Abstract
The recent outbreaks of influenza A H5N1 virus in birds and humans have necessitated the development of potent H5N1 vaccines. In this study, we evaluated the protective potential of an immediate-early promoter-based baculovirus displaying hemagglutinin (BacHA) against highly pathogenic avian influenza (HPAI) H5N1 virus infection in a mouse model. Gastrointestinal delivery of BacHA significantly enhanced the systemic immune response in terms of HA-specific serum IgG and hemagglutination inhibition (HI) titers. In addition, BacHA vaccine was able to significantly enhance the mucosal IgA level. The inclusion of recombinant cholera toxin B subunit as a mucosal adjuvant along with BacHA vaccine did not influence either the systemic or mucosal immunity. Interestingly, an inactivated form of BacHA was able to induce only a negligible level of immune responses compared to its live counterpart. Microneutralization assay also indicated that live BacHA vaccine was able to induce strong cross-clade neutralization against heterologous H5N1 strains (clade 1.0, clade 2.1, and clade 8.0) compared to the inactivated BacHA. Viral challenge studies showed that live BacHA was able to provide 100% protection against 5 50% mouse lethal doses (MLD50) of homologous (clade 2.1) and heterologous (clade 1) H5N1. Moreover, histopathological examinations revealed that mice vaccinated with live BacHA had only minimal bronchitis in lungs and regained their body weight more rapidly postchallenge. Furthermore, immunohistochemistry results demonstrated that the live BacHA was able to transduce and express HA in the intestinal epithelial cells in vitro and in vivo. We have demonstrated that recombinant baculovirus with a white spot syndrome virus (WSSV) immediate-early promoter 1 (ie1) acted as a vector as well as a protein vaccine and will enable the rapid production of prepandemic and pandemic vaccines without any biosafety concerns.
The recent outbreaks of H5N1 avian flu and the current pandemic situation with H1N1 swine-origin influenza A virus (S-OIV) are clear indications of the urgent need for effective vaccines against influenza A viruses (31). Preventive and therapeutic measures against influenza A viruses have received much interest and effort globally to combat the current pandemic and to prevent such a situation in the future. Currently used vaccines for influenza are administered mainly parenterally and include live attenuated reassortant viruses, conventional inactivated whole viral antigens, or split-virus vaccines. Although some of these vaccines have proven to be quite effective, the manufacturing of these vaccines involves several technical and safety issues (21). Furthermore, the production of currently available influenza vaccines often requires high-level biocontainment facilities, an additional hurdle that limits the advancement of present vaccines.
Vaccines containing purified recombinant viral proteins have recently gained special attention due to their ease of production without any safety concerns (25). Recombinant hemagglutinin (rHA) subunit vaccines produced in baculovirus-insect cell expression systems have been extensively tested and evaluated in humans (29, 30). Baculovirus-derived rHA subunit vaccines administered parenterally are safe and immunogenic in animals and humans. Along with its success in recombinant protein vaccines, baculovirus surface display technology allows us to present large complex proteins on the baculovirus envelope in its native antigenic conformation, resulting in good stability and a longer half-life in the host (18, 14, 8).
Along with a suitable antigen, the route of administration of the vaccine has a profound effect in controlling mucosally acquired infections such as influenza. Vaccination via the mucosal route stimulates both systemic and mucosal immune responses (16). Oral and intranasal vaccines are the two main options for mucosal administration. Intranasal vaccines would have a detrimental effect on persons with asthma, reactive airway disease, and other chronic pulmonary or cardiovascular disorders (4). Oral vaccines therefore seem to be the safest alternative (13). Moreover, there is evidence to prove the ability of oral vaccination to prevent infection of the lungs (23) and cause transcytosis of the molecule across the cells into the circulation (24).
In this report, we describe the construction of recombinant baculovirus under control of the immediate-early promoter 1 (ie1) derived from the white spot syndrome virus (WSSV) genome, which enables the expression of hemagglutinin at the early stage of infection in insect cells, thereby enhancing the display of HA on the baculovirus envelope. Incorporation of more HA into the budding baculovirus particles would improve their efficacy as immunogens. We have studied the efficacy of WSSV ie1-based baculovirus displaying hemagglutinin (BacHA) as an oral vaccine in a mouse model of infection. We have also assessed its efficacy with recombinant cholera toxin B (rCTB) as a mucosal adjuvant. This strategy will enable rapid production of prepandemic vaccines with minimal infrastructure around the world, alleviating the need for high-biosafety facilities, risky inactivation of virulent viruses, and meticulous protein purification procedures.
MATERIALS AND METHODS
Influenza viruses.
The highly pathogenic influenza A human H5N1 viruses from clade 2.1 A/Indonesia/CDC/669/Indonesia/2006 and A/Indonesia/CDC/594/2006 were obtained from the Ministry of Health (MOH), Republic of Indonesia. H5N1 viruses from different phylogenetic clades were rescued by reverse genetics (36). Briefly, the hemagglutinin (HA) and neuraminidase (NA) genes of H5N1 viruses from clade 1.0 (A/Vietnam/1203/2004), clade 2.1 (A/Indonesia/CDC1031/2007), and clade 8.0 (A/chicken/Henan/12/2004) were synthesized (GenScript) based on the sequences from the NCBI influenza database. The synthesized HA and NA genes were cloned into a dual-promoter plasmid for influenza A virus reverse genetics (20). The reassortant viruses were rescued by transfecting plasmids containing the HA and NA genes together with the remaining six gene plasmids derived from A/Puerto Rico/8/34 (H1N1) into a coculture of 293T and MDCK cells using Lipofectamine 2000 (Invitrogen Corp.). Stock viruses were propagated in the allantoic cavity, and virus content was determined by standard hemagglutination (HA) assay as described previously (33). All experiments with highly pathogenic viruses were conducted in a biosafety level 3 (BSL-3) containment facility, in compliance with CDC/NIH and WHO recommendations (15, 35). Recombinant cholera toxin B subunit (rCTB) was provided by Shanghai United Cell Biotechnology Co., Ltd. (Shanghai, People's Republic of China).
Generation of recombinant baculovirus vaccine.
For the construction of recombinant baculovirus BacHA, the full-length open reading frame (ORF) of the HA gene (CDC/669/Indonesia/06 and CDC/594/Indonesia/06) was amplified and inserted into pFASTBacHT A (Invitrogen, San Diego, CA) using RsrII and HindIII restriction sites. The ie1 promoter was amplified from WSSV DNA using the primers WSSVie1F (5′-CCTACGTATCAATTTTATGTGGCTAATGGAGA-3′) and WSSVie1R (5′-CGCGTCGACCTTGAGTGGAGAGAGAGCTAGTTATAA-3′) and then inserted into pFASTBacHT A using AccI and RsrII restriction sites.
For the generation of recombinant baculoviruses, the constructs were integrated into the baculovirus genome within DH10Bac (Invitrogen) through site-specific transposition using Bac-To-Bac system (Invitrogen) as described before (19). The recombinant bacmids were then transfected into Sf9 cells, and the budded virus particles released into the medium were harvested at 4 days posttransfection.
Immunofluorescence assay to detect expression of HA in insect cells.
To detect the immunofluorescence signals, Sf9 cells were infected with BacHA and the cells were fixed at 48 h postinfection as described previously (20). The fixed cells were then incubated with guinea pig anti-HA polyclonal antibody at a dilution of 1:100 for 1 h at 37°C. Fluorescein isothiocyanate (FITC)-conjugated rabbit anti-guinea pig antibody (Dako Cytomation, Denmark) at a dilution of 1:100 was subsequently incubated with the cells for 1 h. The fluorescence signal was detected with an inverted fluorescence microscope (Olympus, United Kingdom), and the images were captured by a digital imaging system (Nikon).
Characterization of baculovirus displaying influenza virus HA.
The viral titers were determined by plaque assay, and the virus particles were purified by two rounds of sucrose gradient ultracentrifugation following standard protocols (17). Purified recombinant baculovirus in phosphate-buffered saline (PBS) (pH 7.4) was then mixed with Laemmli sample buffer and resolved by 12% SDS-PAGE. Fractions containing purified baculovirus were then transferred onto a nitrocellulose membrane and blocked with 5% nonfat milk in 1× PBS and 0.1% Tween 20 for 1 h at room temperature. The membrane was incubated with guinea pig anti-HA polyclonal antibodies at a dilution of 1:500, rinsed and subsequently incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-guinea pig antibodies (Dako Cytomation, Denmark) for 1 h at room temperature. The membrane was washed and developed by incubation with 3,3′-diaminobenzidine (DAB) and hydrogen peroxide (6). The vaccine was then prepared based on a log2 hemagglutination titer of 8.
The inactivated BacHA vaccine was prepared by treating baculovirus displaying HA with binary ethylenimine (BEI) as described previously (22). The complete loss of infectivity of the inactivated BacHA was determined by inoculation into Sf9 cell monolayers and observation of cytopathic effects for at least 7 days.
Uptake of recombinant baculovirus by human intestinal cells in vitro.
Human colorectal carcinoma (HCT 116) cells were maintained in McCoy's 5A modified medium (catalog no. M4892; Sigma) supplemented with 10% fetal bovine serum (FBS) and seeded in flat-bottom 24-well plates on the day of the experiment. The cells were incubated with BacHA for 20 h and stained with anti-HA monoclonal antibody, followed by FITC-conjugated goat anti-mouse immunoglobulin (Dako Cytomation, Denmark) (20). In addition, Vero cells maintained in Dulbecco modified Eagle medium (DMEM) (supplemented with 10% FBS) were also incubated with BacHA and used as a reference control.
Oral immunization.
Specific-pathogen-free female BALB/c mice (6 weeks old) were obtained from the Laboratory Animals Centre, National University of Singapore, and maintained at the Animal Holding Unit of the Temasek Life Sciences Laboratory. Prior to immunization, all mice were starved for 2 h; otherwise food and water were supplied ad libitum. Thirty mice per each experimental group (n = 30/group) were immunized intragastrically by oral gavage on days 0, 7, and 21 with 200 μl containing inactivated or live recombinant baculovirus vaccine at a log2 HA titer of 8 suspended in phosphate-buffered saline (PBS), pH 7.4, either adjuvanted with 10 μg rCTB or unadjvanted. Six mice from each experimental group were sacrificed on days 14, 28, and 42, and serum and intestinal lavage fluids were collected as described previously (32). Briefly, the small intestine from each mouse was cut into 4- to 5-cm pieces and transferred to a glass tube. After addition of 1.0 ml of PBS, the tubes were vortexed gently for 30 s and centrifuged at 5,000 rpm for 10 min.
All animal experiments were carried out in accordance with the Guides for Animal Experiments of the National Institute of Infectious Diseases (NIID), and experimental protocols were reviewed and approved by Institutional Animal Care and Use Committee of the Temasek Life Sciences Laboratory, National University of Singapore, Singapore.
Measurement of anti-H5 HA-specific antibodies by indirect ELISA.
The HA-specific serum IgG antibody titer and the HA-specific intestinal mucosal IgA levels were tested separately against purified rHA0 (Protein Sciences Corporation, CT) antigen by indirect enzyme-linked immunosorbent assay (ELISA) according to a previously described method (3). In brief, microtiter well ELISA plates were coated with purified recombinant H5 HA in coating buffer (0.1 mol/liter carbonate-bicarbonate, pH 9.6). Samples of test serum were 2-fold diluted serially in 3% nonfat dry milk in PBS containing 0.05% Tween 20, and mucosal wash samples were diluted directly at 1:20. The color development was then visualized by adding goat anti-mouse IgG (Sigma) and goat anti-mouse IgA (Bethyl Lab) conjugated with horseradish peroxidase to the respective wells, followed by addition of 3,3′,5,5′-tetramethylbenzidine (Sigma). The absorbance was measured at 450 nm using a microwell plate reader.
Hemagglutination inhibition assay.
Hemagglutination inhibition assays were performed as described previously (33). Receptor-destroying enzyme (RDE)-treated (2) sera were serially diluted (2-fold) in V-bottom 96-well plates. Approximately 4 HA units of viral antigen was incubated with the serum for 30 min at room temperature, followed by the addition of 1% chicken red blood cells (RBCs) and incubation at room temperature for 40 min.
Microneutralization assay.
The microneutralization test was performed according to a previously described protocol (27). Briefly, MDCK cells were seeded in 96-well culture plates and cultured at 37°C to form a monolayer. Serial 2-fold dilutions of heat-inactivated (56°C for 45 min) serum samples were mixed separately with 100 50% tissue culture infective doses (TCID50) of H5N1 virus and incubated at room temperature for 1 h, and the mixtures were added to a monolayer of MDCK cells in triplicate wells. The neutralizing titers of mouse antiserum that completely prevented any cytopathic effect at reciprocal dilutions were calculated.
Immunohistochemistry.
The mice were sacrificed on day 28, and intestine samples were collected in 10% (wt/vol) buffered formalin, embedded in paraffin, and sectioned. The sections were then deparaffinized using Histo-choice (Amersco) and rehydrated in sequentially graduated ethanol baths. The sections were treated with trypsin (0.1% [wt/vol] in PBS) for 10 min and washed twice with PBS-Tween 20 (0.01% [vol/vol] with PBS). Slides were blocked in 0.3% nonfat milk in PBS for 30 min, followed by incubation with guinea pig anti-HA polyclonal antibody at a dilution of 1:100 for 1 h at 37°C. FITC-conjugated rabbit anti-guinea pig antibody (Dako Cytomation, Denmark) at a dilution of 1:100 was subsequently incubated with the cells for 1 h. The fluorescence signal was detected with an inverted fluorescence microscope (Olympus, United Kingdom), and the images were captured by a digital imaging system (Nikon).
Disease challenge test against influenza H5N1 virus infection.
The efficacy of the vaccine was assessed by host challenge against highly pathogenic avian influenza (HPAI) H5N1 virus strains. Twenty-one days after final vaccination, mice were transferred into an animal BSL-3 containment facility. Six mice per group were challenged intranasally with 5 50% mouse lethal doses (MLD50) of homologous (CDC/669/Indonesia/06 clade 2.1) and heterologous (Vietnam/1203/2004 clade 1.0) HPAI H5N1 virus strains. The MLD50 of the influenza virus required for intranasal challenge experiments was predetermined. To determine the effect of adjuvant efficacy, animals immunized with vaccines without adjuvant or only with rCTB were also maintained as control groups. Mice were observed daily to monitor body weight and mortality. Monitoring continued until all animals died or until day 14 after challenge. For histopathology, a lung sample was collected in 10% (wt/vol) buffered formalin solution, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin and were analyzed for pathology.
Statistical analysis.
The data are expressed as arithmetic mean ± standard deviation (SD). The unpaired two-tailed Student's t test was performed to determine the level of significance in the difference between means of two groups. One-way analysis of variance (ANOVA) was also used to test for differences between groups, and the Tukey honestly significant difference (HSD) post hoc test was used to determine which groups were significantly different from the rest. All statistical analysis was done with SigmaStat 2.0 (Jandel Corporation) software. A P value of <0.05 was considered significant.
RESULTS
Structural and antigenic conformation of HA0 in insect cells and baculovirus envelope.
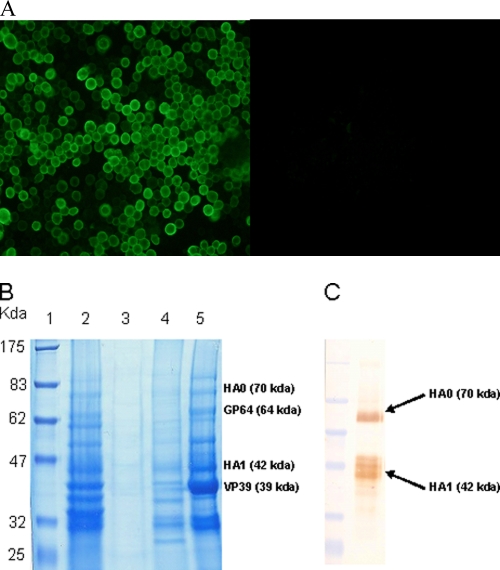
The indirect immunofluorescence assay revealed that HA0 expressed by the recombinant baculovirus was able to successfully translocate to the plasma membrane of infected insect cells (Fig. 1A). SDS-PAGE analysis of budded baculovirus particles from sucrose gradient purification revealed that components of purified baculovirus particles were abundantly present in the third fraction of the gradient (Fig. 1B). Western blot analysis of the fraction containing purified baculovirus indicated that baculovirus surface-displayed HA0 was able to sustain its antigenic conformation and authentic cleavage (Fig. 1C).
FIG. 1.
Characterization of BacHA. (A) Indirect immunofluorescence assay with insect cells infected with recombinant baculovirus expressing H5 HA0. Infected cells were fixed and stained with guinea pig anti-HA antibody and rabbit anti-guinea pig FITC. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of purified baculoviruses, showing the presence of major capsid protein (VP39) and envelope protein (GP64) of baculovirus and influenza HA in the purified sucrose gradient fraction. Lane 1, prestained protein marker; lane 2, infected cell culture supernatant; Lanes 3, 4, and 5, first, second, and third fractions from sucrose density gradient purification, respectively. (C) Western blot. The antigenic conformation of HA incorporated into purified baculoviruses (fraction 3) was probed using anti-HA monoclonal antibody.
Baculovirus-mediated expression of influenza virus HA in human intestinal cells in vitro.
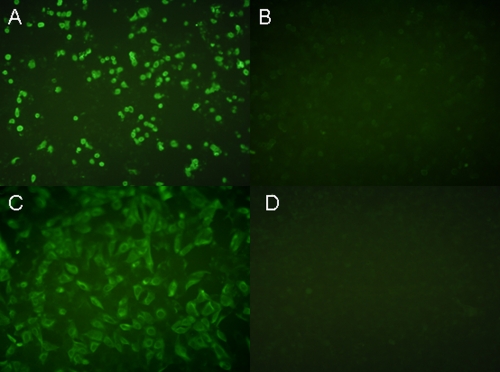
The ability of WSSV ie1 promoter-based BacHA to transduce and express HA in human colorectal carcinoma was evaluated in vitro. Indirect immunofluorescence assay demonstrated that live BacHA was able to successfully transduce intestinal epithelial cells and the expression of HA was effectively driven by the WSSV ie1 promoter (Fig. 2A). In addition, BacHA was also able transduce Vero cells in vitro (Fig. 2C). Intestinal cells incubated with inactivated BacHA did not show any positive fluorescence signal compared to that of live BacHA in both cell lines (Fig. 2B and D).
FIG. 2.
Transduction and expression of HA in human intestinal epithelial cells by WSSV ie1-based BacHA. HCT116 cells were incubated with BacHA at a multiplicity of infection (MOI) of 200 and stained with anti-HA monoclonal antibody followed by FITC-conjugated secondary antibody at 20 h postinfection. Vero cells incubated with BacHA at the same MOI were used as a reference control. (A) Live BacHA-transduced HCT 116 cells. (B) HCT cells incubated with inactivated BacHA. (C) Live BacHA-transduced Vero cells. (D) Vero cells incubated with inactivated BacHA.
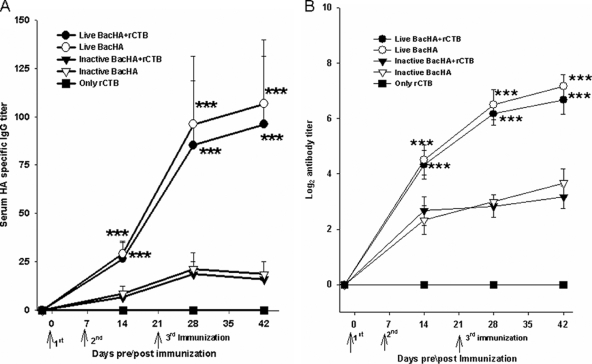
Systemic antibody responses to oral vaccination.
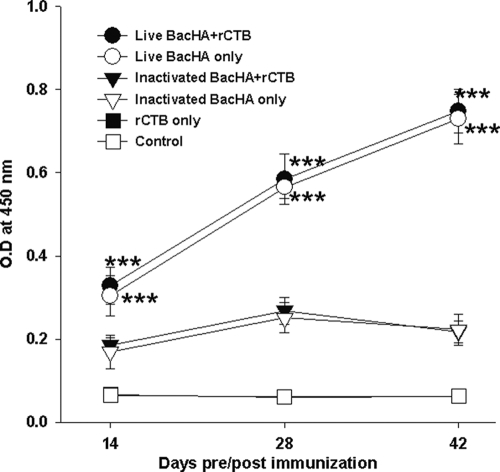
Indirect ELISA was performed to determine the HA-specific serum IgG titers. The groups of mice immunized orally with live BacHA showed significantly (P < 0.001) enhanced HA-specific IgG titers compared to the inactivated BacHA (Fig. 3A). However, the presence of rCTB adjuvant along with the BacHA, either live or inactivated, did not show any significant improvement in antibody titers compared with unadjuvanted BacHA (Fig. 3A). The hemagglutination inhibition titers of the sera were also measured. The HI titers also showed a similar trend, with no significant difference between the groups orally immunized with the vaccine in the presence or absence of the adjuvant. However, the HI titers in the mice immunized with live BacHA titers were significantly (P < 0.001) higher than those in mice immunized with the inactivated BacHA (Fig. 3B).
FIG. 3.
Measurement of systemic immune response. Groups of mice (n = 6) were orally vaccinated three times on days 0, 7, and 21 with 200 μl containing inactivated or live recombinant baculovirus at a log2 HA titer of 8, adjuvanted with or without 10 μg rCTB. (A) HA-specific IgG antibody titers in serum, determined by indirect ELISA. (B) Serum hemagglutination inhibition titer. Each point represents the arithmetic mean value (n = 6) ± SD (***, P < 0.001).
Mucosal immune responses to oral vaccination.
Indirect ELISA was done to determine the HA-specific mucosal IgA levels. The mice immunized with live BacHA showed significantly (P < 0.001) higher mucosal IgA levels than the mice immunized with inactivated BacHA (Fig. 4). However, the presence of the adjuvant rCTB did not cause any increase or decrease in the IgA levels in both cases (Fig. 4).
FIG. 4.
Measurement of mucosal anti-HA specific IgA antibody levels by indirect ELISA. Groups of mice (n = 6) were orally vaccinated three times on days 0, 7, and 21 with 200 μl containing inactivated or live recombinant baculovirus at a log2 HA titer of 8, adjuvanted with or without 10 μg rCTB. Each point represents the arithmetic mean value (n = 6) ± SD (*, P < 0.05; **, P < 0.02; ***, P < 0.001).
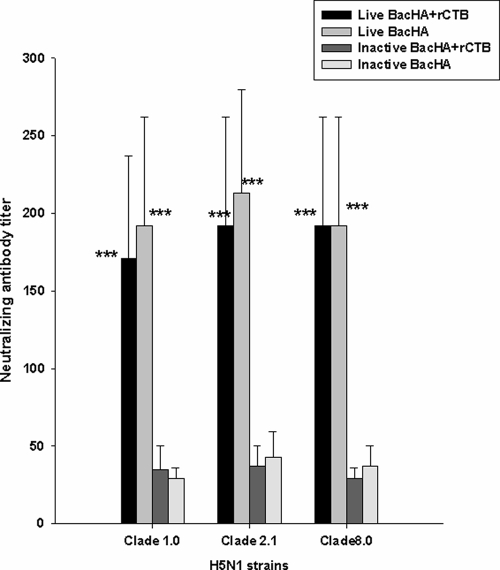
Serum cross-clade neutralizing antibody titer to oral vaccination.
The serum neutralizing antibody titer against 100 TCID50 of different clades of H5N1 strains on day 42 showed that vaccination with live BacHA alone or in the presence of rCTB significantly neutralized (P < 0.001) viruses from clade 1.0, clade 2.1 (circulating strain from 2007), and clade 8.0 compared with inactivated BacHA alone or in the presence of the adjuvant (Fig. 5). However, the presence of the adjuvant rCTB groups does not influence the neutralizing antibody titers compared with those in unadjuvanted vaccination groups administered live BacHA and inactivated BacHA (Fig. 5).
FIG. 5.
Cross-clade serum microneutralization in mice immunized orally with inactivated or live recombinant baculovirus at a log2 HA titer of 8 with or without 10 μg rCTB. Viruses from clade 1.0 (A/Vietnam/1203/2004), clade 2.1 (A/Indonesia/CDC1031/2007), clade 2.3 (A/chicken/Nongkhai/NIAH400802/2007), and clade 8.0 (A/chicken/Henan/12/2004) were used for this study. Sera from the day of peak response, day 21 after the final immunization, were used for the assay. Each point represents the arithmetic mean value (n = 6) ± standard error (*, P < 0.05; **, P < 0.02; ***, P < 0.001).
Baculovirus transduction of intestinal epithelial cells in vivo.
To examine the ability of WSSV ie1-based live baculovirus to mediate gene transduction in the intestinal lumen following oral vaccination, HA expression in the epithelial cells of intestinal villi was examined by immunohistochemistry. The results revealed that live baculovirus was able to transduce the HA gene into the epithelial cells of intestinal villi (Fig. 6A). However, the inactivated form of baculovirus did not show any immunofluorescence signal (Fig. 6B) and appeared similar to the unvaccinated control group (Fig. 6C).
FIG. 6.
Baculovirus transduction of mouse intestinal epithelial cells in vivo. One week after the third immunization, intestinal tissue samples were embedded in paraffin and sectioned. Immunohistochemical staining was carried out using a guinea pig anti-HA antibody and rabbit anti-guinea pig FITC. Intestinal villi of mice orally vaccinated with live baculovirus (A), inactivated baculovirus (B), and PBS (C) are shown.
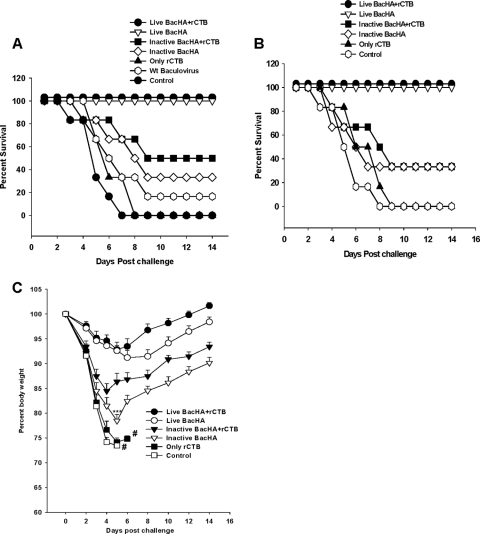
Challenge studies after oral vaccination.
Three weeks after the final immunization, all groups of mice were challenged intranasally with 5 MLD50 of HPAI H5N1 strains from clade 1.0 or clade 2.1. Groups of mice immunized with live BacHA either alone or in the presence of rCTB lost up to 9% of their original body weight by day 5 or 6 after challenge (Fig. 7C) but had 100% protection against both clade 1 and clade 2.1 viruses (Fig. 7A and B). Moreover, the mice that were coadministered with live BacHA and rCTB regained their body weight more rapidly (within 6 days) than the mice that were immunized with unadjuvanted BacHA, which gradually regained only about 6 to 7% of the lost body weight by day 14 after challenge. However, mice coadministered inactivated BacHA and rCTB showed about a 14% loss of body weight (within 5 days) and showed only 49.9% and 33.3% protection against clade 2.1 and clade 1.0 viral challenge, respectively (Fig. 7A and B). The surviving mice from this group gradually regained only about 9% of the lost body weight by day 14. Moreover, mice vaccinated with inactivated BacHA alone showed a significant (P < 0.001) loss in body weight of up to 23%, compared to mice immunized with inactivated BacHA and rCTB on day 5 postchallenge. Only 33.3% of mice immunized with inactivated BacHA survived after challenge with H5N1 virus (Fig. 7C). Mice vaccinated with wild-type baculovirus showed only 16.33% protection against viral challenge (Fig. 7A).
FIG. 7.
Protection of mice from lethal H5N1 virus challenge. Groups of mice (n = 6) were orally vaccinated three times on days 0, 7, and 21 with 200 μl containing inactivated or live recombinant baculovirus at a log2 HA titer of 8, adjuvanted with or without 10 μg rCTB. Wild-type baculovirus at a dose equivalent to BacHA (based on the viral titers determined by plaque assay) served as a negative control. (A and B) Three weeks after the final vaccination, mice were intranasally infected with 5 MLD50 of homologous (CDC/669/Indonesia/06 clade 2.1) (A) and heterologous (Vietnam/1203/2004 clade 1.0) (B) HPAI H5N1 virus strains. Mice were monitored for survival throughout a 14-day observation period. The results are expressed in terms of percent survival. (C) The group of mice challenged with Vietnam/1203/2004 clade 1.0 was also monitored for weight loss throughout a 14-day observation period. The results are expressed as percent body weight (at the beginning of the trial).
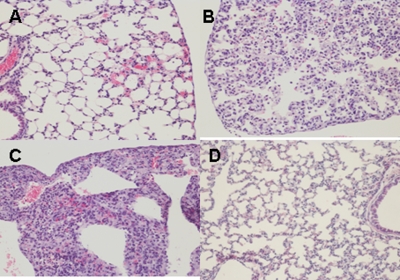
Histopathology.
Histopathology studies were performed with the mice vaccinated and challenged with clade 2.1 virus. On day 6 postinfection, lungs of untreated mice had pulmonary lesions consisting of moderate to severe necrotizing bronchitis and moderate to severe histiocytic alveolitis with associated pulmonary edema (Fig. 8C). The uninfected mice lacked lesions in the lungs (Fig. 8D). Mice vaccinated with live BacHA had only minimal bronchitis (Fig. 8A), while mice vaccinated with inactivated BacHA had moderate bronchitis (Fig. 8B).
FIG. 8.
Photomicrographs of hematoxylin- and eosin-stained lung sections of mice challenged with clade 2.1 H5N1 virus at 6 days postchallenge. (A) Mice vaccinated with live BacHA; (B) mice vaccinated with inactivated BacHA; (C) unvaccinated mice challenged with virus; (D) normal morphology seen in uninfected mice.
DISCUSSION
A recombinant baculovirus with the immediate-early promoter 1 of WSSV was constructed to facilitate high-level expression of influenza virus H5 hemagglutinin in both insect and mammalian cells. The nature of ie1 as an immediate-early promoter supports protein expression at the early phase of the baculoviral life cycle, resulting in an enhanced display of functional hemagglutinin on the baculovirus envelope. HA displayed on the baculovirus surface has retained its native structure as evidenced by the hemagglutination activity and authentic cleavage of HA0 into HA1 and HA2. Earlier, Treanor et al. (29) reported that parenteral immunizations with influenza virus HA expressed in insect cells are safe and immunogenic in humans. However, most studies have attempted only to investigate the efficacy of HA subunit-derived baculovirus-insect cell expression systems as a vaccine for influenza virus. Since recombinant HA proteins expressed in insect cells tend to form monomers (26), it is reasonable to speculate that this may lead to suboptimal immunogenicity, as HA is not being presented in its native trimeric conformation. In fact, Wei et al. (34) demonstrated that oligomeric recombinant HA elicited the strongest immune response in mice, in comparison with that of HA monomer.
Baculovirus surface display technology enables the presentation of large complex proteins in their functional conformation. As oligomerization is required for efficient transport of the HA proteins to the host cell membrane (5), a prerequisite for the baculovirus to acquire the protein, it is presumed that HA displayed on the baculovirus surface should have been presented in its oligomeric form. Hence, we attempted to use this baculovirus displaying HA as an oral vaccine candidate against H5N1 infection in mice. The live BacHA was able to induce both systemic and mucosal immune responses in the orally vaccinated mice, as indicated by the high level of HA-specific IgG and IgA antibodies, respectively. Interestingly, mice vaccinated with inactivated BacHA were able to induce only low-level immune responses compared to live BacHA. The differences between the immune responses of the mice after live BacHA and inactivated BacHA vaccinations could be mainly due to two factors. First, HA displayed on the live baculovirus would have retained its functional oligomeric conformation, resulting in better immunogenicity than inactivated baculovirus. Second, native HA could have played a role in binding to the receptors expressed in the intestinal epithelial cell membrane, resulting in gene delivery and stimulation of the cell-mediated immune response.
Serum hemagglutination inhibition assays and microneutralization assays revealed that live BacHA is superior to inactivated BacHA vaccine, further suggesting that the structural conformation of HA indeed has some effect on its immunogenicity. Determinations of serum neutralization efficiency against 100 TCID50 of heterologous H5N1 strains from different clades revealed that BacHA vaccination induced significantly higher virus neutralization titers against H5N1 strains from clade 2.1 (circulating strain from 2007). This observation shows the efficacy of the live BacHA vaccine against the genetic drift from 2006 to 2007 in clade 2.1 Indonesian strains. Further, the BacHA also efficiently neutralized viruses from clade 1.0, clade 2.3, and clade 8.0 compared with inactivated BacHA. This strong cross-clade immunity could be due to better affinity and avidity of the antibody response generated against conserved epitopes (3, 12). This is remarkable, as cross-clade viral neutralization is indicative of the critical ability of the vaccine in limiting the evolution of escape mutants by mutation and reassortment. However, further experiments need to be done to better understand the nature of the strong cross-clade protection induced by live BacHA vaccination. Our previous observations with mice vaccinated through the intranasal route indicated that baculovirus surface-displayed HA efficiently enhanced both systemic and mucosal immune responses compared to inactivated whole H5N1 viral vaccine. Moreover, rCTB-containing BacHA elicited higher level mucosal and systemic immune responses in a mouse model (19). However, in the present study, we did not observe any increase in the antibody responses in mice orally coadministered with rCTB and BacHA, and the reason why rCTB was not effective is presently unclear.
To evaluate the protective efficacy of BacHA vaccines, vaccinated mice were challenged with both homologous and heterologous H5N1 strains. One hundred percent survival was obtained with the group vaccinated with live BacHA with or without rCTB. Interestingly, mice coadministered live BacHA and rCTB regained their body weight more rapidly than those administered only live BacHA. Further, mice vaccinated with inactivated BacHA had only a 33.3% survival rate, and rCTB adjuvant provided at least 49.9% survival against homologous virus. Moreover, mice coadministered inactivated BacHA and rCTB showed about a 14% loss of body weight, compared to a 23% loss of body weight with unadjuvanted BacHA (P < 0.001).
Though the HA-specific antibody response was lower with inactivated BacHA than with its live counterpart, mice vaccinated with inactivated BacHA also showed moderate protective immunity against 5 MLD50 of H5N1 virus. Earlier reports have shown that intranasal immunization with wild-type baculovirus alone provides sufficient protection against lethal challenge with H1N1 influenza virus (1). This was attributed to the recognition of unmethylated CPG motif of baculoviral DNA by the Toll-like receptor 9 (TLR9) molecule, thus activating the innate immune response (9). Baculovirus is also known to stimulate mammalian cells to secrete interferon (IFN) cytokines and confer in vivo protection of mice from encephalomyocarditis virus infection (7). The partial protection of mice obtained with inactivated BacHA in this study supports the previous findings that baculovirus can trigger innate antiviral mechanisms in a mammalian system.
Baculoviruses are able to transduce several mammalian cells and mediate gene transfer in vitro (11). In the present study, uptake of recombinant baculovirus by human intestinal epithelial cells in vitro was confirmed and the expression of HA by the WSSV ie1 promoter was verified. Moreover, reports have demonstrated that recombinant baculoviruses were also able to deliver genes of interest in vivo in animal models. Tani et al. (28) demonstrated that vesicular stomatitis virus glycoprotein-modified baculovirus was able to transduce a reporter gene into the cerebral cortex and testes of mice by direct inoculation in vivo. Furthermore, intravitreal injection of baculovirus caused expression of vascular endothelial growth factor in the inner retina, photoreceptor cells, and retinal pigment epithelium cells of rabbit eye (10). In the present study, we have also evaluated the potential of live BacHA to transduce the intestinal epithelial cells of orally immunized mice. Immunohistochemical analysis revealed that WSSV ie1-based baculovirus was able to express HA in the epithelial cells of intestines of vaccinated mice. Our results suggest that baculovirus acted as a vectored vaccine as well as a protein vaccine against the H5N1 infection.
In summary, the baculovirus surface-displayed hemagglutinin vaccine is efficacious in inducing mucosal immune responses as well as systemic immune responses and does not require either sophisticated biocontainment infrastructure or downstream purification processes for mass production. In addition, the HA of any given influenza virus isolate could be converted into an efficient vaccine with this technology in a short period of time. Oral vaccination is considered to be a highly desirable form of vaccination, being noninvasive, pain-free, and self-administrable, with improved logistics and good immunization coverage. Despite the recent attention to intranasal administration, oral vaccination is still considered the best approach to increase patient compliance. Parameters such as ease of use, affordability, needle-less administration, and mass coverage during a prepandemic or pandemic situation make oral vaccination an attractive option. Considering the above facts, we conclude that the baculovirus surface-displayed hemagglutinin vaccine could be an ideal choice as a pandemic or prepandemic influenza vaccine.
Acknowledgments
We are grateful for the financial support received from Temasek Life Science Laboratory, Singapore.
We thank the Ministry of Health (MOH), Indonesia, for technical support and collaboration. We thank Ruben Donis, Influenza Division, Centers for Disease Control and Prevention, Atlanta, GA, for providing the plasmids for reverse genetics. We thank Hooi Shing Chuan, Department of Physiology, National University of Singapore, for providing human intestinal cell lines. We also thank Hui Ting Ho for generation of RG-H5N1 (clade 1.0) virus.
Footnotes
Published ahead of print on 13 January 2010.
REFERENCES
- 1.Abe, T., H. Takahashi, H. Hamazaki, N. Miyano-Kurosaki, Y. Matsuura, and H. Takaku. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 171:1133-1139. [DOI] [PubMed] [Google Scholar]
- 2.Bright, R. A., D. K. Shay, B. Shu, N. J. Cox, and A. I. klimov. 2006. Adamantane resistance among influenza A (H3N2) viruses isolated early duringthe 2005-2006 influenza season in the United states. JAMA 295:891-894. [DOI] [PubMed] [Google Scholar]
- 3.Bright, R. A., D. M. Carter, C. J. Crevar, F. R. Toapanta, J. D. Steckbeck, K. S. Cole, N. M. Kumar, P. Pushko, G. Smith, T. M. Tumpey, and T. M. Ross. 2008. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One 3:e1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2004. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 53:1-40. [Google Scholar]
- 5.Copeland, C. S., R. W. Doms, E. M. Bolzau, R. G. Webster, and A. Helenius. 1986. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J. Cell Biol. 103:1179-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallgher, S., S. E. Winston, S. A. Fuller, and J. G. R. Hurrell. 2004. Immunoblotting and immunodetection, p. 10.8.1-10.8.24. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, et al. (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., Newcastle, United Kingdom.
- 7.Gronowski, A. M., D. M. Hilbert, K. C. F. Sheehan, G. Garotta, and R. D. Schreiber. 1999. Baculovirus stimulates antiviral effect in mammalian cells. J. Virol. 73:9944-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He, F., S. Madhan, and J. Kwang. 2009. Baculovirus vector as a delivery vehicle for influenza vaccines. Expert Rev. Vaccines 8:455-467. [DOI] [PubMed] [Google Scholar]
- 9.Hervas-Stubbs, S., P. Rueda, L. Lopez, and C. Leclerc. 2007. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J. Immunol. 9:2361-2369. [DOI] [PubMed] [Google Scholar]
- 10.Kinnunen, K., G. Kalesnykas, A. J. Mahonen, S. Laidinen, L. Holma, T. Heikura, K. Airenne, H. Uusitalo, and S. Yla-Herttuala. 2009. Baculovirus is an efficient vector for the transduction of the eye: comparison of baculovirus- and adenovirus-mediated intravitreal vascular endothelial growth factor D gene transfer in the rabbit eye. J. Gene Med. 11:382-389. [DOI] [PubMed] [Google Scholar]
- 11.Kost, T. A., and J. P. Condreay. 2002. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 20:173-179. [DOI] [PubMed] [Google Scholar]
- 12.Liew, F. Y., S. M. Russell, G. Appleyard, C. M. Brand, and J. Beale. 1984. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T-cell reactivity. Eur. J. Immunol. 14:350-356. [DOI] [PubMed] [Google Scholar]
- 13.Manna, J. F. S., V. A. Ferro, A. B. Mullen, L. Tetley, M. Mullen, K. C. Carter, J. Alexander, and W. H. Stimson. 2004. Optimization of a lipid based oral delivery system containing A/Panama influenza hemagglutinin. Vaccine 22:2425-2429. [DOI] [PubMed] [Google Scholar]
- 14.Mottershead, D., I. van der Linden, C. H. von Bonsdorff, K. Keinanen, and C. Oker-Blom. 1997. Baculoviral display of the green fluorescent protein and rubella virus envelope proteins. Biochem. Biophys. Res. Commun. 238:717-722. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health and Centers for Disease Control and Prevention. 1999. Biosafety in microbiological and biomedical laboratories, 4th ed. U.S. Department of Health and Human Services, Washington, DC.
- 16.Ogra, P. L., H. Faden, and R. C. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14:430-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Reilly, D. R., L. K. Miller, and V. A. Luckow. 1992. Baculovirus expression vectors: a laboratory manual, p. 60-61. W. H. Freeman and Company, New York, NY.
- 18.Peralta, A., P. Molinari, D. Conte-Grand, G. Calamante, and O. Taboga. 2007. A chimeric baculovirus displaying bovine herpesvirus-1 (BHV-1) glycoprotein D on its surface and their immunological properties. Appl. Microbiol. Biotechnol. 75:407-414. [DOI] [PubMed] [Google Scholar]
- 19.Prabakaran, M., S. Velumani, F. He, A. K. Karuppannan, G. Y. Geng, L. K. Yin, and J. Kwang. 2008. Protective immunity against influenza H5N1 virus challenge in mice by intranasal co-administration of baculovirus surface-displayed HA and recombinant CTB as an adjuvant. Virology 380:412-420. [DOI] [PubMed] [Google Scholar]
- 20.Prabakaran, M., H. T. Ho, N. Prabhu, S. Velumani, M. Szyporta, F. He, K. Chan, L. Chen, Y. Matsuoka, R. O. Donis, and J. Kwang. 2009. Development of epitope-blocking ELISA for universal detection of antibodies to human H5N1 influenza viruses. PLoS One 4:e4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan, F., C. Huang, R. W. Compans, and S. Kang. 2007. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 81:3514-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rueda, P., J. Fominaya, J. P. M. Langeveld, C. Bruschke, C. Vela, and J. I. Casal. 2000. Effect of different baculovirus inactivation procedures on the integrity and immunogenicity of porcine parvovirus-like particles. Vaccine 19:726-734. [DOI] [PubMed] [Google Scholar]
- 23.Ruedl, C., M. Fruhwirth, G. Wick, and H. Wolf. 1994. Immune response in the lungs following oral immunization with bacterial lysates of respiratory pathogens. Clin. Diagn. Lab. Immunol. 1:150-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell-Jones, G. J. 2001. The potential use of receptor-mediated endocytosis for oral drug delivery. Adv. Drug Deliv. Rev. 46:59-73. [DOI] [PubMed] [Google Scholar]
- 25.Safdar, A., M. A. Rodriguez, L. E. Fayad, G. H. Rodriguez, B. Pro, M. Wang, J. E. Romaguera, A. H. Goy, F. B. Hagemeister, P. McLaughlin, G. P. Bodey, L. W. Kwak, I. I. Raad, and R. B. Couch. 2006. Dose-related safety and immunogenicity of baculovirus-expressed trivalent influenza vaccine: a double-blind, controlled trial in adult patients with non-Hodgkin B cell lymphoma. J. Infect. Dis. 194:1394-1397. [DOI] [PubMed] [Google Scholar]
- 26.Stevens, J., A. L. Corper, C. F. Basler, J. K. Taubenberger, P. Palese, and I. A. Wilson. 2004. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science 303:1866-1870. [DOI] [PubMed] [Google Scholar]
- 27.Suguitan, A. L., Jr., J. McAuliffe, K. L. Milis, H. Jin, G. Duke, B. Lu, C. J. Luke, B. Murphy, D. E. Swayne, G. Kemble, and K. Subbarao. 2006. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross protection in mice and ferrets. PLoS Med. 3:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tani, H., C. K. Limn, C. C. Yap, M. Onishi, M. Nozaki, Y. Nishimune, N. Okahashi, Y. Kitagawa, R. Watanabe, R. Mochizuki, K. Moriishi, and Y. Matsuura. 2003. In vitro and in vivo gene delivery by recombinant baculoviruses. J. Virol. 77:9799-9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treanor, J. J., G. M. Schiff, F. G. Hayden, R. C. Brady, C. M. Hay, A. L. Meyer, J. Holden-Wiltse, H. Liang, A. Gilbert, and M. Cox. 2007. Safety and immunogenicity of a baculovirus expressed hemagglutinin influenza vaccine. JAMA 297:1577-1582. [DOI] [PubMed] [Google Scholar]
- 30.Treanor, J. J., B. E. Wilkinson, F. Masseoud, J. Hu-Primmer, R. Battaglia, D. O'Brien, M. Wolff, G. Rabinovich, W. Blackwelder, and J. M. Katz. 2001. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 19:1732-1737. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe, T., S. Watanabe, J. H. Kim, M. Hatta, and Y. Kawaoka. 2008. Novel approach to the development of effective H5N1 influenza A virus vaccines: use of M2 cytoplasmic tail mutants. J. Virol. 82:2486-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watarai, S., M. Han, M. Tana, and H. Kodama. 1998. Antibody response in the intestinal tract of mice orally immunized with antigen associated with liposomes. J. Vet. Med. Sci. 60:1047-1050. [DOI] [PubMed] [Google Scholar]
- 33.Webster, R. G., Y. Kawaoka, J. Taylor, R. Weinberg, and E. Paoletti. 1991. Efficacy of nucleoprotein and hemagglutinin antigens expressed in fowlpox virus as vaccine for influenza in chickens. Vaccine 9:303-308. [DOI] [PubMed] [Google Scholar]
- 34.Wei, C. J., L. Xu, W. P. Kong, W. Shi, K. Canis, J. Stevens, Z. Y. Yang, A. Dell, S. M. Haslam, I. A. Wilson, and G. J. Nabel. 2008. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J. Virol. 82:6200-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2004. Laboratory biosafety manual, 3rd ed. World Health Organization, Geneva, Switzerland.
- 36.World Health Organization Global Influenza Program Surveillance Network. 2005. Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Dis. 11:1515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]