Abstract
Forebrain arteries receive nitroxidergic input from parasympathetic ganglionic fibers that arise from the pterygopalatine ganglia. Previous studies have shown that ganglionic stimulation in some species led to cerebral vasodilatation while interruption of those fibers interfered with vasodilatation seen during acute hypertension. Because the ganglionic fibers are quite delicate and are easily damaged when the ganglia are approached with published techniques we sought to develop a method that allowed clear exposure of the ganglia and permitted demonstration of cerebral vasodilatation with electrical stimulation of the ganglia in the rat. We had found that an orbital approach during which the eye was retracted for visualization of the ganglion precluded eliciting vasodilatation with ganglionic stimulation. In the current study approaching the ganglion through an incision over the zygomatic arch provided clear exposure of the ganglion and stimulation of the ganglion with that approach led to vasodilatation.
Keywords: cerebral blood flow, nitric oxide, parasympathetic, rat
Introduction
We have previously shown that neural influences modulate cerebral arterial resistance when arterial blood pressure exceeds the range beyond which cerebrovascular tone and cerebral blood flow (CBF) would break through autoregulation (Talman et al., 1994;Talman & Nitschke Dragon, 1995b;Talman & Nitschke Dragon, 2000;Talman & Nitschke Dragon, 2002). Marked cerebral vasodilatation that occurs with breakthrough is significantly attenuated, if not eliminated, by transection of parasympathetic ganglionic fibers (Talman & Nitschke Dragon, 2000) that originate in the pterygopalatine ganglia (PPG) and project to forebrain cerebral vasculature (Suzuki et al., 1988;Suzuki et al., 1990b). The ganglionic projections pass through the ethmoid foramina that lie on the mesial aspect of the orbital wall (Walters et al., 1986;Hara et al., 1993). Interruption of the arterial baroreflex similarly affects CBF during hypertension (Talman & Nitschke Dragon, 2002). In contrast, chemical stimulation of parasympathetic preganglionic neurons of the superior salivatory nuclei leads to cerebral vasodilatation and consequent increases in CBF (Agassandian et al., 2002;Agassandian et al., 2003). We have shown that the nucleus tractus solitarii, the primary site of termination of arterial baroreceptor afferent fibers, projects directly to the superior salivatory nuclei where its projections synapse with parasympathetic preganglionic neurons within the latter nuclei (Agassandian et al., 2002). These observations suggest that efferent nerves from the PPG are the final limb of the baroreflex pathway to cerebral vessels and effect vasodilatory responses to marked activation of arterial baroreflexes during profound hypertensive events. That suggestion has been supported by studies utilizing dog, monkey, rat and cat as the experimental subject (Okamura et al., 2002;Toda et al., 2000b;Goadsby, 1989;Suzuki et al., 1990a;Seylaz et al., 1988), but preliminary experiments for the current study failed to demonstrate cerebral vasodilatation in the rat when the PPG was exposed after enucleation. Therefore, we hypothesized that stimulation of the PPG would similarly lead to vasodilatation and increases in CBF in rat if the surgical approach we used spared ganglionic fibers from PPG. Through the current study we sought to test that hypothesis by studying effects on cerebral blood flow and arterial blood pressure during electrical stimulation of the PPG in anesthetized rats with intact ganglionic fibers or after transection of those fibers. Exposure of the PPG as described here utilizes minimally invasive techniques that will allow use of chronically prepared animals in future studies.
Methods
Experiments were performed in 12 adult male Sprague Dawley rats that were anesthetized either with halothane (5% induction and 1.5-2.0 % maintenance) or isoflurane (5% induction and 1.5-2.0 % maintenance) delivered with 100% O2 via a nasal cone. A femoral artery was exposed through an inguinal incision and cannulated for recording arterial blood pressure (AP), mean AP (MAP), and heart rate (HR) by means of an electronic data acquisition system (Power Lab/16sp, Minneapolis, MN). In all animals arterial blood gases were assessed at baseline (prior to stimulation) and during stimulation of ganglia. With supplemental oxygen, the arterial PO2 always exceeded 100 TORR throughout each experiment. We set parameters for change of arterial PCO2 such that we would accept data only from experiments in which the PCO2 changed less than 5 TORR from baseline values. Those parameters were not exceeded in any of the studies reported here.
Exposure of the PPG Through a Supraorbital Approach
In 2 animals we exposed the left PPG with an approach modified from that described by Spencer et al. An incision was made above the left eye, and the eye was retracted inferiorly. Nerves and connective tissue lying over the PPG were retracted with the eye and the ethmoid foramen exposed as we have previously described (Agassandian et al., 2002). To achieve and maintain hemostasis blood vessels were cauterized and the bone around the ethmoid foramen was rubbed with a cotton swab. The maxillary division of the trigeminal nerve was retracted with a loop of suture material and a bipolar stimulating electrode, insulated from surrounding tissues with Kwik-Cast silicone sealant (World Precision Instruments, Sarasota, FL, was placed on the PPG).
Exposure of the PPG through a zygomatic approach
In 6 animals one PPG was exposed through an incision made parallel to and immediately above the zygomatic arch, i.e. along the inferior orbit. The extraocular muscles were retracted downward and laterally to expose the maxillary division of the trigeminal nerve, which was itself retracted laterally to expose the PPG. After exposure of the ganglion we applied the electrode as described above. This method provided minimal exposure of the ganglion and complicated electrical stimulation though it could be successfully achieved. We, therefore, subsequently further refined the exposure by using a technique modified from that first described by Rosen et al. We opened the animal's mouth with a 1 cm roll of damp cotton gauze to move the temporal muscle posteriorly; covered the eye with triple antibiotic ointment; made an incision directly over the zygomatic arch; removed a 1 cm length of the zygomatic bone; cut the masseter muscle and retracted it ventrally; retracted the lacrimal gland dorsally; and identified the maxillary division of the trigeminal nerve, which we then retracted dorsally. This technique optimized access to the PPG without extensive retraction of surrounding tissues and facilitated placement of the stimulating electrode on the ganglion. In 4 animals the same approach was used and stimuli were delivered directly to the maxillary division of the trigeminal nerve to control for cerebrovascular effects that might result from spread of current to that nerve when the PPG itself had been stimulated. Stimuli to the maxillary nerve were delivered sequentially both proximal to the retracting suture and distal to that suture. Because cerebrovascular and cardiovascular effects of stimuli to either side of the suture did not differ, data presented here were derived from the proximal stimulus alone.
Recording Cerebral Blood Flow
After instrumenting the PPG we prepared the animal for recording CBF as we have previously described (Talman et al., 1994). We exposed the surface of the parietal cerebral cortex ipsilateral to the exposed PPG and placed a 0.8 mm laser flow probe (Vasamedics Laserflo BPM2 Blood Perfusion Monitor, Eden Prairie, Minnesota) extradurally over the cortical surface for recording CBF in laser Doppler units (LDU). We applied mineral oil onto the dura with the probe tip touching the oil. Care was taken to avoid placing the probe over large arteries. Instead, to avoid artifact, we placed the probe between arteries. Arterial blood pressure was also continuously recorded to allow calculation of changes in cerebrovascular resistance (CVR) determined by the formula MAP/LDU = CVR.
Electrical stimulation of the PPG
After placement of electrodes and instrumentation for recording CBF and cardiovascular variables, we stimulated the PPG for 80 seconds with a continuous current starting at 2 to 4 volts and rapidly ramping to 15 volts, 0.2 msec. pulse duration, and 4 Hz frequency while continuously recording CBF via laser flowmetry. For the maxillary nerve, stimuli were increased to 20 volts to equal and exceed that delivered to the PPG. Measurements of CBF were expressed in laser Doppler units (LDU).
Statistical Analysis of Data
We expressed data as mean ± SEM, subjected data to Wilcoxon analysis or Student's t test as appropriate and considered comparisons statistically significant at a p value ≤ 0.05.
Results
Stimulation of PPG Exposed through a supraorbital approach
Unilateral electrical stimulation of the PPG, exposed through the supraorbital approach, in 2 animals elicited no reproducible changes in CBF. As a result we changed the protocol to the zygomatic approach to the ganglion as described above.
Stimulation of PPG exposed through a zygomatic approach
In 6 animals unilateral electrical stimulation of the PPG, exposed through the zygomatic approach, elicited an increase in CBF but did not significantly change MAP (Fig. 1), which was 81.1 ± 2.1 mmHg prior to stimulation and 82.5 ± 3.0 mmHg during stimulation. At a maximal stimulus (4 volts for 80 seconds) CBF increased 40.4 % from a basal value of 27.7 ± 2.7 LDU to a maximum of 38.9 ± 4.3 LDU (p<0.03) during the stimulus (Fig. 2 & 3). Therefore, the stimulus significantly decreased cerebrovascular resistance (on average by 28%).
Figure 1.
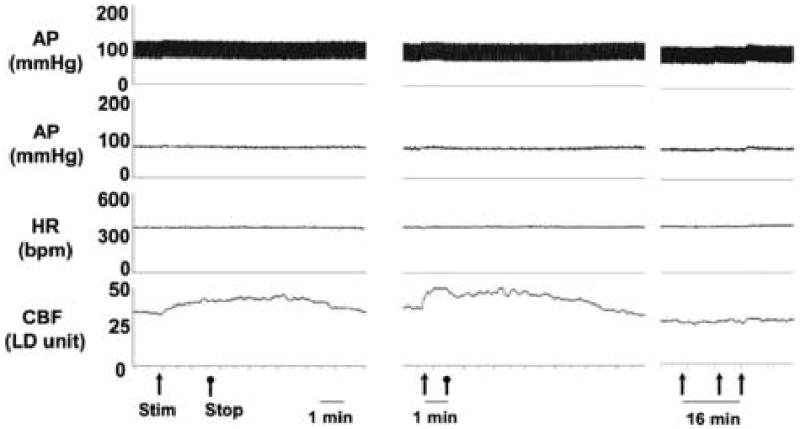
Vasodilatation in response to parasympathetic stimulation. Electrical stimulation of the PPG with intact ganglionic fibers (left) elicited increased CBF as did direct electrical stimulation of ganglionic fibers (center). In contrast, electrical stimulation of the PPG after transection of ganglionic fibers (right) did not affect CBF. Note that the latter recording was made at a slower speed and with repeated stimuli to better demonstrate that CBF changes did not occur late after any stimulus.
Figure 2.
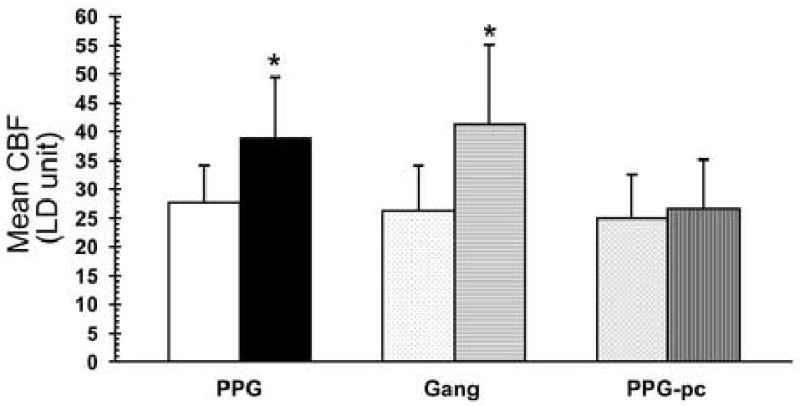
Graphic representation of changes in CBF (LD units) in response to electrical stimulation of the PPG (left), ganglionic fibers from PPG (Gang; center), or the PPG after transection of ganglionic fibers (PPG-pc; right). In each case basal values are shown to the left and responses to electrical stimulation are shown to the right. * p<0.05
Figure 3.
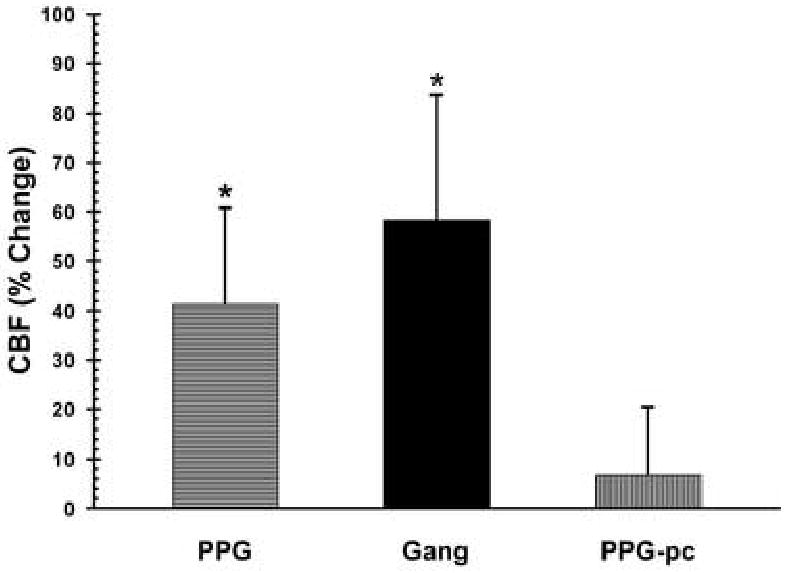
Graphic representation of changes in CBF (% change) in response to electrical stimulation of the PPG (left), ganglionic fibers from PPG (Gang; center), or the PPG after transection of ganglionic fibers (PPG-pc; right). * p<0.05
After transection of ganglionic nerve fibers as they passed through the orbital foramina, identical stimulation of the PPG elicited no change in CBF or MAP (Fig. 2 & 3). With maximal stimulation (4 volts for 80 seconds) CBF increased from 25.0 ± 3.1 LDU prior to stimulation to 26.5 ± 3.4 LDU (p>0.05). Cerebrovascular resistance did not significantly change.
Stimulating the maxillary nerve did not significantly change CBF. Resting CBF was 22.2 ± 1.8 LDU while the maximal change from baseline was 27.1 ± 3.8 LDU (p = 0.2), which was only seen with a stimulus voltage of 20 V. MAP slightly but significantly increased from a basal value of 91± 5 mmHg to a value of 97 ± 4 mmHg (p<0.03) when CBF changes were maximal. Thus, in contrast to the effect of PPG stimulation, cerebrovascular resistance (3.6 ± 0.6) at the maximal MAP did not significantly differ from basal resistance (3.5 ± 0.5).
Discussion
Our previous studies suggested that parasympathetic ganglionic fibers that innervate forebrain cerebral blood vessels are susceptible to injury and that care was needed in approaching those nerves to avoid loss of their influence on cerebrovascular tone (Talman & Nitschke Dragon, 2000). In our hands a supraorbital approach to the PPG, the source of those nerves, may have damaged the nerves and resulted in loss of cerebral vasodilatation during stimulation of the PPG. We acknowledge that others (Suzuki et al., 1990a) have successfully stimulated ganglionic fibers through an approach that included retraction of orbital contents but suggest that inadvertent damage to the nerves is more likely with such an approach. In contrast, an approach (subzygomatic) that avoided damage to the ganglionic nerves allowed expression of vasodilatation during ganglionic stimulation as has been previously reported (Okamura et al., 2002;Toda et al., 2000b;Goadsby, 1989;Seylaz et al., 1988;Suzuki et al., 1990a). Earlier studies in cat (Goadsby, 1989) had shown that vasodilatation occurs independent of any change in cerebral metabolism.
Parasympathetic innervation of cerebral vessels has been shown to provide the nitroxidergic innervation to forebrain cerebral vessels (Yoshida et al., 1993;Kimura et al., 1997), but ganglionic fibers also contain other putative transmitters such as vasoactive intestinal polypeptide and acetylcholine (Suzuki et al., 1988;Suzuki et al., 1990b). In our own studies we have concentrated on nitric oxide influences mediated by the parasympathetics. The current study, showing a prolonged dilator response suggests that actions of nitric oxide alone, with its short duration of action, cannot account dilatation mediated by stimulation of parasympathetic fibers.
Ganglionic parasympathetic nerves to cerebral vessels arise in the PPG, bilateral ganglia that lie posterior to the zygomatic arch and inferior to the orbit. Preganglionic influences to the ganglia arise from the superior salivatory nuclei, bilateral medullopontine nuclei whose neurons modulate several physiological functions including lacrimation, salivation, nasal mucosal secretion, and cerebrovascular tone (Loewy & Spyer, 1990). We have shown that preganglionic neurons of this pathway receive direct projections from the nucleus tractus solitarii and, through those projections, may receive input from peripheral arterial baroreceptor nerves (Agassandian et al., 2002). Interruption of the baroreceptor reflex arc with either peripheral or central lesions leads to profound changes in control of CBF during hypertension (Talman et al., 1994;Talman & Nitschke Dragon, 2002). With such lesions arterial blood pressure can be elevated to very high levels without producing breakthrough of autoregulation, associated marked vasodilatation, or increases in CBF. Interruption of preganglionic signals to the PPG (Agassandian et al., 2003), transection of ganglionic fibers from the PPG (Talman & Nitschke Dragon, 2000), or inhibition of synthesis of the putative transmitter, nitric oxide, released from those ganglionic fibers (Talman & Nitschke Dragon, 1995a) similarly influences autoregulation and breakthrough during hypertension in rats. In our initial attempts to confirm that ganglionic stimulation led to cerebral vasodilatation we found that an established approach did not allow demonstration of that physiological phenomenon. Ganglionic fibers arising from the PPG are very delicate and, we suspected, were susceptible to injury incurred during retraction (or removal) of the eyeball as has previously been done (Spencer et al., 1990;Talman & Nitschke Dragon, 2000). While the latter technique provided a clear exposure of the ganglia and allowed excellent retrograde transport of tracers from the ganglia into the brain stem (Spencer et al., 1990), it had been found to alter cerebrovascular regulation as had intentional interruption of the baroreflex pathway or parasympathetic ganglionic fibers to the cerebral vessels (Talman & Nitschke Dragon, 2000). In contrast, approaches to the ganglion as we describe here allow exposure of the ganglion without compromising ganglionic influences on cerebrovascular tone.
Some studies have suggested that these parasympathetic pathways confer tonic dilatory input to cerebral vessels and that their removal can lead to vasoconstriction (Toda et al., 2000a). Despite these suggestions the role played by parasympathetic nerves in control of cerebral blood flow under normal physiological conditions remains to be demonstrated. However, intriguing studies have shown that the pathway has the potential to attenuate neuronal damage produced by cerebral ischemia in that interruption of the pathway augments the volume of cerebral damage that would result from occlusion of a middle cerebral artery in rat (Kano et al., 1991). If vasoactive substances found in the PPG might serve to attenuate such damage, upregulating expression of those substances could have a beneficial effect in stroke. This study further describes a method that could be used for introducing stimulating electrodes or vectors into the PPG. That method could be used to test such benefit in experimental stroke.
Acknowledgments
This work was supported by a Veterans Affairs Merit Review and, in part, by NIH R01 HL 59593.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Agassandian K, Fazan VPS, Adanina V, Talman WT. Direct projections from the cardiovascular nucleus tractus solitarii to pontine preganglionic parasympathetic neurons: a link to cerebrovascular regulation. J Comp Neurol. 2002;452:242–254. doi: 10.1002/cne.10372. [DOI] [PubMed] [Google Scholar]
- Agassandian K, Fazan VPS, Margaryan N, Nitschke Dragon D, Riley J, Talman WT. A novel central pathway links arterial baroreceptors and pontine parasympathetic neurons in cerebrovascular control. Cell Molec Neurobiol. 2003;23:463–478. doi: 10.1023/A:1025059710382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ. Effect of stimulation of facial nerve on regional cerebral blood flow and glucose utilization in cats. Am J Physiol. 1989;257:R517–R521. doi: 10.1152/ajpregu.1989.257.3.R517. [DOI] [PubMed] [Google Scholar]
- Hara H, Zhang QJ, Kuroyanagi T, Kobayashi S. Parasympathetic cerebrovascular innervation: an anterograde tracing from the sphenopalatine ganglion in the rat. Neurosurgery. 1993;32:822–827. doi: 10.1227/00006123-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Kano M, Moskowitz MA, Yokota M. Parasympathetic denervation of rat pial vessels significantly increases infarction volume following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1991;11:628–637. doi: 10.1038/jcbfm.1991.114. [DOI] [PubMed] [Google Scholar]
- Kimura T, Yu JG, Edvinsson L, Lee TJ. Cholinergic, nitric oxidergic innervation in cerebral arteries of the cat. Brain Res. 1997;773:117–124. doi: 10.1016/s0006-8993(97)00889-5. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Spyer KM. Central regulation of autonomic functions. Oxford; New York: 1990. [Google Scholar]
- Okamura T, Ayajiki K, Fujioka H, Shinozaki K, Toda N. Neurogenic cerebral vasodilation mediated by nitric oxide. Japanese Journal of Pharmacology. 2002;88:32–38. doi: 10.1254/jjp.88.32. [DOI] [PubMed] [Google Scholar]
- Rosen S, Shelesnyak MC, Zacharias LR. Naso-genital relationship. II Pseudopregancy following extirpation of the sphenopalatine ganglion in the rat. Endocrinology. 1940;27:463–468. [Google Scholar]
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab. 1988;8:875–878. doi: 10.1038/jcbfm.1988.145. [DOI] [PubMed] [Google Scholar]
- Spencer SE, Sawyer WB, Wada H, Platt KB, Loewy AD. CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res. 1990;534:149–169. doi: 10.1016/0006-8993(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Kahrstrom J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab. 1990a;10:383–391. doi: 10.1038/jcbfm.1990.68. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular vasoactive intestinal pulypeptide-positive nerves in rat. J Cereb Blood Flow Metab. 1988;8:697–712. doi: 10.1038/jcbfm.1988.117. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and Pathways of choline Acetyltranferase-Positive parasympathetic nerve fibers to cerebral vessels in rat. J Cereb Blood Flow Metab. 1990b;10:399–408. doi: 10.1038/jcbfm.1990.70. [DOI] [PubMed] [Google Scholar]
- Talman WT, Nitschke Dragon D. Inhibition of nitric oxide synthesis extends cerebrovascular autoregulation during hypertension. Brain Res. 1995a;672:48–54. doi: 10.1016/0006-8993(94)01381-q. [DOI] [PubMed] [Google Scholar]
- Talman WT, Nitschke Dragon D. Mechanisms for preserved cerebrovascular autoregulation during hypertension in rats after sinoaortic denervation. Clin Exp Pharmacol Physiol. 1995b;22 1:S77–S79. doi: 10.1111/j.1440-1681.1995.tb02978.x. [DOI] [PubMed] [Google Scholar]
- Talman WT, Nitschke Dragon D. Parasympathetic nerves influence cerebral blood flow during hypertension in rat. Brain Res. 2000;873:145–148. doi: 10.1016/s0006-8993(00)02490-2. [DOI] [PubMed] [Google Scholar]
- Talman WT, Nitschke Dragon D. Inhibiting the nucleus tractus solitarii extends cerebrovascular autoregulation during hypertension. Brain Res. 2002;931:92–95. doi: 10.1016/s0006-8993(02)02264-3. [DOI] [PubMed] [Google Scholar]
- Talman WT, Nitschke Dragon D, Ohta H. Baroreflexes influence autoregulation of cerebral blood flow during hypertension. Am J Physiol Heart Circ Physiol. 1994;267:H1183–H1189. doi: 10.1152/ajpheart.1994.267.3.H1183. [DOI] [PubMed] [Google Scholar]
- Toda N, Ayajiki K, Tanaka T, Okamura T. Preganglionic and postganglionic neurons responsible for cerebral vasodilation mediated by nitric oxide in anesthetized dogs. J Cereb Blood Flow Metab. 2000a;20:700–708. doi: 10.1097/00004647-200004000-00007. [DOI] [PubMed] [Google Scholar]
- Toda N, Tanaka T, Ayajiki K, Okamura T. Cerebral vasodilatation induced by stimulation of the pterygopalatine ganglion and greater petrosal nerve in anesthetized monkeys. Neurosci. 2000b;96:393–398. doi: 10.1016/s0306-4522(99)00557-6. [DOI] [PubMed] [Google Scholar]
- Walters BB, Gillespie SA, Moskowitz MA. Cerebrovascular projections from the sphenopalatine and otic ganglia to the middle cerebral artery of the cat. Stroke. 1986;17:488–494. doi: 10.1161/01.str.17.3.488. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Okamura T, Kimura H, Bredt DS, Snyder SH, Toda N. Nitric oxide synthase-immunoreactive nerve fibers in dog cerebral and peripheral arteries. Brain Res. 1993;629:67–72. doi: 10.1016/0006-8993(93)90482-3. [DOI] [PubMed] [Google Scholar]


