Abstract
Background
Prior research has shown that hypoglycemia is associated with worse outcomes for the elderly, in sepsis, and in children with pneumonia. The purpose of this study was to examine whether hypoglycemia (< 70 mg/dL) is associated with increased 30-day mortality, after adjusting for potential confounders, for adults hospitalized with pneumonia.
Methods
A retrospective cohort study conducted at two tertiary teaching hospitals. Eligible subjects were admitted with a diagnosis of, and had a chest x-ray consistent with, community-acquired pneumonia. Our primary analysis was a multivariable logistic regression with the dependent variable of 30-day mortality with independent variable of hypoglycemia, diabetes, severity of illness utilizing the Pneumonia Severity Index, and pneumonia-related processes of care.
Results
Data were abstracted on 787 subjects at the two hospitals. Mortality was 8.1% at 30-days. At presentation, 55% of subjects were low risk, 33% were moderate risk, and 12% were high risk. In our cohort 2.8% (n=22) had hypoglycemia at presentation. Unadjusted mortality for those who were hypoglycemic was 27.3% vs. 8.6% for those who were not (p=0.0003). In the multivariable analysis, hypoglycemia (odds ratio 4.1, 95% confidence interval 1.4–11.7) was significantly associated with 30-day mortality.
Conclusions
After adjusting for severity of illness and other potential confounders hypoglycemia is significantly associated with 30-day mortality for patients hospitalized with pneumonia. Patients with hypoglycemia should be placed in closely monitored settings even when by pneumonia specific risk systems they would normally be discharged.
Keywords: pneumonia, hypoglycemia, mortality
Introduction
Pneumonia, along with influenza, is the eighth leading cause of death and the leading cause of infectious death in the United States [1]. Although mortality due to pneumonia decreased significantly with the introduction of antibiotics in the 1950s, since that time mortality has been stable or increasing [2]. In recent years there has been considerable interest in individual risk factors, and risk scoring systems, associated with mortality for patients with pneumonia. Although studies of sepsis, bacteremia, pediatric pneumonia, and the elderly have found that hypoglycemia is strongly associated with mortality [3–11], no prior published studies have examined the association of hypoglycemia and mortality for adult patients with pneumonia. In addition, the two most common severity systems for adult pneumonia, the pneumonia severity index [12] and CURB-65 [13], do not identify hypoglycemia as a major risk factor for mortality after pneumonia.
Our study aim was to examine whether hypoglycemia at hospital presentation is associated with increased 30-day mortality, after adjusting for potential confounders and severity of illness at presentation, for patients hospitalized with pneumonia.
Methods
This a retrospective cohort study of patients hospitalized with pneumonia at 2 academic tertiary care hospitals in San Antonio, Texas. Both hospitals are teaching affiliates of the University of Texas Health Science Center at San Antonio. The Institutional Review Board of the University Health Science Center at San Antonio approved the research protocol with exempt status.
Study Sites/Inclusion and Exclusion Criteria
We identified all patients admitted to the study hospitals between January 1, 1999 and December 1, 2002 with a primary discharge diagnosis of pneumonia (ICD-9 codes 480.0–483.99 or 485–487.0) or secondary discharge diagnosis of pneumonia with a primary diagnosis of respiratory failure (518.81) or sepsis (038.xx). Subjects were included if they were 1) greater than 18 years of age, 2) had an admission diagnosis of pneumonia, and 3) had a radiographically confirmed infiltrate or other finding consistent with pneumonia on chest x-ray or CT obtained within 24 hours of admission.
Exclusion criteria included 1) having been discharged from an acute care facility within 14 days of admission, 2) transfer after being admitted to another acute care hospital, and 3) being comfort measures only on this admission. If a subject was admitted more than once during the study period, only the first hospitalization was abstracted.
Data Abstraction
Chart review data included: demographics, comorbid conditions, physical examination findings, laboratory data, and chest radiograph reports. In addition, data on important processes of care measures for patients hospitalized with pneumonia were also abstracted: time to first dose of antibiotics, collection of blood cultures prior to antibiotic administration, and obtaining blood cultures and oxygen saturation measurement within 24 hours of presentation [14]. Antimicrobial therapy was considered guideline-concordant if it agreed with either the 2000 Infectious Diseases Society of America or 2001 American Thoracic Society guidelines [15, 16], which are similar to the recommendations from the 2007 joint guidelines from these societies [17]. Information on all outpatient medications that were either 1) reported as currently being taken by the patient at presentation, or 2) listed in the electronic medical record, were recorded.
Subjects were defined as having hypoglycemia at presentation if their initial serum glucose level (within 24 hours of admission) was <70 mg/dL [18].
Risk Adjustment
The pneumonia severity index was used to assess severity of illness at presentation [12]. The pneumonia severity index is a validated prediction rule for 30-day mortality in patients with community-acquired pneumonia. This rule is based on three demographic characteristics, five comorbid illnesses, five physical examination findings, and seven laboratory and radiographic findings from the time of presentation. Patients are classified into five risk classes with 30-day mortality ranging from 0.1% for class I to 27% for class V for patients enrolled in the PORT cohort study [12].
Outcomes
We used 30-day mortality as the primary outcome for this study. Previous research has demonstrated that 30-day mortality is primarily due to the pneumonia rather than other co-existing co-morbid conditions [19, 20]. In addition, we assessed 90-day mortality, length of hospital stay (excluding those patients who died during the hospitalization), and need for ICU admission. Mortality was assessed using information from the Texas Department of Health and Department of Veteran Affairs clinical database. Mortality status was assessed through December 2002.
Statistical Analyses
Univariate statistics were used to test the association of sociodemographic and clinical characteristics with all-cause 30-day mortality. Categorical variables were analyzed using the Chi-square test and continuous variables were analyzed using Student’s t-test. Statistical significance was set at p≤0.05. To analyze time-to-death by hypoglycemia vs. non-hypoglycemia, a graph was created using Kaplan-Meier estimated probabilities.
A multivariable logistic regression model was derived with 30-day mortality as the dependent variable, and pneumonia severity index risk classes, history of diabetes, process of care measures (initial antibiotics within 8 hours and whether antimicrobial therapy was guideline concordant), and hypoglycemia at presentation as independent variables. Multicollinearity was assessed using the variance inflation factor (VIF) with a VIF of < 5 was regarded as excluding significant interactions, and there was no significant multicollinearity [21]. All analyses were performed using STATA version 9 (STATA Corporation, College Station, Texas).
Results
Data were abstracted on 787 patients at the two hospitals. The mean age was 60 years with a standard deviation of 16 years. The population was 79% male, 84% were admitted through the emergency department, and 20% were admitted to the intensive care unit (ICU) within the first 24 hours after admission. Mortality was 9.2% at 30-days and 13.6% at 90-days. By pneumonia severity index, 52% were low risk (pneumonia severity index classes I-III), 34% were moderate risk (pneumonia severity index class IV), and 14% were high risk (pneumonia severity index class V). Regarding pneumonia-related processes of care, 28% received the initial dose of antibiotics within 4 hours of presentation and an additional 22% received the initial antibiotic dose within 8 hours, 76% of patients had blood cultures obtained within 24 hours and prior to antibiotics, and oxygenation was assessed at presentation in 91%.
In our cohort 2.8% (n=22) had hypoglycemia at presentation. Unadjusted mortality at 48 hours was 18% for those who were hypoglycemic vs. 2% for those who were not (p<0.0001), and 30-day mortality was 27% (hypoglycemic) vs. 9% (p=0.0003) (Figure 1). There was no significant difference in median length of stay (hypoglycemia 4.5 days vs. 5.0 days, p=0.7) or rate of ICU admission (14% vs. 20%, p=0.5). There was no significant difference in the rates of severe sepsis between the hypoglycemic (37%) vs. non-hypoglycemic (29%) groups (p=0.34). Of those subjects hypoglycemic at presentation 41% were diabetic vs. 29% of those who were not hypoglycemic (p=0.2), and 23% of hypoglycemic patients were on at least 1 diabetic medication at presentation vs. 21% of nonhypoglycemic patients (p=0.9). For those patients with hypoglycemia who were on anti-diabetic medications: 3 patients were on insulin only, 1 received metformin only, and 1 received both metformin and glipizide.
Figure 1.
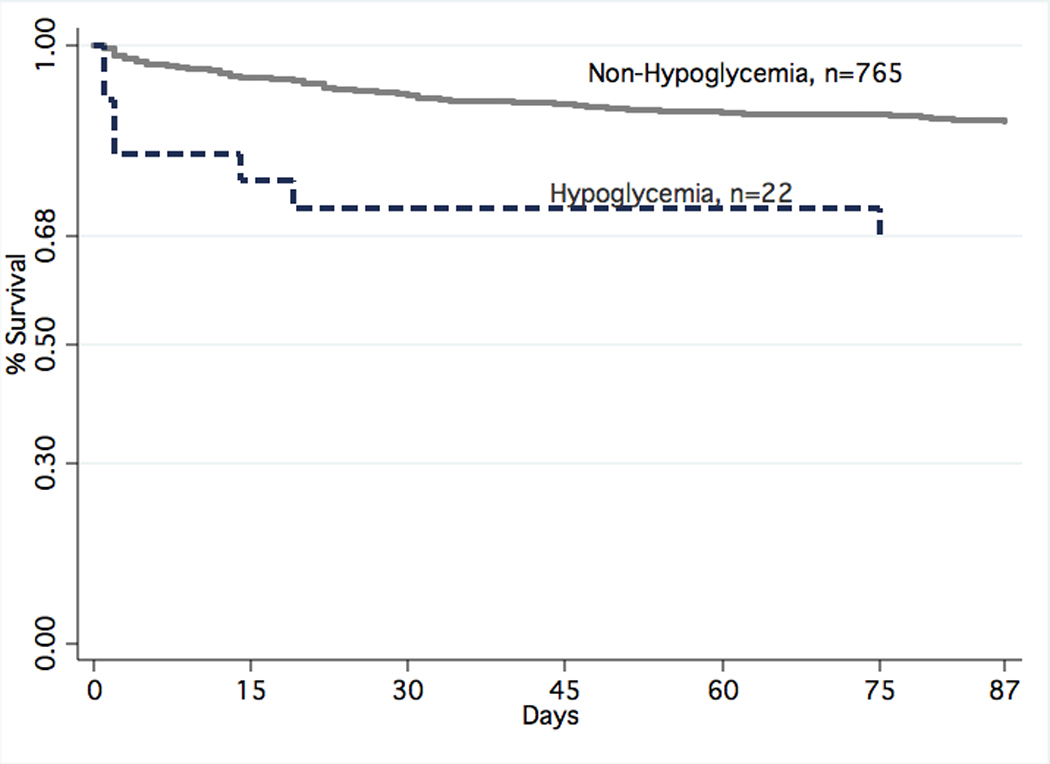
Mortality by Hypoglycemia vs. Non-Hypoglycemia for Subjects with Pneumonia (p=0.0003)
Table 1 shows the demographic factors and clinical characteristics for this population by hypoglycemia at presentation. The only factor significantly different between groups was pleural effusion at presentation (45% for those with hypoglycemia vs. 23%, p=0.02), and there was a borderline result for systolic blood pressure < 90mmHg (9% vs. 2%, p=0.06). In addition, we found no association between etiology and hypoglycemia. For example, Streptococcus Pneumoniae (2.9% vs. 2.8%, p=0.96) and Staphylococcus Aureus (2.8% vs. 0%, p=0.6), were not associated with hypoglycemia.
Table 1.
Subject Demographic and Clinical Characteristics by Hypoglycemia vs. Non-Hypoglycemia at Admission*
| Hypoglycemia | |||
|---|---|---|---|
| Variable | Yes (n=22) |
No (n= 765) |
p- value |
| Age, years mean (standard deviation) |
56.6 (17) | 60.6 (16) | 0.3 |
| Men | 15 (68) | 606 (79) | 0.2 |
| Admitted through emergency department |
18 (82) | 638 (84) | 0.8 |
| Admitted to intensive care within 24 hours |
3 (14) | 151 (19) | 0.5 |
| Preexisting Comorbid Conditions | |||
| Congestive heart failure | 2 (9) | 121 (16) | 0.4 |
| Chronic pulmonary disease | 6 (27) | 212 (27) | 0.96 |
| Stroke | 3 (13) | 102 (13) | 0.97 |
| Chronic liver disease | 4 (18) | 90 (12) | 0.4 |
| History of malignancy | 1 (5) | 38 (5) | 0.9 |
| Renal insufficiency | 4 (18) | 83 (11) | 0.3 |
| Diabetes | 9 (41) | 221 (29) | 0.2 |
| Alcoholism | 3 (13) | 81 (11) | 0.7 |
| History, Physical, Laboratory, and Radiographic Data | |||
| Altered mental status | 4 (18) | 81 (11) | 0.3 |
| Respiratory rate > 30 per minute |
2 (9) | 80 (10) | 0.8 |
| Systolic blood pressure < 90 mmHg |
2 (9) | 19 (2) | 0.06 |
| Heart rate > 125 per minute | 1 (5) | 104 (14) | 0.2 |
| Temperature < 95° or > 104° | 1 (5) | 20 (3) | 0.6 |
| Arterial pH < 7.35 | 3 (14) | 46 (6) | 0.1 |
| Arterial oxygenation saturation < 90% | 4 (19) | 172 (22) | 0.6 |
| Hematocrit < 30% | 4 (19) | 68 (9) | 0.1 |
| Serum blood urea nitrogen > 30 mg/dL |
7 (32) | 161 (21) | 0.2 |
| Serum sodium < 130 meq/L | 1 (5) | 115 (15) | 0.17 |
| Pleural effusion on chest radiograph | 10 (45) | 179 (23) | 0.02 |
| Bacteremia | 6(20) | 109 (14) | 0.39 |
| Pneumonia Severity Index | |||
| Class I-III | 11 (50) | 398 (52) | |
| Class IV | 7 (32) | 259 (34) | |
| Class V | 4 (18) | 108 (14) | 0.9 |
Data are presented as number (%) or mean (standard deviation)
Figure 2 shows outcomes by levels of serum glucose (<70, 71–150, 151–250, >250 mg/dL). Both 30-day and 90-day mortality were significantly higher in the hypoglycemia group however ICU admissions were increased in the higher serum glucose groups (151–250 and >250).
Figure 2.
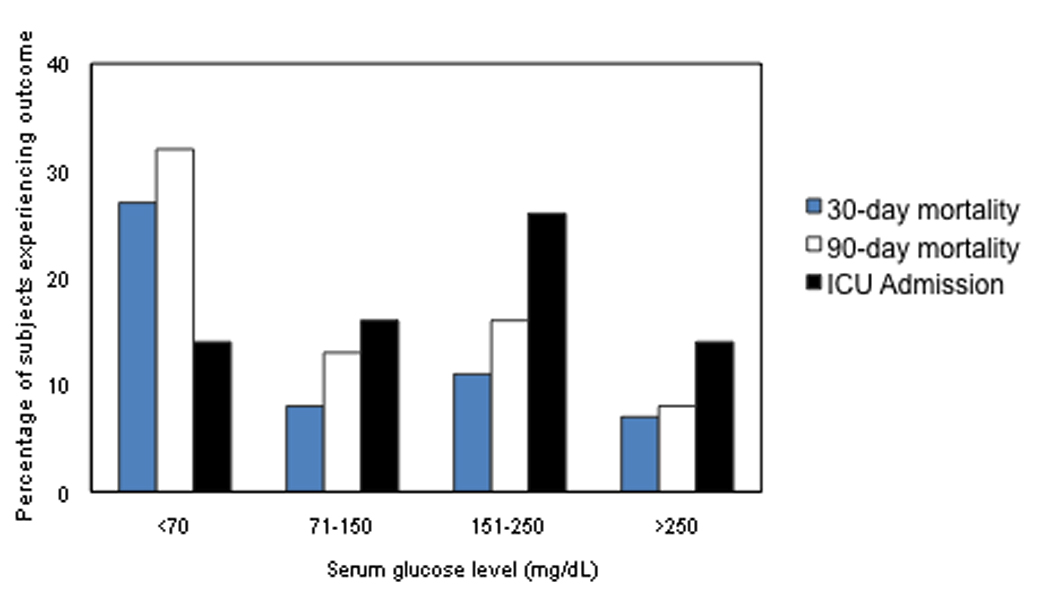
Outcomes by Serum Glucose Level at Presentation
In the multivariable analysis (Table 2), after adjusting for potential confounders including severity of illness and diabetes, hypoglycemia (odds ratio 4.1, 95% confidence interval 1.4–11.7) was significantly associated with 30-day mortality. To exclude those with potential iatrogenic hypoglycemia we excluded those patients (n=4) with hypoglycemia who were taking either insulin or glipizide and repeated the model. We found similar results with hypoglycemia (odds ratio 3.4, 95% confidence 1.3–9.1) still associated with increased mortality.
Table 2.
Results of the multivariable logistic regression model with 30-day mortality as the dependent variable
| Variable | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| PSI class | 2.1 | 1.6–2.6 |
| Initial antibiotics within 8 hours of admission |
0.98 | 0.6–1.6 |
| Use of guideline concordant antibiotics |
0.77 | 0.4–1.4 |
| Diabetes | 0.96 | 0.6–1.7 |
| Hypoglycemia at presentation |
4.1 | 1.4–11.7 |
Discussion
We found that hypoglycemia at hospital presentation was significantly associated with increased 30-day mortality for subjects hospitalized with pneumonia, despite hypoglycemic patients not having significantly different results on most other factors in the pneumonia severity index except for pleural effusion. These results suggest that patients with pneumonia who present with hypoglycemia should be monitored closely (e.g., inpatient observation rather than outpatient care), and that profound hypoglycemia should be a “critical value” that should direct patients to be admitted for close observation.
An association between hypoglycemia and severe infections and mortality has been previously described, including an association among patients who do not have a diagnosis of diabetes. Bacterial infections for which this has been reported include pneumococcus [4, 6], E. coli [18], Haemophilus influenzae type b [6], and Streptococcus pyogenes [6]. In a retrospective study of 70 patients with pneumococcal bacteremia, mortality was 100% in the 3 adult patients presenting with hypoglycemia, compared with 25% (p=0.046) in the non-hypoglycemic patients [4], but other risk factors for hypoglycemia may have confounded these results. A larger study involving 995 patients with E. coli bacteremia, however, did demonstrate an independent association between admission hypoglycemia and in-hospital mortality. In this study hypoglycemic patients were 4.7 times greater risk of dying compared with non-hypoglycemic patients. This association was independent of other variables [18].
Our study is one of the first to show an association between hypoglycemia at presentation and mortality in patients with pneumonia. Among the 2.8% of patients with pneumonia and hypoglycemia, 30-day mortality was 27.3% compared with 8.6% (p=0.0003) in the non-hypoglycemic group. Hypoglycemia was an independent risk factor for mortality even after adjustment for other variables including the pneumonia severity index and other comorbid conditions (e.g., diabetes). In fact, the mean PSI scores were equivalent in the hypoglycemic group compared with the non-hypoglycemic group (89 vs. 91, p=0.8), indicating that this commonly used scoring method might underestimate the mortality risk for this subset of high risk patients.
In our patient population, hypoglycemia was related to diabetes only 41% of the time, and was independent of diabetes as a risk factor for mortality. This result is consistent with a study by Kagansky et al [8] showing that the prevalence of diabetes is less than 50% among elderly hospitalized patients admitted to internal medicine or geriatric services who happen to have a glucose value less than 70 mg/dL. In this group of patients insulin and sulfonylurea use were risk factors for hypoglycemia, but other predisposing factors such as sepsis, malignancy and low albumin levels were shown to have a stronger association. In fact, sepsis was 10 times more common in hypoglycemic patients than non-hypoglycemic controls, compared with a 2-fold risk for insulin or sulfonylurea use. Our study shows the association between hypoglycemia and disease severity in pneumonia even in a younger group of patients, with a median age among hypoglycemic patients of 56.6 years versus 60.6 years in the non-hypoglycemic group.
The pathophysiology of hypoglycemia may be due to a combination of mechanisms. Infections may cause hypoglycemia by increased glucose utilization by macrophage-rich tissues including liver, lung, spleen, ileum, and skin, and from depressed hepatic gluconeogenesis that may result from decreased sensitivity to stress hormones and/or adrenal failure [22].
Our study was retrospective and therefore subject to the recognized limitations of this study design, including bias and incomplete data. We carefully assembled our cohort from complete patient discharge data to avoid ascertainment bias. Additionally, during chart abstraction we encountered a very small amount (<5%) of missing data (e.g., race/ethnicity). Our sample was predominantly men, due to the inclusion of a VA hospital. It is possible that women may have differential responsiveness to hypoglycemia or to treatment for pneumonia as compared to men; however, no suggestion of this has been reported in studies of pneumonia or infection-related hypoglycemia. In addition, we were unable to control for anti-diabetes medications received during the admission. Next, there were only a small percentage of patients with hypoglycemia so it is possible that our findings are due to chance. However as similar findings have been demonstrated in numerous other infectious conditions we believe this is unlikely. In addition, we are unable to examine issues such as whether patients with health care associated pneumonia vs. community-acquired pneumonia have differential risk for hypoglycemia, and we are also unable to examine factors such a adrenal insufficiency, c-peptide or insulin levels. Finally, we were unable to examine serum glucose levels after the first 24 hours, so are unable to assess whether patients became hypoglycemic after admission, and if so, whether they also are at higher risk of mortality.
These results suggest the presence of a sub-group of pneumonia patients previously unidentified by severity of illness scores that are at greatly increased risk of mortality. The association between hypoglycemia and increased mortality suggests that patients with pneumonia who present with hypoglycemia may require a higher level of care than might be suggested by their risk score alone (e.g., hospitalization rather than outpatient care for those who are PSI class I or II or ICU level care rather than ward). Further studies regarding the need to assess hypoglycemia for all patients with pneumonia are warranted.
Acknowledgments
Dr. Mortensen was supported by a Department of Veteran Affairs Veterans Integrated Service Network 17 new faculty grant and a Howard Hughes Medical Institute faculty-start up grant 00378-001. Dr. Anzueto is, in part, supported by the NHLBI grant NO1-HR-16153. Dr. Restrepo is supported by a Department of Veteran Affairs Veterans Integrated Service Network 17 new faculty grant and a CTSA Award Number (KL2 RR025766). This material is the result of work supported with resources and the use of facilities at the South Texas Veterans Health Care System. The funding agencies had no role in conducting the study, or role in the preparation, review, or approval of the manuscript.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kung HC, et al. Deaths: Final data for 2005. Hyattsville, MD: National Center for Health Statistics; 2008. [PubMed] [Google Scholar]
- 2.Gilbert K, Fine MJ. Assessing prognosis and predicting patient outcomes in community-acquired pneumonia. Seminars in Respiratory Infections. 1994;9(3):140–152. [PubMed] [Google Scholar]
- 3.Don M, et al. Hyper- and hypoglycemia in children with community-acquired pneumonia. J Pediatr Endocrinol Metab. 2008;21(7):657–664. doi: 10.1515/jpem.2008.21.7.657. [DOI] [PubMed] [Google Scholar]
- 4.Jan IS, et al. Hypoglycemia associated with bacteremic pneumococcal infections. Int J Infect Dis. 2008 doi: 10.1016/j.ijid.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Luber S, Meldon S, Brady W. Hypoglycemia presenting as acute respiratory failure in an infant. Am J Emerg Med. 1998;16(3):281–284. doi: 10.1016/s0735-6757(98)90103-6. [DOI] [PubMed] [Google Scholar]
- 6.Miller SI, et al. Hypoglycemia as a manifestation of sepsis. Am J Med. 1980;68(5):649–654. doi: 10.1016/0002-9343(80)90250-8. [DOI] [PubMed] [Google Scholar]
- 7.Ali NA, et al. Glucose variability and mortality in patients with sepsis. CritCare Med. 2008;36(8):2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagansky N, et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med. 2003;163(15):1825–1829. doi: 10.1001/archinte.163.15.1825. [DOI] [PubMed] [Google Scholar]
- 9.Mendoza A, Kim YN, Chernoff A. Hypoglycemia in hospitalized adult patients without diabetes. Endocr Pract. 2005;11(2):91–96. doi: 10.4158/EP.11.2.91. [DOI] [PubMed] [Google Scholar]
- 10.Rattarasarn C. Hypoglycemia in sepsis: risk factors and clinical characteristics. J Med Assoc Thai. 1997;80(12):760–766. [PubMed] [Google Scholar]
- 11.Shilo S, et al. Hypoglycemia in hospitalized nondiabetic older patients. J Am Geriatr Soc. 1998;46(8):978–982. doi: 10.1111/j.1532-5415.1998.tb02752.x. [DOI] [PubMed] [Google Scholar]
- 12.Fine MJ, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 13.Lim WS, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meehan TP, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278(23):2080–2084. [PubMed] [Google Scholar]
- 15.Niederman MS, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett JG, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31(2):347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandell LA, et al. Infectious diseases society of america/american thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alamgir S, Volkova NB, Peterson MW. Prognostic value of low blood glucose at the presentation of E. coli bacteremia. Am J Med. 2006;119(11):952–957. doi: 10.1016/j.amjmed.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Mortensen EM, et al. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis. 2003;37(12):1617–1624. doi: 10.1086/379712. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen EM, et al. Causes of death for patients with community- acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162(9):1059–1064. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 21.Meyers LS, Gamst G, Guarino AJ. Applied Multivariate Research: Design and Interpretation. Sage Publications; 2006. [Google Scholar]
- 22.Maitra SR, Wojnar MM, Lang CH. Alterations in tissue glucose uptake during the hyperglycemic and hypoglycemic phases of sepsis. Shock. 2000;13(5):379–385. doi: 10.1097/00024382-200005000-00006. [DOI] [PubMed] [Google Scholar]


