. Clearance of cells pulsed with 10−11 M peptide was almost indistinguishable from clearance of cells pulsed with the control peptide or from mice immunized i.d. with the AdSPCNP virus. As previously observed, AdK14NP did not clear all of the NP peptide-pulsed cells at any concentration used (Fig. 2E, I, G, and K). However, we found that despite the inability to clear all of the peptide-pulsed cells, AdK14NP-primed cells cleared NP peptide-pulsed cells over a similar range of peptide concentrations as AdCMVNP, although there was a minor difference in the lysis of cells at a concentration of 10−9 M peptide. Thus, the failure of AdK14NP-primed cells to exert the complete cytolytic effects seen after priming with AdCMVNP is likely related to limited expansion of cells (Fig. 2B and 3) rather than a lowered affinity of these cells for NP peptide-Db complexes.
Duration of antigen presentation.
We observed no difference in the kinetics with which a TCD8+ response is initiated to peripheral viral antigen versus antigen that can be expressed within pAPC (Fig. 4B to D). To assess the duration of antigen presentation following immunization with each rAd, we immunized mice i.d. and isolated an enriched population of lymph node CD11c+ cells at 48 h postinfection. We incubated these pAPC with CFDA-SE-labeled F5 TCD8+ for 60 h and assessed the ability of the pAPC populations to trigger TCD8+ proliferation. As a positive control, we purified DC from mice immunized 24 h before with rVACV expressing influenza virus NP, a virus to which ex vivo proliferation has previously been demonstrated. DC from mice immunized with rVACV-NP triggered antigen-specific proliferation of F5 TCD8+, but DC from mice immunized with AdCMVNP failed to reproducibly stimulate antigen-specific TCD8+ proliferation in four similar experiments (Fig. 5A).
FIG. 5.
Duration of antigen presentation. (A) Mice were immunized with viruses as shown, and enriched populations of DC were isolated from the draining lymph node at 24 h (VACV) or 48 h (rAd) postinfection. DC were incubated with CFDA-SE-labeled F5 TCD8+ for 60 h, and proliferation of the TCD8+ was measured. In four separate experiments, we observed no proliferation after immunization with AdCMVNP. (B) CFDA-SE-labeled F5 TCD8+ were adoptively transferred into recipients at the days shown after i.d. infection. The proliferation of F5.SJL cells was assessed 3 days after adoptive transfer. The data shown are representative of three independent experiments, but the statistics of pooled data for AdK14NP and AdSPCNP stimulated proliferation are shown. Proliferation in response to AdK14NP was significantly different to that in response to AdSPCNP on 0 and 1 day postinfection (P < 0.01) but was not statistically different on days 3 through 7. Proliferation in response to AdCMVNP infection was statistically different (P < 0.01) from that observed after infection with either Adk14NP or AdSPCNP at all time points examined.
As an alternative approach to measure the duration of antigen presentation, we adoptively transferred CFDA-SE-labeled F5 splenocytes at various times ranging from the day of immunization to 7 days postimmunization and assayed for dye dilution at 3 days after transfer. We previously established that 3 days posttransfer is the optimal day to observe proliferation of F5 TCD8+ after immunization (Fig. 4B). Proliferation of naive F5 TCD8+ requires both costimulation and a “danger” signal (data not shown), so this assay measures antigen presentation by pAPC but not by somatic cells. Despite the fact that the rAd used are replication deficient, antigen presentation was sufficient to initiate proliferation of F5 TCD8+ at least 7 days postimmunization (Fig. 5B). In contrast to the long-lived ability of AdCMVNP-immunized mice to trigger F5 TCD8+ proliferation, pAPC within AdK14NP-immunized mice presented antigen for less than 72 h (Fig. 5B), and at time points after this there was no significant difference between immunization with AdK14NP and AdSPCNP. Thus, although the TCD8+ response is initiated with similar kinetics, the duration of presentation of peripheral viral antigen is markedly reduced compared to systemically expressed antigen.
DC migration.
Previous studies in the lung have described a period after virus infection during which tissue-resident DC are refractory to stimulation of migration to secondary lymphoid organs (19). We hypothesized that the reduced duration of antigen presentation after immunization with AdK14NP, an event that is dependent upon DC migration from the skin, might be a consequence of such a blockade in DC migration. To examine this hypothesis, we used the classic technique of painting the skin with FITC at various time points after rAd infection and then measuring the number of FITC-positive DC in the draining lymph node 18 h later (Fig. 6). Reproducibly, we observed 10 to 15% of CD11c+ cells in the draining lymph node at this point were FITC+ (Fig. 6A), an observation that agrees with previous measurements that non-lymphoid organ-derived DC are typically rare in lymph nodes (15). Immunization with rAd increased the proportion of FITC+ DC in the node substantially, but this increase was transient and lasted for 1 to 2 days before falling back to, or below, steady-state levels (Fig. 6A).
FIG. 6.
DC Migration following rAd infection. (A) The ears of mice were immunized and then painted with FITC at the time periods shown postinfection with rAd. The percentage of FITC+ CD11c+ DC in the draining lymph node was quantified 18 h later. (B) Similar to panel A, but unimmunized mice were painted with FITC, and the numbers of FITC+ CD11c+ DC were quantified at the times shown postpainting.
Antigen presentation after infection with AdK14NP was undetectable as soon as 3 days postinfection (Fig. 5B), suggesting that the migrating DC may have died or exhausted the supplies of antigen that they had taken up by that point. To address the life span of migrating DC, we painted the skin with FITC and examined the persistence of FITC+ DC in the draining lymph node over the following days. Equal numbers of FITC+ DC were observed in the node 24 and 48 h after painting, but at subsequent time points the number of FITC+ DC decreased significantly, in parallel with the decrease in antigen presentation.
DISCUSSION
Many viruses penetrate the body at peripheral sites and are either poorly cleared or establish latency, allowing long-lived infection that can create many complications for the host. In the present study we designed a model system in which viral antigen is expressed at physiologically relevant levels in the periphery in somatic cells but not within pAPC. We observed that the TCD8+ response to peripheral antigen was insufficient to clear cells pulsed with an identical peptide, even when these cells were introduced systemically. The inability to clear all cells presenting a viral peptide could account for the ability of viruses, such as papillomaviruses that infect peripherally, to avoid clearance by the adaptive immune response. This observation was revealed only following a carefully designed system in which antigen is expressed at low levels in a peripheral site. Thus, the conclusions drawn from studies in which large doses of virus are administered systemically may not be relevant for some peripheral human infections.
The lower numbers of TCD8+ responding to peripheral antigen are likely the result of both lower levels of antigen and lower levels of antigen presentation in the draining lymph node. However, there are currently no direct methods available to quantify antigen presentation in a lymph node, and the culture of ex vivo DC from the draining node of mice infected with AdCMVNP with F5 TCD8+ failed to reproducibly induce TCD8+ proliferation. Despite the inability of this widely used assay to detect ex vivo antigen presentation, we found that almost all F5 TCD8+ proliferated in vivo after adoptive transfer. The detection of ex vivo antigen presentation by F5 TCD8+ may result from a difference in sensitivity versus other TCR transgenic lines, such as OT-1 (10). The affinity of activated F5 and OT-1 to peptide-pulsed targets is similar, but naive F5 TCD8+ are not activated by peptide alone in vitro and require some form of inflammatory stimulus, such as virus infection (data not shown). However, it is clear that the relative insensitivity of the ex vivo presentation assay compared to the in vivo assay could lead to misinterpretation of results. A failure to observe ex vivo proliferation triggered by some populations of pAPC could be misinterpreted as an inability of these cells to present antigen in vivo.
Our inability to measure ex vivo antigen presentation from DC prevented us from examining the phenotype and maturation status of the pAPC presenting peripheral or systemic antigen to TCD8+. The TCD8+ response to antigen expressed constitutively in the skin appears to be defined both by the pAPC population that is responsible for antigen presentation and by the form of antigen that is expressed. Initially, it was reported that Langerhans cells presenting a peptide derived from a highly expressed secreted peptide induced TCD8+-mediated autoimmunity (22, 23). However, subsequent studies using full-length antigen indicated that TCD8+ tolerance, rather than autoimmunity, was induced (3, 35). The induction of tolerance was mediated by antigen presentation by a Langerin+ non-Langerhans cell (6, 7, 36). Here we have demonstrated that full-length antigen expressed by virus-infected keratinocytes induces proliferation and functional activation of antigen-specific TCD8+. The discrepancy between our results and those that were previously published likely results from the virus infection, which will activate the innate immune system via the ligation of pattern recognition receptors (17, 32).
One consequence of the activation of the innate immune system is initiation of a burst of DC, likely dermal DC (4) migration, from the skin to the draining lymph node. We confirmed that cell-mediated transport of antigen is required for presentation of peptide derived from NP driven by the K14 promoter, as removal of the infection site within 6 h of infection ablates the TCD8+ response. However, our inability to ablate TCD8+ priming after infection with AdCMVNP is consistent with AdCMVNP particles draining directly to the node, a process that may take seconds (11, 13, 25), wherein these particles could infect lymph node-resident pAPC and trigger naive TCD8+. DC migration to the draining lymph node may occur 10 to 16 h after infection (14, 20), so one might expect the initiation of the TCD8+ response to AdCMVNP to occur more rapidly than that to AdK14NP. However, when we examined the time course of TCD8+ activation after infection with each virus we found similar kinetics. One possible explanation for this observation is that proliferation of F5 TCD8+ could not be detected until 3 days postinfection with any rAd. This is 24 h slower than the time period required for proliferation after infection with either VACV (25) or HSV-1 (24). This delay might be accounted for by the relatively slow expression of antigen by rAd compared to VACV and HSV-1. However, the promiscuous expression of the receptor for rAd particles on parenchymal cells raises the possibility that all available rAd is bound and internalized by these cells and that none are able to drain to the lymph node. Thus, it appears likely that all rAd-encoded antigen must be transported to the node, whether within infected DC (5, 17, 30) or after pAPC-mediated internalization of antigen derived from infected cells.
We did observe a significantly pronounced difference in the duration of antigen presentation after infection with AdK14NP versus AdCMVNP, with presentation after peripheral expression of viral antigen lasting less than 3 days. Antigen presentation after infection with Listeria monocytogenes is transient, likely as a result of TCD8+-mediated killing of pAPC (37). However, the presentation of virus-encoded antigens to TCD8+ can occur for an extended period of time (33, 38) and may potentiate the further expansion of TCD8+ after the initial exposure to antigen that triggers six to seven divisions of these cells (18, 27, 34). Antigen presentation after infection with AdK14NP correlated with a burst of DC migration from the skin that was followed by a refractory period in which migration was reduced significantly. The continued presentation of antigen remains a focus of our laboratory, but these data indicate that this phenomenon may either require the expression of virus genes within pAPC (33) or may require the deposition of large quantities of antigen that can be transferred between DC populations in the draining node (8) for an extended period of time.
Our observation that enhanced DC migration is transient is consistent with similar observations in the lung after influenza virus infection (19) but extend upon the previous observations by providing a functional consequence of the reduced DC migration at later times postinfection. Viruses that infect peripherally and express little antigen initially could utilize the refractory period of DC migration to establish a low-level infection, preventing adequate priming of TCD8+ that can clear all infected cells. This may be the mechanism by which viruses establish latent peripheral infection. Further investigation of the mechanisms responsible for the refractory period of DC migration may yield an intervention that stimulates DC migration and so allows the early phase of peripheral infection to generate a more effective TCD8+ response.
Acknowledgments
We thank Nick Restifo, David Tscharke, Emmy Truckenmiller, and the members of the Norbury lab for critical reviews of the manuscript, helpful comments, and suggestions. We acknowledge the contributions of Nate Sheaffer of the Cell Science/Flow Cytometry Core Facility of the Section of Research Resources, Penn State College of Medicine.
This study was supported by National Institutes of Health grants AI056094, AI076537, and AI0830008 to C.C.N.; by grant C06 RR-15428 to the Penn State College of Medicine Department of Comparative Medicine; and T32 CA60395 training grant to E.L.G. (P. I. R. Courtney).
Footnotes
Published ahead of print on 20 January 2010.
REFERENCES
- 1.Allan, R. S., C. M. Smith, G. T. Belz, A. L. van Lint, L. M. Wakim, W. R. Heath, and F. R. Carbone. 2003. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301:1925-1928. [DOI] [PubMed] [Google Scholar]
- 2.Allan, R. S., J. Waithman, S. Bedoui, C. M. Jones, J. A. Villadangos, Y. Zhan, A. M. Lew, K. Shortman, W. R. Heath, and F. R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25:153-162. [DOI] [PubMed] [Google Scholar]
- 3.Azukizawa, H., H. Kosaka, S. Sano, W. R. Heath, I. Takahashi, X. H. Gao, Y. Sumikawa, M. Okabe, K. Yoshikawa, and S. Itami. 2003. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur. J. Immunol. 33:1879-1888. [DOI] [PubMed] [Google Scholar]
- 4.Bedoui, S., P. G. Whitney, J. Waithman, L. Eidsmo, L. Wakim, I. Caminschi, R. S. Allan, M. Wojtasiak, K. Shortman, F. R. Carbone, A. G. Brooks, and W. R. Heath. 2009. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10:488-495. [DOI] [PubMed] [Google Scholar]
- 5.Brossart, P., A. W. Goldrath, E. A. Butz, S. Martin, and M. J. Bevan. 1997. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J. Immunol. 158:3270-3276. [PubMed] [Google Scholar]
- 6.Bursch, L. S., B. E. Rich, and K. A. Hogquist. 2009. Langerhans cells are not required for the CD8 T-cell response to epidermal self-antigens. J. Immunol. 182:4657-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bursch, L. S., L. Wang, B. Igyarto, A. Kissenpfennig, B. Malissen, D. H. Kaplan, and K. A. Hogquist. 2007. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204:3147-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone, F. R., G. T. Belz, and W. R. Heath. 2004. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 25:655-658. [DOI] [PubMed] [Google Scholar]
- 9.Daeron, M., C. Neauport-Sautes, U. Blank, and W. H. Fridman. 1986. 2.4G2, a monoclonal antibody to macrophage Fc gamma receptors, reacts with murine T-cell Fc gamma receptors and IgG-binding factors. Eur. J. Immunol. 16:1545-1550. [DOI] [PubMed] [Google Scholar]
- 10.den Haan, J. M., S. M. Lehar, and M. J. Bevan. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gretz, J. E., C. C. Norbury, A. O. Anderson, A. E. Proudfoot, and S. Shaw. 2000. Lymph-borne chemokines and other low-molecular-weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192:1425-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez, J., S. Aung, K. Marquardt, and L. A. Sherman. 2002. Uncoupling of proliferative potential and gain of effector function by CD8+ T cells responding to self-antigens. J. Exp. Med. 196:323-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman, H. D., K. Takeda, C. N. Skon, F. R. Murray, S. E. Hensley, J. Loomis, G. N. Barber, J. R. Bennink, and J. W. Yewdell. 2008. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat. Immunol. 9:155-165. [DOI] [PubMed] [Google Scholar]
- 14.Itano, A. A., S. J. McSorley, R. L. Reinhardt, B. D. Ehst, E. Ingulli, A. Y. Rudensky, and M. K. Jenkins. 2003. Distinct dendritic cell populations sequentially present a subcutaneous antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19:47-57. [DOI] [PubMed] [Google Scholar]
- 15.Jakubzick, C., M. Bogunovic, A. J. Bonito, E. L. Kuan, M. Merad, and G. J. Randolph. 2008. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J. Exp. Med. 205:2839-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenne, L., P. Thumann, and A. Steinkasserer. 2001. Interaction of large DNA viruses with dendritic cells. Immunobiology 204:639-648. [DOI] [PubMed] [Google Scholar]
- 17.Jooss, K., Y. Yang, K. J. Fisher, and J. M. Wilson. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 72:4212-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T-cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legge, K. L., and T. J. Braciale. 2003. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18:265-277. [DOI] [PubMed] [Google Scholar]
- 20.Macatonia, S. E., A. J. Edwards, and S. C. Knight. 1986. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology 59:509-514. [PMC free article] [PubMed] [Google Scholar]
- 21.Mamalaki, C., T. Norton, Y. Tanaka, A. R. Townsend, P. Chandler, E. Simpson, and D. Kioussis. 1992. Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T-cell receptor transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 89:11342-11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayerova, D., E. A. Parke, L. S. Bursch, O. A. Odumade, and K. A. Hogquist. 2004. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity 21:391-400. [DOI] [PubMed] [Google Scholar]
- 23.Mayerova, D., L. Wang, L. S. Bursch, and K. A. Hogquist. 2006. Conditioning of Langerhans cells induced by a primary CD8 T-cell response to self-antigen in vivo. J. Immunol. 176:4658-4665. [DOI] [PubMed] [Google Scholar]
- 24.Mueller, S. N., C. M. Jones, C. M. Smith, W. R. Heath, and F. R. Carbone. 2002. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 195:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norbury, C. C., D. Malide, J. S. Gibbs, J. R. Bennink, and J. W. Yewdell. 2002. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 26.Novak, N., and W. M. Peng. 2005. Dancing with the enemy: the interplay of herpes simplex virus with dendritic cells. Clin. Exp. Immunol. 142:405-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obst, R., H. M. van Santen, R. Melamed, A. O. Kamphorst, C. Benoist, and D. Mathis. 2007. Sustained antigen presentation can promote an immunogenic T-cell response, like dendritic cell activation. Proc. Natl. Acad. Sci. U. S. A. 104:15460-15465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad, S. A., C. C. Norbury, W. Chen, J. R. Bennink, and J. W. Yewdell. 2001. Cutting edge: recombinant adenoviruses induce CD8 T-cell responses to an inserted protein whose expression is limited to nonimmune cells. J. Immunol. 166:4809-4812. [DOI] [PubMed] [Google Scholar]
- 29.Smith, C. M., G. T. Belz, N. S. Wilson, J. A. Villadangos, K. Shortman, F. R. Carbone, and W. R. Heath. 2003. Cutting edge: conventional CD8α+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J. Immunol. 170:4437-4440. [DOI] [PubMed] [Google Scholar]
- 30.Song, W., H. L. Kong, H. Carpenter, H. Torii, R. Granstein, S. Rafii, M. A. Moore, and R. G. Crystal. 1997. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 186:1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tindle, R. W. 2002. Immune evasion in human papillomavirus-associated cervical cancer. Nat. Rev. Cancer 2:59-65. [DOI] [PubMed] [Google Scholar]
- 32.Truckenmiller, M. E., and C. C. Norbury. 2004. Viral vectors for inducing CD8+ T-cell responses. Expert Opin. Biol. Ther. 4:861-868. [DOI] [PubMed] [Google Scholar]
- 33.Turner, D. L., L. S. Cauley, K. M. Khanna, and L. Lefrancois. 2007. Persistent antigen presentation after acute vesicular stomatitis virus infection. J. Virol. 81:2039-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423-429. [DOI] [PubMed] [Google Scholar]
- 35.Waithman, J., R. S. Allan, H. Kosaka, H. Azukizawa, K. Shortman, M. B. Lutz, W. R. Heath, F. R. Carbone, and G. T. Belz. 2007. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J. Immunol. 179:4535-4541. [DOI] [PubMed] [Google Scholar]
- 36.Wang, L., L. S. Bursch, A. Kissenpfennig, B. Malissen, S. C. Jameson, and K. A. Hogquist. 2008. Langerin expressing cells promote skin immune responses under defined conditions. J. Immunol. 180:4722-4727. [DOI] [PubMed] [Google Scholar]
- 37.Wong, P., and E. G. Pamer. 2003. Feedback regulation of pathogen-specific T-cell priming. Immunity 18:499-511. [DOI] [PubMed] [Google Scholar]
- 38.Zammit, D. J., D. L. Turner, K. D. Klonowski, L. Lefrancois, and L. S. Cauley. 2006. Residual antigen presentation after influenza virus infection affects CD8 T-cell activation and migration. Immunity 24:439-449. [DOI] [PMC free article] [PubMed] [Google Scholar]