Abstract
Objective
Juvenile idiopathic arthritis (JIA) is a chronic autoimmune arthropathy. β-2 adrenergic receptors are a link between the sympathetic nervous system and the immune system. Associations between variants in the gene encoding the β2-adrenergic receptor (ADRB2) and autoimmune disorders such as rheumatoid arthritis (RA) have been demonstrated. We aimed to investigate ADRB2 variants for association with JIA.
Methods
Genotypes and haplotypes of two ADRB2 variants (G16R and Q27E) were determined in 348 children with JIA and 448 healthy controls, by direct molecular haplotyping using melting-curve analysis of a fluorescently labeled loci-spanning probe. Case-control analysis was performed to investigate whether ADRB2 variants were associated with JIA.
Results
No association was found between JIA and alleles, genotypes or haplotypes of ADRB2. Specifically the haplotype that demonstrated a strong association with RA (R16/Q27) was not associated with JIA. None of the variants demonstrated association after stratification by JIA subtypes or gender.
Conclusions
Our results indicate that ADRB2 variants are not associated with JIA or any of the major JIA subtypes. These observations suggest that although they share several clinical and pathological features, JIA and RA have unique genetic associations.
Keywords: JIA, association, beta adrenergic receptor, genetic, polymorphism, molecular haplotyping
Introduction
Juvenile idiopathic arthritis (JIA), which represents a heterogeneous group of chronic arthropathies of children, is believed to be a complex genetic trait. Associations and linkage between polymorphisms in the human leukocyte antigen (HLA) genes and JIA have been confirmed in multiple cohorts. However, the genetic contribution by HLA-DR, the HLA locus that demonstrates strongest associations with JIA to date, only accounts for ∼17% of susceptibility to JIA.[1] This suggests that other non-HLA variants likely contribute to JIA susceptibility. The arthropathy observed in JIA is similar to that seen in rheumatoid arthritis (RA), and the synovial inflammation in JIA is indistinguishable from that seen in RA.[2] JIA and RA share some common genetic risk factors such as PTPN22.[3] This suggests that genetic variants contributing to risk of RA might also underlie susceptibility to JIA.
Autonomic dysfunction has been demonstrated in individuals with chronic rheumatic diseases.[4] Hormonal and neuronal mediated stress influence levels of inflammation in individuals with RA.[5] Similarly, children with JIA also demonstrate autonomic dysfunction.[6, 7] Beta-2 adrenergic receptors (β2-AR) are important links between the sympathetic nervous system and the immune system.[8] Administration of β2-adrenergic agonists suppresses collagen induced arthritis.[9] Several variants have been reported in ADRB2, the gene encoding β2-AR.[10] Two variants at nucleotide positions 46 (G46A: rs1042713) and 79 (C79G rs1042714) result in substitution of a glycine to arginine at amino acid position 16 (G16R) and a glutamine to a glutamic acid at position 27 (Q27E) respectively. These two variants demonstrate strong associations to RA [11, 12] and to other autoimmune diseases such as myasthenia gravis [13] and Graves disease.[14] To date, the roles of ADRB2 variants have been addressed in only a small JIA cohort who were used as controls in a study of asthma.[15] That study, which failed to find an association with JIA, was underpowered to detect modest associations. Our objective was to investigate a larger JIA cohort for association with ADRB2.
Material and Methods
Subjects
Cases were 348 children (232 females and 116 males) with JIA representing the major subtypes (Table 1) diagnosed according to the ILAR criteria.[16] The mean onset age was 6.4 years. Controls were 448 healthy adult volunteers screened for several common autoimmune diseases. Cases and controls were ascertained from Salt Lake City, UT and were predominantly (>90%) of Northern European ancestry. Subjects were enrolled under protocols approved by the Institutional Review Board at the University of Utah, and provided written informed consents.
Table 1.
Results of case-control analysis of ADRB2 G16R and Q27E variants and JIA
| Variant | Phenotype | N | Genotype1 | Allele1 | P | |||
|---|---|---|---|---|---|---|---|---|
| 1/1 | 1/2 | 2/2 | 1 | 2 | ||||
| G16R | Controls | 448 | 38 | 47 | 15 | 62 | 38 | |
| All JIA | 348 | 43 | 41 | 16 | 63 | 37 | 0.50 | |
| Persistent Oligo | 128 | 44 | 45 | 12 | 66 | 34 | 0.21 | |
| Extended Oligo | 27 | 59 | 19 | 22 | 69 | 31 | 0.32 | |
| Polyarticular | 77 | 44 | 40 | 16 | 64 | 36 | 0.54 | |
| Polyarticular RF+ | 31 | 29 | 52 | 19 | 55 | 45 | 0.28 | |
| Systemic | 30 | 43 | 43 | 13 | 65 | 35 | 0.61 | |
| ERA2 | 28 | 43 | 36 | 21 | 61 | 39 | 0.88 | |
| Other | 27 | 33 | 41 | 26 | 54 | 46 | 0.24 | |
| Q27E | Controls | 448 | 37 | 44 | 19 | 59 | 41 | |
| All JIA | 348 | 34 | 47 | 19 | 58 | 42 | 0.64 | |
| Persistent Oligo | 128 | 34 | 45 | 21 | 57 | 43 | 0.51 | |
| Extended Oligo | 27 | 30 | 40 | 30 | 50 | 50 | 0.20 | |
| Polyarticular | 77 | 30 | 52 | 18 | 56 | 44 | 0.47 | |
| Polyarticular RF+ | 31 | 45 | 39 | 16 | 65 | 35 | 0.39 | |
| Systemic | 30 | 27 | 53 | 20 | 53 | 47 | 0.39 | |
| ERA | 28 | 43 | 39 | 18 | 63 | 37 | 0.60 | |
| Other | 27 | 40 | 56 | 4 | 68 | 31 | 0.16 | |
For the G16R variant, allele 1 was G16 and allele 2 was R16. For the Q27E variant, allele 1 was Q27 and allele 2 was E27. Genotype frequencies have been rounded to the nearest number and hence do not add up to 100 in the case of persistent oligoarticular and systemic subtypes for the G16R variant.
ERA: Enthesitis related arthritis
Allele and genotype percents are shown.
ADRB2 genotyping and haplotyping
DNA was isolated from blood using the Puregene DNA purification kit from Qiagen (Valencia, CA). The two ADRB2 variants, G16R and Q27E, were amplified on a single amplicon in a 96-well plate in a LC480 instrument (Roche Diagnostics, Indianapolis, IN, USA) using previously published primers and conditions.[17] Genotyping at each position and direct molecular haplotyping was established using a method previously validated using a loci spanning probe that interrogates both SNPs simultaneously [17]. Melting curve analysis of the probe identifies the 3 main haplotypes: G16-E27 haplotype melts at 40°C, G16-Q27 haplotype melts at 55°C and R16-Q27 haplotype at 60°C (Figure 1). Genotypes were confirmed for 30 samples, for which the melting temperatures differed from average by +0.5°C
Fig 1. Example of direct molecular haplotyping of the R16G and Q27E polymorphisms in ADRB2.
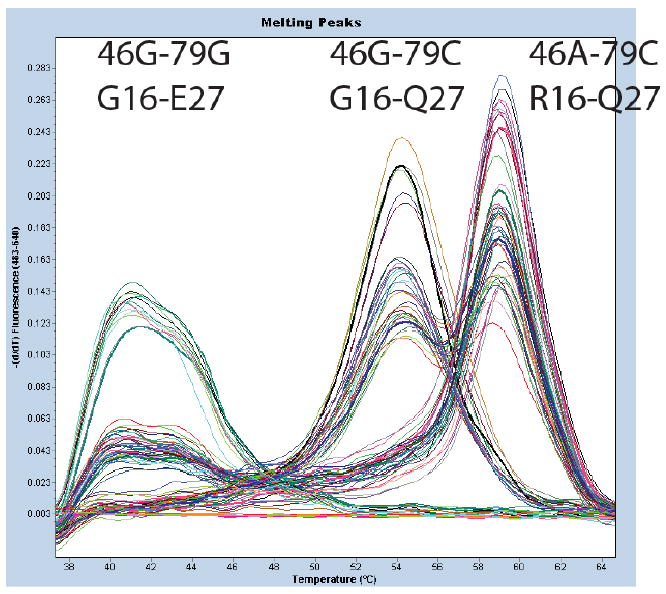
Haplotypes are determined by negative derivative melting curves (fluorescence with respect to temperature,-dF/dT) of a fluorescent probe that analyze both loci simultaneously. In samples with 1 melting peak, both chromosomes contain the same haplotype while in samples with 2 melting peaks the 2 chromosomes have different haplotypes. Multiple examples of each of the possible four combinations of haplotype in an individual are shown. The probe is most stable with the R16-Q27 haplotype (Tm ∼60°C), has one mismatch with the G16-Q27 haplotype (Tm ∼55°C), and has two mismatches with the G16-E27 haplotype (Tm∼40°C).
Statistical analysis
Case-control analyses were performed to investigate if alleles, genotypes or haplotypes of ADBR2 were associated with JIA. The JIA cohort was analyzed as a whole, as well as after stratification by JIA subtypes and by gender. A meta-analysis of the G16R variant was performed under a fixed-effects model, weighting the studies by sample size. Statistical significance was set at p <0.05. All analyses were carried out using SAS 9.1.3 (SAS Institute, Cary, NC, USA)
Results
The distribution of expected and observed frequencies of different genotypes at positions 16 and 27 were in Hardy-Weinberg equilibrium (HWE) among controls. There was a minor deviation from HWE at position 16 among cases (p <0.03), but at position 27 there was no departure from HWE. At position 16, the frequency of the minor allele (R16) was 38% among controls and 37% among cases. At position 27, the frequency of the minor allele (E27) was 41% among controls and 42% among the cases. These are comparable to minor allele frequencies of these two variants among individuals of European ancestry of 33% and 47%, respectively, in the HapMap project. There were no statistically significant differences between cases and controls with respect to allele frequencies at either position (Table 1). Similarly there were no statistically significant differences between cases and controls with regard to genotype frequencies. Specifically, individuals homozygous for the R16 variant were not at an increased risk of JIA. When analyses were repeated after stratifying cases by the different JIA subtypes, again there were no significant differences in the allele or genotype frequencies between cases and controls. We also found no statistically significant differences in allele or genotype frequencies between cases and controls stratified by gender.
The frequencies of the G16-E27, R16-Q27 and G16-Q27 haplotypes were 42%, 37% and 21% respectively among the controls, and 41%, 38% and 21% among the cases, respectively. We did not observe any individuals carrying the R16-E27 haplotype. Overall, the haplotype distributions were very similar between cases and controls. Neither JIA, nor any of the JIA subtypes, were associated with any of the ADBR2 haplotypes.
In a case-control study of children with bronchial asthma, JIA (n=86), and controls (n=270), Schubert et al. investigated two SNPs in the ADRB2 gene, including the G16R variant.[15] Cases and controls were of German descent. The Q27E variant was not typed in that study. The minor allele frequency at position 16 (R16) was 38% among controls and 43% among cases in that study. Including individuals from that study, our pooled analysis of 434 JIA cases and 718 controls found no association between JIA and the R16 allele. The pooled minor allele frequency was identical (38% in both cases and in controls).
Discussion
JIA is an inflammatory arthropathy in which T-cells with a pro-inflammatory cytokine profile play key roles in the perpetuation of immune responses. Alterations in the interactions between the autonomic nervous system and the immune system could be associated with autoimmune disorders. Autonomic dysfunction has been reported in several autoimmune disorders, including RA.[4, 5] Children with active JIA demonstrated higher resting heart rates than controls.[7] This increase was not seen when JIA was inactive, suggesting that JIA is also associated with autonomic dysfunction.
The interaction between the nervous system and the immune system is critical to maintain immune homeostasis.[8] The sympathetic nervous system influences the immune system through the release of norepinephrine. β2-ARs expressed on B-cells, naïve CD4 cells and Th1 cells mediate the interaction between the sympathetic nervous system and the immune system.[8, 18] In a study of β2-adrenergic mechanisms in experimental arthritis, Levine et al found that administration of specific β2-AR antagonists significantly retarded disease onset and reduced the severity of arthritis.[18] This suggests that β2-ARs contribute to joint injury in experimental arthritis. Activation of the β2-ARs results in the production of cAMP, which regulates cellular processes such as production of cytokines. Children with JIA demonstrate altered responses to catecholamines and have decreased cAMP levels compared to healthy controls.[6] Together, these observations suggest that β2-ARs could play important roles in inflammatory arthropathies, including JIA.
ADRB2 variants have been associated with RA in Swedish and German cohorts, [11, 12], as well as with myasthenia gravis [13] and Graves disease.[14] We undertook this study to investigate the same variants in JIA. Our results do not support a major role for these two functional ADRB2 variants in JIA or any of the JIA subtypes. A minor role for these cannot be excluded given that our cohort size was modest. Both RA studies suggest that the R16 variant is associated with increased RA risk, (OR:2-3 for the R allele).[11, 12] Myasthenia gravis also demonstrates an association with the R16 variant (OR:3.6, for the RR genotype).[13] Our JIA cohort is adequately powered to detect an association with an OR of ∼1.5 for both polymorphisms.
Lack of power can be a major limitation of case-control studies. Hence we performed a meta-analysis. The combined cohort of 1152 subjects has 80% power to detect an association with an OR of ∼1.4, but a meta-analysis of the G16R variant did not find an association with JIA. Although our cohort was ascertained in the United States and that of Schubert et al., was from Germany, we felt a meta-analysis was justified because the population of Utah is genetically similar to other Northern European-derived populations.[19] It should be noted that our RF-positive JIA cohort is small, and a larger cohort would be necessary to determine if there is an association with this or other JIA subsets. When we combined all polyarticular JIA subjects and repeated the analyses, we still did not find an association with either variant (data not shown). The minor departure from HWE at position 16 among cases is unlikely to be responsible for the observed lack of association.
JIA is a clinically and genetically heterogeneous disease.[3, 20] The lack of the association observed here might reflect the differences between the different subtypes of JIA. Thus, our cohort is underpowered to detect associations between the individual subtypes of JIA and these variants. While there are some examples of common genetic susceptibility factors between JIA and RA such as PTPN22 and TNFA, others, like CCR5, show conflicting results in different populations.[3, 21, 22]
Although the associations between ADRB2 variants and RA appear to be validated, having been seen in two different cohorts, larger studies are necessary to confirm this association. Recent meta-analyses have failed to confirm several positive associations reported in small initial cohorts of patients with RA. Our results suggest that although RA and JIA share several clinical and pathological features, as well as some common genetic risk factors, ADRB2 variants do not appear to play a major role in susceptibility to JIA.
Acknowledgments
Supported by, The ARUP Institute for Clinical and Experimental Pathology, The National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR50177), The National Center for Research Resources (RR00064), The Arthritis Foundation, and The Val A Browning Charitable Foundation, and The Primary Children's Medical Center Foundation, Salt Lake City, UT.
References
- 1.Prahalad S, Ryan MH, Shear ES, Thompson SD, Giannini EH, Glass DN. Juvenile rheumatoid arthritis: linkage to HLA demonstrated by allele sharing in affected sibpairs. Arthritis Rheum. 2000;43(10):2335–8. doi: 10.1002/1529-0131(200010)43:10<2335::AID-ANR22>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 2.Wynne-Roberts CR, Anderson CH, Turano AM, Baron M. Light- and electron-microscopic findings of juvenile rheumatoid arthritis synovium: comparison with normal juvenile synovium. Semin Arthritis Rheum. 1978 May;7(4):287–302. doi: 10.1016/0049-0172(78)90027-6. [DOI] [PubMed] [Google Scholar]
- 3.Prahalad S, Glass DN. A comprehensive review of the genetics of juvenile idiopathic arthritis. Pediatric rheumatology online journal. 2008 Jul 21;6(1):11. doi: 10.1186/1546-0096-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straub RH, Baerwald CG, Wahle M, Janig W. Autonomic dysfunction in rheumatic diseases. Rheum Dis Clin North Am. 2005 Feb;31(1):61–75. viii. doi: 10.1016/j.rdc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Cutolo M, Straub RH. Stress as a risk factor in the pathogenesis of rheumatoid arthritis. Neuroimmunomodulation. 2006;13(56):277–82. doi: 10.1159/000104855. [DOI] [PubMed] [Google Scholar]
- 6.Kavelaars A, de Jong-de Vos van Steenwijk T, Kuis W, Heijnen CJ. The reactivity of the cardiovascular system and immunomodulation by catecholamines in juvenile chronic arthritis. Ann N Y Acad Sci. 1998 May 1;840:698–704. doi: 10.1111/j.1749-6632.1998.tb09608.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuis W, de Jong-de Vos van Steenwijk C, Sinnema G, Kavelaars A, Prakken B, Helders PJ, et al. The autonomic nervous system and the immune system in juvenile rheumatoid arthritis. Brain Behav Immun. 1996 Dec;10(4):387–98. doi: 10.1006/brbi.1996.0034. [DOI] [PubMed] [Google Scholar]
- 8.Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. Journal of leukocyte biology. 2006 Jun;79(6):1093–104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 9.Malfait AM, Malik AS, Marinova-Mutafchieva L, Butler DM, Maini RN, Feldmann M. The beta2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J Immunol. 1999 May 15;162(10):6278–83. [PubMed] [Google Scholar]
- 10.Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, et al. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000 Sep 12;97(19):10483–8. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malysheva O, Pierer M, Wagner U, Wahle M, Wagner U, Baerwald C. Association between ss2-adrenergic receptor polymorphisms and rheumatoid arthritis in conjunction with HLA-DRB1 shared epitope. Ann Rheum Dis. 2008 Feb 11; doi: 10.1136/ard.2007.083782. [DOI] [PubMed] [Google Scholar]
- 12.Xu BY, Arlehag L, Rantapaa-Dahlquist SB, Lefvert AK. beta2 Adrenoceptor gene single nucleotide polymorphisms are associated with rheumatoid arthritis in northern Sweden. Ann Rheum Dis. 2005 May;64(5):773–6. doi: 10.1136/ard.2004.027532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu BY, Huang D, Pirskanen R, Lefvert AK. beta2-adrenergic receptor gene polymorphisms in myasthenia gravis (MG) Clin Exp Immunol. 2000 Jan;119(1):156–60. doi: 10.1046/j.1365-2249.2000.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jazdzewski K, Bednarczuk T, Stepnowska M, Liyanarachchi S, Suchecka-Rachon K, Limon J, et al. beta-2-adrenergic receptor gene polymorphism confers susceptibility to Graves disease. International journal of molecular medicine. 2007 Jan;19(1):181–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Schubert K, von Bonnsdorf H, Burke M, Ahlert I, Braun S, Berner R, et al. A comprehensive candidate gene study on bronchial asthma and juvenile idiopathic arthritis. Dis Markers. 2006;22(3):127–32. doi: 10.1155/2006/373620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004 Feb;31(2):390–2. [PubMed] [Google Scholar]
- 17.Pont-Kingdon G, Lyon E. Direct molecular haplotyping by melting curve analysis of hybridization probes: beta 2-adrenergic receptor haplotypes as an example. Nucleic Acids Res. 2005;33(10):e89. doi: 10.1093/nar/gni090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine JD, Coderre TJ, Helms C, Basbaum AI. Beta 2-adrenergic mechanisms in experimental arthritis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4553–6. doi: 10.1073/pnas.85.12.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLellan T, Jorde LB, Skolnick MH. Genetic distances between the Utah Mormons and related populations. Am J Hum Genet. 1984 Jul;36(4):836–57. [PMC free article] [PubMed] [Google Scholar]
- 20.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007 Mar 3;369(9563):767–78. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 21.Prahalad S, Bohnsack JF, Jorde LB, Whiting A, Clifford B, Dunn D, et al. Association of two functional polymorphisms in the CCR5 gene with juvenile rheumatoid arthritis. Genes Immun. 2006 Sep;7(6):468–75. doi: 10.1038/sj.gene.6364317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheibel I, Veit T, Neves AG, Souza L, Prezzi S, Machado S, et al. Differential CCR5Delta32 allelic frequencies in juvenile idiopathic arthritis subtypes: evidence for different regulatory roles of CCR5 in rheumatological diseases. Scand J Rheumatol. 2008 Jan-Feb;37(1):13–7. doi: 10.1080/03009740701631935. [DOI] [PubMed] [Google Scholar]


