Abstract
Objective
To evaluate the attainability of tight risk factor control targets for three diabetes risk factors and to assess the degree of polypharmacy required.
Data Sources/Study Setting
National Health and Nutrition Examination Survey-III.
Study Design
We simulated a strategy of “treating to targets,” exposing subjects to a battery of treatments until low-density lipoprotein (LDL)-cholesterol (100 mg/dL), hemoglobin A1c (7 percent), and blood pressure (130/80 mm Hg) targets were achieved or until all treatments had been exhausted. Regimens included five statins of increasing potency, four A1c-lowering therapies, and eight steps of antihypertensive therapy.
Data Collection/Extraction Methods
We selected parameter estimates from placebo-controlled trials and meta-analyses.
Principal Findings
Under ideal efficacy conditions, 77, 64, and 58 percent of subjects achieved the LDL, A1c, and blood pressure targets, respectively. Successful control depended highly on a subject's baseline number of treatments. Using the least favorable assumptions of treatment tolerance, success rates were 11–17 percentage points lower. Approximately 57 percent of subjects required five or more medication classes.
Conclusions
A significant proportion of people with diabetes will fail to achieve targets despite using high doses of multiple, conventional treatments. These findings raise concerns about the feasibility and polypharmacy burden needed for tight risk factor control, and the use of measures of tight control to assess the quality of care for diabetes.
Keywords: Quality measurement, Monte Carlo simulation, outcomes research, diabetes mellitus
The cornerstone of diabetes care is management of risk factors for vascular complications, particularly control of blood pressure, cholesterol, and blood glucose. Most treatment guidelines call for lowering hemoglobin A1c below 7 percent, blood pressure below 130/80, and low-density lipoprotein (LDL)-cholesterol below 100 mg/dL (2.59 mmol/L) (Grundy et al. 2004; American Diabetes Association 2008;). Despite demonstrating substantial health benefits, trials that have compared tight control strategies to conventional approaches have also consistently shown that a large proportion of patients fail to reach targets for A1c (Abraira et al. 1995; UKPDS Study Group 1998a; Patel et al. 2008;) and blood pressure (Hansson et al. 1998; UKPDS Study Group 1998b;) even after a battery of treatments. In one of the largest trials of blood glucose control, the United Kingdom Prospective Diabetes Study (UKPDS) (UKPDS Study Group 1998a), for example, the median A1c in the intensive treatment group over the course of the study was 7.5 percent, meaning that most patients were unable to achieve or maintain the 7 percent target, even though these patients were early in the course of diabetes. In the Hypertension Optimal Treatment (HOT) trial (Hansson et al. 1998), over half of subjects randomized to the lowest diastolic blood pressure (DBP) target failed to reach it.
Successful control of diabetes risk factors depends on a number of factors, including a patient's baseline value of the risk factor, the efficacy of treatment, pathophysiological factors, treatment contraindications and side effects, and each patient's willingness to accept multiple and potentially burdensome treatments. Guidelines often emphasize the benefits of treating to targets without consideration of their costs and side effects, and often without reference to the potential diminishing effects of adding a third, fourth, or fifth medication. Discontinuation rates from A1c-lowering therapies in the UKPDS ranged from 10 to 25 percent over a 3-year period (UKPDS Study Group 1995), suggesting that the side effects or inconvenience of these treatments are significant even among clinical trial volunteers. Little quantifiable information exists on the necessary resources and potential harms associated with a strategy of treating to target levels of risk factors. For patients having multiple uncontrolled risk factors, the overall treatment burden could be substantial.
Although trials of intensive risk factor control often report improved health outcomes on average, these trials often fail to provide information that is critical for guiding decision making and policy making in the real world (Hayward, Hofer, and Vijan 2006). First, these studies rarely report the incremental efficacy of each successive treatment in the intensification regimen, and without these data, the benefits of repeatedly adding or titrating medications cannot meaningfully be weighed against their harms. Second, because trial patients tend to be healthier, more adherent, and more likely to be screened for nonresponse and intolerance to treatment (Nallamothu, Hayward, and Bates 2008), the rate at which targets are reached in real-world settings is likely to be significantly lower than that observed in the clinical literature, and the polypharmacy requirements could be much higher. We therefore developed a simulation model that integrates three of the key determinants of attaining targets—efficacy of treatment, individual variation in treatment response, and treatment tolerance—to estimate the impact of a treat-to-target strategy for a nationally representative diabetic population. We assess the attainability of targets across three risk factors and the level of polypharmacy required. We conclude with a discussion of the implications of our findings for patient care and quality measurement.
METHODS
Data
We created a cohort of subjects aged 30–75 with a self-reported diagnosis of diabetes from the third wave of the National Health and Nutrition Examination Survey (NHANES-III). We used the third wave, fielded from 1988 to 1994, because during this period, statins, metformin, and thiazolidinediones did not exist or were rarely used, and antihypertensive medications were used much less intensely (Psaty et al. 2002; McAlister et al. 2006;). These factors lessen the need to make assumptions about a subject's medication history (e.g., contraindications and prior intolerances and treatment failures), or about dosing information for a subject's current medications, neither of which are reported in NHANES. We used multivariate imputation by chained equations (Van Buuren and Oudshoorn 2007), implemented in the R language to impute missing data (see Table SA1 for missing data rates). We accounted for the complex sampling design of NHANES using the “Survey” package in R (Lumley 2008).
Treatments
We specified risk factor–specific intensification regimens that differed according to a subject's baseline medications (Table 1). Subjects having LDL levels above target at baseline (100 mg/dL [2.59 mmol/L]) and receiving either no treatment or treatment with lipid-lowering drugs other than statins were started on a low-dose statin that was sequentially titrated (a maximum of five treatment steps). We assumed those who were already taking a statin at baseline were on a low dose. Treatments used to lower A1c below 7 percent included metformin, sulfonylurea, glitazone, and insulin. Although the 7 percent threshold is no longer recommended in the elderly population, it was a common target for all populations at the beginning of this study. We assumed subjects who were taking sulfonylurea or insulin at baseline were on submaximal doses, so we included titration steps for both therapies. Subjects having either a systolic blood pressure (SBP) above 130 mm Hg or a diastolic blood pressure (DBP) above 80 mm Hg and who were untreated at baseline were consecutively treated with a thiazide, ACE inhibitor, beta blocker, and calcium channel blocker. Subjects self-reporting antihypertensives at baseline were assumed to be receiving standard doses, and drug classes were added in the aforementioned order as needed. Those remaining above the target after receiving standard doses of each medication were intensified with double doses.
Table 1.
Treatment Intensification Regimens
| Intensification Treatment |
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Treatment | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | Step 6 | Step 7 | Step 8 |
| LDL | ||||||||
| None | Add SMV20 | Intensify to SMV40 | Switch to ATV40 | Intensify to ATV80 | Switch to SMV/EZE | — | — | — |
| Nonstatin | Add SMV20 | Intensify to SMV40 | Switch to ATV40 | Intensify to ATV80 | Switch to SMV/EZE | — | — | — |
| Low-dose statin | Intensify to SMV40 | Switch to ATV40 | Intensify to ATV80 | Switch to SMV/EZE | — | — | — | — |
| A1c | ||||||||
| None | Add MET | Add SUL | Add TZD | Add INS | — | — | — | — |
| Sulfonylurea (SUL) | Intensify SUL | Add MET | Add TZD | Add INS | — | — | — | — |
| Insulin (INS) | Add MET | Add TZD | Intensify INS | — | — | — | — | — |
| Sulfonylurea+insulin | Add MET | Add TZD | Intensify INS | — | — | — | — | — |
| BP | ||||||||
| None | Add THI | Add ACE | Add BBL | Add CCB | Intensify THI | Intensify ACE | Intensify BBL | Intensify CCB |
| Thiazide (THI) | Add ACE | Add BBL | Add CCB | Intensify THI | Intensify ACE | Intensify BBL | Intensify CCB | — |
| ACE Inhibitor (ACE) | Add THI | Add BBL | Add CCB | Intensify THI | Intensify ACE | Intensify BBL | Intensify CCB | — |
| Beta blocker (BBL) | Add THI | Add ACE | Add CCB | Intensify THI | Intensify ACE | Intensify BBL | Intensify CCB | — |
| Calcium channel blocker (CCB) | Add THI | Add ACE | Add BBL | Intensify THI | Intensify ACE | Intensify BBL | Intensify CCB | — |
Notes. Metformin therapy was considered contraindicated for subjects having a standardized creatinine level exceeding 150 μmol/L or a diagnosis of congestive heart failure (12.8% of the population) (Jones, Macklin, and Alexander 2003).
Patients who reported taking insulin without concomitant sulfonylurea were assumed to have had a history of prior treatment with sulfonylurea and then discontinued. For patients treated with multiple antihypertensive classes at baseline, drugs were added in the following order as needed: THI, ACE, BBL, and CCB.ATV, Atorvastatin; MET, Metformin; SMV/EZE, Simvastatin 80 mg/Ezetimibe 10 mg; SMV20, Simvastatin 20 mg; TZD, Thiazolidinedione.
Model Parameters
Mean Treatment Efficacy
The bulk of our treatment efficacy parameters came from two published meta-analyses of randomized placebo-controlled trials, and a meta-analysis that we performed ourselves (Tables SA2–SA4). Law, Wald, and Rudnicka (2003b) pooled 72 simvastatin treatment groups and 24 atorvastatin groups, and a second meta-analysis by Law et al. (2003a) included 104 thiazide treatment groups, 217 ACE inhibitor groups, 136 beta blocker groups, and 209 calcium channel blocker groups. For each treatment we abstracted difference-in-difference estimates (mean absolute or relative reductions in each risk factor for the treatment group relative to placebo) along with their standard errors. Because the efficacy of A1c-lowering treatments depended on the types of drugs a subject was already taking, we conducted our own meta-analysis to estimate the efficacy of A1c-lowering treatments for a broad range of two-drug combinations. To determine the efficacy of adding a third or fourth antihyperglycemic therapy to a subject's existing regimen, for which few reliable estimates were found in the literature, we compared the efficacy of the treatment in question when added as the second drug in a two-drug combination with each treatment in the existing regimen. We then selected the minimum estimate to serve as an upper bound for the efficacy of the three-drug combination. For example, the efficacy of metformin added to a regimen consisting of insulin and sulfonylurea was estimated to be 6.4 percent, since patients on sulfonylurea and those on insulin had reductions of 6.4 and 12.5 percent, respectively, when metformin was added to each monotherapy.
Among antihypertensives, Wu et al. (2005) demonstrated that combination therapy, on average, resulted in a 16/35 percent lower efficacy (systolic/diastolic) relative to monotherapy. We extended this finding to incorporate a diminishing marginal efficacy for each additional agent used, so that, whereas a second agent added would have 84 percent of the expected (systolic) efficacy, a third and fourth would have 71 percent (0.842) and 59 percent (0.843) of the efficacy of monotherapy. In sensitivity analyses, we used a constant relative efficacy—16/35 percent lower efficacy for all antihypertensives.
We modeled treatment efficacy as a percentage change in each risk factor from baseline to account for larger treatment effects for subjects with elevated risk factors at baseline. Studies typically reported absolute changes in risk factors for A1c and blood pressure treatments, so we estimated relative changes by dividing each efficacy estimate by the baseline risk factor level in each treatment group.
Variance in Treatment Efficacy
We found no meta-analyses that pooled estimates of between-subject variance in treatment efficacy. For the variance in statin efficacy, we pooled variance estimates from a large number of individual studies. For A1c and blood pressure treatments, however, variance estimates were reported only on the absolute scale, so we adopted an ecological approach and estimated the variance in relative reductions at the study level to serve as a surrogate for the patient-level variance. Because the between-study variance will underestimate the between-patient variance, our base case analysis assumed that the range in efficacy estimates from the contributing studies was approximately equal to 1 standard deviation in patient-level variability, or in other words, about 70 percent of the patient-level variation was contained in the interval defined by the range of study-level estimates. In sensitivity analyses we defined the patient-level variance to be equal to the study-level variance. We derived coefficients of variation (CV) for each treatment, defined as the ratio of the standard deviation in efficacy to the mean treatment efficacy to allow us to crosswalk any simulated mean efficacy parameter to a variance estimate by simply taking the product of the mean efficacy and the CV.
Treatment Discontinuation
We used treatment discontinuation rates to measure patients' intolerance to treatment. Discontinuation rates reflect both the side effects and burdens of treatment, including polypharmacy, the inconvenience of injection therapy, and potentially cost burdens. We used all-cause discontinuation rates from several large statin trials, our own meta-analysis of A1c-lowering treatments, and a meta-analysis of discontinuation rates for antihypertensives (Ross et al. 2001). Using results from the IDEAL trial (back-titration rate from atorvastatin 80 mg of 13 percent and discontinuation rate of 5.4 percent), we specified back-titration and discontinuation rates following the initiation of both atorvastatin 40 and 80 mg to be 6.5 and 2.7 percent, respectively (Pedersen et al. 2005). We found no relationship between dose and discontinuation rates for antihypertensives (Materson et al. 1993). We assumed that subjects who were titrated from submaximal doses of sulfonylurea and insulin would not discontinue treatment on a higher dose but would only back-titrate to the baseline dose at half the discontinuation rate reported for those initiating each therapy.
Simulation
We used Monte Carlo simulation to integrate the treatment efficacy and discontinuation parameters into a model of the effectiveness of a treat-to-target strategy. First, we sampled a mean efficacy estimate for each treatment and then calculated the standard deviation as the product of the mean and CV. Using these estimates and assuming that treatment responses in the population were normally distributed, we randomly sampled percentage reductions for each subject for each step 1 therapy. We then computed each subject's new risk factor level and assessed whether each target was attained. We repeated these steps for each subject until the target was reached or until all treatments had been exhausted. We simulated discontinuation from each drug class and assumed subjects who discontinued received no treatment benefit. We ran 500 simulations of the model to capture uncertainty in all parameters.
By using discontinuation rates and adherence levels from randomized controlled trials, our base case analysis provides the most favorable estimates of target attainment. In sensitivity analyses, we increased discontinuation rates by 5 and 10 percentage points and limited risk factor reductions to 90 and 80 percent of their simulated values to mimic poorer adherence. We then varied both parameters simultaneously to compare levels of attainment for a “less favorable” and a “least favorable” scenario.
RESULTS
The weighted NHANES-III data provide nationally representative estimates for the diabetic population of the early 1990s, comprised of nearly 8 million individuals between the ages of 30 and 75. Baseline characteristics of the population are reported in Table SA1, and all efficacy and discontinuation parameters are listed in Tables SA2–SA4. The incremental efficacy of each statin dose beyond step 1 decreased monotonically (with the exception of simvastatin 40 mg), ranging from a 32.1 percent reduction for simvastatin 20 mg, to a reduction of 5.7 percent for combination therapy with ezetimibe. Relative reductions in A1c tended to be much larger for treatments used as monotherapy (range: 11.8 percent [glitazone] to 14.9 percent [sulfonylurea]), compared with the efficacy of each drug when used in combination (range: 6.4 percent [adding metformin to sulfonylurea] to 12.9 percent [adding glitazone to sulfonylurea]). Adding insulin to oral therapy had the greatest efficacy, reducing A1c levels by 17.6 percent. When used as monotherapy, each standard dose of antihypertensive therapy had comparable efficacy in lowering SBP, 5.5–6.0 percent (8.5–9.2 points), while DBP reductions ranged from 4.5 to 6.9 percent (4.4–6.7 points). Double doses of each drug had on average less than one-third the efficacy of standard doses. When accounting for the diminishing benefit of combination therapy, however, the effective reductions in SBP were smaller: 5.7, 4.6, 4.2, and 3.4 percent, for thiazides, ACE-inhibitors, beta blockers, and calcium channel blockers, for a subject on no medications at baseline. DBP reductions were 4.5, 3.1, 2.9, and 1.7 percent, respectively.
The between-subject variation in treatment efficacy, based on our own meta-analysis (Tables SA2–SA4) varied between treatments for each risk factor as well as between risk factors. The coefficient of variation, which expresses the ratio of the standard deviation in efficacy to the mean efficacy, was 40 percent for statin therapy. For a treatment that lowers LDL by 32 percent on average, this implies that roughly 20 percent of subjects would experience a reduction of 21 percent or less and 20 percent would have more than a 43 percent reduction. Coefficients of variation for A1c-lowering treatments ranged from 23 percent (adding glitazone to sulfonylurea) to 76 percent (adding metformin to sulfonylurea). Response heterogeneity was larger for the blood pressure treatments. Coefficients of variation ranged from 44 to 66 percent for SBP and 54 to 72 percent for DBP.
In Table 2, we report the results of our base case analysis. At the time of NHANES-III, 90 percent of subjects with elevated LDL levels were on no cholesterol-lowering treatment, and the mean LDL of these subjects was 150 mg/dL. About 46 percent of subjects were taking only a sulfonylurea (mean A1c, 9.2 percent), while 30 percent took only insulin (mean A1c, 9.4 percent). Forty-three percent took no antihypertensives (mean BP, 142/79 mm Hg), 32 percent were being treated with a single drug class (mean BP, 145/78 mm Hg), and 22 percent were on two classes (mean BP, 146/81 mm Hg). Treatment was generally effective in reducing risk factor levels, but many subjects did not achieve targets even when titrated to maximal doses. Overall target attainment was 77.0 percent for LDL, 63.6 percent for A1c, and 57.9 percent for blood pressure. The mean reduction in LDL among naïve subjects was 55 points, but 22 percent of these subjects failed to achieve targets. Those already on lipid-lowering therapy had lower levels of attainment (51–77 percent), even with maximal doses of potent statins. A1c treatment was effective in achieving targets for fewer subjects; those on no therapy at baseline achieved targets 80 percent of the time, but the majority of subjects who were already on glucose-lowering agents achieved targets only 32–68 percent of the time. Similarly, blood pressure targets were achieved between 0.3 percent of the time (those on four agents at baseline) and 78 percent of the time (those on no agents at baseline). Among subjects undergoing intensification for at least one risk factor 44.3 percent ultimately achieved all three targets.
Table 2.
Base Case Simulation Results
| Absolute Treatment Effect |
Target Attainment |
|||||
|---|---|---|---|---|---|---|
| Baseline Treatment | Prevalence (%) | Baseline Level | Mean | SD* | Mean (%) | SD* (%) |
| LDL | ||||||
| No treatment | 89.8 | 149.8 mg/dL | 54.9 | 1.8 | 78.2 | 2.7 |
| Non statin | 5.9 | 153.0 mg/dL | 57.4 | 5.6 | 77.0 | 8.5 |
| Low-dose statin | 4.3 | 159.6 mg/dL | 47.0 | 8.4 | 50.5 | 13.1 |
| Overall | 150.4 mg/dL | 54.7 | — | 77.0 | — | |
| A1c | ||||||
| No treatment | 21.9 | 9.0% | 2.3 | 0.1 | 80.0 | 4.2 |
| Sulfonylurea only | 45.9 | 9.2% | 2.1 | 0.1 | 68.4 | 3.4 |
| Insulin only | 29.8 | 9.4% | 1.9 | 0.1 | 47.0 | 6.2 |
| Sulfonylurea and insulin | 2.5 | 9.7% | 1.5 | 0.3 | 31.5 | 8.5 |
| Overall | 9.2% | 2.1 | — | 63.6 | — | |
| BP | ||||||
| No treatment | 42.5 | 141.6, 78.7 mm Hg | −16.1, −6.6 | 0.4, 0.2 | 78.2 | 2.3 |
| 1 class | 31.7 | 145.4, 77.8 mm Hg | −15.0, −4.9 | 0.5, 0.2 | 56.8 | 2.6 |
| 2 classes | 21.6 | 146.1, 80.7 mm Hg | −12.2, −3.6 | 0.7, 0.2 | 27.4 | 3.5 |
| 3 classes | 3.5 | 154.8, 80.1 mm Hg | −8.7, −2.1 | 1.0, 0.2 | 19.2 | 4.0 |
| 4 classes | 0.6 | 141.6, 87.9 mm Hg | −4.4, −1.2 | 1.0, 0.3 | 0.3 | 2.3 |
| Overall | 144.2, 78.9 mm Hg | −14.6, −5.2 | — | 57.9 | — | |
Note. Targets were 100 mg/dL (2.59 mmol/L) (LDL), 7% (A1c), and 130/80 mm Hg (blood pressure). Measurement scale for absolute treatment effect is same as that of baseline level. Results for blood pressure are systolic, diastolic.
Across 500 model simulations.
We found significantly lower rates of attainment when we changed our assumptions about the expected level of tolerance patients might exhibit in nonexperimental settings. As shown in Table 3, when we inflated discontinuation rates by an additional 5 and 10 percentage points, target attainment rates fell by about 4 and 8 percentage points, respectively, for both LDL and A1c but less so for blood pressure. Lower adherence rates had a large effect on target attainment for all three risk factors. When the least favorable assumptions were incorporated, attainment decreased by 11–17 percentage points across the three risk factors, giving success rates of 62 percent for LDL, 46 percent for A1c, and 46 percent for blood pressure.
Table 3.
Sensitivity Analysis Results
| Incremental Target Attainment (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Discontinuation |
Adherence |
||||||
| Baseline Treatment | Base Case Attainment (%) | +5% | +10% | 90% | 80% | Less Favorable* | Least Favorable† |
| LDL | |||||||
| No treatment | 78.2 | −4.8 | −9.3 | −3.1 | −7.2 | −7.7 | −15.6 |
| Nonstatin | 77.0 | −5.0 | −9.7 | −4.0 | −7.7 | −7.7 | −16.2 |
| Low-dose statin | 50.5 | −1.0 | −0.6 | −4.8 | −9.8 | −5.6 | −11.5 |
| Overall | 77.0 | −4.6 | −9.0 | −3.2 | −7.4 | −7.6 | −15.5 |
| A1c | |||||||
| No treatment | 80.0 | −3.0 | −6.1 | −4.2 | −9.9 | −7.5 | −15.8 |
| Sulfonylurea only | 68.4 | −3.7 | −7.5 | −5.0 | −10.4 | −8.3 | −17.4 |
| Insulin only | 47.0 | −3.1 | −7.0 | −6.6 | −13.5 | −9.6 | −18.8 |
| Sulfonylurea and insulin | 31.5 | −2.6 | −5.6 | −3.3 | −6.2 | −6.0 | −10.4 |
| Overall | 63.6 | −3.3 | −7.0 | −5.2 | −11.1 | −8.5 | −17.3 |
| BP | |||||||
| No treatment | 78.2 | −2.1 | −4.2 | −4.1 | −8.7 | −5.8 | −13.1 |
| 1 class | 56.8 | −2.0 | −3.9 | −4.1 | −8.0 | −5.6 | −11.9 |
| 2 classes | 27.4 | −1.4 | −2.5 | −3.4 | −6.9 | −4.2 | −8.9 |
| 3 classes | 19.2 | −0.5 | −1.3 | −1.2 | −2.1 | −1.8 | −3.2 |
| 4 classes | 0.3 | 0.2 | 0.0 | −0.3 | −0.3 | −0.3 | −0.3 |
| Overall | 57.9 | −1.8 | −3.6 | −3.8 | −7.8 | −5.3 | −11.4 |
Note. Alternative coefficient of variation parameters resulted in incremental target attainment rates of 0.7 percentage points (A1c) and 1.2 percentage points (BP). When we incorporated a constant 16%/35% lower systolic/diastolic efficacy for any blood pressure treatment used in combination, target attainment for blood pressure increased by 9.0 percentage points.
Includes 5 percentage points additional discontinuation and 90% adherence.
Includes 10 percentage points additional discontinuation and 80% adherence.
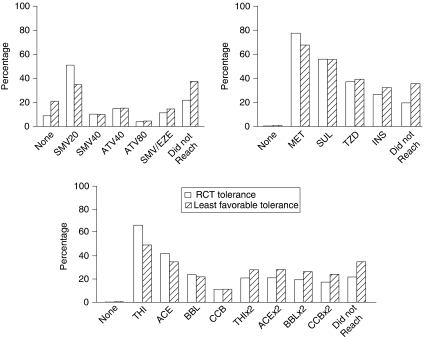
Among those undergoing intensification for at least one elevated risk factor, over half of the population required five or more medications to sustain their final levels (results not shown). Thirteen percent of the population required combination statin therapy, 21, 26, and 38 percent required 2, 3, and 4 or more A1c-lowering drug classes, respectively, while 38 percent needed all four antihypertensive treatments. Poor tolerance caused subjects undergoing intensification to need greater amounts of treatments downstream in the regimen, particularly high-dose medications (Figure 1). When we used our least favorable assumptions about tolerance, utilization of statin-combination therapy was 3.2 percentage points higher, use of glitazones and insulin increased by 2.1 and 5.9 percentage points, respectively, and use of high-dose antihypertensives rose between 6.7 and 7.1 percentage points.
Figure 1.
Distribution of Treatments Following Intensification for Two Levels of Assumed Tolerance to Treatment
Note. Simulation results that assumed tolerance levels of randomized controlled trials are depicted with solid bars. Striped bars reflect the least favorable tolerance assumptions (10 percentage points higher discontinuation and 80 percent adherence relative to randomized trials). All results reflect the subpopulation of subjects on no treatments at baseline and assume that metformin is not contraindicated for any subject.
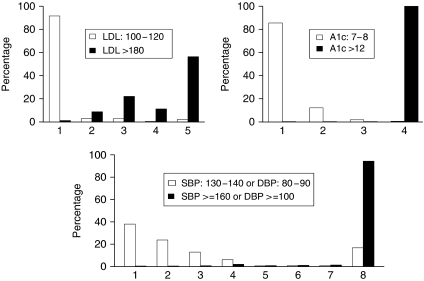
Individual variation in response to treatment also affected the level of polypharmacy required, albeit for a much smaller fraction of the population. In Figure 2, we display the average number of intensifications required by those subjects untreated at baseline for each risk factor. For patients with a baseline LDL ranging from 100 to 120, 91.6 percent of subjects required only one intensification to reach targets, while 5.5 percent required three or more. Among those subjects with a baseline A1c between 7 and 8, 2.2 percent needed three or more agents because of variance in response to treatment. The majority of subjects having mild hypertension needed four or fewer intensifications, but 19.3 percent required five or more.
Figure 2.
Differences in the Number of Intensifications Required Due to Between-Subject Variability in Treatment Response for Subjects with Moderate and Poor Risk Factor Control at Baseline
Note. White bars correspond to subjects with moderate risk factor control. Black bars correspond to subjects with poor control. All results reflect the subpopulation of subjects on no treatments at baseline, assume zero discontinuation and 100 percent adherence, and assume that metformin is not contraindicated for any subject.
DISCUSSION
Using results from clinical trials, we developed a simulation model to assess the likelihood a nationally representative diabetes cohort could achieve commonly cited risk factor targets through the repeated intensification of medications. Our model accounted for three factors likely to determine the success of treating to targets—the efficacy of stepped combination therapy in lowering the risk factor, and heterogeneity in treatment response and treatment tolerance. Each of these factors is rarely reported in the results of treat-to-target trials, but all are key to generalizing their findings to larger populations. In our review of the literature, we found that almost all treatments had a diminishing relative efficacy when combined with other medications. For an untreated subject at baseline, ezetimibe combination therapy had only 8 percent of the absolute reduction of simvastatin 20 mg, adding a glitazone as a third oral medication was 52 percent as effective as metformin monotherapy, and adding a fourth antihypertensive medication provided only 51 percent of the systolic efficacy of the first medication. The diminishing absolute reductions of treatment intensification result from the dual effects of the second, third, and fourth medications having a progressively inferior relative impact on the patient's risk factor as well as a patient's diminishing pretreatment levels of each risk factor. We also found that variation in patients' responses to treatment and poor tolerance of treatment, even assuming levels of discontinuation and adherence in randomized controlled trials, caused a large fraction of subjects to fail to reach tight control targets. Finally, a treat-to-target strategy required lifetime treatment with substantial polypharmacy just for treating these three conditions.
Target attainment rates were low despite a number of assumptions we made that were favorable to larger risk factor reductions. First, we most likely overstated the benefits of combination therapy involving three or more A1c-lowering treatments by assuming a third drug added to a two-drug regimen had the same efficacy as when added to monotherapy. The paucity of evidence in the literature on these effect sizes and the lack of any data on combinations of three or more antihypertensive drugs is concerning given the widespread recommendation to treat-to-target without consideration of the amount of risk factor reduction achieved with progressively greater polypharmacy. Second, we assumed that any medication not reported as being currently taken by a subject in NHANES had never been taken and was therefore likely to have the level of efficacy and tolerance observed in clinical trials. Some subjects, however, might have used medications in the past but discontinued them due to a poor response or poor tolerance. Third, we assumed that subjects who were taking medications at baseline did not discontinue their use over the intensification period. Fourth, we did not account for lower adherence and higher discontinuation rates that might be expected for subjects with higher levels of polypharmacy. Finally, though our base case results assumed adherence levels of patients enrolled in trials, we did not account for case management strategies that are commonly used in clinical practice today. If these interventions improve adherence rates beyond those typically found in trials, we might have underestimated levels of goal attainment and overstated our polypharmacy results.
In the absence of certain parameter estimates or conventions, we were forced to make several simplifications that might affect our model's validity. First, there is no one standard treatment protocol for patients having elevated blood pressure, A1c, and LDL levels, and using different treatments or a greater number of therapies beyond what we considered could impact our results. We might have included additional classes of antihyperglycemics, such as GLP-1 analogs (e.g., Bayetta), or antihypertensives, such as minoxidil, particularly for subjects who could not tolerate standard treatments, but we restricted our focus to treatments considered to be part of standard care in most practice settings. We excluded fibrate therapy because these agents generally are prescribed to lower triglycerides and increase high-density lipoprotein (HDL), and they have limited efficacy in lowering LDL (Rubins et al. 1999) or reducing cardiovascular disease mortality (Saha et al. 2007).
Second, rather than assuming that patients' therapies are always intensified, we might have incorporated treatment substitutions, particularly for those subjects having a poor clinical response to a new therapy. Doing so would have resulted in lower levels of polypharmacy but not lower levels of risk factor control if the set of available treatments remained fixed. Modeling the substitution of treatments is complicated, however, and in practice, there could be wide variation between physicians in the rates with which drugs are switched, added, and intensified.
Third, lacking individual-level data to more fully understand the shape of treatment efficacy distributions, we used a standard assumption of normality. And because we did not have variance estimates on the relative scale, we used an ecological estimate of variance that likely caused us to underestimate the true level of variability. Nevertheless, we showed that some patients with moderate control required a disproportionate amount of treatment due to individual variability in response to treatment.
The absolute level of tight control attained by the NHANES-III cohort might differ from levels based on more recent waves of the survey. Without detailed information on patients' treatment histories or their current doses, however, estimating the expected level of attainment in the current U.S. diabetic population would require considerable extrapolation. Furthermore, because of changes in the composition of the diabetic population over time the implications for the level of goal attainment are unclear. While our results apply to a specific population at a specific point in time, the factors that contribute to the attainment of targets—diminishing effectiveness of combination therapy and variation in treatment response and tolerance—are generalizable across time.
These results have important implications for both patient care and quality measurement. First, patients will require significant levels of polypharmacy to reach tight control targets. Since nearly half of the NHANES-III cohort required five or more medications, the side effect rate and overall treatment burden for patients are likely to be high, though our analysis did not explicitly measure these effects. There is little data on the relationship between polypharmacy and a patient's side effect burden for combinations of three or more drugs, let alone five or more. Although tight control targets are critical elements of practice guidelines and can help guide clinical practice, our results suggest that the risks of treating to targets should be carefully weighed with the benefits at the individual level. For patients who are already on multiple medications and remain slightly above risk factor targets—arguably the most common scenario—the net benefit of intensifying treatment is unclear. It also remains unclear whether patients in typical clinic settings will be willing to tolerate the out-of-pocket costs, time costs, risks and burdens of polypharmacy, and indeed whether our health system can support the personnel and treatment costs required for monitoring risk factor levels, titrating medications, and managing side effects. Combination medications would lower the polypharmacy burden, but not the incidence of side effects and other safety risks such as drug–drug interactions.
Second, these results highlight potential limitations to the use of intermediate outcomes as quality measures. We have shown that the attainment of targets depends in large part on three factors that are unrelated to the quality of care, and that the attainment of targets in nonexperimental settings will be much more difficult despite the use of a broad range of highly potent treatments. Subjects receiving multiple treatments at baseline had a much lower likelihood of reaching targets despite having sizable absolute reductions in risk factors. Under such circumstances, current quality measures could create incentives to avoid these patients and cherry pick patients that are more likely to reach targets. Risk adjustment models developed to allow “fair” comparisons of performance have so far proven inadequate (Zhang et al. 2000; Thompson et al. 2005; Kaplan et al. 2009;). Although few have looked at the unintended consequences of publicly reporting intermediate outcomes, evidence from other clinical areas raises concerns about the potential for risk selection to avoid patients likely to have poor outcomes (Werner, Asch, and Polsky 2005).
Alternative quality measures such as “linked action measures” (Kerr et al. 2001, 2003) have been proposed to compensate for the limitations of intermediate outcome measures of quality. These measures define quality standards as either the attainment of a risk factor target or the timely intensification of therapy for an uncontrolled risk factor, while also accounting for a limited set of exceptions, such as the use of high-dose medications at baseline and allowing follow-up measurements to account for measurement error. Others have argued that quality measures should require more explicit considerations of the risks and benefits of the treatments required (Krumholz and Lee 2008).
We limited our intensification regimen to common treatments—statins, four classes of A1c-lowering agents, and four classes of antihypertensives—but these treatments were ineffective in helping a large share of the population attain targets. A particular concern in light of recent safety problems (Nissen and Wolski 2007) and efficacy questions (Kastelein et al. 2008) is that providers might be encouraged to rely more heavily on the expanding list of newer medications—beyond those considered in this analysis—for patients who remain refractory to standard treatments. New drugs generally have poorly established safety profiles (Food and Drug Administration 2000; Lasser et al. 2002;), lack evidence of benefit for the most clinically important outcomes (Fleming and DeMets 1996), and are much more costly. While these treatments may have some role in the care of patients who are at highest risk of adverse outcomes, the pursuit of tight risk factor control targets will require that they be used at much higher rates, and the relative benefits and risks of doing so should first be clearly defined.
CONCLUSION
The diminishing efficacy of polypharmacy and heterogeneity in patients' clinical response to and tolerance of common treatments to control diabetes risk factors implies that the average patient will require multiple classes of medications, and a significant fraction will fail to achieve tight control with standard treatments even under ideal efficacy conditions. In practice, a far greater share of the population will remain above targets in spite of intensive treatment, increasing the likelihood that patients will be exposed to the burdens and risks of polypharmacy. Performance measures that define standards by the attainment of tight risk factor control ignore potential treatment efficacy constraints, biological variability, and the escalating side effects and burdens associated with polypharmacy.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This work was supported in part by the VA Health Services Research & Development Service's (IIR 06#253) and by the Measurement Core of the Michigan Diabetes Research & Training Center (NIDDK of The National Institutes of Health [P60 DK#20572]). An earlier version of this paper was presented at the Veterans' Affairs Health Services Research & Develop annual meeting in Baltimore, MD on February13, 2009.
Disclaimers: The funders played no direct role in the design, analysis, or reporting of these findings.
Disclosures: Dr. Vijan discloses that he is a member of a scientific advisory board for Sanofi-Aventis to assist the company in developing tools for comparative effectiveness research. Sanofi-Aventis played no role in the design, analysis, interpretation, or reporting of these findings, and no specific products manufactured by the company were mentioned in the paper. There are no other disclosures.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Table SA1. Baseline Characteristics of the NHANES-III Diabetic Population.
Table SA2. Model Parameters, LDL.
Table SA3. Model Parameters, A1c.
Table SA4. Model Parameters, Blood Pressure.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Abraira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, Emanuele NV, Levin SR, Henderson W, Lee HS. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes (VA CSDM). Results of the Feasibility Trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995;18(8):1113–23. doi: 10.2337/diacare.18.8.1113. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of Medical Care in Diabetes–2008. Diabetes Care. 2008;31(suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- Fleming TR, DeMets DL. Surrogate End Points in Clinical Trials: Are We Being Misled? Annals of Internal Medicine. 1996;125(7):605–13. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. From the Food and Drug Administration. Journal of the American Medical Association. 2000;283(17):2228. [Google Scholar]
- Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr., Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr., Stone NJ. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110(2):227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of Intensive Blood-Pressure Lowering and Low-Dose Aspirin in Patients with Hypertension: Principal Results of the Hypertension Optimal Treatment (HOT) Randomised Trial. HOT Study Group. Lancet. 1998;351(9118):1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- Hayward RA, Hofer TP, Vijan S. Narrative Review: Lack of Evidence for Recommended Low-Density Lipoprotein Treatment Targets: A Solvable Problem. Annals of Internal Medicine. 2006;145(7):520–30. doi: 10.7326/0003-4819-145-7-200610030-00010. [DOI] [PubMed] [Google Scholar]
- Jones GC, Macklin JP, Alexander WD. Contraindications to the use of Metformin. British Medical Journal. 2003;326(7379):4–5. doi: 10.1136/bmj.326.7379.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SH, Griffith JL, Price LL, Pawlson LG, Greenfield S. Improving the Reliability of Physician Performance Assessment: Identifying the “Physician Effect” on Quality and Creating Composite Measures. Medical Care. 2009;47(4):378–87. doi: 10.1097/MLR.0b013e31818dce07. [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E. Simvastatin with or without Ezetimibe in Familial Hypercholesterolemia. New England Journal of Medicine. 2008;358(14):1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- Kerr EA, Krein SL, Vijan S, Hofer TP, Hayward RA. Avoiding Pitfalls in Chronic Disease Quality Measurement: A Case for the Next Generation of Technical Quality Measures. American Journal of Managed Care. 2001;7(11):1033–43. [PubMed] [Google Scholar]
- Kerr EA, Smith DM, Hogan MM, Hofer TP, Krein SL, Bermann M, Hayward RA. Building a Better Quality Measure: Are Some Patients with ‘Poor Quality’ Actually Getting Good Care? Medical Care. 2003;41(10):1173–82. doi: 10.1097/01.MLR.0000088453.57269.29. [DOI] [PubMed] [Google Scholar]
- Krumholz HM, Lee TH. Redefining Quality–Implications of Recent Clinical Trials. New England Journal of Medicine. 2008;358(24):2537–9. doi: 10.1056/NEJMp0803740. [DOI] [PubMed] [Google Scholar]
- Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of New Black Box Warnings and Withdrawals for Prescription Medications. Journal of the American Medical Association. 2002;287(17):2215–20. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- Law MR, Wald NJ, Morris JK, Jordan RE. Value of Low Dose Combination Treatment with Blood Pressure Lowering Drugs: Analysis of 354 Randomised Trials. British Medical Journal. 2003a;326(7404):1427–31. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MR, Wald NJ, Rudnicka AR. Quantifying Effect of Statins on Low Density Lipoprotein Cholesterol, Ischaemic Heart Disease, and Stroke: Systematic Review and Meta-Analysis. British Medical Journal. 2003b;326(7404):1423–27. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T. 2008. Analysis of Complex Survey Samples (release 3.10), Available at http://cran.r-project.org.
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J, Ramirez EA, Henderson WE for the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Single-Drug Therapy for Hypertension in Men. A Comparison of Six Antihypertensive Agents with Placebo. New England Journal of Medicine. 1993;328(13):914–21. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- McAlister FA, Campbell NR, Duong-Hua M, Chen Z, Tu K. Antihypertensive Medication Prescribing in 27,822 Elderly Canadians with Diabetes over the Past Decade. Diabetes Care. 2006;29(4):836–41. doi: 10.2337/diacare.29.04.06.dc05-1875. [DOI] [PubMed] [Google Scholar]
- Nallamothu BK, Hayward RA, Bates ER. Beyond the Randomized Clinical Trial: The Role of Effectiveness Studies in Evaluating Cardiovascular Therapies. Circulation. 2008;118(12):1294–303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. New England Journal of Medicine. 2007;356(24):2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. New England Journal of Medicine. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J. High-Dose Atorvastatin vs Usual-Dose Simvastatin for Secondary Prevention after Myocardial Infarction: The Ideal Study: A Randomized Controlled Trial. Journal of the American Medical Association. 2005;294(19):2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Manolio TA, Smith NL, Heckbert SR, Gottdiener JS, Burke GL, Weissfeld J, Enright P, Lumley T, Powe N, Furberg CD. Time Trends in High Blood Pressure Control and the Use of Antihypertensive Medications in Older Adults: The Cardiovascular Health Study. Archives of Internal Medicine. 2002;162(20):2325–32. doi: 10.1001/archinte.162.20.2325. [DOI] [PubMed] [Google Scholar]
- Ross SD, Akhras KS, Zhang S, Rozinsky M, Nalysnyk L. Discontinuation of Antihypertensive Drugs Due to Adverse Events: A Systematic Review and Meta-Analysis. Pharmacotherapy. 2001;21(8):940–53. doi: 10.1592/phco.21.11.940.34520. [DOI] [PubMed] [Google Scholar]
- Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the Secondary Prevention of Coronary Heart Disease in Men with Low Levels of High-Density Lipoprotein Cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. New England Journal of Medicine. 1999;341(6):410–8. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- Saha SA, Kizhakepunnur LG, Bahekar A, Arora RR. The Role of Fibrates in the Prevention of Cardiovascular Disease—a Pooled Meta-Analysis of Long-Term Randomized Placebo-Controlled Clinical Trials. American Heart Journal. 2007;154(5):943–53. doi: 10.1016/j.ahj.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Thompson W, Wang H, Xie M, Kolassa J, Rajan M, Tseng CL, Crystal S, Zhang Q, Vardi Y, Pogach L, Safford MM. Assessing Quality of Diabetes Care by Measuring Longitudinal Changes in Hemoglobin A1c in the Veterans Health Administration. Health Services Research. 2005;40(6, Part 1):1818–35. doi: 10.1111/j.1475-6773.2005.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKPDS Study Group. United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative Efficacy of Randomly Allocated Diet, Sulphonylurea, Insulin, or Metformin in Patients with Newly Diagnosed Non-Insulin Dependent Diabetes Followed for Three Years. British Medical Journal. 1995;310(6972):83–8. [PMC free article] [PubMed] [Google Scholar]
- UKPDS Study Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998a;352(9131):837–53. [PubMed] [Google Scholar]
- UKPDS Study Group. Tight Blood Pressure Control and Risk of Macrovascular and Microvascular Complications in Type 2 Diabetes: UKPDS 38. UK Prospective Diabetes Study Group. British Medical Journal. 1998b;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- Van Buuren S, Oudshoorn CGM. 2007. Multivariate Imputation by Chained Equations (release 1.16).
- Werner RM, Asch DA, Polsky D. Racial Profiling: The Unintended Consequences of Coronary Artery Bypass Graft Report Cards. Circulation. 2005;15(10):1257–63. doi: 10.1161/01.CIR.0000157729.59754.09. [DOI] [PubMed] [Google Scholar]
- Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, Heiss G, Lalouel JM, Turner ST, Hunt SC, Province MA, Rao DC. A Summary of the Effects of Antihypertensive Medications on Measured Blood Pressure. American Journal of Hypertension. 2005;18(7):935–42. doi: 10.1016/j.amjhyper.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Safford M, Ottenweller J, Hawley G, Repke D, Burgess JF, Jr., Dhar S, Cheng H, Naito H, Pogach LM. Performance Status of Health Care Facilities Changes with Risk Adjustment of HbA1c. Diabetes Care. 2000;23(7):919–27. doi: 10.2337/diacare.23.7.919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.