Abstract
Background
Androgen deprivation is increasingly being used for the treatment of biochemically recurrent prostate cancer. Thalidomide has been shown to have activity in metastatic prostate cancer.
Methods
159 patients enrolled in a double-blind randomized trial to determine if thalidomide can improve the efficacy of gonadotropin receptor hormone agonist (GnRH-A) in hormone-responsive patients with a rising PSA after primary definitive therapy for prostate cancer. Patients were randomized to GnRH-A for six months followed by oral thalidomide 200 mg per day or placebo (Oral Phase A, OPA). At the time of PSA progression, GnRH-A was restarted for six additional months. Patients were then crossed over to the opposite drug and were treated until PSA progression (Oral Phase B, OPB). Testosterone (T) and dihydroxytestosterone (DHT) were likewise monitored throughout the study.
Results
During OPA, the median time to PSA progression was 15 months for thalidomide group compared to 9.6 months on placebo (P=0.21). The median time to PSA progression during OPB for the thalidomide group was 17.1 months versus 6.6 months for the placebo group (P= 0.0002). No differences were observed in time to serum T normalization between the thalidomide arm and placebo arm during both OPA and OPB. Thalidomide was tolerable although dose reductions occurred in 47% (58 of 124 patients).
Summary
While thalidomide had no effect on T normalization, there was an observed effect on PSA progression during OPB. This is the first study to demonstrate effects of thalidomide and feasibility of intermittent hormonal therapy for biochemically recurrent prostate cancer.
Keywords: prostate cancer, hormonal therapy, thalidomide, angiogenesis, placebo, randomized, progression free survival, prostate specific antigen
INTRODUCTION
Although definitive treatment with primary surgery or radiotherapy affords cure in a majority of patients with prostate cancer, an increasing number present with rising prostate-specific antigen (PSA) in the absence of radiographic evidence of metastasis, so-called Stage D0 prostate cancer or biochemical recurrence. Biochemical recurrence occurs in approximately 30 – 40% of patients undergoing definitive local therapy.1–4 Several salvage options have been offered to these patients but unfortunately, all patients eventually progress to overt metastatic disease. Definite recommendations on how to treat this subset of patients are currently lacking.5
Angiogenesis is important in the pathogenesis, aggressiveness, and potential for metastasis in prostate cancer.6,7 We have previously demonstrated clinical activity of thalidomide in phase II clinical trials of heavily pretreated patients with metastatic castration resistant prostate cancer (CRPC) alone8 and in combination with docetaxel.9 Although the exact mechanisms by which thalidomide controls prostate cancer is still unknown, inhibition of angiogenesis has been widely postulated.10 In addition, anti-angiogenic therapy has been hypothesized to achieve maximal benefit when tumor burden is low.
Intermittent ADT is increasingly being utilized in patients with biochemical recurrence of prostate cancer.11 Patients who manifest with only biochemical recurrence have the least burden of disease and hormone-responsive prostate cancer implies a better prognosis than that of castration resistant prostate cancer. As such, we conducted a randomized, double-blind, multi-institutional, placebo-controlled, cross-over trial design using thalidomide in patients with stage D0 prostate cancer. This report describes the results of the first randomized trial evaluating the time to PSA progression using thalidomide in biochemically recurrent, androgen dependent prostate cancer.
PATIENTS AND METHODS
The primary objective of this study was to determine if thalidomide could improve the efficacy of limited hormonal ablation in androgen-sensitive prostate cancer in patients with biochemical recurrence. Secondary objectives included safety and toxicity evaluation, pharmacokinetic characterization, and analysis of testosterone (T) and dihydroxytestosterone (DHT).
Patient Eligibility
All patients had PSA-only (biochemical recurrence), androgen-dependent adenocarcinoma of the prostate and had failed previous local definitive therapy with radical prostatectomy, radiation therapy, or cryosurgery. To be eligible, patients were required to meet the following criteria: (1) histopathological documentation of prostate cancer, (2) negative evidence of disease other than PSA rise (negative CT scan or bone scan), (3) progressive prostate cancer as evidenced by two consecutively rising PSAs above the post-definitive therapy PSA nadir with an absolute value greater than 1.0 ng/mL separated by at least 2 weeks, (4) life expectancy of more than 12 months, (5) Eastern Cooperative Oncology Group criteria performance status of 0 to 2. Patients with abnormal hematologic and biochemical parameters (as defined by a granulocyte count of < 1,000/mm3, platelet count of < 75,000/mm3, creatinine of > 2.0 mg/dL, or total bilirubin of > 1 mg/dL), concurrent malignancies (with the exception of CLL Stage 0 or non-melanoma skin cancer), unstable angina, recent myocardial infarction, or other uncontrolled cardiac problems, were excluded.
Study Design
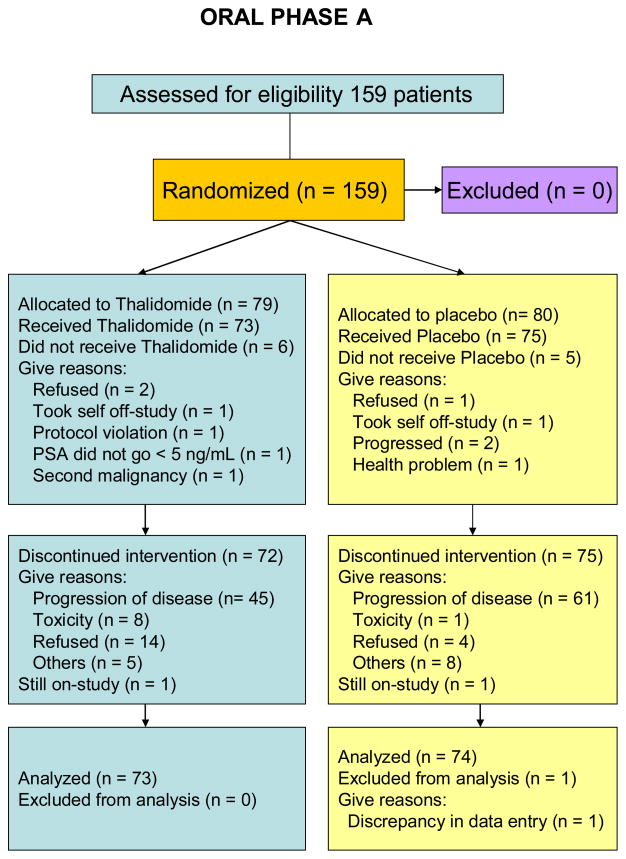
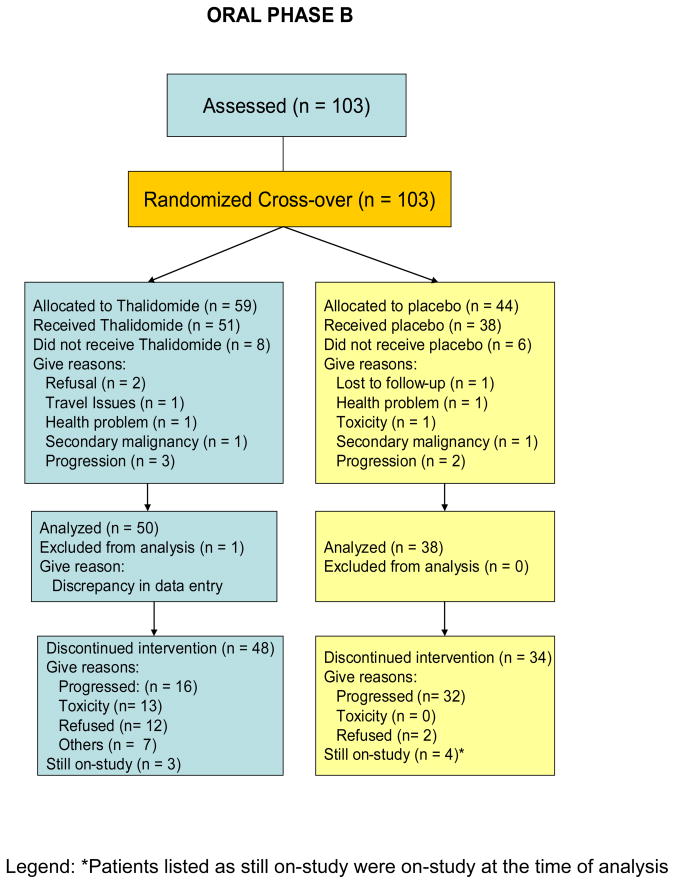
This was a phase III two-arm randomized, double-blind placebo-controlled trial of thalidomide in patients with biochemical recurrence of androgen-sensitive prostate cancer. This study was approved by the institutional review board of the National Cancer Institute and 7 other institutions including Louisiana State University, University of Washington, Columbia University, Wayne State University, University of Minnesota, University of Pittsburgh, and Holy Cross Hospital, Fort Lauderdale, Florida. Patients were initially administered a Gonadotropin-Releasing Hormone-Agonists (GnRH-A) for 6 months, and received either thalidomide or placebo, depending on their randomization schedule (Fig. 1 and Fig. 2). This constituted oral phase A (OPA). Once patients progressed by PSA, as defined by a rising PSA concentration of >5 ng/mL or reaching on-study value (minimum 1 ng/mL), whichever occurred first, they were retreated with a GnRH-A for another 6 months and crossed-over to the opposite treatment. This constitutes oral phase B (OPB). While patients were crossed-over to the other treatment, the time until cross-over was determined by individual patients’ time to progression. An initial sample size of 140 patients on each arm was planned for this study, in order to provide 80% power to be able to detect a difference between progression free survival curves with a median of 10 and 14 months, using a 0.05 one-tailed alpha level test, assuming 18 months are required to accrue patients and 18 additional months of follow-up after the entry of the last patient.
Fig 1.
CONSORT diagram for Oral Phase A (OPA)
Fig 2.
CONSORT diagram for Oral Phase B (OPB)
Treatment Plan
All patients signed informed consent prior to starting drug therapy. The blinded study drugs were provided by the Pharmaceutical Management Branch of the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI). Thalidomide was given orally, at 200 mg dose every evening. This dose was based upon our previous experience using thalidomide in patients with prostate cancer.8,9 Treatment continued as long as patients tolerated the drug without significant toxicity or evidence of disease progression. PSA was monitored monthly and patients were monitored in clinic monthly for the duration of the study. Radiographic studies (CT and bone scan) were obtained at the time of PSA progression.
Treatment dose was reduced to 100 mg (50%) whenever drug-related peripheral neuropathy of grade 2 or more, or any toxicity of grade 3 or more occurred. No further dose reductions beyond 50% were allowed. Patients requiring further dose reductions were crossed over to the opposite drug treatment if it occurred during cycle 1 or taken off-study if it occurred in cycle 2.
Toxicity Evaluation and Response Evaluation
This study utilized the Cancer Therapy Evaluation Program/National Cancer Institute Common Toxicity Criteria (version 2.0) for toxicity grading. Response was evaluated by measuring monthly PSA concentrations. Patients who failed to achieve a PSA concentration of < 5 ng/mL at the end of either treatment cycles of GnRH-A was not allowed to proceed to drug treatment. Patients who commenced drug treatment and developed progressively rising PSA concentrations with absolute value of > 5.0 ng/ml or return to their baseline value were crossed over to the opposite drug treatment (during cycle 1) or taken off study if it occurred during cycle 2. Development of any bone or soft tissue lesions which were attributed to prostate cancer were considered progressive disease and patients were taken off-study.
Thalidomide Pharmacokinetics
Complete pharmacokinetics of thalidomide in patients with prostate cancer has previously characterized.12 However, no clear pharmacodynamic associations have been made with regards to efficacy or toxicity. Therefore, limited pharmacokinetic analysis was performed on NCI patients to assess steady-state concentrations. Blood was drawn at each monthly visit into a tube containing sodium heparin as an anticoagulant (time of collection varied based on clinic appointment). Following centrifugation, plasma was transferred to a cryovial and stored at −80 °C until the time of analysis. A total of 39 samples from 15 patients (median samples per patient, 2) were analyzed using a validated HPLC-UV analytical assay for thalidomide.
Androgen Assessment
Initial results of T and DHT data have been published.13 Briefly, T was measured using the Immulite 2000 solid-phase competitive chemiluminescent enzyme immunoassay (Diagnostic Products Corporation, Los Angeles, California) while DHT was measured by radioimmunoassay after oxidation and extraction (Mayo Clinic Rochester, Minnesota), both assays were done as previously described.13 The normal range for the T assay is 212 to 742 ng/dL, while normal range for DHT is 150 to 980 pg/mL. Both T and DHT were obtained every 3 months while on the GnRH-A therapy and monthly on oral study drug, data mostly available for patients enrolled at the NCI.
Statistical Analysis
All patients who received any oral study medication were assessable for response and toxicity. Progression-free survival (PFS) was calculated from on-study date until progression or at the time of last follow-up. Analyses were also performed beginning from the date at which the blinded study drug was first administered. The probability of PFS was determined using the Kaplan-Meier method,14 and the statistical significance of the overall difference between a pair of Kaplan-Meier curves was determined by the log-rank test.15 All p-values are two-tailed. In addition, the probability of normalization of T and DHT levels, where available, were statistically analyzed using Kaplan-Meier curves. All results were expressed from the last 3-month GnRH-A minus 12 weeks to account for the activity of the GnRH-A therapy. Patients were censored if serum T or DHT has not returned to normal by the cut-off PSA progression.
RESULTS
Patient Data
A total of 159 patients were accrued beginning March 2000 until January 2005. Accrual was slow, and the study was closed to further patient entry because of the poor accrual. The baseline demographics and clinical characteristics are presented in Table 1. The median age of patients was 68 years, with a range of 49 to 87 years. One hundred thirty-one patients (82%) enrolled were Caucasians. The median Gleason score was 7 and on-study PSA concentration was 5.1 ng/mL (range 0.9 to 311.8 ng/mL). All patients had received and failed prior local therapy as shown in Table 1. The ECOG performance status was 0 in most patients (n=142) and 1 in others (n=17).
Table 1.
Patient Characteristics
| Demographics | Number of patients |
|---|---|
| Number of patients | 159 |
| Age, years | |
| Median | 68 |
| Range | 49 – 87 |
| Race | |
| Caucasians | 131 |
| African-Americans | 28 |
| Gleason score | |
| Median | 7 |
| Range | 3 – 10 |
| Gleason score | |
| ≤ 6 | 53 |
| 7 | 62 |
| 8 – 10 | 42 |
| Indeterminate | 2 |
| On-study PSA, ng/mL | |
| Median | 5.1 |
| Range | 0.9 – 311.8 |
| Prior Therapy | |
| Surgery alone | 31 |
| Radiotherapy alone | 33 |
| Surgery and Hormonal Therapy | 2 |
| Radiotherapy and Hormonal Therapy | 17 |
| Surgery and Radiation Therapy | 59 |
| Surgery, Radiation and Hormonal Therapy | 17 |
| ECOG Performance Status | |
| 0 | 142 |
| 1 | 17 |
| Evaluable patients with baseline T | 129 |
| Baseline T (ng/mL) prior to OPA, median | 311.5 |
| Range | 10 – 1000 |
| Baseline T (ng/mL) prior to OPB, median | 326 |
| Range | 20 – 1400 |
Exposure to Study Medication
Of the 159 patients enrolled, 11 patients received neither oral phase A nor B medications. Of the 148 patients who were randomized to either thalidomide or placebo, 34 (23%) patients received only thalidomide and did not cross-over to the placebo arm while 24 (16%) patients received only placebo and did not cross-over to the thalidomide arm. Of the 124 patients who received any thalidomide, the median number of cycles received was 6 (range <0.5 – 56 cycles). Of the 113 patients who received any placebo, the median number of cycles received was 6 (range 1 – 60 cycles). However, dose reduction occurred in 58 out of 124 of patients (47%) in the thalidomide arm, while dose reduction occurred in only 7 out of 113 patients (6.2%) in the placebo arm. The median number of cycles received prior to the first dose reduction was 2 cycles for the patients in the thalidomide arm and 3 cycles for the patients in the placebo arm. Among the most prevalent conditions necessitating protocol-required, principal investigator-initiated or patient requested dose reductions which occurred in the thalidomide arm includes peripheral neuropathy, dyspnea, dizziness, fatigue, and alteration in consciousness, including depression or cognitive disturbances. Most of the symptoms improved after dose reduction, without requiring any treatment although 24 out of the 58 patients (41%) eventually discontinued thalidomide before reaching the cross-over PSA progression.
Response to Therapy
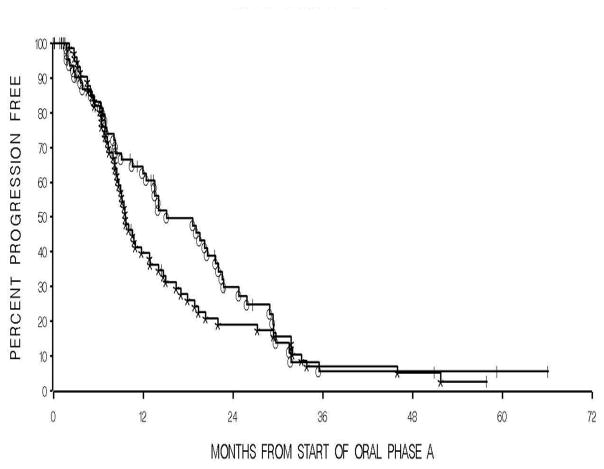
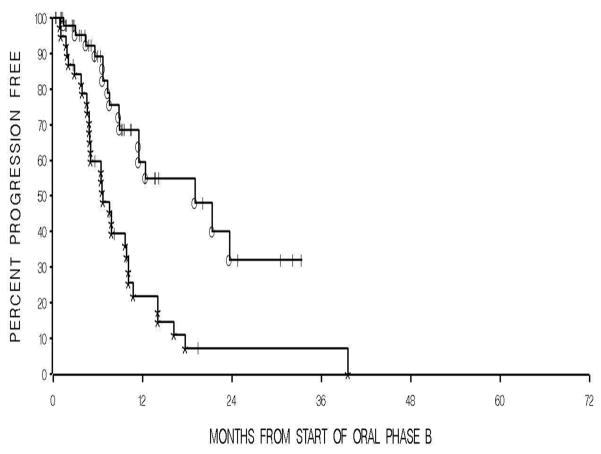
Of the 159 patients, 79 were randomized to thalidomide during OPA and 80 patients to placebo. Only patients who received any oral study medication were included in the analysis for both OPA (n=147) and OPB (n=88), see CONSORT diagrams. The overall median progression-free time for all patients in OPA and OPB was 12 months and 9.9 months, respectively. For patients on OPA, the median time to progression for the patients on thalidomide was 15 months, compared to those on placebo of 9.6 months (p=0.21), Fig. 3. The median time to PSA progression during OPB for the thalidomide arm was 17.1 months versus 6.6 months for patients on the placebo arm (p= 0.0002), Fig. 4.
Fig 3.
A Kaplan-Meier graph showing times to PSA-based progression between thalidomide (°) and placebo (*) during oral phase A. The median time for thalidomide was 15 months compared to 9.6 months for placebo, P=0.21.
Fig 4.
A Kaplan-Meier graph showing times to PSA-based progression between thalidomide (°) and placebo (*) during oral phase B. The median time for thalidomide was 17.1 months compared to 6.6 months for placebo, P=0.0002.
Toxicity
All patients who received any treatment were evaluated for toxic effects. The observed grade 3 and 4 adverse events, as well as grade 2 events that occurred in > 10% of patients after a total of 1,346 cycles of thalidomide and 1,323 cycles of placebo are summarized in Table 2. There were no grade 3 or 4 toxicities that occurred in > 5% of patients. The most prevalent grade 2 complications occurring in > 10% of patients were constipation, fatigue, dizziness or lightheadedness, change in consciousness, dyspnea, as well as sensory neuropathy. Hot flashes occurred frequently between both arms but probably secondary to the previous GnRH-A therapy. The incidence of grade 3 and 4 adverse events was only slightly higher for the thalidomide arm compared to the placebo arm. The grade 3 hematologic toxicities were mild in the thalidomide arm. Only 3 patients developed grade 3 neutropenia and another patient had grade 3 leukopenia. Of the non-hematologic toxicities, the most common grade 3 toxicities occurring in the thalidomide arm includes depressed level of consciousness, dyspnea, syncope, and dizziness. Grade 3 cardiovascular events occurred mostly in the thalidomide arm and most of the grade 4 adverse events in the thalidomide arm were related to cardiac or thromboembolic events, with an incidence of <2%.
Table 2.
Incidence of Treatment-Related Adverse Events
| Grade 2 | Grade 3 | Grade 4 | ||||
|---|---|---|---|---|---|---|
| Adverse Events | Thalidomide n = 124 (%) | Placebo n = 113 (%) | Thalidomide n = 124 (%) | Placebo n = 113 (%) | Thalidomide n = 124 (%) | Placebo n = 113 (%) |
| Allergic reaction | 1 (0.8) | |||||
| Blood/Bone Marrow | ||||||
| Leukopenia | 6 (4.8) | 2 (1.8) | 1 (0.8) | |||
| Neutropenia | 5 (4) | 2 (1.8) | 3 (2.4) | |||
| Lymphopenia | 5 (4) | 2 (1.8) | 1 (0.9) | |||
| Cardiovascular | ||||||
| Arrythmia | 2 (1.6) | |||||
| Bradycardia | 4 (3.3) | 2 (1.6) | 1 (0.8) | |||
| Supraventricular arrythmia | 1 (0.9) | 1 (0.8) | 1 (0.9) | 1 (0.8) | ||
| Edema | 6 (4.8) | 4 (3.5) | 1 (0.8) | |||
| Hypertension | 1 (0.8) | 1 (0.8) | ||||
| Ischemia/Infarct | 1 (0.8) | 1 (0.8) | ||||
| Thrombosis | 1 (0.8) | 1 (0.8) | 1 (0.9) | |||
| Constitutional symptoms | ||||||
| Fatigue | 23 (18.5) | 12 (10.6) | 6 (4.8) | 1 (0.9) | ||
| Weight gain | 1 (0.8) | 1 (0.8) | ||||
| Dermatology/skin | ||||||
| Rash/desquamation | 5 (4) | 2 (1.8) | 1 (0.8) | |||
| Endocrine | ||||||
| Hot flashes | 59 (47.6) | 51 (45.1) | ||||
| Gastrointestinal | ||||||
| Gastritis/Colitis | 1 (0.9) | |||||
| Constipation | 51 (41.1) | 16 (14.2) | 2 (1.6) | |||
| Diarrhea | 1 (0.8) | |||||
| Dyspepsia | 1 (0.8) | |||||
| Nausea | 2 (1.6) | 1 (0.8) | ||||
| Vomiting | 1 (0.8) | 1 (0.8) | ||||
| Metabolic/Laboratory | ||||||
| CPK | 1 (0.8) | |||||
| Hyperglycemia | 3 (2.4) | 5 (4.4) | 1 (0.8) | |||
| Hypophosphatemia | 1 (0.8) | 1 (0.9) | ||||
| SGOT | 1 (0.9) | |||||
| SGPT | 1 (0.9) | |||||
| Musculoskeletal | ||||||
| Arthritis | 1 (0.8) | |||||
| Neurology | ||||||
| Anxiety | 4 (3.2) | 1 (0.8) | ||||
| Ataxia | 3 (2.4) | 1 (0.9) | 1 (0.8) | |||
| CNS ischemia | 1 (0.8) | 2 (1.8) | 2 (1.6) | |||
| Cognitive disturbance | 1 (0.8) | |||||
| Dizziness | 17 (13.7) | 2 (1.8) | 5 (4) | 1 (0.9) | ||
| Depressed level of consciousness | 15 (12.1) | 1 (0.9) | 3 (2.4) | |||
| Insomnia | 1 (0.9) | 2 (1.8) | ||||
| Neuropathic Motor | 2 (1.6) | 2 (1.6) | ||||
| Neuropathic Sensory | 14 (11.3) | 5 (4.4) | ||||
| Syncope | 1 (0.8) | 3 (2.4) | ||||
| Ocular/Visual | ||||||
| Ocular-other | 1 (0.9) | |||||
| Pain | ||||||
| Headache | 2 (1.6) | 2 (1.8) | 1 (0.8) | 1 (0.9) | ||
| Pulmonary | ||||||
| Dyspnea | 20 (16.1) | 10 (8.8) | 2 (1.6) | 1 (0.9) | 1 (0.8) | |
| Pleural effusion | 1 (0.8) | |||||
| Renal | ||||||
| Incontinence | 3 (2.4) | 3 (2.7) | 1 (0.8) | |||
| Sexual/Reproductive | ||||||
| Impotence | 2 (1.6) | 3 (2.7) | 5 (4) | 3 (2.7) | ||
| Secondary Malignancy | 1 (0.8) | 1 (0.9) | ||||
Pharmacokinetics of Thalidomide
The mean steady-state plasma concentration of thalidomide was 352.9 ± 219.5 ng/mL. There was no apparent correlation between plasma concentrations of thalidomide and time to progression (R2 = 0.05).
Androgen Concentrations
Of the 159 patients on-study, only 107 had baseline evaluable T concentrations, with an additional 21 patients who had no baseline T levels but with follow-up T data available. Median baseline values prior to OPA and OPB are shown in Table 1. During OPA, the median time to normalization of T was 15.4 weeks and to DHT normalization was 15.2 weeks. Time to T and DHT normalization was defined as time from the 2nd 3-month depot of GnRH-A until the time when normal values of T (212 ng/dl) or DHT (150 pg/ml) were reached, minus 12 weeks. Analysis of the secondary T endpoints of this study has been previously reported.13,16 There was no difference during OPA between the median time to serum T normalization in the thalidomide group (14.5 weeks) versus 16.7 weeks in the placebo group (P = 0.20) while the median time to serum DHT normalization in the thalidomide group was 15.2 weeks versus 14.8 weeks in the placebo group (P = 0.31). During OPB, the median time to serum T normalization was 18.3 weeks while DHT normalization was 18.7 weeks. Similar to OPA, thalidomide did not affect time to serum normalization of T, with a median of 18.0 weeks versus 19.2 weeks for placebo (P = 0.70).
DISCUSSION
In 1994, the laboratory of Dr. Judah Folkman found that thalidomide, an agent originally synthesized in 1954 and used as a sedative until it was linked to over 10,000 severe malformation in infants, had antiangiogenic activity. Based on those preclinical observations, we conducted several studies using monotherapy or combining thalidomide with chemotherapy in patients with CRPC. A phase II trial combined docetaxel with or without thalidomide (n=50 and n=25, respectively) in patients with metastatic CRPC,9 demonstrated that the addition of thalidomide to docetaxel resulted in an encouraging PSA decline and overall median survival rate for patients in the combination arm (docetaxel plus thalidomide – median survival, 25.9 months versus docetaxel alone – median survival, 14.7 months; p=0.0407).17 This clinical trial confirmed that thalidomide had a role in treatment of solid tumors, beyond the well documented activity in multiple myeloma.
Intermittent ADT is increasingly being utilized in patients with biochemical recurrence. Although the efficacy of applying immediate ADT as compared to deferring therapy until emergence of metastatic disease is a subject of ongoing controversy,18 there may be a role for instituting early treatment. The increasing use of ADT is based on studies suggesting clinical benefit in patients with early stage prostate cancer treated earlier with ADT compared to those receiving it later in the disease course.19,20 It is postulated that the efficacy of anti-angiogenic agents, such as thalidomide, will be greatest in the setting of minimal disease burden. Patients found to have a rising PSA following definitive therapy for prostate cancer as their only evidence of disease are felt to have a minimal disease state. The presence of hormone responsive disease also implies a better prognosis than those who fail to respond to androgen ablation. Therefore, it is in this setting that we proposed to evaluate the clinical efficacy of thalidomide. The results of this trial support this hypothesis. Although time to PSA-based progression was not significantly different during OPA, time to PSA-based progression was substantially longer during OPB, 17.1 months versus only 6.6 months for patients on the placebo arm (p= 0.0002). This is independent of the effects on T normalization brought about by the intermittent androgen deprivation therapy since thalidomide had no apparent effects on T normalization by itself. The effects seen during the first course of GnRH-A could be postulated to occur secondary to the natural history of hormone-sensitive disease, where majority would respond well to ADT regardless of any additional further therapy. Therefore, the benefit of thalidomide may not be readily apparent in this setting. However, in OPB, the possible emergence of castration resistance, coupled with a therapy such as thalidomide that is perhaps most effective when disease burden is low, provides the rationale for the observed prolongation of PSA-based progression among men with biochemically recurrent prostate cancer. Although we did not reach the intended accrual goal, the trial did enroll enough patients in order to be able to detect a reasonably large difference between PSA-based progression between the thalidomide treated group versus placebo. These results have implications not only for this disease state in general, but also for clinical trial design, where agents that would have modest activity as monotherapy, such as thalidomide, may have a more pronounced effect in early disease states, after intermittent hormonal therapy. Intermittent ADT is increasingly employed for a greater number of patients and provides certain advantages over continuous ADT.21 Given the deleterious effects of ADT,22 and possible long natural history of men with biochemical recurrence prior to evidence of metastatic disease,23 it is essential to weigh the risks and benefits of commencing ADT. Administering hormonal therapy intermittently may obviate the potential long-term effects, improve cost benefits, as well as delay the progression to castrate-resistant disease. The addition of thalidomide, which also incurs certain side effects, should also be considered. It is important to note that about 46% of men had to be dose-reduced from the original 200 mg/day dose. However, most men are able to continue at half the dose (dose reduction beyond 50% was not allowed) without requiring any additional therapy while still maintaining an effect on PSA-based progression.
In summary, thalidomide is associated with an in increase in PSA-based progression in men with biochemically recurrent prostate cancer after intermittent GnRH-A that was independent of effects on testosterone. Thalidomide appears promising in this clinical state. Larger studies are warranted to determine the clinical utility of this approach.
Acknowledgments
We thank the following individuals for contributing to the trial: Drs. Mike Hamilton, Gurkamal Chatta, Leonard Siegel, Hikaru Nakajima, Jon L. Hopkins, John Wright, Howard Streicher, David Kohler, Howard Parnes, Jim Pluda, Ulka Vaishampayan, Joseph Fontana, and Eddie Reed; Nurses David Draper, Mary Lewis, Alisa Trout, Jane Carter, Kathy Fedenko, Cathy Parker, and Lea Latham; Maya Goldfarb for data management; Matthew Danish and Erin Gardner for pharmacokinetic analysis; the medical oncology fellows and nurses; and most of all our patients who took part in this study.
Footnotes
Clinical Trials identifier: NCT00020085
References
- 1.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, Coen JJ, Dallow KC, et al. The treatment of prostate cancer by conventional radiation therapy: an analysis of long-term outcome. Int J Radiat Oncol Biol Phys. 1995;32:287–92. doi: 10.1016/0360-3016(95)00123-G. [DOI] [PubMed] [Google Scholar]
- 3.Han M, Partin AW, Piantadosi S, et al. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001;166:416–9. [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. Jama. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 6.Wakui S, Furusato M, Itoh T, et al. Tumour angiogenesis in prostatic carcinoma with and without bone marrow metastasis: a morphometric study. J Pathol. 1992;168:257–62. doi: 10.1002/path.1711680303. [DOI] [PubMed] [Google Scholar]
- 7.Weidner N, Carroll PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Figg WD, Dahut W, Duray P, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001;7:1888–93. [PubMed] [Google Scholar]
- 9.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2532–9. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 10.Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363:1802–11. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 12.Figg WD, Raje S, Bauer KS, et al. Pharmacokinetics of thalidomide in an elderly prostate cancer population. J Pharm Sci. 1999;88:121–5. doi: 10.1021/js980172i. [DOI] [PubMed] [Google Scholar]
- 13.Gulley JL, Figg WD, Steinberg SM, et al. A prospective analysis of the time to normalization of serum androgens following 6 months of androgen deprivation therapy in patients on a randomized phase III clinical trial using limited hormonal therapy. J Urol. 2005;173:1567–71. doi: 10.1097/01.ju.0000154780.72631.85. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 16.Aragon-Ching JB, Gulley JL, Steinberg SM, et al. Kinetics of Serum Androgen Normalization after Limited Androgen-Deprivation Therapy in Nonmetastatic Prostate Cancer. Society of Urologic Oncology Annual Meeting; Bethesda, MD. November 29–December 1, 2007. [Google Scholar]
- 17.Figg WD, Retter A, Steinberg SM, et al. In Reply. J Clin Oncol. 2005;23 2113-a-2114. [Google Scholar]
- 18.Walsh PC, DeWeese TL, Eisenberger MA. A structured debate: immediate versus deferred androgen suppression in prostate cancer-evidence for deferred treatment. J Urol. 166:508–15. doi: 10.1016/s0022-5347(05)65972-1. discussion 515-6, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 20.Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol. 1997;79:235–46. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- 21.Wright JL, Higano CS, Lin DW. Intermittent androgen deprivation: clinical experience and practical applications. Urol Clin North Am. 2006;33:167–79. vi. doi: 10.1016/j.ucl.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. Jama. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 23.Makarov DV, Humphreys EB, Mangold LA, et al. The Natural History of Men Treated With Deferred Androgen Deprivation Therapy in Whom Metastatic Prostate Cancer Developed Following Radical Prostatectomy. J Urol. 2007 doi: 10.1016/j.juro.2007.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]