Abstract
The tryptophan photooxidation product 6-formylindolo[3,2-b]carbazole (FICZ) has been proposed as a physiological ligand for the mammalian aryl hydrocarbon receptor (AHR), which it binds with high affinity, inducing expression of cytochrome P450 1A1 (CYP1A1). We investigated whether the response to FICZ is evolutionarily conserved in vertebrates by measuring FICZ binding to two zebrafish AHRs (AHR1B and AHR2) and its ability to induce zebrafish CYP1 genes (CYP1A, CYP1B1, CYP1C1, CYP1C2, and CYP1D1) in vivo. Exposure of zebrafish embryos (48 hours-post-fertilization; hpf) to 10 nM FICZ for 6 hours caused strong induction of CYP1A mRNA and a statistically significant but modest induction of CYP1B1 and CYP1C1. Neither CYP1C2 nor CYP1D1 expression was induced by FICZ under the conditions of dose, time or developmental stage examined here. CYP1A induction was significantly greater after 6 hours than after 12 hours of exposure to FICZ, suggesting a rapid degradation of inducer. The 6-hr EC50 values for induction of CYP1A and CYP1B1 by FICZ were 0.6 and 0.5 nM compared to 72-hr EC50 values of 2.3 and 2.7 nM for PCB126, indicating that in zebrafish embryos FICZ is a more potent inducer than PCB126. FICZ at 10 nM was able to completely displace binding of 2,3,7,8-tetrachloro-1,6[3H]-dibenzo-p-dioxin to in vitro-expressed zebrafish AHR2 and AHR1B. Inhibition of AHR2 translation in zebrafish embryos by an AHR2-specific morpholino antisense oligonucleotide decreased the induction of CYP1A and CYP1B1 by FICZ and by PCB126. Together, these results demonstrate that FICZ is a potent AHR agonist in zebrafish, inducing expression of multiple CYP1 genes largely through AHR2. Evolutionary conservation of the response to FICZ is consistent with a possible role as an endogenous signaling molecule acting through the AHR.
Introduction
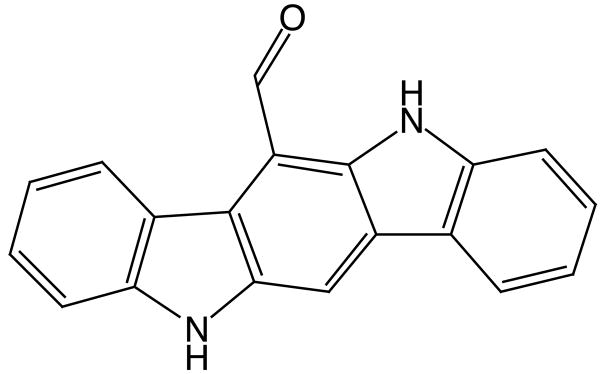
The aryl hydrocarbon receptor (AHR), in addition to its well known role mediating effects of various xenobiotics, has been suggested to participate in a variety of biological processes, including cell cycle control, growth, apoptosis, differentiation, and light-regulated biological rhythms [1–3]. Activation of the AHR transcription factor by diverse chemicals alters the expression of genes, most prominently cytochrome P450 1 (CYP1) family genes. AHR agonists include exogenous chemicals such as polynuclear aromatic hydrocarbons, planar halogenated aromatic hydrocarbons and a variety of natural products, as well a diversity of endogenous compounds [1, 4, 5]. There may be endogenous ligand(s) involved in regulation of physiological pathways, and perhaps environmental sensing, but such ligands have been elusive. 6-Formylindolo[3,2-b]carbazole (FICZ; Fig. 1), a tryptophan oxidation product formed by exposure to ultraviolet (UV) or visible irradiation, binds with high affinity to the AHR in mammalian cells [6], inducing expression of CYP1A1 [2]. FICZ has been proposed as a physiological ligand for the AHR, possibly involved in light response in animals [2, 7–9].
Fig 1.
Structure of 6-Formylindolo[3,2-b]carbazole
Studies of AHR activation by FICZ have been carried out primarily in mammals, and a consequent question is whether FICZ can activate AHRs in other vertebrate groups. We have begun to address the evolutionary conservation of the response to FICZ by assessing its ability to interact with AHRs and induce CYP1 gene expression in zebrafish, a teleost model. Zebrafish possess three AHR genes, designated AHR1A, AHR1B and AHR2. AHR1B and AHR2 proteins both are capable of high-affinity binding of TCDD and are transcriptionally active [10, 11], whereas AHR1A does not appear to be capable of binding (or becoming activated by) TCDD [11, 12]. Zebrafish also have five CYP1 genes: CYP1A, 1B1, 1C1 and 1C2 are induced by halogenated AHR agonists 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [13–15], while a fifth gene, CYP1D1, is not induced by TCDD or PCB [16]. Induction of the four inducible CYP1 genes by TCDD and PCB126 is regulated primarily by AHR2 [14, 15, 17].
The objective of the present studies was to determine whether FICZ is an agonist for AHRs and induces CYP1 genes in zebrafish. Thus, we determined whether FICZ binds avidly to zebrafish AHR2 and AHR1B in vitro, how the five CYP1 genes respond to FICZ in vivo in zebrafish embryos, and whether there are differences in response among these multiple CYP1s. AHR2 has been found to be most prominent in mediating CYP1 induction by halogenated AHR ligands in zebrafish [14]. Thus, to determine whether there might be a similar role of AHR2 in FICZ effects, we measured the ability of FICZ to induce CYP1 genes in embryos in which AHR2 expression had been suppressed using morpholino antisense oligonucleotides.
Materials and methods
Animals
Zebrafish of the Tup/Long fin (TL) type were used in the experiments. Fertilized eggs were obtained by breeding multiple groups of 30 female and 15 male fish as previously described [13]. The day after fertilization unfertilized eggs and dead embryos were removed. Generally, no mortality was observed subsequent to this. Embryos and larvae (up to 7 days post-fertilization; dpf) were held in 0.3× Danieau’s solution and juveniles in zebrafish system water at 28°C [13]. Procedures used in these experiments were approved by the Animal Care and Use Committee of the Woods Hole Oceanographic Institution.
Exposure to FICZ
The effect of FICZ on CYP1 gene expression was studied in developing zebrafish in a series of experiments that were designed to examine CYP1 inducibility, the dose-dependence of induction, and the developmental time course of inducibility. Depending on time after fertilization (i.e., age and size of the fish), glass petri dishes or small beakers were used for the exposure. FICZ (Biomol International, L.P., Plymouth Meeting, PA) was dissolved in DMSO and mixed into the 0.3× Danieau’s solution or system water. Exposure was performed at 28 °C during the light period of the 14 h light/10 h dark diurnal cycle. The experiments were terminated immediately after the exposure, at which time 3–6 replicates (each of pools of embryos, as specified below) were collected from each treatment group, frozen in liquid nitrogen, and stored at −80 °C until analyzed.
Experiment 1
This experiment was performed to determine if FICZ is able to induce CYP1 gene expression in zebrafish embryos. Groups of 200 embryos in their chorions were placed in two dishes containing 150 ml 0.3× Danieau’s solution. Starting 48 hours post-fertilization (hpf) the embryos were exposed for 6 hours to 10 nM FICZ and carrier (100 ppm DMSO) or carrier only. After exposure, six replicates of 33–34 embryos each were sampled from each dish (6-h DMSO and 6-h FICZ).
Experiment 2
In order to study whether the CYP1A gene inducibility increases by removal of chorion and to look at sustainability of induction, 48-hpf embryos were dechorionated and exposed to 10 nM FICZ including carrier (100 ppm DMSO) or carrier only for either 6 or 12 hours. Exposure was performed in glass petri dishes (10 cm diameter) containing 25 ml of 0.3× Danieau’s solution and 33–34 embryos. Four replicate pools of embryos were analyzed from each exposure group (i.e., 6-h DMSO, 6-h FICZ, 12-h DMSO, and 12-h FICZ).
Experiment 3
The concentration-response relationship for CYP1 gene expression and FICZ was examined in groups of 48-hpf embryos (100 per concentration) exposed to various concentrations of FICZ (0.1, 0.3, 1, 3, 10, or 30 nM) in carrier (150 ppm DMSO), or carrier only for 6 hours. Triplicate pools of 33–34 embryos were sampled for each concentration.
Experiment 4
Zebrafish at different developmental stages (6, 24, 48, 72, and 96 hpf, and 7, 14, and 28 dpf) were exposed for 6 hours to 10 nM FICZ in carrier (100 ppm DMSO) or carrier only. At the end of exposure the fish were examined for phenotypic changes, and then five replicates (1–30 individuals each, depending on age) were sampled per treatment group, for analysis of CYP1 expression.
AHR2 knock-down
To examine whether AHR2 is involved in CYP1 induction by FICZ, we treated zebrafish embryos with a morpholino antisense oligonucleotide (MO) targeting AHR2, as previously described [14, 17, 18]. MOs targeting the transcriptional start site of AHR2 (5′-TGTACCGATACCCGCCGACATGGTT-3′) and negative control morpholinos (5′-CCTCTTACCTCAGTTACAATTTATA-3′) were obtained from Gene Tools (Philomath, OR). The morpholinos were fluorescein-tagged to enable selection of successfully injected embryos for the experiments. Two to four-cell stage embryos were injected with morpholinos at approximately 3.3 ng per egg [14]. Embryos were screened at 3 hpf by fluorescence microscopy to verify incorporation of MO. Any damaged embryos or those not displaying homogenous fluorescence were removed. At 48 hpf, groups of embryos injected with the AHR2-MO, the control-MO, or non-injected embryos were placed in glass petri dishes containing 100 ml of 0.3× Danieau’s solution and exposed for 6 hours to either carrier (200 ppm of DMSO), 10 nM FICZ in carrier, or 30 nM PCB126 in carrier (i.e., 9 dishes total: control-MO DMSO, control-MO FICZ, control-MO PCB126, AHR2-MO DMSO, AHR2-MO FICZ, AHR2-MO PCB126, no-MO DMSO, no-MO FICZ, and no-MO PCB126). After exposure, 4 replicates of 20 to 25 embryos were sampled from each dish. The samples were frozen in liquid nitrogen and then stored at −80°C until analyzed.
In vitro protein synthesis and ligand-binding assay
The expression construct for zebrafish AHR1B was prepared as described previously [11]. The AHR2 expression construct pBKCMV-zfAHR2 was generously provided by Dr. R. Tanguay (Oregon State University, Corvallis, OR) and Dr. Richard E. Peterson (University of Wisconsin, Madison, WI). The in vitro expression and ligand-binding assays were performed as described previously [11, 19]. Briefly, the TNT-Quick Coupled Reticulocyte Lysate System (Promega, Madison, WI) was used to synthesize AHR proteins. In vitro-synthesized AHRs were incubated with [3H]TCDD (2 nM nominal; 1.75–2.02 nM measured) in the presence or absence of FICZ (1 or 10 nM), overnight at 4°C. The amount of [3H]TCDD specific binding was measured by velocity sedimentation on sucrose gradients in a vertical tube rotor [20]. Nonspecific binding was determined by incubating [3H]TCDD with TNT reactions containing an empty vector (unprogrammed lysate (UPL)).
Real-time, quantitative RT-PCR
RNA was isolated using RNA STAT-60™ (Tel-Test Inc. Friendswood, TX, USA) and the isolates were DNase treated by the TURBO DNA-free™ kit (Ambion). The RNA quantity and quality were determined spectrophotometrically (NanoDrop ND-1000; NanoDrop Technologies, Wilmington, DE, USA). cDNA was synthesized using the Omniscript™ Reverse Transcriptase kit (Qiagen Inc., Valencia, CA, USA), random hexamer primers (Operon Biotechnologies Inc.) and the RNasin® RNase inhibitor (Promega).
Gene specific real time PCR primers for zebrafish CYP1A, CYP1B1, CYP1C1, CYP1C2, CYP1D1, and ARNT2 cDNAs were synthesized by Operon Biotechnologies Inc (Table 1). (The ARNT2 primers were designed to amplify a sequence common to ARNT2a, b, and c.) Real time PCR was performed using the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) as previously described [13, 14]. To ensure that a single product was amplified, melt curve analysis was performed on the PCR products at the end of each PCR run.
Table 1.
Real time PCR primer sequences
| Primer | Sequences |
|---|---|
| ZFCYP1A Fa | GCATTACGATACGTTCGATAAGGAC |
| ZFCYP1A Ra | GCTCCGAATAGGTCATTGACGAT |
| ZFCYP1B1 Fb | CTGCATTGATTTCCGAGACGTG |
| ZFCYP1B1 Rb | CACACTCCGTGTTGACAGC |
| ZFCYP1C1 F | AGTGGCACAGTCTACTTTGAGAG |
| ZFCYP1C1 R | TCGTCCATCAGCACTCAG |
| ZFCYP1C2 Fb | GTGGTGGAGCACAGACTAAG |
| ZFCYP1C2 Rb | TTCAGTATGAGCCTCAGTCAAAC |
| ARNT2 Fb | CACCTTTGGATCACATCTCATTG |
| ARNT2 Rb | TCACCCTCCTTAGACGGACC |
| CYP1D1 Fc | TCAACTTCGACACGAACTGTATC |
| CYP1D1 Rc | TGTGAACGATCTGGGAGTTG |
Relative mRNA expression of the CYP1 genes was calculated for each reaction according to the method of Livak and Schmittingen, EΔΔCt[21] using ARNT2 as the reference gene [14]. For each target gene EΔCt values of the samples were divided by the mean EΔCt value of the controls (EΔCt[sample]/mean EΔCt[control]). PCR efficiencies (E) for within-experiment amplicon groups were determined by the new LinRegPCR program [22]. Outliers were excluded based on the Grubbs test [23]. The statistical analyses were performed using Prism 4 by GraphPad Software Inc. (San Diego, CA, USA). Data were log-transformed when the variation differed between groups. In the figures data are shown as mean + standard deviation of the mean (SD).
Results
CYP1 gene expression in 48-hpf zebrafish embryos after exposure to FICZ
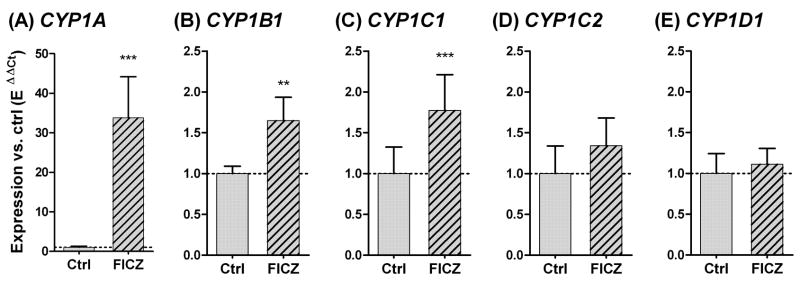
Initially we determined whether FICZ added in the water could pass the chorion and induce CYP1 gene expression in intact zebrafish embryos. Embryos that were exposed to 10 nM FICZ for 6 hours when exposure started at 48 hpf responded with a 34-fold increase in CYP1A mRNA expression compared to the DMSO control (Fig. 2A). The same exposure produced significant induction of CYP1B1 and CYP1C1, although the induction was weak for both, only1.6-fold and 1.8-fold, respectively (Fig. 2B and 2C). There was no statistically significant change in expression of CYP1C2 (1.3-fold; p = 0.09 with t test) (Fig. 2D) or CYP1D1 (1.1-fold; p = 0.38 with t test) (Fig 2E).
Fig 2.
Relative CYP1A, 1B1, 1C1, 1C2, and 1D1 gene expression (A-E) in 48-hpf zebrafish embryos after exposure to 10 nM 6-formylindolo[3,2-b]carbazole (FICZ; hatched bars) or 100 ppm DMSO (Ctrl; filled bars) for 6 hours. Relative expression was calculated by EΔCt[sample]/mean EΔCt[control]. A significant statistical difference compared with the control (n=6) was determined with student’s t test and is shown by stars (*** = p<0.001 and ** = p<0.01).
To determine whether the presence of the chorion affected the response to FICZ, and to assess the persistence of the CYP1A induction, 48-hpf embryos were dechorionated and exposed for 6 or 12 hours to 10 nM FICZ and then analyzed. CYP1A was significantly induced after both 6 and 12 hours of exposure to FICZ (p < 0.001), but the level of CYP1A induction was considerably less after 12 hours than after 6 hours (6-fold versus 26-fold; p < 0.01; Fig. 3). The absence of the chorion had no effect on the degree of induction (Fig. 3 versus Fig. 2).
Fig 3.
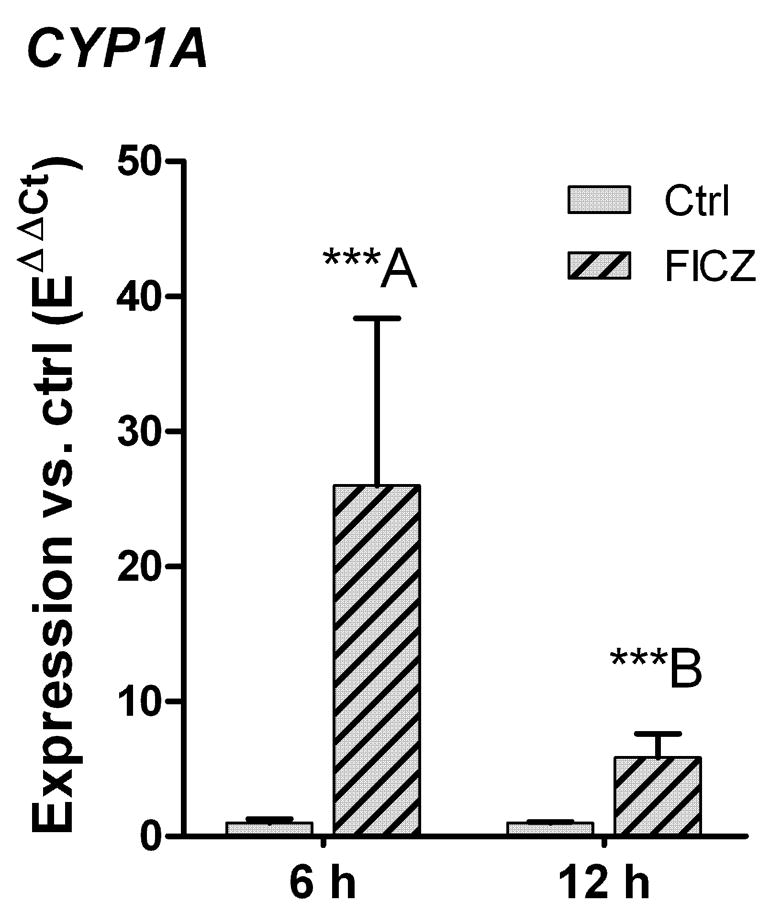
Effect of 6 or 12 hours of exposure to 6-formylindolo[3,2-b]carbazole (FICZ) on CYP1A expression in dechorinated 48-hpf zebrafish embryos. Embryos were exposed to 10 nM FICZ (hatched bars) and 100 ppm DMSO, or 100 ppm DMSO only (Ctrl; filled bars) for 6 or 12 hours. Relative level of CYP1A gene expression was calculated by EΔCt[sample]/mean EΔCt[control]. Significant statistical differences between groups (n=4) were determined using one-way ANOVA followed by Bonferroni’s Multiple Comparison Test of selected pairs (post hoc test) with log transformed data. Differences between the FICZ-exposed groups and the corresponding controls and between the groups exposed to FICZ for 6 or 12 hours are shown by stars (*** = p < 0.001) and different letters (p < 0.01), respectively.
FICZ concentration-response relationship in 48-hpf zebrafish embryos
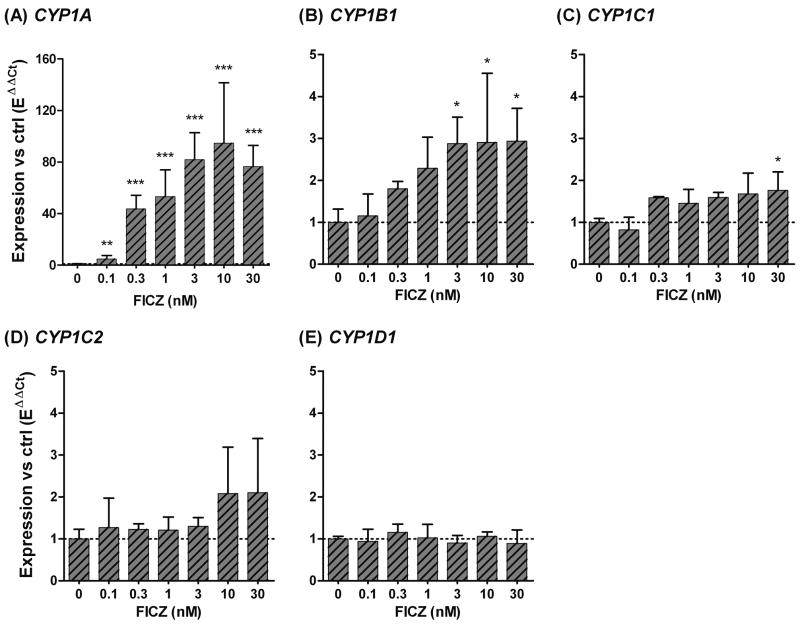
A 6 hour exposure to a range of FICZ concentrations (0.1 nM to 30 nM) caused a concentration-dependent induction of CYP1A, CYP1B1, and CYP1C1 (Fig. 4). A significant induction of CYP1A mRNA was observed at 0.1 nM, the lowest FICZ concentration tested (Fig. 3A). The induction of CYP1A peaked at 10 nM FICZ, although the differences in the degree of induction (expressed as fold increase in FICZ-exposed versus DMSO-exposed fish) were not statistically significant in the concentration range of 0.3–30 nM FICZ. The lowest FICZ concentration that caused a significant induction of CYP1B1 was 3 nM, and higher concentrations caused no further induction (Fig. 4B). CYP1C1 was induced at 10 nM FICZ in the first experiment (Fig. 2C); however, in the concentration-response experiment induction was statistically significant only at the 30 nM FICZ dose (Fig. 4C). This is probably because triplicates were used in the concentration-response experiment, while in the first experiment there were six replicates. There was a trend toward higher CYP1C2 expression in embryos exposed to 10 and 30 nM FICZ, but the levels were not significantly different from that of the control (Fig. 4D). CYP1D1 expression was not induced in the 48-hpf embryos at any of the FICZ concentrations tested (Fig. 4E). For CYP1A and CYP1B1 the EC50 values for induction were almost the same, 0.6 and 0.5 nM FICZ, respectively.
Fig 4.
6-Formylindolo[3,2-b]carbazole (FICZ) concentration response relationships for expression of CYP1A, 1B1, 1C1, 1C2, and 1D1 (A-E) in zebrafish embryos. At 48 hpf embryos were exposed for 6 hours to 0.1, 0.3, 1, 3, 10, or 30 nM FICZ, or 150 ppm DMSO (“0”). Relative expression was calculated by EΔCt[sample]/mean EΔCt[control]. Statistically significant differences between the control and exposed groups (n=3) were determined with one-way ANOVA followed by Dunnett’s post hoc test (* = p < 0.05, ** = p < 0.01, and *** = p < 0.001).
Developmental profile of CYP1A inducibility by FICZ
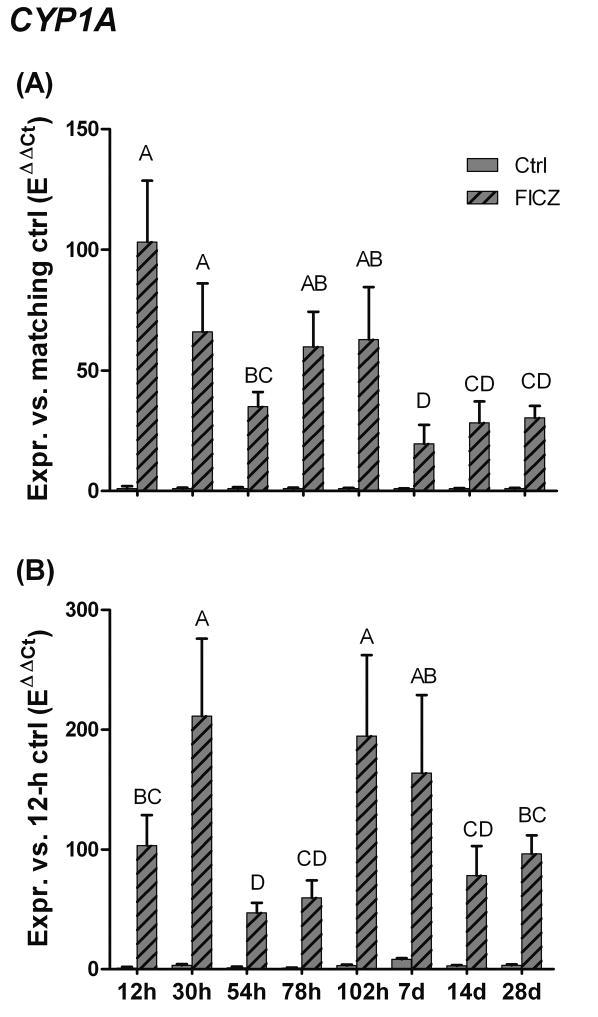
To determine whether sensitivity to CYP1 induction by FICZ varies with developmental stage, zebrafish embryos or larvae were exposed to 10 nM FICZ for a 6-hour period starting at various times after fertilization. Exposures began at 6, 24, 48, 72, and 96 hours, and 7, 14, or 28 days post-fertilization. (The times listed in Table 2 and plotted in Figure 5 refer to the times when the experiment was terminated.) The relative induction of CYP1A by FICZ was greatest at 12 hpf (100-fold) and least at 7.25 dpf (20-fold) (Fig 5A; Table 2). To compare the levels of FICZ-induced CYP1A among developmental stages, we also calculated the change in CYP1A expression for the same data, after the data had been normalized to the mean value for 12-hpf control embryos (Fig. 5B and Table 2). Calculated this way, the highest levels of FICZ-induced CYP1A expression were observed in 30- and 102-hpf zebrafish (210-fold and 200-fold greater than the 12-hpf DMSO control value, respectively; Fig 5B and Table 2). The lowest levels of FICZ-induced CYP1A expression were recorded in groups exposed to FICZ in the period between these time points, that is, at 54 and 78 hpf (47-fold and 60-fold greater than the 12-hpf DMSO control value, respectively).
Table 2. CYP1A expression in zebrafish exposed to 10 nM FICZ or 100 ppm of DMSO for 6 hours at various times in development.
The time post-fertilization (pf) is the age at termination of the experiment, FICZ exposure started 6 hours prior to this. The first set of data are normalized to the mean values of the time-matched controls, the second set are normalized to the mean value of the control at 12 hpf.
| Time pf | N | Expression vs control | Expression vs 12-hpf control | ||
|---|---|---|---|---|---|
| Ctrl | FICZ | Ctrl | FICZ | ||
| 12 h | 5 | 1.0 ± 1.0 | 100 ± 25 | 1.0 ± 1.0 | 100 ± 25 |
| 30 h | 5 | 1.0 ± 0.4 | 66 ± 20 | 3.2 ± 1.2 | 210 ± 65 |
| 54 h | 5 | 1.0 ± 0.6 | 35 ± 6 | 1.4 ± 0.9 | 47 ± 8 |
| 78 h | 5 | 1.0 ± 0.5 | 60 ± 15 | 1.0 ± 0.5 | 60 ± 15 |
| 102 h | 5 | 1.0 ± 0.3 | 63 ± 22 | 3.1 ± 0.9 | 200 ± 68 |
| 7.25 d | 4 | 1.0 ± 0.1 | 20 ± 8 | 8.4 ± 1.0 | 160 ± 65 |
| 14.25 d | 5 | 1.0 ± 0.2 | 28 ± 9 | 2.8 ± 0.7 | 78 ± 25 |
| 28.25 d | 5 | 1.0 ± 0.3 | 30 ± 5 | 3.2 ± 1.1 | 100 ± 16 |
Fig 5.
Developmental profile of CYP1A inducibility by 6-formylindolo[3,2-b]carbazole (FICZ) in zebrafish embryos, larvae, and juveniles. Zebrafish at different developmental stages (6 hpf, or 1, 2, 3, 4, 7, 14 or 28 dpf) were exposed to FICZ (10 nM) or DMSO for 6 hours. CYP1A induction A) versus the matching control (EΔCt[sample]/mean EΔCt[control]) and B) versus the 12-hour control (EΔCt[sample]/mean EΔCt[12-h control]). Statistically significant differences between groups (n=5) were determined by one-way ANOVA followed by Tukey’s post hoc test and are shown by different letters (p < 0.05).
Assessment of embryo toxicity in FICZ-exposed zebrafish embryos
Planar halogenated aromatic hydrocarbons (e.g. TCDD and PCB126) cause a variety of pathological effects in early life stages of fish, e.g., lack of swimbladder inflation, edemas, craniofacial and heart malformations, and hemorrhages [24, 25]. Zebrafish embryos exposed to PCB126 show lack of inflation of the swim bladder at 1 nM, and edemas and malformations at 10 nM (or higher concentrations) [14]. Zebrafish are most sensitive in the period before hatching. However, in the present study no phenotypic changes due to FICZ were observed in a group of zebrafish embryos that were exposed to FICZ (10 nM) starting at 48 hpf (with renewal of the FICZ solution after 6 hours), and followed for 48 hours.
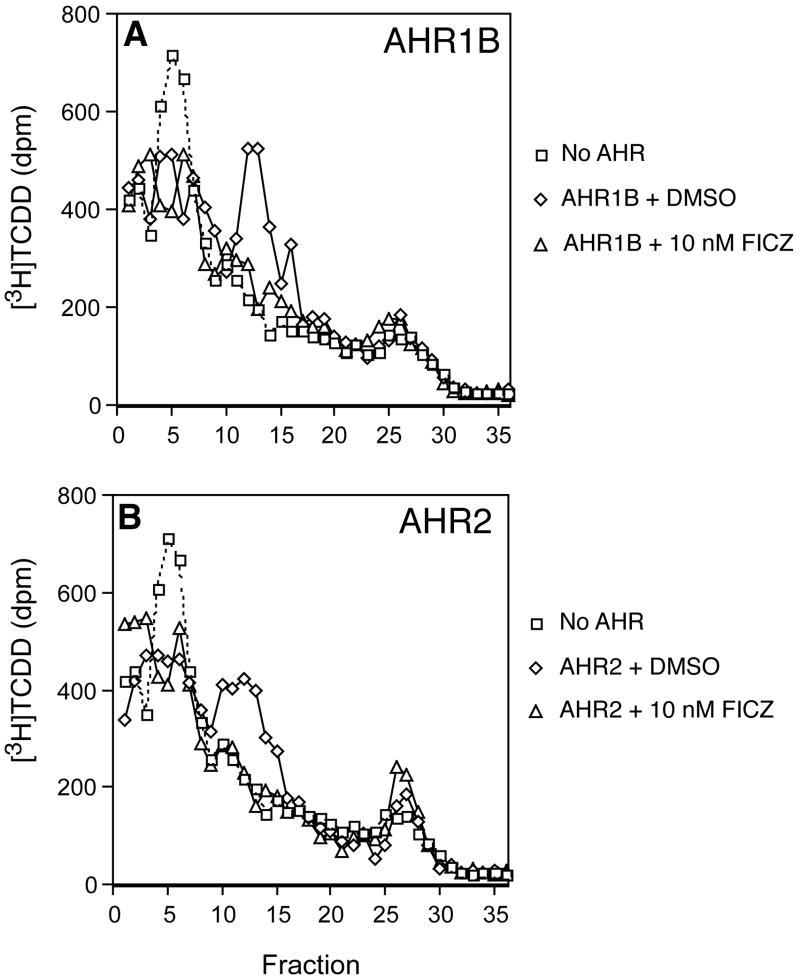
Competitive binding of FICZ to AHR1B and AHR2
To determine whether FICZ is capable of binding to both AHR2 and AHR1B of zebrafish, and whether these two AHRs might differ in this regard, we measured the ability of FICZ to displace the specific binding of [3H]TCDD to each AHR, using a sucrose gradient assay. For both AHRs, FICZ at 1 nM had little or no effect on the specific binding of [3H]TCDD. However, at 10 nM, FICZ completely inhibited the binding of [3H]TCDD to both AHR1B and AHR2 (Fig. 6). These results suggest that FICZ is a high-affinity ligand for both zebrafish AHRs.
Fig 6.
Competitive binding assay using in vitro-expressed zebrafish AHR1B or AHR2 proteins. AHR1B (A) and AHR2 (B) proteins were expressed by in vitro transcription and translation, incubated with [3H]TCDD (2 nM) ± 10 nM FICZ or DMSO, and analyzed by velocity sedimentation on sucrose gradients, as described in Materials and Methods. No AHR: Unprogrammed lysate incubated with [3H]TCDD (2 nM), showing nonspecific binding; AHR1B or AHR2 protein incubated with [3H]TCDD (2 nM) + DMSO, showing total binding in the absence of FICZ; AHR1B or AHR2 protein incubated with 2 nM [3H]TCDD + 10 nM FICZ, showing displacement of [3H]TCDD binding by FICZ.
Effect of AHR2 knock-down on FICZ-induced CYP1A expression
In mammals, the induction of CYP1A1 by FICZ in vivo is dependent on the presence of the single mammalian AHR [9]. The ability of FICZ to interact with zebrafish AHR2 and AHR1b in vitro suggests that they may be involved in mediating the response to FICZ in zebrafish, but does not reveal which of these AHRs regulates CYP1 induction by this compound in vivo. Using the morpholino knock-down technique, we and others have shown previously that transcriptional induction of CYP1A, 1B1, 1C1, and 1C2 by PCB126 and TCDD is regulated primarily by AHR2 in zebrafish embryos [14, 15, 17]. To determine whether CYP1 induction by FICZ also involves AHR2, we microinjected zebrafish embryos with a morpholino targeting AHR2 translation. Non-injected embryos (data not shown) and embryos treated with a standard control morpholino were used as controls. Embryos of each treatment group were exposed to DMSO (200 ppm), FICZ (10 nM), or PCB126 (30 nM). Both the non-injected and the control MO-injected embryos exposed to FICZ or PCB126 responded with a strong induction of CYP1A, which was similar for the two compounds (Fig. 7A). In the AHR2 MO-injected FICZ- and PCB126-exposed embryos the induction of CYP1A was reduced to 11% and 7%, respectively, of the levels seen in the group injected with control morpholino and exposed to the same compounds (p < 0.001; Fig. 7A). The induction of CYP1B1 by FICZ, although weak, also was similar for FICZ and PCB-126, and was decreased in the AHR2 MO-injected embryos (p < 0.001) to 36% and 43% of the corresponding levels in control morpholino-injected embryos (Fig. 7B). Both CYP1Cs were strongly induced by PCB126 and this induction was suppressed by the AHR2-MO (Fig. 7C and 7D). However, in this experiment neither CYP1C was significantly induced by FICZ, precluding detection of an effect of AHR2 knockdown on the expression of these two genes.
Fig 7.
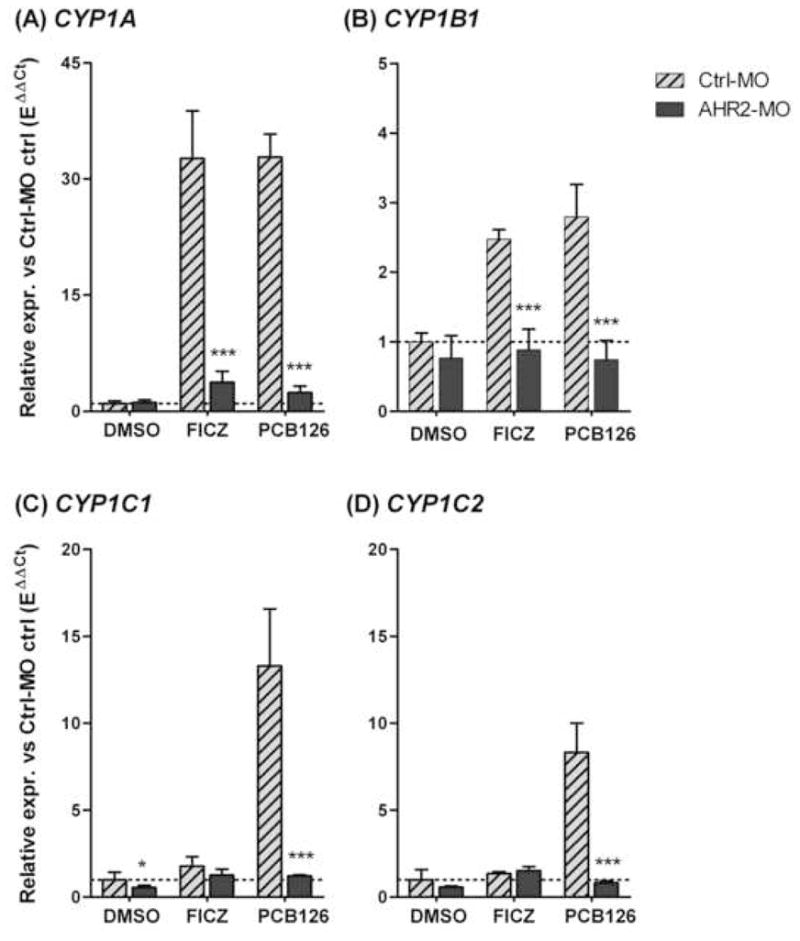
Effect of AHR2 knock-down on CYP1 induction by FICZ or PCB126 in zebrafish embryos. Embryos (2–4 cell stage) were injected with an AHR2 morpholino (AHR2-MO; dark bars), a negative control morpholino (Ctrl-MO; hatched bars), or not injected by a morpholino (Not shown). At 48 hpf these embryos were exposed to 200 ppm of DMSO, 10 nM FICZ, or 30 nM PCB126 for 6 hours (n=3–4). Relative expression was calculated by EΔCt[sample]/mean EΔCt[control MO control]. A) CYP1A. B) CYP1B1. C) CYP1C1. D) CYP1C2. Differences among groups treated with Ctrl-MO or AHR2-MO and with the same exposure (DMSO, FICZ, or PCB126) were examined by one-way ANOVA followed by Bonferroni’s Multiple Comparison Test of selected pairs (* = p < 0.05 and *** = p < 0.001).
Discussion
The tryptophan oxidation product FICZ has been suggested as a physiological ligand for the AHR. Studies have shown that FICZ is formed in mammalian cells upon exposure to ultraviolet (UV) radiation and that it has a high affinity for rodent and human AHR [6, 7]. FICZ also induces CYP1s in human and rodent, and these CYP1s are able to metabolize FICZ very rapidly [8, 26]. The results of the present study indicate that the features of FICZ interaction with AHR and induction of CYP1s seen in mammals apply to zebrafish as well. At low concentrations, FICZ completely displaced TCDD from AHR2 and AHR1B, and thus apparently is an avid ligand for both. FICZ was a potent inducer of CYP1 genes in zebrafish embryos, particularly CYP1A, and as in mammals this induction of CYP1A appeared to be transient, declining in a matter of hours, consistent with rapid metabolism of an inducer. Our results also indicate that FICZ induction at least of CYP1A and CYP1B1 in zebrafish is mediated largely by AHR2.
We found that FICZ was a more potent CYP1 inducer than PCB126 in zebrafish, i.e., for induction of CYP1A and 1B1 by FICZ the 6 hour EC50 values were 0.5–0.6 nM, whereas for PCB126 the 72 hour EC50 values are in the range of 2.3–2.7 nM [14]. However, while PCB126 can strongly induce the four CYP1 genes (CYP1A, 1B1, 1C1, and 1C2) [14], FICZ strongly induced only CYP1A (>30-fold; Fig. 2, Fig. 4, Fig 7A). CYP1B1 was induced only about 3-fold by 6 hours of exposure to FICZ or PCB126 (Fig. 7B). Although the levels of induction differed greatly between CYP1A and CYP1B1, 6 hours of exposure to either of the two compounds (FICZ and PCB126) induced each gene to a similar level. In contrast, responses of CYP1C1 and CYP1C2 to FICZ not only were quite weak (at most 2- to 3-fold), the responses to FICZ were much less than that after 6 hours of exposure to PCB126 (13-fold and 8-fold, respectively; Fig 7C-D), or to that after 48 hours of exposure to PCB126 (19- and 34-fold, respectively) [14]. CYP1D1 was not induced by FICZ, consistent with findings that zebrafish CYP1D1 is not induced by other AHR agonists [16]. However, the reason for the much weaker induction of CYP1Cs by FICZ than by PCB126 is not known.
Weak induction of the CYP1Cs could be a consequence of rapid degradation of the inducer. In studies performed with recombinant human enzymes, FICZ was shown to be an exceptionally good substrate for CYP1A1 and to be metabolized also by CYP1A2 and CYP1B1 [8, 27]. Furthermore, hydroxylated FICZ metabolites were good substrates for sulfotransferases, and human urine was found to contain a variety of FICZ-derived metabolites [8]. FICZ might induce its own metabolism in zebrafish via transcriptional activation of CYP1 genes. Although the capability of zebrafish CYP1 enzymes to metabolize FICZ has not yet been studied, the decline in CYP1A expression after 12 hours compared to 6 hours of exposure is consistent with a rapid degradation of the inducer. The half-lives of the CYP1C mRNAs are not known; if they are longer than the half-life of CYP1A message, then a rapid metabolism could reduce concentrations of FICZ to ineffective levels before induction of the other CYP1s could be detected. However, the relatively strong response of the CYP1Cs after a 6-hour exposure to PCB126 is not consistent with that (Fig. 7). CYP1A protein is strongly induced in the endothelium of fish, and if FICZ is a substrate for zebrafish CYP1A that could reduce the amounts of FICZ reaching other parts of the organism. That could in turn affect induction of the other CYP1s, if their expression is predominantly extravascular; cell specific expression of all the CYP1s is yet to be determined in zebrafish. This situation would be different with exposure to PCB126, which is slowly metabolized and penetrates thoroughly. A possible alternative or additional explanation for the relatively weak induction of CYP1Cs by FICZ is that FICZ-AHR complexes may interact differently with the promoters of these genes, in comparison to its interaction with the CYP1A promoter. Experiments using chromatin immunoprecipitation will be required to examine the possibility of ligand- and gene-specific AHR-promoter interactions.
Looking at FICZ-induced CYP1A expression at different times during development in zebrafish we found a strong induction as early as 12 hpf (49-fold; Fig 4A). In fact, the largest increase relative to the control was recorded at this time point. The high relative induction is partially explained by the low basal level of CYP1A expression early in zebrafish embryo development, which results in a high ratio between induced and control. The present study confirms our previous finding that the basal level of CYP1A expression is lowest early in zebrafish embryo development and increases after hatching [14].
In order to compare CYP1A gene expression over time we normalized all data to the 12-hpf control level. This revealed that the highest FICZ-induced CYP1A expression occurred during the first week. However, there was a temporal drop in level of FICZ-induced CYP1A expression (Fig 4B) from over 200-fold at day 1 post-fertilization, to about 50- and 60-fold at day 2 and day 3, and then an increase to 200-fold at day 4, all versus the 12-hpf control value. Interestingly, the decrease in FICZ-induced CYP1A at day 2–3 coincides with the time for hatching in zebrafish, but at present we do not know what this implies physiologically.
In mammals, induction of CYP1A by FICZ occurs in an AHR-dependent fashion [2, 9], and FICZ has been shown to be a ligand for the AHR [6]. Unlike mammals, which have a single AHR, fish have multiple AHR paralogs, classified within the AHR1 and AHR2 subfamilies. Zebrafish possess three AHRs, one of which (AHR1A) is unable to bind typical AHR ligands such as TCDD and BNF and lacks a functional transactivation domain [11, 12]. In the present study, FICZ was able to bind to both of the other two AHRs, as indicated by its ability to displace the binding of [3H]TCDD to in vitro-expressed AHR2 and AHR1B. AHR2 has been shown previously to be the major AHR form involved in CYP1A induction and embryo toxicity in response to TCDD, PCB126, and some PAHs [17, 28–30], while the function of AHR1B is not yet well understood [11]. Similar to results with xenobiotic ligands, we showed that CYP1A and CYP1B1 induction by FICZ is largely dependent on AHR2. The residual level of induced CYP1A or CYP1B1 in embryos in which AHR2 expression had been knocked down could result from low levels of AHR2 protein remaining in these embryos. It also could represent a contribution of AHR1B to CYP1A or CYP1B induction by FICZ, for example in specific tissues or cell types. Studies of organ and cell-specific differences in the expression of the two AHRs, in relation to cell-specific expression of CYP1s are underway. Likewise, studies to clarify the role of AHR1B in vivo in the response to a variety of AHR ligands (including FICZ as well as TCDD and PAHs) are underway and will be reported separately. The results of such studies could shed light on the possible physiological roles of FICZ and AHRs, taking advantage of the multiple AHR paralogs found in zebrafish and the possibility that they may have partitioned the subfunctions of the single mammalian AHR.
Conclusion
Together, the results here demonstrate that FICZ is a potent AHR agonist in zebrafish, inducing expression of multiple CYP1 genes. CYP1A expression was strongly induced by FICZ, but as in mammals this induction appeared to be transient. Interestingly, FICZ induced the expression of other inducible CYP1s very slightly compared to CYP1A, or to the effect of PCB126, for reasons not yet understood. We also found that while FICZ has a high affinity for two of the three AHRs in zebrafish, i.e., AHR1B and AHR2, the induction of CYP1A and CYP1B1 (in whole embryos) was primarily through the AHR2. It appears that the high potency of FICZ to bind and activate the AHR is evolutionarily conserved from fish to mammals, consistent with a possible role as an endogenous signaling molecule, acting through the AHR. Our knowledge of the endogenous roles of mammalian and fish AHRs is evolving. If AHRs are involved in physiological sensing of UV and visible light that involves formation of FICZ in vivo, this may be important in aquatic vertebrates, including those such as zebrafish with embryos that are transparent. Studies of in vivo gene expression responses of zebrafish to UV light are underway.
Acknowledgments
This work was supported by the Swedish research council Formas and Carl Trygger’s foundation (to MEJ) and in part by a grant from the Superfund Basic Research Program at Boston University (NIH grant P42ES007381), and by NIH grants R01ES015912 and R01ES006272. The sponsors had no involvement in performing or in the decision to publish this study. The U.S. Government is authorized to produce and distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei YD, Rannug U, Rannug A. UV-induced CYP1A1 gene expression in human cells is mediated by tryptophan. Chem-Biol Interact. 1999;118:127–140. doi: 10.1016/s0009-2797(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 3.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- 4.Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem-Biol Interact. 2002;141:3–24. doi: 10.1016/s0009-2797(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 5.Sinal CJ, Bend JR. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol Pharmacol. 1997;52:590–599. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- 6.Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafström AK. Certain Photooxidized Derivatives of Tryptophan Bind with Very High Affinity to the Ah Receptor and Are Likely to be Endogenous Signal Substances. J Biol Chem. 1987;262:15422–15427. [PubMed] [Google Scholar]
- 7.Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz LO, Rannug A, Furst P, Hanenberg H, Abel J, Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 2009;284:2690–2696. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- 9.Mukai M, Tischkau SA. Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci. 2007;95:172–181. doi: 10.1093/toxsci/kfl126. [DOI] [PubMed] [Google Scholar]
- 10.Tanguay RL, Abnet CC, Heideman W, Peterson RE. Cloning and characterization of the zebrafish (Danio rerio) aryl hydrocarbon receptor. Biochim Biophys Acta. 1999;1444:35–48. doi: 10.1016/s0167-4781(98)00252-8. [DOI] [PubMed] [Google Scholar]
- 11.Karchner SI, Franks DG, Hahn ME. AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: tandem arrangement of ahr1b and ahr2 genes. Biochem J. 2005;392:153–161. doi: 10.1042/BJ20050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreasen EA, Hahn ME, Heideman W, Peterson RE, Tanguay RL. The zebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol Pharmacol. 2002;62:234–249. doi: 10.1124/mol.62.2.234. [DOI] [PubMed] [Google Scholar]
- 13.Jönsson ME, Orrego R, Woodin BR, Goldstone JV, Stegeman JJ. Basal and 3,3, ′4,4′,5-Pentachlorobiphenyl-induced expression of Cytochrome P450 1A, 1B and 1C Genes in Zebrafish. Toxicol Appl Pharmacol. 2007;221:29–41. doi: 10.1016/j.taap.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jönsson ME, Jenny MJ, Woodin BR, Hahn ME, Stegeman JJ. Role of AHR2 in the expression of novel cytochrome P450 1 family genes, cell cycle genes, and morphological defects in developing zebrafish exposed to 3,3′,4,4′,5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2007;100:180–193. doi: 10.1093/toxsci/kfm207. [DOI] [PubMed] [Google Scholar]
- 15.Yin HC, Tseng HP, Chung HY, Ko CY, Tzou WS, Buhler DR, Hu CH. Influence of TCDD on zebrafish CYP1B1 transcription during development. Toxicol Sci. 2008;103:158–168. doi: 10.1093/toxsci/kfn035. [DOI] [PubMed] [Google Scholar]
- 16.Goldstone JV, Jönsson ME, Behrendt L, Woodin BR, Jenny MJ, Nelson DR, Stegeman JJ. Cytochrome P450 1D1: a novel CYP1A-related gene that is not transcriptionally activated by PCB126 or TCDD. Arch Biochem Biophys. 2009;482:7–16. doi: 10.1016/j.abb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- 18.Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 19.Karchner SI, Powell WH, Hahn ME. Identification and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleost Fundulus heteroclitus: Evidence for a novel subfamily of ligand-binding basic helix loop helix-Per-ARNT-Sim (bHLH-PAS) factors. J Biol Chem. 1999;274:33814–33824. doi: 10.1074/jbc.274.47.33814. [DOI] [PubMed] [Google Scholar]
- 20.Tsui HW, Okey AB. Rapid vertical tube rotor gradient assay for binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to the Ah receptor. Can J Physiol Pharmacol. 1981;59:927–931. doi: 10.1139/y81-143. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 23.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 24.Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol Appl Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- 25.Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, Kirby ML. PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol Sci. 2008;106:193–205. doi: 10.1093/toxsci/kfn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei YD, Helleberg H, Rannug U, Rannug A. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo(3,2-b)carbazole. Chem-Biol Interact. 1998;110:39–55. doi: 10.1016/s0009-2797(97)00111-7. [DOI] [PubMed] [Google Scholar]
- 27.Bergander L, Wincent E, Rannug A, Foroozesh M, Alworth W, Rannug U. Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-b]carbazole. Chem-Biol Interact. 2004;149:151–164. doi: 10.1016/j.cbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol Pharmacol. 2006;70:549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- 30.Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- 31.Evans BR, Karchner SI, Franks DG, Hahn ME. Duplicate aryl hydrocarbon receptor repressor genes (ahrr1 and ahrr2) in the zebrafish Danio rerio: structure, function, evolution, and AHR-dependent regulation in vivo. Arch Biochem Biophys. 2005;441:151–167. doi: 10.1016/j.abb.2005.07.008. [DOI] [PubMed] [Google Scholar]