Abstract
The hepatitis C virus NS3 protein contains a N-terminal serine protease and a C-terminal helicase that unwinds RNA or DNA duplexes. The HCV NS3 protein is the target for several antiviral drugs in clinical trials, which inhibit the protease function. A method is reported to simultaneously monitor the helicase and protease function of the NS3 protein in a single reaction using fluorescence spectroscopy and a single chain recombinant protein where NS3 is fused to its protease activator NS4A. The method monitors both activities together in real time and is amenable to high throughput screening. This new procedure could be used to identify compounds that inhibit both the helicase and protease activity of NS3.
Keywords: Helicase, protease, ATPase, high throughput screening, Antiviral agents
1. Introduction
The hepatitis C virus (HCV) causes a common liver disease affecting 2-3% of the world's population and is frequently called a “silent” killer because patients show few signs of infection (1). As the disease progresses over a period of decades, when patients might unknowingly transmit the blood-borne positive sense RNA virus to others, hepatitis C patients frequently develop fibrosis, cirrhosis, or liver cancer. At this late stage of the disease, a liver transplant is the only option for survival, and as a result, HCV infection is presently the most common cause for liver transplantation in many parts of the world. Current HCV therapies, which combine pegylated interferon and ribavirin, are quite effective for patients infected with certain HCV strains, but they are costly and produce debilitating side effects that are usually worse than the symptoms caused by the virus itself. In addition, patients infected with HCV genotypes most common in North America, patients with advanced disease, transplant recipients and patients co-infected with HIV frequently do not respond to current therapies.
Compounds that inhibit HCV encoded enzymes are being developed as new antivirals to treat HCV infection. Two of the leading candidates in clinical trials are the protease inhibitors telaprevir (VX-950, Vertex) and boceprevir (SCH 503034, Schering-Plough). Telaprevir and boceprevir inhibit a protease formed from two of ten proteins encoded by the 9,600 nucleotide long HCV RNA genome – nonstructural protein 3 (NS3) and nonstructural protein 4A (NS4A). The HCV genome encodes one main open reading frame, which is translated into a single ~3,000 amino acid long polyprotein. The NS3/NS4A protease, one other viral protease and cellular proteins process the polyprotein into the mature peptides needed for replication. The catalytic triad of this serine protease resides near the N-terminus of NS3 and NS4A activates the enzyme by binding NS3.
In addition to being a protease, NS3 is also an ATP-fueled helicase capable of separating duplex RNA or DNA. The two catalytic activities can be expressed separately as recombinant proteins containing truncated NS3 fragments, with the protease residing in the N-terminal NS3 fragment and the helicase residing in the C-terminal fragment. Mutants lacking helicase function still cleave the polyprotein but the virus is no longer viable (2). There are three lines of evidence that the two activities are linked. First, without the protease, NS3 unwinds RNA less efficiently. Second, RNA binds more tightly to NS3 when the protease is present possibly because the protease adds positively charged surfaces that may stabilize RNA (3). Third, mutations on the helicase domain alter binding of various protease inhibitors (4). It is therefore reasonable to believe that small molecules can be discovered that simultaneously inhibit both NS3 helicase and protease activities, and as proof of this concept, some RNA aptamers have already been designed with such properties (5).
To aid in the discovery and analysis of dual NS3 helicase/protease inhibitors, we have developed a method to simultaneously monitor both peptide cleavage and DNA unwinding catalyzed by NS3 in a single reaction. The simultaneous measurement of NS3 protease and helicase is conceptually simple because continuous fluorescence-based assays have been developed to separately measure either NS3 protease (6) or NS3 helicase (7). Combining these assays is technically challenging for two reasons. First, purification of the full-length NS3 protein in combination with NS4A is difficult because of their highly insoluble nature; in cells NS4A transverses membranes. Second, helicase catalyzed unwinding is only apparent at low ionic strength, and NS3 protease assays must be performed in high salt concentrations because NS4A binds NS3 through hydrophobic interactions. The use of a protein in which a His-tagged truncated NS4A peptide is covalently fused with full-length NS3 (8) essentially solved these problems. This single chain complex, called here “scNS3-4A,” differs from the native complex because NS4A, which is normally attached via a cleavable linker to the NS3 C-terminus, is instead attached to the N-terminus of NS3 with a stable peptide. We have found that under conditions where NS3 helicase is most active (without salt at slightly acidic pH), the protease of scNS3-4A can be monitored if glycerol, dithiothreitol (DTT) and the detergent β-octyl glucoside (β-OG) are added to the reactions. These components do not drastically alter detection sensitivity in helicase assays.
In the assay below, both the helicase and protease substrates are designed to directly mimic parts of the HCV genome (Fig. 1A). The depsipeptide used to measure protease is based on the junction between the NS4A and NS4B proteins, and the molecular beacon helicase substrate is based on a stem loop found at the end of NS5B (Fig. 1A). While the protease substrate is widely used and now part of an assay Kit (SensoLyte™ 490 HCV Protease Assay Kit, Anaspec, San Jose, CA), our new molecular beacon based helicase assay is noteworthy because it differs from other fluorescent helicase assays in three critical ways. First, all modifications are made on only one strand of the helicase substrate. Second, the assay monitors a decrease in fluorescence, which is noteworthy because data can be easily converted to percentage duplex remaining simply by calculating fractional fluorescence (F/Fo) after subtracting background fluorescence. Third, the products form hairpins, making the reaction essentially irreversible. Intramolecular hairpin formation is thermodynamically favored even at relatively high DNA concentrations. Only at DNA concentrations above ~50 nM is the intermolecular reaction favored. This last point is a key advantage over helicase assays described elsewhere in this volume because the molecular beacon assay does not require high concentrations of substrate traps, which could influence observed reaction rates. The simultaneous assay can be performed either in cuvettes or in 96 or 384-well microplates.
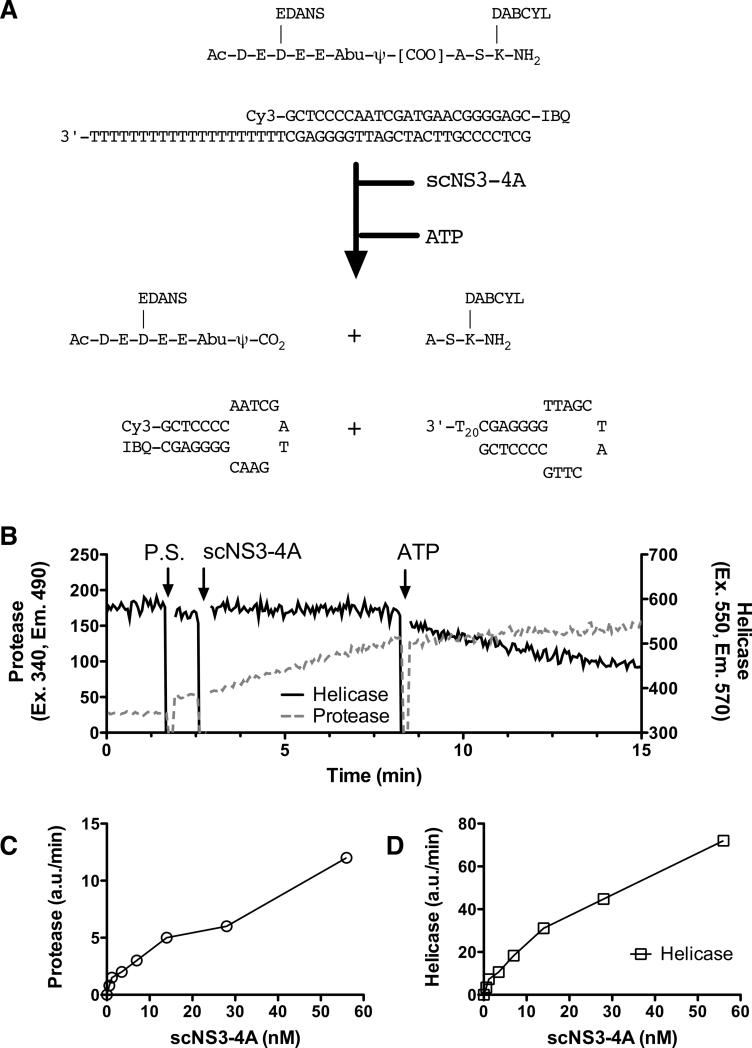
Fig. 1.
The simultaneous HCV protease/helicase assay. (A) Schematic diagram of the assay. The protease assay (6) and the helicase assay (7) have been previously reported. (B) Assay performed as describe in Methods. At the indicated times, protease substrate (P. S., 2 μM final concentration), scNS3-4A (10 nM) and ATP (1 mM) were added to the solution. The reaction was monitored at the excitation wavelength of 355 nm and the emission wavelength of 485 nm (protease substrate) and at excitation 550 nm and emission 570 nm (helicase substrate) using a Varian Cary Eclipse Fluorescence spectrophotometer. (C) Initial rates (arbitrary units/minute) of protease reactions as a function of scNS3-4A concentration. (D) Initial rates of helicase reactions as a function of scNS3-4A concentration.
Numerous potent specific inhibitors for NS3 protease are commercially available, like the NS3 product-based inhibitor Ac-Asp-D-Gla-Leu-Ile-β-cyclohexyl-Ala-cys-OH (Bachem, Torrance, CA), which gives an IC50 of 8.9 nM in this assay. However, only a few HCV helicase inhibitors are available at this time. Typical non-hydrolyzable nucleotide analogs are weak binders, and only a few nucleotide-like compounds display any potent inhibitory activity. Examples with IC50's in the micromolar range include a nucleoside analog with a diaminodihydro-triazine substituent replacing the 5-amino group of the nucleoside metabolite 5-aminoimidazole-4-carboxamide-1- β-D -ribofuranoside (9), a nucleotide mimic called QU663, (10), 4,5,6,7-Tetrabromobenzotriazole (TBBT), 5,6-dichloro-1-(β-D-ribofuranosyl)benzotriazole (DRBT) (11), and a short peptide (HCVpep) that mimics a conserved helicase motif (12). Of these compounds, only NS3pep (12), DRBT and TBBT (13) have been confirmed to have antiviral activity in assays using HCV replicons.
We have used the assay below to evaluate the potency of many of these available HCV helicase inhibitors (7), but we have yet to find any inhibitors that bind more tightly than the helicase substrate, which binds with nanomolar affinity. Compared with various known inhibitors, the oligonucleotide, dT20, a representative compound that binds in place of the RNA or DNA substrate, is still the most potent inhibitor we know with an IC50 of 30 nM. Only NS3pep (12) is comparable with an IC50 of 100 nM. By comparison, non-hydrolyzable ATP analogs bind much weaker. For example, the IC50 of α,β-methylene-ATP is 140 μM, and β,γ-imido-ATP binds even weaker with an IC50 of 1.8 mM (7).
It is tempting to speculate that compounds could be found that influence both the helicase and protease activities of NS3, and this assay was designed to help discover such compounds. The many atomic structures available for NS3, in particular, the structure of scNS3-4A (14) and the structure of HCV helicase bound to an oligonucleotide (15) support this contention. In the full-length NS3-NS4A complex, two domains compose the protease core, and three other domains form the helicase core. If the helicase is viewed like a “Y,” one strand of RNA binds below two RecA-like domains, and ATP likely binds between the two upper RecA-like domains. The protease domains pack with the helicase domains to bury the catalytic triad in a cleft formed behind the ATP and DNA-binding clefts. Based on electrostatic analyses of NS3 structures, our laboratory has suggested that in addition to binding below the RecA-like domains, RNA might also bind in the cleft separating the helicase from the protease. If RNA binds near the protease active site, then compounds binding to this region could affect both the helicase and protease activities.
2. Materials
2.1 Cloning scNS3-4A
A recombinant DNA clone of HCV containing the entire NS3 region. The primers below have been used successfully with HCV genotype 1b strains J4 and con1. Primer sequences may need to be adjusted if a distantly related HCV genotype is used as the source of the NS3 protein (see Note 1).
Plasmid vector pET28b (Novagen, Madison, WI).
An upstream PCR primer, which encodes an NdeI restriction enzyme site, NS4A residues 21-32, and a gly-ser-gly-ser linker. 5'-GC GAT ATA CAT ATG GGT TCT GTT GTT ATT GTT GGT AGA ATT ATT TTA TCT GGT AGT GGT AGT ATC ACG GCC TAC TCC CAA-3'.
A downstream primer, which contains an EcoRI site. (5'-GCG CGC GAA TTC GGT CAA GTG ACG ACC TCC AGG TCA GCC GAC ATG C-3').
Molecular biology reagents including Nde1 and EcoR1 (New England Biolabs, Ipswich, MA), PCR reagents, thermocycler, DNA agarose gel electrophoresis apparatus, gel extraction kit (Qiagen), and a DNA ligation kit (Novagen).
2.2 Purification of scNS3-4A
Buffer A: 25 mM HEPES, 500 mM NaCl, 10 mM β-mercaptoethanol, 20% Glycerol, pH 8.
Sonifier Cell Disruptor (Branson, Danbury, CT).
Pre-charged nickel-nitrilotriacetic acid (NiNTA) resin pre-equilibrated with buffer A.
An ~200 ml Sephacryl S-300 HR column (GE Healthcare Bio-Sciences, Piscataway, NJ) pre-equilibrated with buffer A.
Polyacrylamide gel electrophoresis apparatus.
2.3 The simultaneous helicase/protease assay
2X reaction buffer: 50 mM MOPS pH 6.5, 3 mM MgCl2, 60% glycerol, 20 mM DTT, 0.4% β-octyl glucoside: prepare fresh.
HCV Protease substrate (Anaspec, San Jose, CA).
Helicase substrate short strand: 5’-Cy3-GCTCC CCAAT CGATG AACGG GGAGC-IBQ-3’. IBQ is Iowa Black quencher from Integrated DNA Technologies (Coralville, Iowa).
Helicase substrate long strand 5’-GCTCC CCGTT CATCG ATTGG GGAGC TTTTT TTTTT TTTTT TTTTT-2’.
3. Methods
3.1 Cloning and expression of scNS3-4A
Set up and perform four 25 μl PCRs using the HCV primers and templates using standard protocols (see Note 1).
Combine PCRs, add 20 μl of 6X agarose gel loading buffer and load into one large well of a 1% DNA agarose gel. After electrophoresis, visualize DNA with ethidium bromide staining, excise the 1,964 bp band and extract DNA from the gel with the Qiagen Kit.
Digest purified amplicon with NdeI and EcoRI and in a second tube digest 2 μg of pET28. After 2 hrs at 37 °C, purify digested DNA again using gel electrophoresis.
Ligate purified vector and insert using DNA ligase, and transform competent cells that lack the T7 RNA polymerase (e.g. Novablue or BL21).
Purify DNA from transformed colonies and confirm the presence of the inserted DNA using restriction enzyme analysis. Confirm the sequence of clones bearing the insert using DNA sequencing.
After sequence verification, use the resulting plasmid (p28scNS3-4A) to transform a strain carrying a functional T7 polymerase (e.g. BL21(DE3)).
Pick a single kanamycin resistant colony from a fresh plate and inoculate 2 ml of LB media with 50 μg/ml kanamycin.
When the 2 ml culture is slightly turbid (OD600 ~ 0.1), transfer to a 4 L flask containing 1 L of LB-Kan. Incubate with vigorous shaking at 37 °C. Monitor OD600.
When OD600 reaches 1.0, transfer the flask to a room temperature (23 °C) shaker.
Add 5 ml of 200 mM IPTG, and continue shaking for 3 hours at room temperature. Harvest cells using centrifugation. Store pellet at −80 °C.
3.1 Purification of scNS3-4A
Thaw frozen cells and suspend in 23.9 mls of Buffer A. Add 0.25 ml of 10% β-OG (0.1% final concentration) and 1.0 ml of 500 mM imidazole (20 mM final concentration). All subsequent purification step should be performed at 4 °C.
Sonicate cell suspension 30 seconds, rest 1 minute, and repeat twice.
Spin at 14,000 rpm for 15 min, and filter supernatant through glass fiber pre-filter.
Take ~1ml of NiNTA beads, place in ~25 ml column and wash with 10 ml of buffer A.
Suspend beads in 1 ml of buffer A and transfer to the crude extract. Mix gently for 30 min at 4 °C, and pour solution into a small column (~25 ml). Discard the flow through.
Wash NiNTA column with 10 mls of Buffer A supplemented with an additional 0.5 M NaCl.
Wash NiNTA column with 10 mls of Buffer A supplemented with 40 mM imidazole.
Elute scNS3-4A with 1.5 ml of Buffer A supplemented with 500 mM imidazole (see Note 2). Load eluent on Sephacryl S-300 HR column. Wash column with buffer A at ~0.2 ml/min.
Collect 4 ml fractions, while monitoring absorbance at 280 nm.
Analyze 10 μl aliquots of peak A280 fractions using 10% SDS PAGE (see Note 3).
Combine fractions containing the 71 kDa scNS3-4A protein (see Note 4).
Dialyze into 50 mM Tris, 0.1 M NaCl, 1 mM EDTA, 30 % Glycerol, 0.1 mM DTT (pH 7.4). Calculate concentration from A280 of using an extinction coefficient of 68.4 mM−1 cm−1, (see Note 5). Concentrate if desired (see Note 6), and store aliquots at −80 °C.
3.3 The simultaneous helicase/protease assay
Prepare helicase substrate by diluting molecular beacon and long oligonucleotide to 20 μM in 20 mM TrisCl, pH 7. Heat to 95 °C, and cool slowly to room temperature. Although not absolutely necessary, the best results are obtained if the annealed substrate is purified from the free oligonucleotides using non-denaturing polyacrylamide gel electrophoresis. The free Cy3 molecular beacon and annealed helicase substrate are clearly visible without staining.
Reactions can be performed in microplates, or cuvettes at temperatures up to 45 °C. For a 100 μl reaction, add appropriate volumes of diluted helicase and protease substrate to 50 μl of 2X reaction buffer and water such that the final concentration of helicase substrate is 5 nM and the protease substrate is 2 μM.
Initiate protease reactions were by adding scNS3-4A protein (1- 250 nM).
Initiate helicase reactions by adding ATP to a final concentration of 1 mM.
Continuously monitor fluorescence using a fluorescence spectrophotometer at the excitation wavelength of 355 nm and the emission wavelength of 485 nm (protease substrate) and at excitation 550 nm and emission 570 nm (helicase substrate) (Fig. 1).
Inhibitors of NS3 protease are commercially available and can be used as positive controls in high throughput screens. For the helicase, simple oligonucleotides (e.g. dT20: 5’-TTTTT TTTTT TTTTT TTTTT TTTTT-3’) are potent inhibitors with IC50 values in the nanomolar range (see Note 7).
Acknowledgement
This study was supported by a grant from the National Institutes of Health (AI052395).
Footnotes
Upstream and downstream primers may need to be adjusted if they do not amplify DNA from the desired HCV isolate. Use appropriate computer software to align the primer to the desired sequence. If the 3’ ends of the primers sequences do not completely match the desired HCV sequence, adjust them accordingly. The desired NS3 sequence should also be checked for internal NdeI and EcoRI sites, and if they are present other restriction sites should be incorporated into primers. An alternative upstream site is NheI, which is immediately downstream from the NdeI site of pET28. Alternate downstream sites are BamHI, SacI, SalI, HindIII, EagI, NotI, and XhoI.
Some investigators regularly use NS3 proteins purified in a single step using immobilize metal affinity chromatography, but in our experience such preparations are typically contaminated with RNase and other nucleases, which can confound helicase assays. We recommend assaying all stages of the purification with a commercial RNAse assay, such as RNaseAlert® Kit (Ambion, Austin, TX).
Further purification of scNS3-4A might be necessary, but is challenging because the protein tends to aggregate in the absence of high salt. Affinity chromatography using poly(U)-agarose (Sigma, St. Louis, MO) is recommended.
The His-tag can be removed after purification if desired using Thrombin. We have not yet found any impact of the His-tag in any assays, however.
The extinction coefficient was calculated based on the sequence of the scNS3-4A protein from the HCV genotype 1b(con1) strain using the program Sequence Analysis (http://informagen.com/SA/). Adjust if another HCV strain is used as the source.
Care should be taken with concentration because the protein tends to aggregate. Ultra-filtration and centrifugal concentrators are not recommended. We typically concentrate the protein by surrounding a dialysis bag with high molecular weight polyethylene glycol until a desired amount of liquid is removed.
Many compounds in libraries quench one of the two fluorophore used here. To assay such compounds other fluorescently labeled protease substrates are commercially available (Anaspec). Similarly a different molecular beacon helicase substrate in which Cy3 is substituted with a different fluorophore could be employed.
References
- 1.McHutchison JG. Understanding hepatitis C. Am J Manag Care. 2004;10:S21–9. [PubMed] [Google Scholar]
- 2.Lam AM, Frick DN. Hepatitis C virus subgenomic replicon requires an active NS3 RNA helicase. J. Virol. 2006;80:404–411. doi: 10.1128/JVI.80.1.404-411.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frick DN. The hepatitis C virus NS3 protein: a model RNA helicase and potential drug target. Curr Issues Mol Biol. 2007;9:1–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl G, Sandstrom A, Akerblom E, Danielson UH. Effects on protease inhibition by modifying of helicase residues in hepatitis C virus nonstructural protein 3. FEBS J. 2007;274:5979–5986. doi: 10.1111/j.1742-4658.2007.06120.x. [DOI] [PubMed] [Google Scholar]
- 5.Umehara T, Fukuda K, Nishikawa F, Kohara M, Hasegawa T, Nishikawa S. Rational design of dual-functional aptamers that inhibit the protease and helicase activities of HCV NS3. J Biochem (Tokyo) 2005;137:339–347. doi: 10.1093/jb/mvi042. [DOI] [PubMed] [Google Scholar]
- 6.Taliani M, Bianchi E, Narjes F, Fossatelli M, Urbani A, Steinkuhler C, De Francesco R, Pessi A. A continuous assay of hepatitis C virus protease based on resonance energy transfer depsipeptide substrates. Anal. Biochem. 1996;240:60–67. doi: 10.1006/abio.1996.0331. [DOI] [PubMed] [Google Scholar]
- 7.Belon CA, Frick DN. Monitoring Helicase Activity With Molecular Beacons. BioTechniques. 2008;45:433–440. doi: 10.2144/000112834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe AY, Chase R, Taremi SS, Risano C, Beyer B, Malcolm B, Lau JY. A novel recombinant single-chain hepatitis C virus NS3-NS4A protein with improved helicase activity. Protein Sci. 1999;8:1332–1341. doi: 10.1110/ps.8.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ujjinamatada RK, Baier A, Borowski P, Hosmane RS. An analogue of AICAR with dual inhibitory activity against WNV and HCV NTPase/helicase: synthesis and in vitro screening of 4-carbamoyl-5-(4,6-diamino-2,5-dihydro-1,3,5-triazin-2-yl)imidazole-1-beta -D-ribofuranoside. Bioorg Med Chem Lett. 2007;17:2285–2288. doi: 10.1016/j.bmcl.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maga G, Gemma S, Fattorusso C, Locatelli GA, Butini S, Persico M, Kukreja G, Romano MP, Chiasserini L, Savini L, Novellino E, Nacci V, Spadari S, Campiani G. Specific Targeting of Hepatitis C Virus NS3 RNA Helicase. Discovery of the Potent and Selective Competitive Nucleotide-Mimicking Inhibitor QU663. Biochemistry. 2005;44:9637–9644. doi: 10.1021/bi047437u. [DOI] [PubMed] [Google Scholar]
- 11.Borowski P, Deinert J, Schalinski S, Bretner M, Ginalski K, Kulikowski T, Shugar D. Halogenated benzimidazoles and benzotriazoles as inhibitors of the NTPase/helicase activities of hepatitis C and related viruses. Eur. J. Biochem. 2003;270:1645–1653. doi: 10.1046/j.1432-1033.2003.03540.x. [DOI] [PubMed] [Google Scholar]
- 12.Gozdek A, Zhukov I, Polkowska A, Poznanski J, Stankiewicz-Drogon A, Pawlowicz JM, Zagorski-Ostoja W, Borowski P, Boguszewska-Chachulska AM. NS3 peptide, a novel potent Hepatitis C virus NS3 helicase inhibitor, its mechanism of action and antiviral activity in the replicon system. Antimicrob. Agents Chemother. 2008;52:393–401. doi: 10.1128/AAC.00961-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paeshuyse J, Vliegen I, Coelmont L, Leyssen P, Tabarrini O, Herdewijn P, Mittendorfer H, Easmon J, Cecchetti V, Bartenschlager R, Puerstinger G, Neyts J. Comparative in vitro anti-hepatitis C virus activities of a selected series of polymerase, protease, and helicase inhibitors. Antimicrob. Agents Chemother. 2008;52:3433–3437. doi: 10.1128/AAC.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao N, Reichert P, Taremi SS, Prosise WW, Weber PC. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Structure Fold Des. 1999;7:1353–1363. doi: 10.1016/s0969-2126(00)80025-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim JL, Morgenstern KA, Griffith JP, Dwyer MD, Thomson JA, Murcko MA, Lin C, Caron PR. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure. 1998;6:89–100. doi: 10.1016/s0969-2126(98)00010-0. [DOI] [PubMed] [Google Scholar]