Abstract
It has been shown that mechanical stimulation affects the physical properties of multiple types of engineered tissues. However, the optimum regimen for applying cyclic radial stretch to engineered arteries is not well understood. To this end, the effect of mechanical stretch on the development of engineered blood vessels was analyzed in constructs grown from porcine vascular smooth muscle cells. Cyclic radial distension was applied during vessel culture at three rates: 0 beats per minute (bpm), 90 bpm, and 165 bpm. At the end of the 7-week culture period, harvested vessels were analyzed with respect to physical characteristics. Importantly, mechanical stretch at 165 bpm resulted in a significant increase in rupture strength in engineered constructs over nonstretched controls. Stress–strain data and maximal elastic moduli from vessels grown at the three stretch rates indicate enhanced physical properties with increasing pulse rate. In order to investigate the role of collagen cross-linking in the improved mechanical characteristics, collagen cross-link density was quantified by HPLC. Vessels grown with mechanical stretch had somewhat more collagen and higher burst pressures than nonpulsed control vessels. Pulsation did not increase collagen cross-link density. Thus, increased wall thickness and somewhat elevated collagen concentrations, but not collagen cross-link density, appeared to be responsible for increased burst strength.
Keywords: Mechanical stretch, Tissue engineering, Blood vessels, Rupture strength, Collagen cross-links
INTRODUCTION
Surgical approaches to remedy cardiovascular disease often employ vascular grafts. Currently, these replacement vessels may be derived from either autologous tissue or synthetic materials, or, in the future, they may be engineered from donor cells (19). However, in order for a graft to be clinically useful, it must be able to withstand the in vivo hemodynamic environment. The vessel walls should be of sufficient thickness and tensile strength to resist dilatation and prevent rupture (16). The medial layer of native blood vessel walls is comprised primarily of smooth muscle cells and the extracellular matrix (ECM). The smooth muscle cells deposit the collagen, elastin, and other ECM components (5). Vessel wall thickness, which distributes applied forces to the wall and modulates wall stress, results partly from the ability of the vascular cells to produce matrix proteins. Much of the rupture strength and maximal elastic modulus of blood vessels relates to the amount and quality of the collagen within the vessel wall (7).
Methods to improve the physical strength of vessels by affecting the engineered tissue matrix include biochemical additives, cell source optimization, and selection of cell donor age (25,30). Studies have shown mechanical stretch to be an important factor in extracellular protein synthesis and expression (6,20,26). Indeed, cyclic stretch has been shown to upregulate collagen and other extracellular matrix protein production in vascular smooth muscle (17,21,31). Application of mechanical stretch likely improves the physical properties of engineered blood vessels by enhancing collagen matrix expression, but the precise impact of pulse rate on matrix components is poorly understood (33).
The impact of cyclic mechanical stretch per se on posttranslational modification of collagen molecules is not well studied. Collagen matrix is strengthened by intrafibrillar cross-links between the collagen chains (4). Covalent collagen cross-links serve to bolster the mechanical integrity of the entire vessel wall in response to physiological forces. The posttranslational modification of the collagen network occurs as a result of the actions of several enzymes, including copper-dependent lysyl oxidase (12). However, the specific impact of cyclic mechanical forces on cross-link formation in engineered tissues has not been studied in detail.
These experiments were designed to analyze the impact of mechanical stretch on the physical properties of engineered vessels. In particular, these studies attempted to modulate vessel strength by applying different rates, in terms of cycles per minute, of cyclic radial distension during in vitro culture. Vessel characteristics were analyzed in terms of burst strength, stress–strain characteristics, collagen production, and cross-link densities. These results will provide guidance for the future production of improved tissue engineered arteries. Mechanical stimulation may represent a means to impact the engineering of vascular and other connective tissues, by providing a means of impacting collagen synthesis and mechanics.
MATERIALS AND METHODS
Engineered Vessel Culture
Vascular smooth muscle cell (VSMC) populations were isolated from the carotid arteries of Yucatan miniature swine (Sinclair Research Farms) using nonenzymatic methods (28). Culture medium for VSMC contained DMEM, 10% fetal bovine serum (FBS), 10% porcine serum (PS), 2.6 mg/ml HEPES, 50 μg/ml ascorbic acid, 100 U/ml penicillin G, 50 μg/ml proline, 20 μg/ml alanine, 50 μg/ml glycine, 3 ng/ml CuSO4, 10 ng/ml basic fibroblastic growth factor (bFGF), and 10 ng/ml platelet-derived growth factor (PDGF-bb), as previously described (25).
Polymeric scaffolds consisting of 1 N NaOH-treated polyglycolic acid (PGA, Albany International, Mansfield, MA) were used for vessel construction (13,15). Using a degradable Dexon suture (US Surgical), the mesh was sewn around silicone tubing (Norton Performance Plastics) into a tube that was 7 cm long, 3 mm in diameter, and 1 mm thick, as previously described (24). Silicone tubing with mesh was placed into a glass bioreactor and sterilized by immersion in 70% ethanol followed by rinses in tissue culture grade water.
A suspension of 3 × 106 porcine VSMC at passage 3 was used to seed the polymeric tube. The cells were allowed to adhere for 15 min and then the bioreactor was filled with VSMC culture medium. Bioreactors were maintained in a humidified incubator in 10% CO2 and at 37°C for approximately 7 weeks. During vessel growth, culture medium was changed once a week and 50 μg/ml ascorbic acid was added to culture every 2 days.
Cyclic Mechanical Stretching
Cyclic radial distension was applied to the engineered vessels during culture (29). The silicone tube in the lumen of the PGA scaffold was connected to a continuous flow loop. A pulsatile pump (Cole Parmer) forced a solution of phosphate buffered saline (PBS; Gibco) through the silicone tubing. The movement of PBS distended the silicone tubing 1.44 ± 0.46% in a radial direction. Two different pulse rates, 90 beats per minute (bpm) or 165 bpm, were chosen to simulate an adult or an infant pulse rate, respectively (3,32). As a control, we also studied a third, nonstretched (0 bpm) condition.
Mechanical Testing
At the end of culture, harvested vessel segments were inserted into a flow-loop for measuring mechanics, and a solution of PBS was injected through the lumen of the vessel segment (23). Intraluminal pressure was applied in 30-mmHg increments and maintained for 5 s at each increment. A Canon XL1 digital video camcorder with a 180 mm/3.5 F-stop lens recorded the corresponding change in vessel diameter at each pressure (29).
Vessels were inflated in this manner until a final “burst” pressure was recorded. The vessel wall thickness, diameter change, and corresponding pressures at each point were used to determine the values for vessel wall stress (σ) and midwall strain (∈) (1,2,25). The maximal elastic modulus (E) was calculated as the slope of the last five points of the stress–strain curves.
Histology
Tissue samples were fixed in 10% (v/v) neutral-buffered formalin for 1 h, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E) and Masson's trichrome stain, which stains collagen blue and cell bodies red.
Collagen Density
The collagen content of engineered vessels was determined by measuring the levels of hydroxyproline as described previously (38). Briefly, vessel segments that were digested in papain buffer were further digested in 6 N HCl at 110°C overnight. Samples were neutralized, incubated with chloramine T (Mallinckrodt Baker, Phillipsburg, NJ) followed by an incubation in p-dimethyl-aminobenzaldehyde (Mallinckrodt Baker) for 20 min at 60°C. Hydroxyproline content was measured at a wavelength of 530 nm. Collagen was calculated as 10 times the amount of hydroxyproline. For cross-link density, moles of collagen were calculated per dry tissue weight using 300,000 g/mol as the molecular weight of collagen.
Cross-Link Determination
The covalent collagen cross-link hydroxylysylpyridinoline (HP) was quantified in segments of known dry tissue weight using high performance liquid chromatography (HPLC) (12). Frozen vessel segments were lyophilized for 12 h, weighed, and then digested in 6 N HCl (Sigma) at 115°C for 24 h. Prepared samples were solubilized in 1% (v/v) n-heptafluorobutyric acid (HFBA) (Pierce) and loaded onto an analytical octadecylsilane (ODS) column (Beckman Coulter). Linear gradients of HFBA and acetonitrile eluted the samples and the fluorescence was measured. Cross-link densities were reported as mole HP per mole collagen.
Statistics
Data are presented as the average ± SEM. Comparisons between vessels were performed using a one-way ANOVA with a Bonferroni post hoc comparison. In all cases, significance levels were set to be less than 0.05.
RESULTS
Histology of Engineered Vessels
Histologic examination of engineered vessels was performed using H&E and Masson's trichrome staining (in which collagen stains blue) (Fig. 1). Dense tissue formation was evident at all pulse rates: 0, 90, and 165 bpm. However, vessel wall thickness was greater in vessels grown under pulsatile conditions, compared to nonpulsed controls. Masson's trichrome staining, which highlights collagen, revealed that collagen density in engineered tissues appeared to be similar in the three experimental groups. Hence, although wall thickness was increased, collagen content in tissues did not appear to be grossly different between groups by histology.
Figure 1.
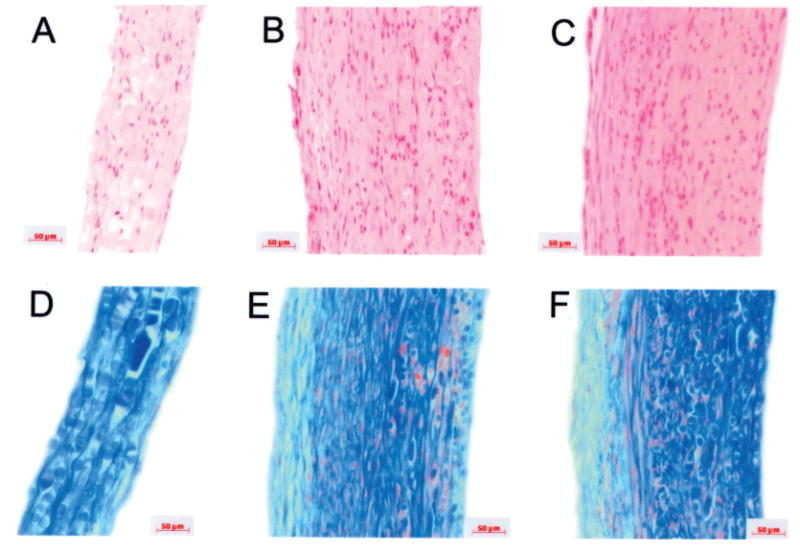
Histologies of engineered vessels. Vessels were grown at three radial distension rates: 0 beats per minute (bpm; nonstretched control), 90 bpm, and 165 bpm. (A, D) Nonstretched control vessels; (B, E) vessels cultured at 90 bpm; (C, F) vessels cultured at 165 bpm. (A–C) H&E stains; (D–F) Masson's trichrome, where collagen stains blue, cell bodies stain red, and glycosaminoglycans stain green. PGA polymer fragments more noticeable as oval or rectangular objects (D–F), staining blue. Wall thickness is increased in pulsed vessels. Scale bars: 50 μm.
Rupture Strength
Harvested engineered vessels were inflated until they reached a final, rupture pressure. The effects of mechanical stretch on vessel burst pressure are shown in Figure 2. Nonstretched controls ruptured at average pressures much lower than values reported for saphenous vein (348 ± 78 mmHg for controls vs. 1680 ± 307 mmHg for saphenous vein) (22). Pulsed vessels, by comparison, had higher average rupture strengths (458 ± 69 mmHg for 90 bpm and 670 ± 127 mmHg for 165 bpm). Burst pressures of vessels cultured at 165 bpm were significantly greater than those of nonpulsed controls (p < 0.05). Although mechanical pulsation produced engineered vessels with burst strengths less than those of native human vein, the mechanical characteristics over nonstretched controls were improved.
Figure 2.
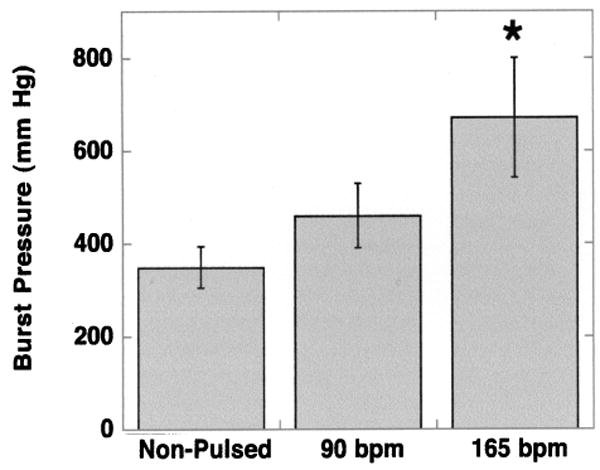
Burst pressure measurements of engineered porcine vessels. Rupture strengths were measured from engineered vessels harvested at the end of culture. Vessels were grown at three radial distension rates: 0 beats per minute (bpm; nonpulsed control; n = 6), 90 bpm (n = 7), and 165 bpm (n = 8). Error bars represent SEM. *Statistically significant differences in comparing average burst pressures of engineered vessels grown at 165 bpm compared to nonstretched controls (p < 0.05).
Stress–Strain Characteristics
Engineered vessel wall stress and strain characteristics were ascertained based upon changes in diameter in response to intraluminal pressure, assuming vessel wall incompressibility. Representative stress–strain curves of vessels grown under different mechanical conditions (nonstretched, 90 bpm, 165 bpm) are shown in Figure 3. Vessels grown with mechanical stretch showed similar stress–strain curves, whereas the control vessels were less stiff, as demonstrated by the lower slopes of the stress–strain curve (Fig. 3). Interestingly, stress–strain curves for these vessels were fairly linear, and did not display the typical viscoelastic properties of native arteries. In native vessels, a low-modulus behavior regime at physiological pressures is produced by the presence of elastin and contractile smooth muscle cells. As previously reported, our engineered constructs do not contain appreciable amounts of insoluble elastin and the contractility of SMC in these vessels, while measurable, is less than native (8,9,25). Hence, stress–strain curves for these constructs reflect primarily the stress–strain behavior of extracellular collagen, which if stretched has a fairly linear stress–strain response.
Figure 3.
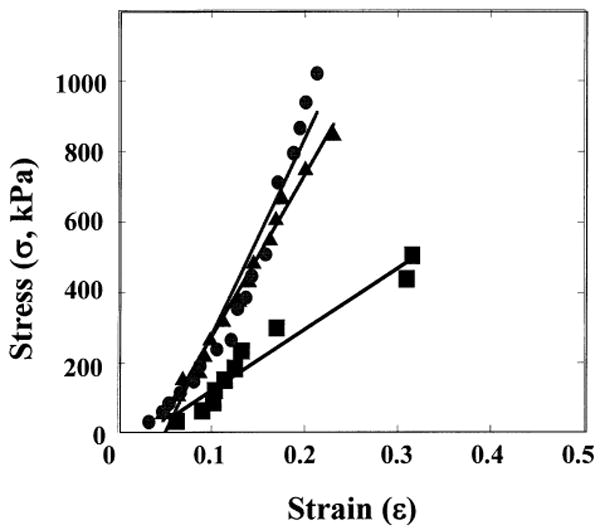
Representative stress–strain curves of engineered porcine vessels. Representative stress–strain curves for vessels grown with mechanical stimulation at 0 bpm (squares), 90 bpm (triangles), and 165 bpm (circles).
As a measure of vessel physical properties, the maximal elastic modulus was calculated in vessels grown with and without mechanical stretch (Fig. 4). Engineered constructs grown at pulse rates of 90 bpm (4.0 ± 0.8 MPa) and 165 bpm (5.6 ± 1.1 MPa) exhibited improved maximal elastic moduli. Although the average maximum modulus for the nonstretched controls (3.4 ± 0.8 kMPa) was less than the values for the stretched vessels, this difference was not significant. These modulus values are comparable to moduli of fibrin-based engineered vessels that were cultured with distensions ranging from 2.5% to 20% (33). All of these values compare favorably to those reported for physiologic properties of native saphenous veins, which are 1–2 MPa near 100 mmHg (14). However, it is important to note that these moduli for engineered vessels are maximal values, whereas values for saphenous vein are at arterial pressures but may be submaximal values.
Figure 4.
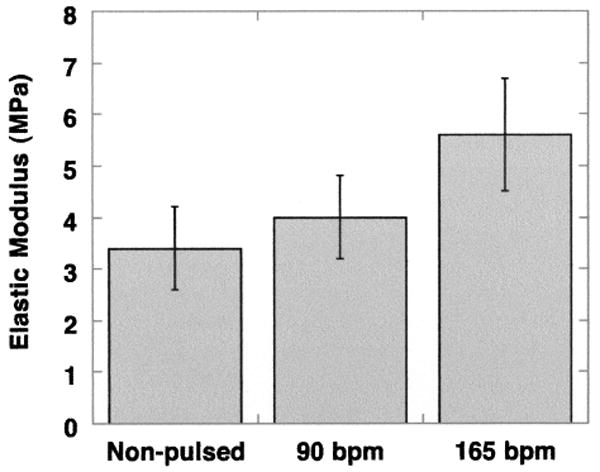
Maximal elastic modulus for engineered porcine vessels. Maximum moduli were measured from engineered vessels harvested at the end of culture. Vessels were grown at three radial distension rates: 0 beats per minute (bpm; nonpulsed control; n = 6), 90 bpm (n = 4), and 165 bpm (n = 8). Error bars represent SEM.
Collagen Contents and Cross-Links
Consistent with previous reports, application of pulsatile stretch to engineered blood vessels served to increase collagen content as a fraction of dry weight compared to nonstretched controls (Fig. 5A), although the difference did not reach significance in this particular study (25,29). Collagen cross-link densities were determined at the end of culture for vessels grown at 0, 90, and 165 bpm (Fig. 5B). Statistically, there was no difference in cross-link densities between these groups. Interestingly, weaker static controls contained no fewer cross-links than the pulsed vessels (0.61 ± 0.47 vs. 0.28 ± 0.13 vs. 0. 26 ± 0.06 mol HP/mol collagen for static, 90 bpm and 165 bpm, respectively). While there was no significant trend, it did not appear that increases in collagen cross-linking had any role in the increased strength of tissue engineered vessels grown with pulsatile stretch. Combined with previous studies that looked at the impact of copper ion supplementation on collagen crosslink formation in vitro (10), the results presented here imply that cross-linking is not a major contributor to engineered vessel mechanical properties.
Figure 5.
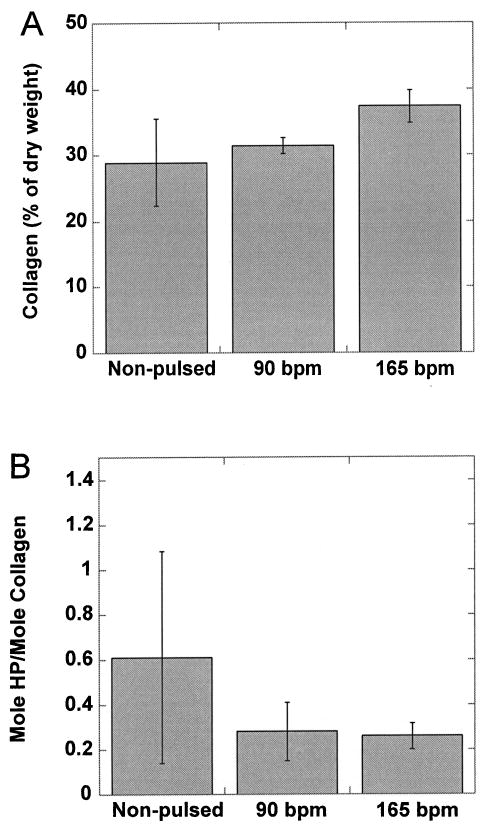
Collagen and cross-link densities measured in engineered porcine vessels. Collagen (A) and cross-link densities (B) were measured in engineered vessels at the end of culture using hydroxyproline assay and HPLC, respectively. Vessels were grown at three radial distension rates: 0 beats per minute (bpm; nonpulsed control; n = 4), 90 bpm (n = 6), and 165 bpm (n = 8). Error bars represent SEM. While the trend in collagen accumulation in response to pulsation mirrors observations regarding mechanics, the trend in cross-link formation does not. HP, hydroxylysylpyridinoline.
DISCUSSION
The mechanical ability of a vascular graft to resist dilatation and aneurysm formation is critically important to long-term clinical use (36). Although synthetic grafts possess the physical properties necessary for in vivo use, they also have significant negative attributes, including rapid intimal hyperplasia, propensity to thrombose, and low resistance to infection (34). Tissue engineered blood vessels present a unique opportunity to custom-build vascular grafts using autologous cells and tissues. However, in order to be a feasible alternative to native materials, the physical properties of tissue engineered conduits must be designed to meet the physical requirements of the in vivo hemodynamic environment.
The rupture strength is arguably the most important physical characteristic of a successful engineered vessel. The ability to withstand intraluminal pressures is attributable to the vessel wall composition. In particular, extracellular matrix deposition and collagen structural alignment correlate with improved physical properties. Our previous work, and results published by others, show that most of the collagen that is secreted by cultured smooth muscle in engineered vessels is type I, with a minority of type III (24). This distribution of collagen subtypes is slightly different from native arteries, wherein type III predominates over type I. We observed no differences in the distribution of subtypes of collagen in engineered vessels as a function of mechanical stretch.
With respect to degradation of collagen matrix and impact on mechanics, we have previously examined the impact of both donor age and pulsatile culture on MMP-1 and TIMP-1 protein levels (29,30). We have shown that, somewhat contrary to expectations, increased levels of MMP-1 activity correlate with increased vessel strength in this system, and that pulsatile culture increases levels of active MMP-1 and TIMP-1 (the endogenous inhibitor of MMP-1) measured in culture medium. Because MMP-1 is known to degrade fibrillar collagen, this result may seem somewhat counterintuitive. However, we postulate that collagen deposition in the engineered tissue is an iterative and ongoing process, and that a highly organized collagen matrix can only be achieved after multiple cycles of deposition and re-organization that occur gradually during culture.
Mechanical pulsation during culture created vessels that burst at higher pressures than nonpulsed control vessels. Engineered vessels grown with pulsation showed increased burst pressures, enhanced stress–strain behavior, and greater average maximum moduli. However, there were no significant differences observable between the two pulse rates examined: 90 bpm and 165 bpm (29). This implies that pulsation per se, as opposed to exact pulse rate, is important for increasing collagen production and vessel stiffness.
Recently, other investigators have examined the impact of cyclic strain on engineered vessel constructs. Syedain and colleagues (33) showed, in fibrin gels cultured with valvular interstitial cells at a pulse rate of 30 bpm, that cyclic distension of 10–15% improved modulus, while 15% strain increased collagen accumulation. There was also a uniform trend toward increasing cell density in pulsed cultures, though this trend did not often reach significance. One difference in outcomes between the work of Syedain and our study is the impact of amplitude: while Syedain did not observe significant effects from 2.5% or 5% stretch, we observed significant effects at 1.4% stretch. Differences that may explain this discrepancy are pulse rates (30 bpm as opposed to 90 or 165 bpm) and cell type (valvular interstitial cell as opposed to vascular smooth muscle).
We also investigated whether pulsed vessels and pulsation rate had an impact on levels of collagen crosslinks. Collagen fibers are strengthened and stabilized by intrafibrillar cross-links (4). However, we found no correlation between mechanical pulsation and collagen crosslink formation. Indeed, cross-links per mole of collagen were lower (nonsignificantly) in pulsed compared to nonstretched control vessels. Hence, improved burst strength in pulsed engineered vessels was not due to stiffening of collagen by cross-linking. But while cross-linking did not contribute to improved elastic modulus of pulsed vessels, it is likely that changes in collagen fiber orientation in response to stretch did influence modulus (11). Indeed, pulsatile culture conditions were recently shown to be associated with orientation of collagen fibers in a circumferential direction, which may improve load bearing and elastic modulus of pressurized engineered vessels.
Mechanical stretch of the ECM is sensed by transmembrane integrins, which relay information to the intracellular region (18). One of the affected downstream processes is activation of the MAP kinase pathway and induction of cellular proliferation (35). Previous studies have also established a positive correlation between cyclic stretch and vascular smooth muscle cell proliferation (27,37). Syedain and colleagues showed significant increases in ERK activation caused by pulsatile culture (33). Consistent with these observations, we noted substantial increases in wall thickness in pulsed vessels as compared to nonstretched controls. Pulsatile culture also stimulated increased collagen density as a function of dry tissue weight, which is consistent with our previous observations (25,29). While wall thickness does not impact modulus, thicker walls are able to distribute stress across a larger area, resulting in higher burst strengths. Increased collagen density within the wall also improves ultimate mechanical properties. In the future, careful study of VSMC behavior in the engineered model may reveal a mechanistic role for integrin-mediated signaling in enhanced cellular mitosis and collagen production under conditions of mechanical stretch.
These studies, combined with previous reports, lead to several overall conclusions. Consistent with previous findings, it appears that pulsatile stretch increases collagen accumulation, wall thickness, burst pressure, and elastic modulus of engineered blood vessels. Some of the increase in modulus may be due to preferential deposition of collagen fibers along a circumferential direction in pulsed tissues. However, it does not appear that changes in covalent cross-linking of collagen underlie any of the improvements in mechanics that have been observed in this and other studies. In short, it is wall thickness and total collagen deposition, as opposed to inherent collagen stiffness as modulated by cross-link formation, that is the basis for improved mechanics of pulsed engineered blood vessels derived from vascular smooth muscle cells.
The precise value of mechanical stiffness or burst strength that is required for long-term arterial implantation is not known. Anecdotal studies from our group have shown that vessels having rupture strengths of 600–700 mmHg are implantable in the arterial system without significant dilatation, while vessels rupturing at 300–400 mmHg dilate rapidly in porcine models (Nikla-son, unpublished observations). Hence, vessels cultured in these experiments at higher pulse rates may be functional in the arterial system in vivo, even though the burst strengths and moduli of engineered tissues are less than those of native vessels. Further in vivo testing of the impact of modulus on mechanical function in vivo is necessary to firmly resolve this question.
Acknowledgments
This work was supported by NIH grant R01HL-063766 and R01HL-083895 (both to L.E.N.).
References
- 1.Armentano RL, Levenson J, Barra JG, Cabrera Fischer EI, Breitbart GJ, Pichel RH, Simon A. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am J Physiol. 1991;260:H1870–1877. doi: 10.1152/ajpheart.1991.260.6.H1870. [DOI] [PubMed] [Google Scholar]
- 2.Barra JG, Armentano RL, Levenson J, Cabrera Fischer EI, Pichel RH, Simon A. Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res. 1993;73:1040–1050. doi: 10.1161/01.res.73.6.1040. [DOI] [PubMed] [Google Scholar]
- 3.Berne RM, Levy MN. Physiology. 1st. St. Louis, MO: C.V. Mosby Co.; 1983. [Google Scholar]
- 4.Byers PH. Inherited disorders of collagen gene structure and expression. Am J Med Genet. 1989;34:72–80. doi: 10.1002/ajmg.1320340114. [DOI] [PubMed] [Google Scholar]
- 5.Campbell GR, Campbell JH. The Development of the Vessel Wall: overview. In: Schwartz SM, Mecham RP, editors. The vascular smooth muscle cell: Molecular and biological responses to the extracellular matrix. San Diego, CA: Academic Press Inc.; 1995. pp. 1–10. [Google Scholar]
- 6.Cheng GC, Briggs WH, Gerson DS, Libby P, Grodzinsky AJ, Gray ML, Lee RT. Mechanical strain tightly controls fibroblast growth factor-2 release from cultured human vascular smooth muscle cells. Circ Res. 1997;80:28–36. doi: 10.1161/01.res.80.1.28. [DOI] [PubMed] [Google Scholar]
- 7.Cox RH. Regional variation of series elasticity in canine arterial smooth muscles. Am J Physiol. 1978;234:H542–551. doi: 10.1152/ajpheart.1978.234.5.H542. [DOI] [PubMed] [Google Scholar]
- 8.Dahl SLM, Chen Z, Solan AK, Brockbank KGM, Niklason LE, Song YC. Feasibility of vitrification as a storage method for tissue engineered blood vessels. Tissue Eng. 2006;12:291–300. doi: 10.1089/ten.2006.12.291. [DOI] [PubMed] [Google Scholar]
- 9.Dahl SLM, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348–355. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahl SLM, Rucker RB, Niklason LE. Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant. 2005;14:861–868. [PubMed] [Google Scholar]
- 11.Dahl SLM, Vaughn ME, Niklason LE. An ultra-structural analysis of collagen in tissue engineered arteries. Ann Biomed Eng. 2007;35:1749–1755. doi: 10.1007/s10439-007-9340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyre D. Collagen cross-linking amino acids. Methods Enzymol. 1987;144:115–139. doi: 10.1016/0076-6879(87)44176-1. [DOI] [PubMed] [Google Scholar]
- 13.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (NY) 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 14.Fung YC. Biomechanics: Mechanical properties of living tissue. 2nd. New York: Springer-Verlag; 1993. [Google Scholar]
- 15.Gao J, Niklason LE, Langer RS. Surface modification of polyglycolic acid meshes increases the seeding density and spreading of smooth muscle cells. J Biomed Mater Res. 1998;42:417–424. doi: 10.1002/(sici)1097-4636(19981205)42:3<417::aid-jbm11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton G, Megerman J, L'Italien GJ, Warnock DF, Schmitz-Rixen T, Brewster DC, Abbott WM. Prediction of aneurysm formation in vascular grafts of biologic origin. J Vasc Surg. 1988;7:400–408. [PubMed] [Google Scholar]
- 17.Heydarkhan-Hagvall S, Esguerra M, Helenius G, Soderberg R, Johansson BR, Risberg B. Production of extracellulalr matrix components in tissue-engineered blood vessels. Tissue Eng. 2006;12:831–842. doi: 10.1089/ten.2006.12.831. [DOI] [PubMed] [Google Scholar]
- 18.Ingber DE. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 19.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 20.Kolpakov V, Rekhter MD, Gordon D, Wang WH, Kulik TJ. Effect of mechanical forces on growth and matrix protein synthesis in the in vitro pulmonary artery. Circ Res. 1995;77:823–831. doi: 10.1161/01.res.77.4.823. [DOI] [PubMed] [Google Scholar]
- 21.Leung DYM, Glagov S, Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976;191:475–477. doi: 10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- 22.L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell SL, Niklason LE. Requirements for growing tissue engineered vascular grafts. Cardiovasc Pathol. 2003;12:59–64. doi: 10.1016/s1054-8807(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 24.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, Conroy N, Jones R, Vasanawala A, Sanzgiri S, Langer R. Morphologic and mechanical characteristics of bovine engineered arteries. J Vasc Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 25.Niklason LE, Gao J, Abbott WM, Hirschi K, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 26.Owens GK. Role of mechanical strain in regulation of differentiation of vascular smooth muscle cells. Circ Res. 1996;79:1054–1055. doi: 10.1161/01.res.79.5.1054. [DOI] [PubMed] [Google Scholar]
- 27.Predel H, von Segresser L, Buhler FR, Yang Z, Turina M, Luscher TF. Implications of pulsatile stretch ongrowth of saphenous vein and mammary artery smooth muscle. Lancet. 1992;340:878–879. doi: 10.1016/0140-6736(92)93287-w. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971;50:172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solan A, Mitchell S, Moses M, Niklason L. Effect of pulse rate on collagen deposition in the tissue-engineered blood vessel. Tissue Eng. 2003;9:579–586. doi: 10.1089/107632703768247287. [DOI] [PubMed] [Google Scholar]
- 30.Solan A, Niklason LE. Age effects on vascular smooth muscle: an engineered tissue approach. Cell Transplant. 2005;14:481–488. doi: 10.3727/000000005783982918. [DOI] [PubMed] [Google Scholar]
- 31.Sumpio BE, Banes AJ, Link WG, Johnson G. Enhanced collagen production by smooth muscle cells during repetitive mechanical stretching. Arch Surg. 1988;123:1233–1236. doi: 10.1001/archsurg.1988.01400340059010. [DOI] [PubMed] [Google Scholar]
- 32.Swindle MM, Moody DC, Phillips LD. Swine as models in biomedical research. 1st. Ames, IA: Iowa State University Press; 1992. [Google Scholar]
- 33.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA. 2008;105:6537–6542. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teebken OE, Haverich A. Tissue engineering of small diameter vascular grafts. Eur J Vasc Endovasc Surg. 2002;23:475–485. doi: 10.1053/ejvs.2002.1654. [DOI] [PubMed] [Google Scholar]
- 35.Vuori K. Integrin signaling: Tyrosine phosphorylation events in focal adhesions. J Membr Biol. 1998;165:191–199. doi: 10.1007/s002329900433. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 37.Wilson E, Mai Q, Krishnankutty S, Weiss RH, Ives HE. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 1993;123:741–747. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]


