Abstract
OBJECTIVES
There is increasing evidence that depressive symptoms are associated with the development of cognitive impairment and dementia in late life. We sought to examine whether depression increased the risk of incident cognitive impairment in a longitudinal study of older women.
DESIGN
observational study, up to 6 examinations spanning up to 9 years.
SETTING
university-based Division of Geriatric Medicine
PARTICIPANTS
community-based sample of 436 older, non-demented women
MEASUREMENTS
Participants were followed with regular medical and neuropsychiatric evaluations. Cognitive assessment included episodic immediate and delayed memory, psychomotor speed, and executive functioning. Participants were characterized as having incident impairment on a cognitive test when scores fell below the tenth percentile on age-adjusted norms. Baseline depressive symptoms were measured using the Geriatric Depression Scale (GDS) (30-item). Discrete-time Cox Proportional hazards regression with generalized linear models were used to determine whether baseline risk factors predicted incident impairment on each cognitive test, defined as performance below the tenth percentile on age-adjusted norms.
RESULTS
Baseline GDS was highly associated with incident impairment on all cognitive tests (p <.03). These associations were unaffected by vascular conditions except diabetes, which was associated with incident impairment in delayed recall and psychomotor speed.
CONCLUSIONS
These data suggest that depression may be risk factors for cognitive decline, and thus a potential target for diagnostic and therapeutic interventions.
Keywords: cognitive decline, depression, cognitive impairment, Mild Cognitive Impairment
Background
There is increasing evidence for an association between depression and dementia. The prevalence of clinical depression in Alzheimer’s patients is estimated to be 25–30% (1), and late-life depression is often found to be a prodrome to dementia (2). For example, a diagnosis of a current major depressive episode increases the risk of progression from mild cognitive impairment (MCI) to Alzheimer’s Disease (AD) four-fold (3). Similarly, a recent study suggests that depressive symptoms approximately double the risk of incident MCI in cognitively normal older persons (4, 5). Additional evidence suggests that late-life depression is associated with executive dysfunction, which persists even after remission of the depressive episode (6-8).
It is important to determine whether depressive symptoms may be early symptoms of dementia and whether they might eventually be identifiable risk factors for future dementia. The abovementioned studies used cutoffs for depression that were validated for diagnosis of major depression, a valid approach for assessing major depression as a risk factor; but the studies did not examine the spectrum of depressive symptoms to include “subsyndromal” depression that would not likely meet DSM-IV critieria but yet is of clinical significance.
Since patients with MCI often convert to AD (9), depressive symptoms may be an important clinical marker of future cognitive and functional impairment. Thus, we sought to examine baseline depressive symptoms as a risk factor for incident cognitive impairment across domains of memory and executive function in a population-based sample of community dwelling older women enrolled in the Women’s Health and Aging Study (WHAS) II. Given prior studies suggesting that vascular conditions are also associated with incident cognitive impairment (5), we controlled for vascular conditions (including diabetes diagnosis) as covariates.
Methods
Participants
The Women’s Health and Aging Study (WHAS) II is a prospective study of physical and cognitive functioning among the least disabled two-thirds of 70–80 year-old, community-dwelling women in eastern Baltimore, Maryland. Sampling and recruitment of this cohort is described in detail elsewhere (10, 11) The sampling frame for WHAS II was drawn from female Medicare beneficiaries on Health Care Financing Administration Medicare eligibility lists. Trained interviewers determined eligibility at sampling according to whether individuals were: 1) aged 70–79 years at screening, 2) had sufficient hearing and proficiency in English to be interviewed, 3) could be contacted by telephone, 4) had a Mini-Mental State Exam (MMSE; (12)) score >23, and 5) reported difficulty in no more than one of four functional domains: mobility and exercise tolerance; upper extremity; higher functioning (e.g., shopping); and basic self-care (10). Of 880 eligible individuals, 436 agreed to participate in the baseline examination. Five follow-up exams were conducted at approximately 1.5 year intervals, with the exception of a 3-year interval between exams three and four, yielding a 9-year study period. This study was approved by the Johns Hopkins IRB and each participant gave informed, written consent before completing a standardized interview at each exam. Exams included a medical history ascertaining 14 physician-diagnosed chronic conditions or diseases (10) and vision or hearing difficulties, and a cognitive exam, as part of a one-day evaluation in the Functional Status Laboratory at the Johns Hopkins Outpatient General Clinical Research Center, or in the home, as needed. Over the 9-year follow-up, 90 participants died (20.6%) and an additional 103 participants (23.6%) were lost to follow-up.
Study Measures
Standardized cognitive testing by a trained technician included a global cognitive screen, the MMSE. Verbal immediate and delayed recall memory of 12 common objects were assessed using the Hopkins Verbal Learning Test-Revised (HVLT; (13)) and its six alternate forms. Participants heard and recalled words over three successive learning trials for a score of immediate recall (HVLT-Imm) (maximum= 36), and again after a filled, 20-minute interval for a score of delayed recall (HVLT-Del). The Trail Making Test (TMT;(14)) was used to evaluate psychomotor speed via Part A (TMT-A), and executive function via Part B (TMT-B). Participants were allotted a maximum time of 240 sec. on TMT-A and 360 sec. TMT-B. Longer times (higher scores) reflected worse performance on TMT-A and TMT-B.
Depressive symptoms were assessed at baseline using the Geriatric Depression Scale (GDS) (15-17), a 30-item scale in which each item is endorsed “yes” or “no” by the participant. Responses reflecting depressed mood are summed with higher score reflecting more depressive sxs. The GDS is widely used in studies of older persons and its reliability and validity vs. other depression measures are well established (18). We examined the GDS as a continuous variable and also dichotomized at ≥9 to reflect significant baseline depressive symptoms; this cutoff includes many participants with “minor” or “subsyndromal” depression as well as more severe symptomatology (19).
Medical diagnoses of “possible” angina, MI, stroke, peripheral arterial disease (PAD), and diabetes were adjudicated by the WHAS-II study team in multidisciplinary case conference as previously described (10, 11).
Study outcomes
The study outcomes were defined as incident impairment in each cognitive domain. Impairment was conservatively defined according to its first onset, based on performance at or below tenth percentile cut-offs for each cognitive test using published age- and education- matched norms: HVLT-Imm≤ 16; and HVLT-Del≤ 4 (13); TMT-A ≥ 81 sec. TMT-B≥ 225 sec.(20). These cut-points matched well with internal norms, corresponding to 1.4 to 1.8 standard deviations below internal norms at baseline, and between the fifth and twelfth percentiles (21). Cases coded on a given test as incomplete due to cognitive impairment were defined as impaired at that exam. If data were missing at a timepoint but there were data available at later timepoints, the person treated as not impaired in that cognitive domain at that timepoint. If data were missing for the final timepoint available, the case was censored at that timepoint.
Data Analysis
The baseline cross-sectional associations between depressive symptoms, vascular conditions, and cognitive impairment on each test was examined using logistic regression. To examine the predictive effect of these baseline variables on the risk of developing cognitive impairment on each cognitive test over the 9-year follow-up, discrete time Cox proportional hazards regression with generalized linear modeling was used to estimate the hazard ratio (HR; (22)) Subjects without baseline cognitive impairment were included in the analyses if they had a baseline evaluation and had at least one additional follow-up evaluation for each outcome. Participants with baseline impairment on a cognitive test were excluded for that specific outcome, resulting in cohorts of different sizes for each of the four cognitive test outcomes. Participants were censored at incident impairment, death, or last followup visit without incident impairment. The timing of incident impairment was defined as the time of first occurrence of impairment during the study, and all intermittent missing events were conservatively treated as no impairment. Time was calculated as number of days from baseline evaluation.
Univariate analyses were first performed to examine the effect of individual covariates on each cognitive outcome. Covariates examined included baseline age, education (years of schooling), GDS, systolic blood pressure (SBP), diastolic blood pressure (DBP), and adjudicated diagnoses of “possible” angina, MI, stroke, peripheral arterial disease (PAD), and diabetes. In univariate analyses, GDS, age, education, diabetes were associated (p<0.05) with incident cognitive impairment on at least one outcome (data not shown), and therefore included in multivariate analyses. Diagnosis of hypertension, SBP, DBP, angina, MI, stroke, or peripheral arterial disease was not significantly associated with incident cognitive impairment on any outcome and therefore excluded from the multivariate analyses. We repeated the analyses using more restrictive adjudicated diagnoses of “probable” angina, etc.; using these alternative definitions did not change the results (data not shown). Statistical analysis was performed with the software package Stata 9.2 (College Station, TX).
Results
Description of cohort
Mean duration of follow-up was 7.23 years. Demographic variables, baseline cognitive performance, and prevalence of vascular conditions are shown in table 1. The participants averaged 75 years of age at baseline, had 12.5 years of education, and had mild levels of depression (mean GDS = 4.04 [SD 3.8]). 10.8% of the participants had baseline GDS scores ≥9. The prevalence of each adjudicated vascular condition ranged from approximately 5 to 15%. While approximately half the participants had a diagnosis of hypertension, their blood pressure was reasonably, though not tightly, controlled
TABLE 1. Baseline Demographics and Covariates (N=436).
Medical diagnoses are adjudicated diagnoses that were considered “possible” by the interdisciplinary consensus team
| Variable | Mean or N (S.D or %) |
|---|---|
| Age at baseline (years) | 74.6 (2.81) |
| Age at visit 5(years) | 82.4 (2.92) |
| Education (years) | 12.5 (3.34) |
| Nonwhite | 83 (19.0%) |
| Married | 166 (38.1%) |
| GDS total | 4.0 (3.8) |
| Baseline depression (GDS≥9) | 47 (10.8%) |
| Mini-Mental State Exam | 28.1 (1.8) |
| HVLT-Immediate (baseline) | 22.6 (5.1) |
| HVLT-Immediate (visit 5) | 21.9 (6.6) |
| HVLT-Delayed (baseline) | 8.1 (2.7) |
| HVLT-Delayed (visit 5) | 7.1 (3.5) |
| Trail Making Test, Part A (sec) | 48.8 (29.9) |
| Trail Making Test, Part A (sec) | 59.6 (33.5) |
| Trail Making Test, Part B (sec) | 132 (77.2) |
| Trail Making Test, Part B (sec) | 195 (112) |
| Hypertension | 212 (48.7%) |
| Systolic blood pressure (mm Hg) | 152 (21.7) |
| Diastolic blood pressure (mm Hg) | 77 (16.0) |
| Serum total cholesterol (mg/dl) | 233 (39) |
| Angina | 65 (14.9%) |
| History of Myocardial infarction | 21 (4.8%) |
| Peripheral arterial disease | 42 (9.6%) |
| History of Stroke | 22 (5.1%) |
| Diabetes | 41 (9.4 %) |
The associations of depression and vascular conditions with baseline cognitive impairment
Forty-five of 412 participants with available baseline HVLT-Imm scores were impaired (10.9%), 36 of 401 participants with baseline HVLT-Del scores were impaired (8.9%), 71 of 395 participants with baseline TMT-A scores were impaired (17.9%), and 92 of 393 participants with baseline TMT-B scores were impaired (23.4%). The probability of being impaired in one cognitive domain was strongly associated with being impaired in the other 3 domains (χ2 [1df] ranged from 50.5 to 346.6, p<.001 for these comparisons [data not shown]). A one-point increase in baseline GDS was associated with greater risk of impairment in baseline HVLT-Del (OR=1.10 [95% CI 1.02, 1.19], Wald test with 1df, z=2.51, p=.012) after adjustment for age and education. Baseline vascular factors were not associated with impairment on any domain with the exception of diabetes being associated with greater risk of impairment on TMT-B (OR=2.28 [95% C.I. 1.1, 4.7], likelihood ratio χ2 = 47.80, df=3, p=.026) after adjustment for age and education.
The associations of depression and vascular conditions with incident cognitive impairment
Participants with baseline impairment in a cognitive domain were excluded from longitudinal analyses for that domain. For HVLT-Imm, 103 of 367 remaining participants (28.0%) developed impairment during follow-up; 77 of 365 remaining participants (21.0%) developed impairment on the HVLT-Del; 175 of 324 participants developed impairment (54.0%) on TMT-A; and 92 of 301 participants (30.6%) developed impairment on TMT-B.
The multivariate analysis is presented in Table 2. Higher age and GDS scores were associated with greater risk of incident impairment on all cognitive outcomes (Wald test with 1df, z = 2.00–2.41, p<0.05), while higher education levels were protective. The association of higher baseline GDS score with incident impairment in TMT-B was not altered by controlling for TMT-A at each timepoint. Diabetes was the only vascular condition associated with greater risk of incident impairment in any cognitive outcome; a baseline diabetes diagnosis was associated with greater risk of incident impairment in HVLT-Del (Wald test with 1 df, z= 2.81, p = .005) and TMT-A (Wald test with 1 df, z = 3.77, p <.001). There were no significant interactions between GDS and diabetes in altering the risk of incident cognitive impairment in any domain (data not shown).
Table 2. Multivariate models.
Cognitive outcome was modeled with adjusted discrete time series as described in the Methods. The statistical significance of the contribution of each covariate to cognitive outcome was tested with Wald test (1df)
| Cognitive outcome | ||||||||
|---|---|---|---|---|---|---|---|---|
| HVLT-Imm <17 | HVLT-Delayed <5 | TMT-A > 80 sec | TMT-B > 224 sec | |||||
| Covariate | HR | C.I. | HR | C.I. | HR | C.I. | HR | C.I. |
| GDS | 1.07* | 1.02, 1.12 |
1.06^ | 1.01, 1.11 | 1.07* | 1.03– 1.13 |
1.07* | 1.02, 1.12 |
| Diabetes | 1.34 | .73, 2.47 | 2.38* | 1.33, 4.25 | 3.65* | 2.00, 6.70 |
1.60 | .82, 3.11 |
| Age | 1.09* | 1.02, 1.17 |
1.14* | 1.06, 1.22 | 1.18* | 1.08, 1.28 |
1.15* | 1.07, 1.22 |
| Education | .88* | .83, .94 | .93^ | .87, .99 | .94 | .87, 1.01 | .89* | .84, .94 |
p<.05
p<.01
HR= hazard ratio C.I. = 95% confidence interval limits.
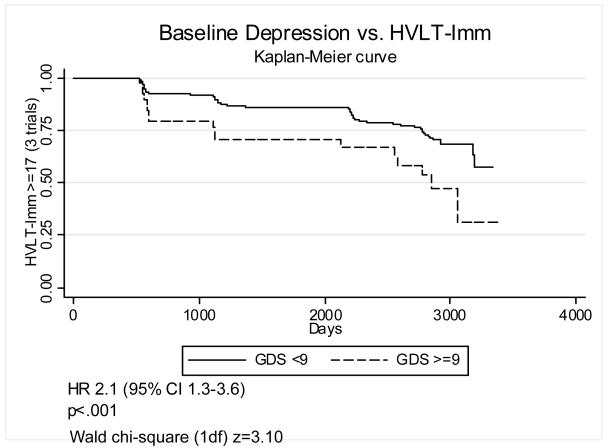
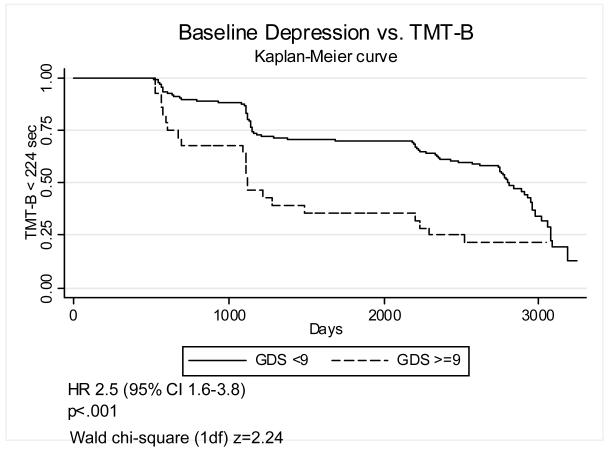
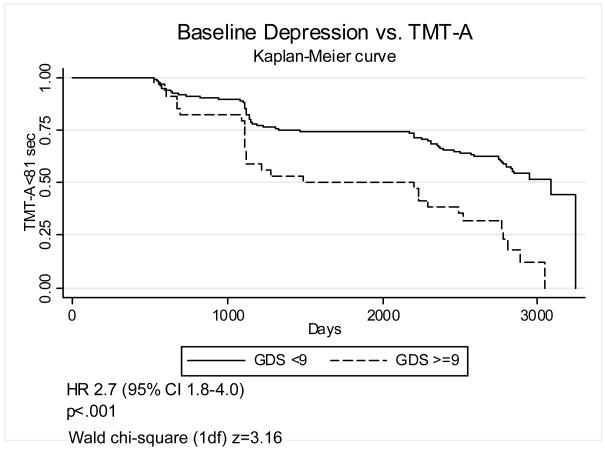
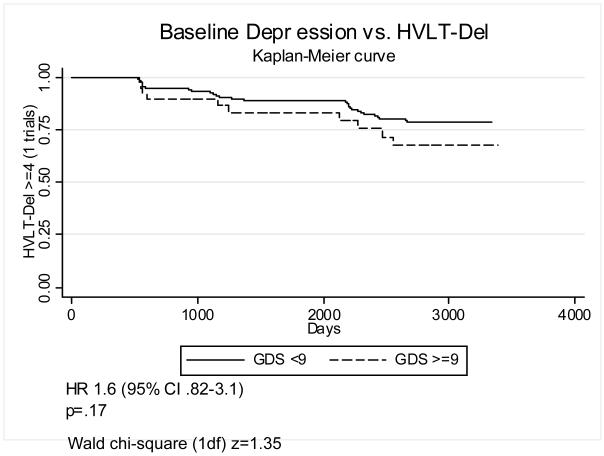
In order to improve the clinical interpretability of these results we also defined baseline depression as GDS≥9, a fairly low cutoff chosen to include subsyndromal depression. Figures 1-4 graphically display Kaplan-Meier survival curves for incident cognitive impairment dichotomized by baseline depression. Baseline GDS≥9 significantly increased the risk of incident impairment in HVLT-Imm 2.1-fold (95% C.I. 1.3–3.6) (figure 1), TMT-A by 2.7-fold (95% C.I. 1.8–4.0) (figure 3), and TMT-B (figure 4) by 2.5-fold (95% C.I. 1.6–3.8), but did not significantly increase the risk of incident impairment in HVLT-Del (figure 2). There were no significant interactions between GDS≥9 and diabetes on incident impairment in any domain (data not shown).
Figure 1. Baseline depression and incident impairment in immediate recall.
Kaplan-Meier survival curves graphing the proportion of participants with HVLT-Imm > 16 (3 trials) vs. time (days), dichotomized by baseline GDS<9 vs. GDS>=9.
Figure 4. Baseline depression and incident impairment in executive function.
Kaplan-Meier survival curves graphing the proportion of participants with TMT-B < 224 sec vs. time (days), dichotomized by baseline GDS<9 vs. GDS>=9.
Figure 3. Baseline depression and incident impairment in psychomotor speed.
Kaplan-Meier survival curves graphing the proportion of participants with TMT-A < 81 sec vs. time (days), dichotomized by baseline GDS<9 vs. GDS>=9.
Figure 2. Baseline depression and incident impairment in delayed recall.
Kaplan-Meier survival curves graphing the proportion of participants with HVLT-Del > 4 (1 trial) vs. time (days), dichotomized by baseline GDS<9 vs. GDS>=9.
Discussion
We report that depression is a risk factor for the development of cognitive impairment, across domains of cognition, in a longitudinal study of older, community-dwelling women. The effect of depressive symptoms was considerable: for each 1-point increase in GDS, we found a 6–7% increase in annual risk of cognitive impairment in each cognitive domain, and baseline depression (defined as GDS ≥9) effectively doubled this risk for three of four domains. These results imply that the association of depression with incident cognitive impairment generalizes over a variety of cognitive domains.
These results replicate and extend prior findings, importantly reporting a very similar effect size. There are several studies examining the effect of depressive symptoms on incident MCI. Geda et al. reported a hazard ratio for incident MCI of 2.2, using a cutoff of 6 on the 15-item form of the GDS (4). Barnes et al. reported an odds ratio for incident MCI of >2 for participants with Center for Epidemiological Studies Depression Scale (CES-D) >7 followed over 6 years (5). Cross-sectional studies, while not directly comparable, report similar risk. Lopez et al. reported an odds ratio for prevalent MCI of 1.5 for participants with CES-D>7 (23). Green et al reported an odds ratio of 2.8 for prevalent AD for participants with a history of clinically significant depression (24); although the data collection was retrospective and subject to recall bias, they noted higher odds ratios when the depression was quite recent (within one year of interview) suggesting that depression was a prodrome to AD in their cohort. Our analysis differs from these prior studies in that we examined subsyndromal depressive syndromes, while the abovementioned studies used cutoffs for depression severity similar to Major Depressive Episode. We chose this approach because, if depressive symptoms are a prodrome or risk factor for incident cognitive impairment, there is likely to be a clinical spectrum of severity in this prodrome; to pick up the earliest symptoms requires using a more sensitive threshold, likes less severe than major depression. Additionally, our analysis examines incident cognitive impairment rather than incident diagnosis of MCI as an outcome. Even with these differences in analysis, our findings support these prior results and suggest that clinically significant depression approximately doubles the risk of developing clinically significant cognitive impairment.
Observational data cannot directly address the crucial mechanistic question of whether depression is a symptom of cognitive impairment (i.e., whether depression and dementia are different symptoms of the same underlying process) or whether depression adds independently to risk of incident cognitive impairment. Future studies can address these questions by examining the association of depressive symptoms and biomarkers in prodromal dementia states. One potential mechanism is the growing evidence that inflammatory mechanisms are important in the neurotoxicity of Alzheimer’s disease (25) and in mood disorders (26).
Other replicative findings include: 1) increased age and less education were associated with increased risk of incident cognitive impairment as has previously reported (5, 21). 2) the baseline (cross-sectional) association of depression with slower psychomotor speed has been observed in community-dwelling older persons (27).
A diagnosis of diabetes was associated with increased risk of incident cognitive impairment (delayed recall and processing speed) paralleling prior studies of AD and MCI incidence (28) and MCI prevalence (23). However, other vascular conditions did not add significantly to the risk of incident cognitive impairment. This partly replicates the findings of two longitudinal studies examining similar vascular factors (5, 29) but is at odds with others (23, 30, 31), particularly in the lack of added risk we found for hypertension despite a high prevalence of the diagnosis. However, our participants had adequate though not excellent control of blood pressure on average, and it is possible that medication treatment of hypertension modified the effect (not assessable with these data). There is not strong concordance in the literature concerning which vascular risk factors affect cognitive decline. One possibility is that some risk factors (diabetes) are associated with microvascular ischemia, some risk factors (angina, MI, and stroke) largely reflect the outcome of macrovascular disease, and some risk factors (hypertension and hypercholesterolemia) likely affect both. Diabetes has such pleiotropic mechanisms, affecting many organ systems, that it may be difficult to discern the specific mechanisms increasing risk of cognitive impairment. Given the discordance with the effects of other vascular conditions, our results would suggest that vascular mechanisms did not have a major effect on the cognitive outcomes of our participants. Another possibility is that the effect of risk factors varies according to age and disease stage; for example, higher serum cholesterol in mid-life is a risk factor increases the risk of late-life dementia (30) whereas higher serum cholesterol in late-life decreases the risk of late-life dementia (32). Our results only reflect the effect of late-life vascular risk factors and are unable to address effects more remote in time.
Strengths of the study include use of a population-based sample, availability of multiple cognitive outcomes, consensus diagnosis of medical conditions, low attrition, and long duration of follow-up. Limitations include a cohort limited to women and lack of clinical consensus diagnoses or MCI and/or dementia. The latter is especially important in terms of generalizability, because our analysis is of incident cognitive impairment not dementia, and the risk factor profile may be different for this important clinical outcome. An additional limitation is the lack of apoE genotyping: it is possible that the presence of an apoE4 allele underlies both depressive symptoms and incident cognitive impairment.
These results provide further support for the role of depressive symptoms as a prodromal marker of future cognitive impairment, and for the incorporation of depressive symptoms into profiles of clinical and biological markers for the identification of persons at risk for dementia. This has direct relevance to identifying candidates for disease-modifying therapies for dementia prevention.
Acknowledgements
This work was supported by grants 5K08AG029157-02, RO1 AG19825-02 and R01 AG11703-10 (National Institute of Aging) and 1U01 MH 66136 (National Institute of Mental Health). We gratefully acknowledge the assistance of Marilyn Albert PhD and Ho-Chang Lee MD MHS in study design and interpretation of results.
Footnotes
Disclosure: The authors report no conflict of interest.
References
- 1.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: Findings from the cache county study on memory in aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 2.Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 3.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of alzheimer type: A prospective cohort study. Arch Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 4.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: A prospective cohort study. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 5.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: Findings from the cardiovascular health study. Arch Gen Psychiatry. 2006;63:273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: The women’s health and aging study. J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 11.Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol B Psychol Sci Soc Sci. 1999;54:S262–70. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test - revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 14.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York City, NY: 1991. [Google Scholar]
- 15.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 16.Yesavage JA. Geriatric depression scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 17.Parmelee PA, Lawton MP, Katz IR. Psychometric properties of the geriatric depression scale among the institutionalized aged. Psychol Assess. 1989;1:331–338. [Google Scholar]
- 18.Lichtenberg PA, Marcopulos BA, Steiner DA, Tabscott JA. Comparison of the hamilton depression rating scale and the geriatric depression scale: Detection of depression in dementia patients. Psychol Rep. 1992;70:515–521. doi: 10.2466/pr0.1992.70.2.515. [DOI] [PubMed] [Google Scholar]
- 19.Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the geriatric depression scale: A systematic review. Acta Psychiatr Scand. 2006;114:398–410. doi: 10.1111/j.1600-0447.2006.00888.x. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ. Mayo’s older americans normative studies: Age- and IQ-adjusted norms for the trail-making test, the stroop test, and MAE controlled oral word association test. Clin Neuropsychol. 2005;19:329–377. doi: 10.1080/13854040590945210. [DOI] [PubMed] [Google Scholar]
- 21.Carlson MC, Xue QL, Zhou J, Fried LP. Executive decline and dysfunction precedes that in memory and increases risk for functional difficulty and global cognitive impairment. 2007. [Google Scholar]
- 22.Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 23.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: Part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 24.Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for alzheimer disease: The MIRAGE study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg PB. Clinical aspects of inflammation in alzheimer’s disease. Int Rev Psychiatry. 2005;17:503–514. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- 26.Capuron L, Miller AH. Cytokines and psychopathology: Lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Baune BT, Suslow T, Engelien A, Arolt V, Berger K. The association between depressive mood and cognitive performance in an elderly general population - the MEMO study. Dement Geriatr Cogn Disord. 2006;22:142–149. doi: 10.1159/000093745. [DOI] [PubMed] [Google Scholar]
- 28.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident alzheimer disease and vascular dementia: The cache county study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 29.Monastero R, Palmer K, Qiu C, Winblad B, Fratiglioni L. Heterogeneity in risk factors for cognitive impairment, no dementia: Population-based longitudinal study from the kungsholmen project. Am J Geriatr Psychiatry. 2007;15:60–69. doi: 10.1097/01.JGP.0000229667.98607.34. [DOI] [PubMed] [Google Scholar]
- 30.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and alzheimer’s disease in later life: Longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tervo S, Kivipelto M, Hanninen T, et al. Incidence and risk factors for mild cognitive impairment: A population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 32.Mielke MM, Zandi PP, Sjogren M, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]