Abstract
Background
Erythropoietin mitigates myocardial damage and improves ventricular performance after experimental ischemic injury. This study assessed safety and efficacy markers relevant to the biological activity of recombinant human erythropoietin (rHuEpo) in patients with acute myocardial infarction.
Methods
We conducted a prospective, placebo-controlled, randomized, double-blind trial to determine the effects of intravenous rHuEpo (200 U/kg daily for 3 consecutive days) on measures of platelet and endothelial cell activation, soluble Fas ligand, and peripheral blood mononuclear cell (PBMC) expression of angiogenesis signaling proteins in 44 subjects with acute myocardial infarction (MI) treated with aspirin and clopidogrel after successful percutaneous coronary intervention.
Results
rHuEpo did not alter bleeding time, platelet function assay closure time, von Willebrand factor levels, soluble P-selectin, or soluble Fas ligand levels when compared with placebo. By contrast, rHuEpo significantly increased expression of erythropoietin receptor, vascular endothelial growth factor receptor Flt-1, and phosphorylated phosphatidylinositol 3-kinase in PBMC’s when compared with placebo (all p<0.05).
Conclusions
In acute MI patients treated with aspirin and clopidogrel, short-term administration of rHuEpo did not alter markers of platelet and endothelial cell activation associated with thrombosis, yet did increase expression of angiogenesis signaling proteins in PBMC’s when compared with placebo. These data provide preliminary evidence of safety and biologic activity of rHuEpo at this dosing and support continued enrollment in ongoing efficacy trials.
Keywords: platelet function, clinical trial, angiogenesis, erythropoiesis stimulating proteins, acute coronary syndrome
Eyrythropoietin, a glycoprotein growth hormone secreted by renal juxtaglomerular cells in response to reduced oxygen tension, plays a critical role in the homeostatic regulation of red cell mass.1, 2 Erythropoietin promotes red blood cell maturation in the bone marrow by binding to homodimeric receptors coupled to anti-apoptotic Akt and JAK-STAT signaling pathways in erythroid precursors.1
Erythropoietin receptors are also expressed in non-hematopoietic tissues, including human adult myocardium and vascular endothelial cells.3, 4 Administration of a single dose of exogenous erythropoietin in rodent, rabbit, and canine models of myocardial infarction and ischemia reperfusion injury is associated with reduced infarct size, decreased post-injury ventricular remodeling, and preservation of ventricular pump function.5–7 These salutary effects are attributed to erythropoietin-mediated reduction of apoptotic cell death, increased mobilization of marrow-derived circulating endothelial progenitor cells, and increased angiogenesis in the peri-infarct ischemic zone.5–9
Other known biological effects of erythropoietin include platelet and endothelial cell activation and increased risk of thrombotic events during chronic use in anemic populations.10–12 We demonstrated previously that administration of recombinant human erythropoietin (rHuEpo) at doses up to 200 U/kg daily for 3 consecutive days did not inhibit the anti-platelet effects of aspirin or clopidogrel in normal subjects.13 The effects of short-term administration of rHuEpo on platelet and endothelial cell activation in patients with acute myocardial infarction are unknown.
The current study was undertaken to investigate the safety and potential mechanisms of action of rHuEpo in acute coronary syndrome patients treated with aspirin and clopidogrel. We hypothesized that clinically-indicated anti-platelet therapy would mitigate potential pro-thrombotic effects of short-term rHuEpo administration and thus enhance the risk/benefit ratio in this clinical setting. We further hypothesized that short-term administration of rHuEpo would be associated with measurable changes in surrogate markers of the biological activity of erythropoietin related to apoptosis and angiogenesis signaling. To test these hypotheses, we conducted a prospective randomized double-blind single center trial in subjects with acute myocardial infarction treated with aspirin and clopidogrel to determine the effects of rHuEpo vs. placebo on: (1) in vivo and in vitro measures of platelet and endothelial cell activation, (2) soluble Fas ligand levels as a marker of myocyte apoptosis, and (3) peripheral blood mononuclear cell expression of proteins associated with angiogenesis signaling.
Methods
Study Population
Adults >21 years of age with ST segment elevation myocardial infarction (electrocardiographic changes with elevated cardiac enzymes and/or compatible history), treated with successful percutaneous coronary intervention of the culprit lesion (post-procedure TIMI grade 3 flow), and clinically indicated aspirin (325 mg daily) and clopidogrel therapy (300–600 mg loading dose determined by the attending cardiologist) were eligible for the study. Key exclusion criteria included hemodynamic instability, time from chest pain onset to percutaneous coronary intervention >16 hours or to screening >24 hours, use of intravenous thrombolytic agents, target lesion at vessel bifurcation or within previous coronary stent, cocaine-associated myocardial infarction, planned additional revascularization procedures, ongoing treatment with an erythropoiesis stimulating protein, history of deep venous thrombosis or pulmonary embolism, or history of cancer within 2 years. The study protocol (clinicaltrials.gov Identifier NCT00367991) was approved by the Human Investigation Committee of Yale University. All subjects provided written informed consent.
Study Protocol
Forty-four eligible subjects were randomized to receive intravenous rHuEpo (200 U/kg daily for 3 consecutive days, Amgen Inc., Thousand Oaks CA) or matching placebo. The study drug dose was selected based on safety and efficacy reports in experimental models, pilot studies in human subjects with stroke or myocardial infarction, and our published report demonstrating that this dose of rHuEpo did not mitigate the anti-platelet action of either aspirin or clopidogrel in healthy human subjects.7, 13–16 The Yale Investigational Drug Service generated and held the randomization code and allocated double-blinded study drug through an electronic ordering system (2:1 randomization scheme (active treatment:placebo), stratified by gender and by the time from chest pain to percutaneous coronary intervention (<6 hours vs. ≥6 hours).
The primary safety outcome variables, bleeding time and platelet function assay closure time, were determined before the first dose of study drug, immediately after the last dose of study drug, and 7 days after the last dose of study drug. Secondary safety measures (soluble P-selectin, von Willebrand factor, complete blood count, and vital signs) and soluble Fas ligand levels were determined at the same time points. Additional venous blood (10 ml) was collected after the last dose of study drug for isolation of peripheral blood mononuclear cells. At 30 days after discharge, the study subject was contacted by telephone and questioned about any recurrent hospitalization.
Bleeding Time
Bleeding time (sec) was performed as an integrated measure of in vivo platelet function and tissue hemostasis in a standardized fashion by one operator on the volar aspect of the forearm using a sterile, disposable automated incision device (Surgicutt Adult, Cardinal Health Inc., Montgomery, NY) as previously described. 13 Bleeding time for healthy subjects in our laboratory is 388±64 seconds.
Platelet Function Assay
In vitro assessment of high-shear platelet aggregation, determined as closure time (seconds), was measured with the PFA-100 (Dade Behring Co. LLC, Newark, DE) according to the manufacturer’s instructions. PFA closure times are closely correlated with in vitro optical platelet aggregometry measurements and have been tentatively linked to clinical outcomes in some disease populations.18–20 Two cartridges were used to determine the closure time, each holding a membrane with a central aperture and coated with collagen and either epinephrine (PFA-EPI) or adenosine diphosphate (PFA-ADP).
Laboratory Studies
Complete blood cell count (ABX MICROS 60 CS/CT-16 Hematology Analyzer, High Technology, Inc) was measured in the clinical laboratory at Yale-New Haven Hospital. Serum erythropoietin (R & D Systems, Inc. Minneapolis, MN), plasma soluble Fas ligand, plasma soluble P-selectin (R & D Systems, Inc. Minneapolis, MN), and plasma von Willebrand factor (American Diagnostics Inc., Stamford CT), were measured with commercially available enzyme-linked immunosorbent assays (ELISA) in the Yale General Clinical Research Center core laboratory. Peripheral blood mononuclear cells were isolated by density gradient centrifugation. Total protein from isolated peripheral blood mononuclear cells was extracted in 400 μL ice-cold lysis buffer [7 mol/L urea, 2 mol/L thiourea, 1% ditheiothreitol (DTT), 2%3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 0.8% Pharmalyte (pH 3–10)] and protease inhibitor cocktail on ice. Lysed cells were centrifuged for 15 min at 10,000 × g at 4 °C, and the supernatant fluid (cell lysate) was used for protein concentration measurement with the Bio-Rad protein assay (Bio-Rad, Munich, Germany) using bovine serum albumin as a standard.21 The final protein concentration of our samples was 1–2mg/ml. The concentration of erythropoietin receptor, vascular endothelial growth factor receptor Flt-1 (R & D Systems, Inc. Minneapolis, MN) and phosphorylated phosphatidylinositol 3-kinase protein (Panomics, Inc. Redwood City, CA) was measured by ELISA and normalized to the total protein (ng/μg).
Data Analysis
Continuous variables are summarized as mean±SD or median (interquartile range (IQR)) depending on the normality of the distribution (determined with Shapiro Wilk test). Baseline characteristics were compared between treatment groups with Student’s unpaired t-test, Rank-sum test, or Pearson’s chi-squared test. Changes between treatment groups over time were analyzed with general estimated equations with adjustment of standard errors for the clustered data of the repeated measures design (Stata Version 8.2, College Station TX). For all analyses, a two-tailed p<0.05 was used to infer statistical significance. The pre-specified primary endpoint was change in bleeding time. The study sample size provided >80% power to detect a clinically meaningful 20% decrease in bleeding time (240 seconds) between active treatment and placebo with one-tailed alpha=0.05.22
Study Support
This study was supported solely by grants from the American Heart Association and National Heart Lung and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Study sample characteristics
All randomized subjects received 3 doses of study drug according to protocol and completed study measurements before and immediately after the last dose of study drug (Figure 1). Two subjects from each treatment group did not undergo planned studies 7 days after study drug. No subjects were lost to follow-up for determination of adverse events at 1 month after study drug administration. Pre-treatment clinical characteristics of the study sample did not differ between groups (Table 1).
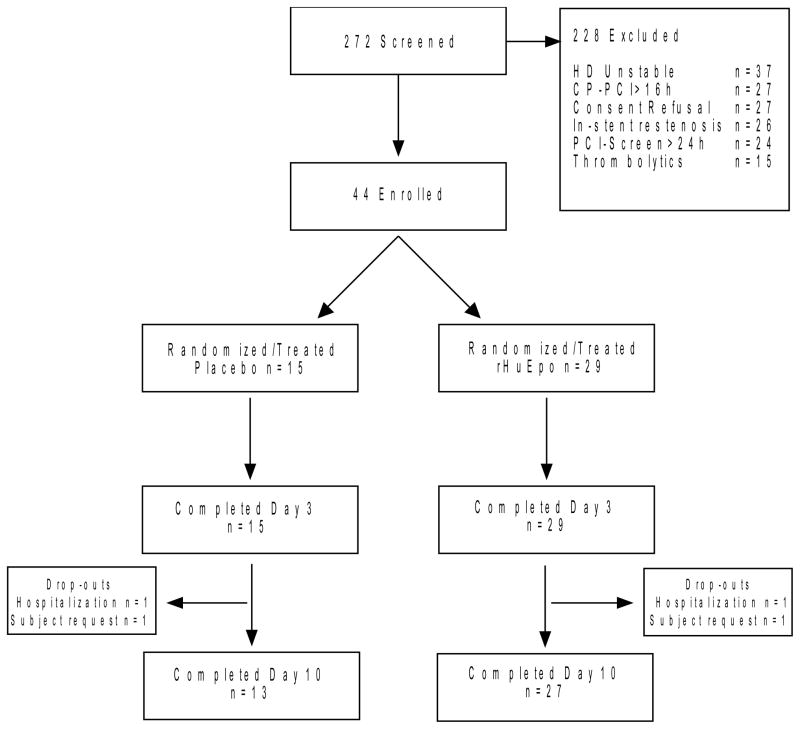
Figure 1.
Summary of study screening, most common criteria for exclusion, study enrollment, randomization and study drug allocation, and reasons for withdrawal from study. Legend CP-PCI=Time elapsed between onset of chest pain and percutaneous coronary intervention; HD=Hemodynamically; PCI-Screen=Time elapsed between percutaneous coronary intervention and study screening.
Table 1.
Pre-treatment clinical characteristics of study subjects. Values are expressed as proportions, mean±SD for continuous variables with normal distribution and median (interquartile range) for continuous variables with non-normal distributions.
| All (n=44) | Placebo (n=15) | rHuEPO (n=29) | p-value | |
|---|---|---|---|---|
| Age (yrs) | 56±11 | 53±9 | 58±12 | 0.21 |
| Gender (% male) | 70 | 67 | 72 | 0.69 |
| Body mass index (kg/m2) | 28.4±4.9 | 27.6±5.5 | 28.8±4.9 | 0.71 |
| Systolic blood pressure (mmHg) | 123±12 | 123±15 | 123±10 | 0.85 |
| Diastolic blood pressure (mmHg) | 69±9 | 70±9 | 68±9 | 0.37 |
| Heart rate (min−1) | 70±8 | 70±8 | 69±8 | 0.71 |
| Time chest pain to PCI (hrs) | 5.0 (9.0) | 5.5 (10) | 5.0 (9.5) | 0.49 |
| Peak cardiac enzyme level | ||||
| Total creatine kinase (U/L) | 714 (744) | 817 (1926) | 652 (489) | 0.56 |
| Troponin-I (ng/ml) | 18 (40) | 31 (73) | 17 (29) | 0.42 |
| PCI target coronary artery (%) | 0.42 | |||
| Left anterior descending | 32 | 47 | 24 | |
| Left circumflex | 23 | 20 | 23 | |
| Right/Posterior descending | 45 | 33 | 52 | |
| Drug-eluting stent (%) | 42 | 50 | 27 | 0.14 |
| Stent diameter (mm) | 3 (0.8) | 3 (0.8) | 2.9 (0.8) | 0.72 |
| Intravenous agents (%) | ||||
| Heparin | 66 | 62 | 68 | 0.69 |
| Eptifibatide | 58 | 62 | 57 | 0.79 |
| Bivalirudin | 34 | 38 | 32 | 0.69 |
| Concomitant Medications (%) | ||||
| Aspirin | 100 | 100 | 100 | - |
| Clopidogrel | 100 | 100 | 100 | - |
| RAS inhibition | 73 | 73 | 72 | 0.93 |
| Beta-Blocker | 98 | 93 | 100 | 0.19 |
| Statin | 95 | 93 | 96 | 0.71 |
Legend: PCI=percutaneous coronary intervention; RAS (renin-angiotensin system) inhibition includes angiotensin converting enzyme inhibitor, angiotensin receptor blocker, and/or aldosterone receptor blocker.
Erythropoietin levels
The time (expressed as median (IQR)) from percutaneous intervention to administration of the first dose of study drug did not differ between groups (placebo 18 (16) vs. rHuEpo 16 (9) hrs, p=0.54). Pre-treatment serum erythropoietin levels also did not differ between study groups (placebo 13.6 (11.0) vs. rHuEpo 19.0 (18.2) mIU/ml, p=0.22). In subjects who received placebo, erythropoietin levels declined significantly compared to pre-treatment values immediately after the last dose of study drug and at 7 days after study drug (8.4 (13.8) and 11.1 (11.7) mIU/ml respectively, both p<0.05). In subjects who received rHuEpo, erythropoietin levels were significantly increased immediately after the last dose of study drug, and then fell significantly at 7 days after the last dose of study drug when compared to pre-treatment values (4790 (2331) and 12.6 (7.3) mIU/ml respectively, both p<0.001 vs. pre-treatment values).
Platelet function
Bleeding time was increased above normal values prior to study drug administration in both treatment groups, consistent with the effects of concomitant treatment with aspirin, clopidogrel, and other agents (Table 2). Bleeding time remained above normal values at each post-study drug measurement time point in both groups, but did not differ between treatment groups after study drug administration (Table 2). Likewise, platelet function assay closure times, plasma soluble P-selectin levels and plasma von Willebrand factor levels did not differ between groups at any time point (Table 2).
Table 2.
In vivo and in vitro measures of platelet and endothelial cell activation (median (interquartile range)) by treatment group before study drug, immediately after the last dose of study drug, and 7 days after the last dose of study drug.
| Placebo | rHuEpo | |
|---|---|---|
| Bleeding Time (sec) | ||
| Before study drug | 1050 (150) | 1080 (120) |
| Immediately after study drug | 1020 (150) | 1020 (150) |
| 7 days after study drug | 990 (150) | 1020 (120) |
| PFA-Epi closure time (sec) | ||
| Before study drug | 272 (81) | 274 (83) |
| Immediately after study drug | 251 (116) | 281 (128) |
| 7 days after study drug | 293 (53) | 279 (166) |
| PFA-ADP closure time (sec) | ||
| Before study drug | 105 (203) | 152 (171) |
| Immediately after study drug | 83 (22) | 81 (43) |
| 7 days after study drug | 94 (42) | 96 (45) |
| Plasma von Willebrand Factor (mU/ml) | ||
| Before study drug | 1018 (1508) | 857 (1154) |
| Immediately after study drug | 566 (1383) | 855 (1123) |
| 7 days after study drug | 600 (923) | 824 (898) |
| Plasma P-selectin (ng/ml) | ||
| Before study drug | 90 (23) | 82 (30) |
| Immediately after study drug | 82 (19) | 81 (46) |
| 7 days after study drug | 90 (31) | 90 (31) |
Legend: PFA-Epi=Platelet function assay with collagen/epinephrine; PFA-ADP=Platelet function assay with collagen/adenosine diphosphate
Soluble Fas ligand levels
Soluble Fas ligand levels did not differ between treatment groups before study drug administration (placebo 45±12 vs. rHuEpo 56±19 pg/ml, p=0.07), immediately after the last dose of study drug (placebo 47±12 vs. rHuEpo 58±20), and at 7 days after the last dose of study drug (placebo 60±18 vs. rHuEpo 63±22 pg/ml, p=0.12 for treatment group by time interaction).
Peripheral blood mononuclear cell protein expression
Expression of erythropoietin receptor, vascular endothelial growth factor receptor Flt-1, and phosphorylated phosphatidylinositol 3-kinase were significantly increased in subjects who received rHuEpo when compared with placebo (erythropoietin receptor: placebo 88 (152) vs. rHuEpo 472 (356) ng/μg, p<0.001; Flt-1: placebo 17 (16) vs. rHuEpo 30 (23) ng/μg, p=0.016; phosphorylated phosphatidylinositol 3-kinase: placebo 0.25±0.06 vs. rHuEpo 0.32±0.11 ng/μg, p=0.045).
Tolerability and adverse events
Hematocrit, platelet count, systolic blood pressure, diastolic blood pressure, and resting heart rate did not differ between treatment groups before or after administration of study drug (Table 3). There were no clinically detected In-stent thrombosis events or deaths reported during the 1-month follow-up period. One subject in the rHuEpo group with a previous history of heart failure was hospitalized for recurrent heart failure 7 days after completion of study drug. One subject in the placebo group developed stent thrombosis just distal (<5 mm) to the site of previous stent placement after cessation of anti-platelet therapy 3 weeks after administration of study drug.
Table 3.
Safety measures by treatment group (mean±SD) before study drug, immediately after the last dose of study drug, and 7 days after the last dose of study drug.
| Placebo | rHuEpo | |
|---|---|---|
| Hematocrit (%) | ||
| Before study drug | 38.7±4.1 | 37.9±5.2 |
| Immediately after study drug | 39.8±4.7 | 38.5±5.4 |
| 7 days after study drug | 40.1±3.6 | 40.8±4.5 |
| Platelet count (x103/mm3) | ||
| Before study drug | 260±72 | 229±53 |
| Immediately after study drug | 254±65 | 232±49 |
| 7 days after study drug | 343±72 | 274±79 |
| Systolic Blood Pressure (mmHg) | ||
| Before study drug | 123±15 | 123±10 |
| Immediately after study drug | 120±12 | 119±10 |
| 7 days after study drug | 120±10 | 122±8 |
| Diastolic Blood Pressure (mmHg) | ||
| Before study drug | 70±9 | 68±9 |
| Immediately after study drug | 67±8 | 67±7 |
| 7 days after study drug | 67±8 | 70±7 |
| Heart Rate (min−1) | ||
| Before study drug | 70±8 | 69±8 |
| Immediately after study drug | 69±6 | 69±7 |
| 7 days after study drug | 69±7 | 69±7 |
Discussion
Administration of rHuEpo 200 U/kg for 3 consecutive days to patients with acute myocardial infarction treated with aspirin and clopidogrel after successful percutaneous coronary intervention was not associated with changes in either in vivo or in vitro measures of platelet or endothelial cell activation, changes in soluble Fas ligand, or increased early incidence of thrombotic events. Consistent with its known spectrum of biological activity, rHuEpo significantly increased levels of erythropoietin receptor protein, vascular endothelial growth factor receptor Flt-1 protein, and phosphorylated phosphatidylinositol 3-kinase in peripheral blood mononuclear cells when compared with placebo.
The current study is the first to investigate the potential interaction between rHuEpo and anti-platelet agents in patients with acute coronary syndromes. rHuEpo therapy is known to reverse uremic platelet dysfunction and decrease bleeding time in patients with end-stage kidney disease and during chronic use is associated with increased risk of myocardial infarction in patients with end-stage renal disease and after coronary artery bypass surgery.10, 11, 23–27 The mechanisms that contribute to pro-thrombotic effects of rHuEpo are thought to be mediated by increased red cell mass, increased marrow release of reticulated platelets, endothelial cell activation, activation of tyrosine kinase-dependent signal transduction pathways, and increased basal and stimulated cytosolic calcium levels in platelets.23, 24, 28–31 The absence of detectable effects of rHuEpo on selected measures of platelet and endothelial cell activation in the current study suggests that pro-thrombotic effects of rHuEpo may be dose- and/or time-dependent, or may be offset by the combined use of aspirin and clopidogrel.
rHuEpo administration significantly increased expression of erythropoietin receptor, vascular endothelial growth factor receptor Flt-1, and phosphorylated phosphotidylinositol 3-kinase in peripheral blood mononuclear cells when compared with placebo. These findings demonstrate that the selected dose of rHuEpo is associated with measurable effects consistent with its known biological activity.32, 33 In experimental models of myocardial ischemia reperfusion injury and infarction, the beneficial effects of rHuEpo on ventricular remodeling and pump function are strongly associated with evidence of increased mobilization of endothelial progenitor cells and increased angiogenesis in the peri-infarction myocardium.7, 34–36 These cytoprotective effects of erythropoietin are mediated in part by Akt activation and increased expression of erythropoietin and vascular endothelial growth factor receptors in myocytes and vascular endothelial cells, and are blocked by pharmacological inhibition of phosphatidylinositol 3-kinase.37–41 The pattern of specific protein expression after rHuEpo observed in peripheral blood mononuclear cells in our study is in accord with these previous reports.
We selected bleeding time as the primary outcome safety measure since erythropoietin has previously been reported to decrease bleeding time in uremic patients and normal subjects.13, 23, 25, 26 Although we observed no significant effects of rHuEpo on bleeding time or other in vitro measures of platelet and endothelial cell activation, it is uncertain to what extent such markers can predict the thrombotic risk of short-term rHuEpo in acute coronary syndrome patients. The PFA closure time is relatively insensitive to clopidogrel as compared to optical aggregometry; thus, although the bleeding time and P-selectin results were consistent with the lack of a rHuEpo antagonist effect, the PFA results cannot be used to determine whether rHuEpo specifically inhibited the clopidogrel response.20 In accord with 2 previous open-label pilot studies in acute MI populations, we observed no evidence of clinical thrombosis or change in hemoglobin levels in association with rHuEpo.15, 42 Larger study populations with sufficient power to assess clinical outcomes are needed to estimate thrombotic risk. Such data are forthcoming as there are currently four active worldwide studies registered on the clinicaltrials.gov website (registry numbers NCT00390832, NCT00449488, NCT00378352, NCT00648089) that are investigating the clinical safety and efficacy of rHuEpo (total doses ranging 60,000U–100,000U over 1–3 days) in acute myocardial infarction populations.43 In a separate randomized clinical trial of 525 post-stroke patients, a preliminary report indicated an increased mortality rate in subjects treated with rHuEpo (40,000U intravenously daily for 3 days) when compared with placebo (16% vs. 9%, respectively).44 Information regarding concomitant use of thrombolytic agents, anti-platelet agents, and anticoagulant agents in this trial is not available. Although this preliminary report is of concern, we found no evidence of a pro-thrombotic effect of rHuEpo at a lower dose (mean total dose 51,000U) in acute myocardial infarction patients treated with aspirin and clopidogrel. The current data support continued enrollment in ongoing clinical trials with comparable rHuEpo dosing to further characterize the safety and therapeutic potential of rHuEpo in patients with acute coronary syndromes.
Acknowledgments
This work was supported by Grant-In-Aid 0555844T from the American Heart Association Heritage Affiliate, National Institutes of Health HL K24 04024, and National Center of Research Resources Clinical and Translational Science Award Grant Number UL1 RR024139. There was no pharmaceutical industry support for this study.
Footnotes
Conflict of Interest Disclosures
Dr. Katz has received consulting fees from Amgen, Inc. No other author reports any financial or other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jelkmann W. Molecular biology of erythropoietin. Intern Med. 2004;43(8):649–59. doi: 10.2169/internalmedicine.43.649. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38(2):415–25. doi: 10.1053/ajkd.2001.26111. [DOI] [PubMed] [Google Scholar]
- 3.Depping R, Kawakami K, Ocker H, Wagner JM, Heringlake M, Noetzold A, et al. Expression of the erythropoietin receptor in human heart. J Thorac Cardiovasc Surg. 2005;130(3):877–8. doi: 10.1016/j.jtcvs.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91(9):3974–8. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, et al. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci U S A. 2003;100(20):11612–7. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112(7):999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirata A, Minamino T, Asanuma H, Fujita M, Wakeno M, Myoishi M, et al. Erythropoietin enhances neovascularization of ischemic myocardium and improves left ventricular dysfunction after myocardial infarction in dogs. J Am Coll Cardiol. 2006;48(1):176–84. doi: 10.1016/j.jacc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Bogoyevitch MA. An update on the cardiac effects of erythropoietin cardioprotection by erythropoietin and the lessons learnt from studies in neuroprotection. Cardiovasc Res. 2004;63(2):208–16. doi: 10.1016/j.cardiores.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141(1):14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 10.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369(9559):381–8. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 11.Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006;113(20):2454–61. doi: 10.1161/CIRCULATIONAHA.105.583666. [DOI] [PubMed] [Google Scholar]
- 12.Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–14. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 13.Tang YD, Rinder HM, Katz SD. Effects of recombinant human erythropoietin on antiplatelet action of aspirin and clopidogrel in healthy subjects: results of a double-blind, placebo-controlled randomized trial. Am Heart J. 2007;154(3):494 e1–7. doi: 10.1016/j.ahj.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Moon C, Krawczyk M, Paik D, Lakatta EG, Talan MI. Cardioprotection by recombinant human erythropoietin following acute experimental myocardial infarction: dose response and therapeutic window. Cardiovasc Drugs Ther. 2005;19(4):243–50. doi: 10.1007/s10557-005-3189-6. [DOI] [PubMed] [Google Scholar]
- 15.Lipsic E, van der Meer P, Voors AA, Westenbrink BD, van den Heuvel AF, de Boer HC, et al. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20(2):135–41. doi: 10.1007/s10557-006-7680-5. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8(8):495–505. [PMC free article] [PubMed] [Google Scholar]
- 17.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 18.Frossard M, Fuchs I, Leitner JM, Hsieh K, Vlcek M, Losert H, et al. Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation. 2004;110(11):1392–7. doi: 10.1161/01.CIR.0000141575.92958.9C. [DOI] [PubMed] [Google Scholar]
- 19.Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110(19):e489–93. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 20.Favaloro EJ. Clinical utility of the PFA-100. Semin Thromb Hemost. 2008;34(8):709–33. doi: 10.1055/s-0029-1145254. [DOI] [PubMed] [Google Scholar]
- 21.Westmark CJ, Malter JS. Up-regulation of nucleolin mRNA and protein in peripheral blood mononuclear cells by extracellular-regulated kinase. J Biol Chem. 2001;276(2):1119–26. doi: 10.1074/jbc.M009435200. [DOI] [PubMed] [Google Scholar]
- 22.Payne DA, Hayes PD, Jones CI, Belham P, Naylor AR, Goodall AH. Combined therapy with clopidogrel and aspirin significantly increases the bleeding time through a synergistic antiplatelet action. J Vasc Surg. 2002;35(6):1204–9. doi: 10.1067/mva.2002.122027. [DOI] [PubMed] [Google Scholar]
- 23.Cases A, Escolar G, Reverter JC, Ordinas A, Lopez-Pedret J, Revert L, et al. Recombinant human erythropoietin treatment improves platelet function in uremic patients. Kidney Int. 1992;42(3):668–72. doi: 10.1038/ki.1992.333. [DOI] [PubMed] [Google Scholar]
- 24.Zhou XJ, Vaziri ND. Defective calcium signalling in uraemic platelets and its amelioration with long-term erythropoietin therapy. Nephrol Dial Transplant. 2002;17(6):992–7. doi: 10.1093/ndt/17.6.992. [DOI] [PubMed] [Google Scholar]
- 25.Moia M, Mannucci PM, Vizzotto L, Casati S, Cattaneo M, Ponticelli C. Improvement in the haemostatic defect of uraemia after treatment with recombinant human erythropoietin. Lancet. 1987;2(8570):1227–9. doi: 10.1016/s0140-6736(87)91849-6. [DOI] [PubMed] [Google Scholar]
- 26.el-Shahawy MA, Francis R, Akmal M, Massry SG. Recombinant human erythropoietin shortens the bleeding time and corrects the abnormal platelet aggregation in hemodialysis patients. Clin Nephrol. 1994;41(5):308–13. [PubMed] [Google Scholar]
- 27.Johnson & Johnson Pharmaceutical Research & Development LLC. Safety of Erythropoietin Receptor Agonists (ERAs) in Patients With Cancer (available for public disclosure without redaction) 2004 In; http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037B2_02_JohnsonJohnson-Procrit.pdf.
- 28.Van Geet C, Van Damme-Lombaerts R, Vanrusselt M, de Mol A, Proesmans W, Vermylen J. Recombinant human erythropoietin increases blood pressure, platelet aggregability and platelet free calcium mobilisation in uraemic children: a possible link? Thromb Haemost. 1990;64(1):7–10. [PubMed] [Google Scholar]
- 29.Diaz-Ricart M, Etebanell E, Cases A, Lopez-Pedret J, Castillo R, Ordinas A, et al. Erythropoietin improves signaling through tyrosine phosphorylation in platelets from uremic patients. Thromb Haemost. 1999;82(4):1312–7. [PubMed] [Google Scholar]
- 30.Marrero MB, Venema RC, Ma H, Ling BN, Eaton DC. Erythropoietin receptor-operated Ca2+ channels: activation by phospholipase C-gamma 1. Kidney Int. 1998;53(5):1259–68. doi: 10.1046/j.1523-1755.1998.00887.x. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe PC, Desai ZR, Morris TC. Increase in mean platelet volume in patients with chronic renal failure treated with erythropoietin. J Clin Pathol. 1994;47(2):159–61. doi: 10.1136/jcp.47.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102(4):1340–6. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 33.Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2003 doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 34.Prunier F, Pfister O, Hadri L, Liang L, Del Monte F, Liao R, et al. Delayed erythropoietin therapy reduces post-MI cardiac remodeling only at a dose that mobilizes endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2007;292(1):H522–9. doi: 10.1152/ajpheart.00357.2006. [DOI] [PubMed] [Google Scholar]
- 35.van der Meer P, Lipsic E, Henning RH, Boddeus K, van der Velden J, Voors AA, et al. Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infarction. J Am Coll Cardiol. 2005;46(1):125–33. doi: 10.1016/j.jacc.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Westenbrink BD, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28(16):2018–27. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 37.Nakano M, Satoh K, Fukumoto Y, Ito Y, Kagaya Y, Ishii N, et al. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ Res. 2007;100(5):662–9. doi: 10.1161/01.RES.0000260179.43672.fe. [DOI] [PubMed] [Google Scholar]
- 38.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, et al. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308(4):990–4. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- 39.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98(11):1405–13. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 40.Hirata A, Minamino T, Asanuma H, Sanada S, Fujita M, Tsukamoto O, et al. Erythropoietin just before reperfusion reduces both lethal arrhythmias and infarct size via the phosphatidylinositol-3 kinase-dependent pathway in canine hearts. Cardiovasc Drugs Ther. 2005;19(1):33–40. doi: 10.1007/s10557-005-6895-1. [DOI] [PubMed] [Google Scholar]
- 41.George J, Goldstein E, Abashidze A, Wexler D, Hamed S, Shmilovich H, et al. Erythropoietin promotes endothelial progenitor cell proliferative and adhesive properties in a PI 3-kinase-dependent manner. Cardiovasc Res. 2005;68(2):299–306. doi: 10.1016/j.cardiores.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Liem A, van de Woestijne AP, Bruijns E, Roeters van Lennep HW, de Boo JA, van Halteren HK, et al. Effect of EPO administration on myocardial infarct size in patients with non-STE acute coronary syndromes; results from a pilot study. Int J Cardiol. 2009;131(2):285–7. doi: 10.1016/j.ijcard.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 43.Belonje AM, Voors AA, van Gilst WH, Anker SD, Slart RH, Tio RA, et al. Effects of erythropoietin after an acute myocardial infarction: rationale and study design of a prospective, randomized, clinical trial (HEBE III) Am Heart J. 2008;155(5):817–22. doi: 10.1016/j.ahj.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 44.United States Food and Drug Administration. [Accessed on April 27, 2009];Early Communication about an Ongoing Safety Review: Epoetin alfa. at http://www.fda.gov/Cder/drug/early_comm/epoetin_alfa.htm.