Abstract
Throughout history, human kind has won many battles against deadly diseases, including small pox, polio, tuberculosis and more recently severe acute respiratory syndrome. All of these diseases were defeated by prevention. Achieving cancer prevention is a global priority, but history tells us that the pathway to achievement is difficult and full of detours and roadblocks. Epidemiology and clinical evidence clearly indicate that specific factors are associated with an increased risk for cancer development. What can we learn from the past that is applicable to the reality of successful cancer prevention?
Introduction
Concentrated efforts to prevent cancer date back to antiquity. Positive associations between environmental factors or behaviors and increased risk of cancer development have been alleged as risk factors for at least 200 years. However, until the late 20th century, very little progress was made in understanding the underlying mechanisms. The current hypothesis is that prevention is a highly feasible approach to cancer control. The question is, if prevention works, why does cancer still exist and still pose a huge human and economic burden all over the world? The World Health Organization (WHO) predicts that cancer deaths will increase to 14% of all deaths in 2008 and by 2030, 18% of all deaths worldwide will be due to cancer (http://www.who.int/mediacentre/factsheets/fs297/en/index.html). Importantly, WHO indicates that at least 30% of all cancer deaths are preventable 1. This means, for example, that of the 7.4 million cancer deaths (or 13% of all deaths worldwide) in 2004, 2.2 million could have been prevented.
Historically, research efforts have focused heavily on ‘early detection and treatment’ with little emphasis on environmental and lifestyle causes. Cancer prevention research per se has seemingly never been a major health research emphasis until recently. But in spite of the renewed interest, only about 2% of the National Cancer Institute's (NCI's) total budget in the US appears to be directed toward cancer prevention and early detection (http://plan2009.cancer.gov/pdf/At_a_Glance_Budget_2009.pdf).
This Timeline will examine past discoveries and evaluate the cancer prevention research climate over time. The various ideas, driving forces, and key players that shaped the field of cancer prevention research will be considered. The means by which scientific discoveries, concepts, or techniques have shaped the development of cancer prevention research will also be discussed. The big questions that need to be addressed over the next few years in cancer prevention and cancer prevention research will be introduced and the techniques that need to be improved or developed to answer these questions will be presented.
Earliest observations and cancer prevention—1700-1800
Cancer prevention research was virtually ignored through the ages, although the idea of the importance of cancer prevention was basically realized early on. The association of an unhealthy diet with cancer was recognized as early as 168 B.C. by the Roman physician Galen 2 (see Timeline). In the 18th century, Bernardino Ramazzini, an Italian doctor, reported in1713 the almost complete absence of cervical cancer and relatively high incidence of breast cancer in nuns and hypothesized that this might be related to their lifestyle of abstinence from sex 3. During this period, tobacco use was first recognized as a health risk 4 and exposure to soot by chimney sweeps was associated with a high risk for scrotal cancer 5. In the 19th century, arsenic 6, aromatic amines 7 and various other chemicals were added to the list of environmental cancer risk factors 8. In 1902, X-rays were found to induce tumors 9, 10 and in 1911 Peyton Rous showed that viruses can cause cancer in chickens11. The presence of carcinogens in coal tar was established in 1915 when laboratory animals exposed to this agent developed tumours12. These types of observations directed most cancer prevention activities to avoidance of industrial, occupational or environmental carcinogens 13. Notably, these carcinogenic agents were identified by observational or epidemiologic studies, but cancer prevention research in the 18th and 19th centuries seemed to be almost nonexistent. Even though environmental and lifestyle factors were recognized as being associated with cancer, not everyone exposed developed the disease, which made recommendations of lifestyle changes difficult, if not impossible.
Early 20th century detection and treatment versus prevention—1900-1960
The politics and key players in the US
Because statistical evidence suggested that cancer death rates were increasing steadily in the late 19th and early 20th centuries (http://www.infoplease.com/ipa/A0922292.html) 14, much of the research interest was focused on detecting and treating the disease. In 1907, the American Association for Cancer Research was established and held their first scientific meeting 15; and in 1913 The Ladies Home Journal published the first popular article about the warning signs for cancer 16. In the early 20th century, cancer was rarely publicized because it was so greatly feared (http://www.cancer.org/docroot/AA/content/AA_1_4_ACS_History.asp). Although cancer research was becoming more of a priority, cancer prevention research was still not recognized as very important. The idea that dietary changes, such as less meat and reduction of alcohol consumption, could prevent cancer was favored by some 17. However, for much of the 20th century, cancer prevention was not viewed separately from the practice of early detection and treatment.
The next major US organization that was established to focus on cancer was the American Society for the Control of Cancer (ASCC) in 1913 (http://www.cancer.org/docroot/AA/content/AA_1_4_ACS_History.asp). The ASCC was established by 10 prominent physicians and 5 business leaders in New York City, and later (1944) became the American Cancer Society (ACS). The ASCC became a highly influential organization whose goal was to encourage Americans to seek early detection and treatment with the idea that cancer was curable if this practice was followed 18. This concept was the standard policy and practice for cancer control for the first half of the 20th century 18, which very likely contributed to the lack of organized research focusing on cancer prevention. In fact, the physician-dominated ASCC suggested that the idea of diet causing or curing cancer was quackery or food faddism and that changes in diet would do nothing to prevent or treat cancer 17. At this time the focus was on cancer patient care for whom physicians seemed to be able to do little except diagnose and try to treat the symptoms. During the 1960s and 1970s, interest in environmental and lifestyle causes of cancer was eventually revived and these factors were emphasized as potential health issues. Even then, the idea of lifestyle changes posed a major threat to therapeutics as a driving force in cancer control or prevention even though therapeutics had not increased cancer survival 17.
Despite the fact that cancer prevention research was not recognized as a separate discipline, other key events occurred during the first half of the 20th century that would eventually lead to the emergence of a major emphasis on cancer prevention research during the 2nd half of the century. In particular, the National Institutes of Health (NIH) was established in 1930 followed in 1937 by the passage of the National Cancer Institute Act signed by President Franklin D. Roosevelt that authorized annual funding to support research related to the causes, diagnosis and treatment of cancer (http://www.cancer.gov/aboutnci/). In 1939 the National Cancer Institute (NCI) was formed and the first research on the association of smoking with lung cancer was initiated. NCI later (1955) formed the Cancer Chemotherapy National Service Center to focus on screening of natural and synthetic compounds for their anticancer properties (http://www.cancer.gov/aboutnci/). Since its inception, the NCI has been a key driving force in cancer research, including, most recently, cancer prevention research.
Throughout the early and mid 20th century, the boundaries between science, industry and government were quite unclear. Academic scientists with industrial ties appeared to exert major influence on cancer research direction 19. The American Health Foundation (AHF; also known as the Institute for Cancer Prevention) was founded in 1969 by Dr. Ernst L. Wynder, whose work in the 1950s clearly established the link between tobacco smoking and cancer 20. The AHF was established as a nonprofit private research organization devoted primarily to the prevention of chronic diseases, especially cancer (http://www.sourcewatch.org/index.php?title=American_Health_Foundation). The establishment of an organization to focus on prevention of chronic disease should have moved the field forward rapidly. However, the history of the AHF is overshadowed by controversy surrounding funding from tobacco and food industries for research related to dietary and lifestyle causes of lung cancer (http://www.cspinet.org/integrity/nonprofits/institute_for_cancer_protection.html). The Foundation eventually declared bankruptcy and closed its doors circa 1980.
Other organizations founded with a focus in cancer prevention research in the US included the American Institute for Cancer Research (AICR), which was established in 1982 (http://www.aicr.org/site/PageServer?pagename=abt_index_2). The AICR supports research into the role of diet, physical activity and obesity in the prevention and treatment of cancer. This organization has worked with the World Cancer Research Fund (WCRF) International to publish two international reports 21, 22, which based on an extensive review of the cancer research literature, offers its own series of recommendations for everyday cancer prevention.
Identification of cancer links and technological developments 1900-1970
For many past decades as now, cancer research continues to be driven by what are considered to be the most common and deadly cancers. The revival of the interest in cancer prevention research in the 1980s up to the present day was certainly accelerated by the discovery of important associations between cancer development and environment, lifestyle, and genetics. The advent of new technologies and other discoveries associated with detecting and treating lung cancer, breast cancer, cervical cancer, skin cancer and colon cancer through the 1950s, 60s, and 70s all facilitated the identification of cancers and cancer risks much earlier than was possible previously.
Beginning in the 1950s, the relationship between smoking and lung cancer drove the evolution of epidemiology and clinical cancer research. In 1950, the link between cigarette smoking and cancer was confirmed 20, 23. Notably, in 1954 the Hammond-Horn Study (USA) 24-26 and the British Doctor's Study (UK) 27-29 further confirmed the link between smoking and lung cancer. The ACS initiated its first cancer prevention study (CPS I) in 1959, which also eventually linked cigarette smoking with early death from lung cancer 30, 31. The 1962 Royal College of Physicians 32 and the 1964 U.S. Surgeon General report 33 linked smoking to lung cancer. These two reports probably marked the beginning of a change in attitude toward lifestyle and environmental causes and especially the role of smoking in cancer 17. In 1971, asbestos exposure was linked to mesothelioma, a rare lung cancer 34. In 2002, the International Agency for Research on Cancer (IARC) classified second-hand smoke as carcinogenic to humans 35 and this was affirmed by the Surgeon General in 2006 36.
Early mammogram instrumentation for breast cancer detection was presented in 1951 37 and the first randomized controlled trial of periodic breast cancer screening with mammography began in 1963 38, 39. In 1956, the relationship between age at menopause and risk of breast cancer was recognized 40. Tamoxifen was shown to prevent mammary cancer in the dimethylbenzanthracene (DMBA)-induced rat mammary carcinoma model in 1974 41 and was approved by the US Food and Drug administration (FDA) to treat estrogen receptor positive breast cancer in 1978. In 1994, two genes, breast cancer 1 (BRCA1) and BRAC2, were identified as risk factors for developing breast cancer 42. This discovery further highlighted the need for genetic testing to determine risk for certain inherited cancers and might also have strengthened the idea of using surgery, such as mastectomy and oophorectomy, as a means of preventing cancer occurrence.
The discovery by George Papanicolaou that vaginal cell smears can reveal the presence of cervical cancer in 1928 43, 44 was followed in 1943 by the introduction of the Pap smear as a method of detecting carcinomas in the female genital tract. Later, Wynder reported that barrier contraceptives were associated with a lower risk of cervical cancer, implicating a transmissive agent in the etiology of cervical cancer 45. Despite the early work of Peyton Rous, the viral theory of carcinogenesis was not taken seriously until the 1940s and became a dominant theory through the 1970s 13. In 1974, the idea that cervical cancer might be caused by a viral agent was suggested 46. The concept that certain cancers might be induced by viral infection lead to a great deal of funding being spent on the development of vaccines47. The first human viral vaccine that can prevent cancer — the hepatitis B virus vaccine to prevent liver cancer — was introduced in 1981 48. The human papillomavirus (HPV) was identified as a risk factor for cervical cancer in 1987 49, 50. The Hybrid Capture II human papillomavirus (HPV) DNA test was approved by the FDA in 1999 as a test that can be used in conjunction with the Pap smear in screening for cervical cancer 51, 52. The first HPV vaccine clinical trial was completed in 2002 with positive results 40 and those results were further confirmed more recently 53. In 2006, the FDA approved the vaccine Gardasil 54, which protects against persistent infection by two types (16 and 18) of HPV that cause approximately 70 percent of cervical cancers worldwide 55. Administering HPV vaccine to HPV-naïve women and sexually active women has been suggested as a means to substantially reduce the incidence of HPV16/18-related cancers 56.
Humans have been fascinated with sunlight since antiquity and Hippocrites reportedly prescribed sunbathing (heliotherapy) for medical and psychological purposes. The existence of ultraviolet (UV) radiation in sunlight was well established by 1920 and interest in understanding the effect of UV radiation on humans was increasing 57. Even though early scientific research studies in animals 58 suggested that UV might be dangerous or harmful, the primary opinion at that time was that sunlight had a positive influence on health. However, by the end of the 20th century epidemiological evidence supported by strong experimental evidence suggested that solar UV irradiation is an important environmental carcinogen and a major etiologic factor in human skin cancer 59-66. Sunscreens alone appear to be imperfect in preventing skin cancers 67-71 because an increased incidence of human skin cancer even with the application of sunscreen continues to be observed 69-71. The most effective prevention appears to be moderation or avoidance of UV exposure.
Finally during in the late 1960s, Greegor introduced the fecal occult blood test (FOBT) as a tool to screen for colorectal cancers 72 and this was followed by colonoscopy of the entire colon in 1969 73. All of these associations and technologies have moved the field of cancer prevention research forward into the 21st century.
Late 20th century and early 21st century, cancer prevention revival in the US
The 1970s
The 1970s saw a major change in the amount of interest in cancer detection and prevention beginning with the signing of the National Cancer Act of 1971 by President Richard Nixon on December 23 to declare war on cancer with the goal of eliminating cancer by 1976 (http://www.cancer.gov/aboutnci/national-cancer-act-1971/allpages). This was the beginning of a change in NCI's research funding emphasis from treatment to prevention. Epidemiological evidence continued to suggest that even though 30-40% of cancer deaths were related to smoking, perhaps just as many were connected to diet 74, 75. NCI's concentration shifted to support for research focusing on tobacco, diet and environmental and occupational causes of cancer.
The 1980s
The early 1980s seemed to indicate a change in the way we viewed cancer. In 1982, the second ACS prevention study (CPS II) was initiated to study cancer risk and prevention factors in 1.2 million American men and women (http://www.cancer.org/docroot/RES/content/RES_6_2_Study_Overviews.asp). Also, in 1982, a large working party formed by the U.S. National Research Council Committee published a report on diet, nutrition and cancer and proposed what they called ‘interim guidelines’ on diet and cancer 76. This terminology was used mainly because they felt that to make firm scientific pronouncements about the association between diet and cancer was not yet possible because knowledge of diet and cancer was in an interim stage similar to that for cigarettes 20 years previously 76. Based on their review of the scientific literature, they concluded that various cancers occurring in different human populations appeared to correspond with differences in diet 76. This idea was based on observations indicating that individuals who move from countries where a particular cancer incidence is low, to countries where the incidence of that cancer is high, acquire the cancer incidence of their new home 77. In general, they concluded that some types of diets and some dietary components (such as high fat diets or the frequent consumption of salt-cured, salt-pickled, and smoked foods) tend to increase the risk of cancer (BOX1), whereas others (such as low fat diets or the frequent consumption of certain fruits and vegetables) tend to decrease it. Based on these results, they recommended a decreased fat intake, especially animal fat, to less than 30% of total energy intake 76. Other recommendations included decreasing sugar, salt and alcohol consumption, avoid being overweight, and increase the consumption of fruit, vegetables and fiber 76. Notably, the 1988 Surgeon General's Report on Nutrition and Health in the US indicated that the three most important personal habits that influence health are smoking, alcohol consumption and diet 78.
BOX 1. The impact of refrigeration on gastric cancer incidence.
Before mechanical refrigeration systems were introduced, people cooled their food with ice and snow, which was the only means of refrigeration for most of human history. The first patent for mechanical refrigeration was issued (1834) in Great Britain to the American inventor Jacob Perkins 123. Domestic mechanical refrigerators appeared in the U.S. around 1911 (http://www.history.com/exhibits/modern/fridge.html). By the 1950s and 1960s, more and improved refrigerators reached the consumer market. Today, the refrigerator is America's most used appliance, found in more than 99.5% of American homes (http://www.history.com/exhibits/modern/fridge.html). In Western countries the incidence of gastric cancer, once a very common cancer, fell dramatically between 1950 and 1990 124, seemingly without any specific intervention but corresponding with the availability of refrigeration.
Since the turn of the century, new methods of processing and refrigeration have resulted in a huge variety in the kinds and numbers of food items available in developed countries. Gastric cancer is one of the most common cancers in underdeveloped countries and appears to be associated with diet, especially with high intake of salted foods 125. The use of refrigeration was suggested to be inversely correlated with the use of salting, with other methods of food preservation using salt, such as curing and smoking, and with the volume of salt in diets 21.
Increased refrigeration and decreased use of older food preservation methods have been attributed to the decline in deaths from stomach, liver, and rectal cancers 126. Long-time refrigerator use was shown to halve the incidence of stomach cancer risk and risk was high in subjects, who as children did not have a means of cool storage of food 127. The evaluation of cohort and case-control studies (1980-1990) revealed a consistently increased risk for stomach cancer for later availability of refrigeration facilities in the household, non-centralized water supply (especially well water), and high salt intake 128.
The consensus view is that the decline in gastric cancer in developed countries is attributable to improved food hygiene and increasingly available facilities for refrigeration 129 and perhaps also by the transition in food preservation methods from salting to refrigeration 125.
1990s until now–success of chemoprevention trials
The 1990s and early 21st century marked the beginning of a keen awareness of the effects of diet on health and especially more interest in cancer prevention and cancer prevention research. The NCI established the current NCI Division of Cancer Prevention (DCP) in October 1997 (http://prevention.cancer.gov/about/mission). The mission of the DCP “is to plan, direct, implement, and monitor cancer research and training that is focused on early detection, cancer risk, chemoprevention, and supportive care.” NIH currently defines cancer prevention as the reduction of cancer death through the reduction of cancer incidence and suggests that cancer can be prevented by avoiding known risk factors such as carcinogens or smoking or by chemoprevention to reverse preneoplastic changes (http://www.cancer.gov/cancertopics/pdq/prevention/overview/healthprofessional). The term chemoprevention (initially referred to as “chemoprophylaxis of carcinogenesis” 79) was coined in 1976 80, 81. Chemoprevention is defined as the use of chemical agents, drugs, or dietary supplements to prevent disease 82.
A number of NCI-funded chemoprevention clinical trials were initiated or completed during this period, including the 1993 Prostate Cancer Prevention Trial (PCPT), the Breast Cancer Prevention Trial (BCPT; 1998), and the Study of Tamoxifen and Raloxifene (STAR) trial (1999), which was a follow-up to the BCPT. These three trials were considered very successful in identifying drugs that could prevent prostate or breast cancer in high risk individuals. Results from the PCPT showed that men who took finasteride (an anti-androgen agent) daily for seven years had about a 25% reduced risk of developing prostate cancer compared with men taking a placebo 83. However, finasteride treatment was also associated with a slight increased risk for developing high-grade prostate tumors. The first results of the BCPT showed that women on tamoxifen exhibited about a 50% decrease in invasive and noninvasive breast cancers 84. However, tamoxifen was associated with increased risk for blood clots and endometrial cancer in postmenopausal women. Updated results 85 confirmed tamoxifen's ability to reduce the risk of breast cancer in high-risk women. In October 1998, based on the initial results of the BCPT, the FDA approved the use of tamoxifen for the prevention of breast cancer in women at high risk of developing the disease. Initial results from the STAR trial 86 showed that raloxifene and tamoxifen were equally effective in reducing breast cancer risk in postmenopausal women who are at increased risk of the disease. The effectiveness of raloxifene was confirmed in two additional trials, the Multiple Outcomes of Raloxifene Evaluation (MORE) in 1999 87 and the Raloxifene Use for The Heart (RUTH) 88 trials. Based on the results of these three trials, the FDA (September 2007) approved raloxifene to reduce the risk of invasive breast cancer in postmenopausal women.
Results from the ACS CPS II trial suggested that colon cancer risk could be decreased in persons who took aspirin regularly 89. The effect of celecoxib, a selective cyclooxygenase-2 (COX2) inhibitor, on colorectal polyps in patients with familial adenomatous polyposis was evaluated in a double-blind, placebo-controlled study and results indicated that 6 months of twice-daily treatment with 400 mg of celecoxib led to a significant reduction in the number of colorectal polyps 90. These results were confirmed in the 5-year Adenoma Prevention with Celecoxib (APC) trial; but unfortunately, the use of celecoxib was associated with an increased risk of adverse cardiovascular effects 91. Most recently, results of a phase III trial using low doses of the anti-inflammatory sulindac and an investigational compound difluoromethylornithine (DFMO), showed significant efficacy to prevent colon polyp recurrence by 70% 92. Most notably, the results showed that the treatment was most effective in preventing the recurrence of the highest-risk polyps, advanced adenomas, demonstrating a 92% reduction. These results are suggested to comprise the first major clinical success with combination chemoprevention and are considered a major clinical advance93.
Diet based intervention and the lows of dietary prevention trials
The idea that nutrition is an important factor in cancer causation is not new. Williams in 1908 94 observed that excessive eating and lack of exercise are predisposing factors for cancer and in 1815 Lambe 95 warned against the danger of excess consumption of food in general and meat in particular. Early nutritionists demonstrated that many diseases could be dramatically cured, not by removing a harmful toxin, but by correcting a nutrient deficiency 13. In the early 1980s, Doll 75 reported that diet might be responsible for 10-70% of upper aero digestive tract, esophagus, stomach, large intestine and breast cancers. However, the relationship between diet and cancer is still a mystery because cancer is a multifaceted disease and diet is a complex factor.
Since the 1990s the public interest in the effect of diet on health and its role in preventing cancer have expanded exponentially. The “5 A Day for Better Health Program” in the US was initiated in 1991 in a collaborative effort between the NCI and Produce for Better Health Foundation (PBH). Its purpose was to use every communication means possible to encourage Americans to improve their eating habits to reduce the risk of diet-related cancers and other chronic diseases. The goal was to increase the average per capita consumption of fruits and vegetables in the U.S. to at least 5 servings a day. Unfortunately, based on the data presented online (http://apps.nccd.cdc.gov/5ADaySurveillance/), more Americans eat none or less than 1 serving (2.9% in 1996 compared to 4.4% in 2007) and the number eating 5 servings a day has increased by only 0.3%. The “5 A Day” program is going to be replaced by the Fruits & Veggies—More Matters™ strategy by January 1, 2009 (www.pbhfoundation.org). The replacement program's goal is essentially the same–to direct American attitudes toward “wanting to eat more fruits and vegetables” as opposed to “should eat more fruits and vegetables.”
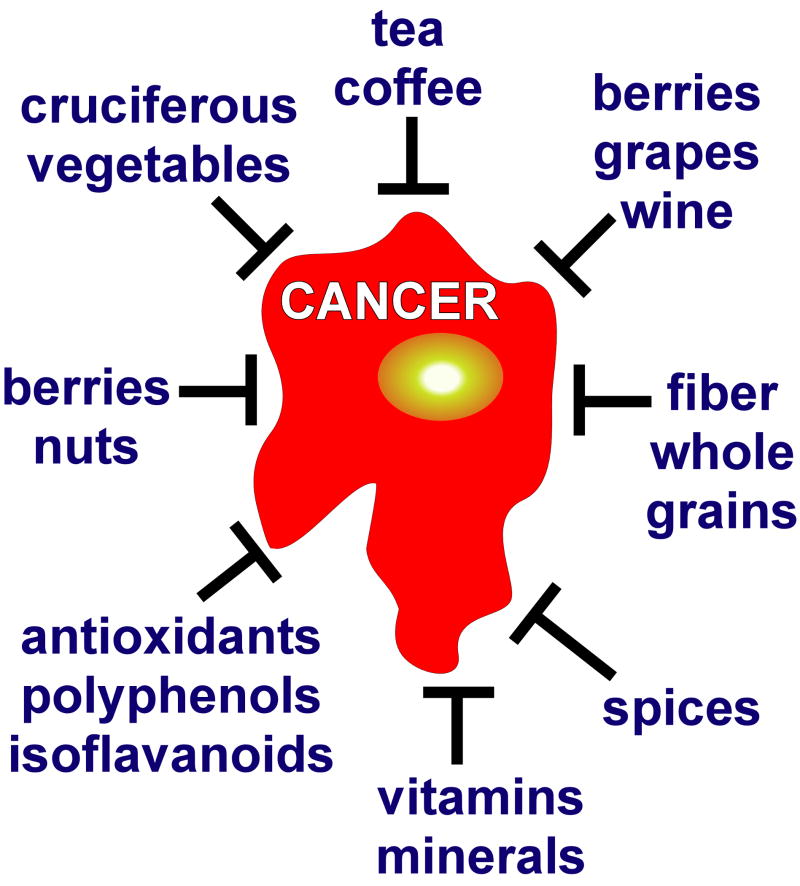
Numerous dietary epidemiologic observations and animal studies for a plethora of dietary factors from fruits and vegetables consistently suggest possible protection against various cancers (Figure 1); but in general, these findings have not yet been validated in randomized trials. In fact, results of most dietary intervention clinical trials have been extremely disappointing. In the mid 1990s, dietary-based cancer prevention suffered a major setback based on the results of the β-carotene cancer prevention trial in which β-carotene actually was associated with an increased risk of lung cancer in smokers 96, 97. Results from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC Study) in Finland indicated that alpha-tocopherol had no effect on lung cancer incidence, but was associated with decreased prostate cancer risk 96. Unexpectedly, a higher incidence of lung cancer was also observed in men receiving β-carotene compared to those who did not. Another study examining the incidence of lung cancer across subgroups of participants in the ATBC supported these findings, but also indicated that the β-carotine effect might be associated with heavier smoking and higher alcohol intake 98. The statistical significance of the beneficial and adverse effects of supplemental alpha-tocopherol and β-carotene disappeared during post-intervention follow-up studies 99. Concurrently, in the U.S., the Beta Carotene and Retinol Efficacy Trial (CARET) was initiated and involved smokers, former smokers, and workers exposed to asbestos and its purpose was to examine the effect of daily β-carotene and retinol (vitamin A) supplementation on the incidence of lung cancer 100. Similar to the ATBC, the β-carotene group had a higher risk of lung cancer compared with the placebo group. The trial was stopped and follow-up continued for an additional 5 years 97. A later report indicated that plant foods have an important preventive influence in a population at high risk for lung cancer, but the effect is diminished with β-carotene supplements 101. In another follow-up CARET report, the adverse effects of β-carotene and vitamin A on lung cancer incidence in these high-risk individuals persisted after drug administration was stopped, but no longer reached statistical significance 102.
Figure 1. Anticancer effects of diet.
Hundreds of whole foods and dietary factors, especially fruits, vegetables and their diverse components, have been suggested to exert potent anticancer activities, but as yet none have been verified to be effective in clinical trials.
In contrast with earlier research suggesting that dietary supplementation with selenium and vitamin E may lower the risk of prostate cancer 96, 99, 103-105, initial results from the largest-ever prostate cancer prevention study, the Selenium and Vitamin E Cancer Prevention Trial (SELECT), showed that these substances did not help prevent prostate cancer 106. The data also showed a nonstatistically significant increase in the number of prostate cancer cases in men taking only vitamin E, but more analysis of the data is needed before a final conclusion can be made. Participants stopped taking supplements in October of 2008 and will continue to be monitored for another 3 years.
A number of epidemiology studies suggest that fiber can reduce cancer risk. The Women's Healthy Eating and Living (WHEL) Study was initiated in 2002 to determine the effectiveness of an increased consumption of vegetables, fruits and fiber, and a decreased consumption of total fat to reduce additional breast cancer events and death in women who had been treated for early-stage invasive breast cancer within the previous 4 years 107. The outcome measure was any invasive breast cancer event (recurrence or new primary) or death from any cause. Results indicated that the intervention group achieved and maintained a statistically significant increased consumption of vegetables, fruits and fiber and decreased total fat. Among survivors of early-stage breast cancer, adoption of a diet that was very high in vegetables, fruit, and fiber and low in fat did not reduce additional breast cancer events or mortality during a 7.3-year follow-up period 108.
The findings appear to conflict with interim results from the Women's Intervention Nutrition Study (WINS), which showed that a low-fat diet may help prevent breast cancer recurrence in postmenopausal women 109, 110. For a direct comparison, a recent review re-examined data for only postmenopausal women from the two studies. The results of the review indicated that the evidence does not convincingly support the idea that changing dietary patterns will improve prognosis for most women with early stage breast cancer. However, changes in diet appear to be important for some subgroups, but more investigations are needed 111.
The NIH announced the Women's Health Initiative (WHI) in the spring of 1991, with the purpose to address three of the leading health problems for women: cardiovascular disease, breast and colon cancer, and osteoporosis 112 and the program was initiated in 1992 with a planned completion date of 2007. Women were enrolled in either a clinical trial or observational study. For the clinical trial the intervention for cancer included a low-fat eating pattern hypothesized to prevent breast and colorectal cancer or calcium and vitamin D supplementation hypothesized to prevent colorectal cancer 113. Results indicated that dietary fat intake was significantly lower in the dietary modification intervention group compared with the comparison group. Among postmenopausal women, a low-fat dietary pattern did not result in a statistically significant reduction in invasive breast cancer risk over an 8.1-year average follow-up period 114. On the other hand, a low-fat dietary pattern was suggested to reduce the incidence of ovarian cancer among postmenopausal women 115. Later analyses suggested that a modest reduction in fat intake and increase in fruit, vegetable, and grain intake do not alter the risk of benign proliferative breast disease 116. Finally, daily supplementation of calcium with vitamin D for seven years had no effect on the incidence of colorectal cancer among postmenopausal women 117.
Future emphasis on preventable causes and strategies to achieve effective, realistic prevention
Based on the almost total lack of the success of dietary intervention clinical trials, one might ask where did we go wrong? How can we move forward? Some suggest that we have moved from a period in which cancer causes were identified with very little understanding of the underlying mechanisms, and now we have entered a period in which the understanding of mechanisms has progressed extremely rapidly but has not yet contributed to effective strategies for cancer prevention 118. Historically, the urgency to treat patients with diagnosed cancer understandably has been the dominant focus of cancer research. However, a boarder view is necessary because as with other diseases, prevention is likely a relatively straightforward and effective approach to control cancer. NCI's current approach to cancer prevention is defined by the use of advanced tools and technologies, including those used in genomics, proteomics and metabolomics, to determine the molecular events associated with the molecular mechanisms and early signs of cancer development (http://plan2009.cancer.gov/The_Promise_of_Prevention_and_Early_Diagnosis.htm). Notably, in 2003 the human genome was sequenced 119 and now the Cancer Genome Atlas Project is seeking to produce a list of all mutations associated with cancer and will be used to develop new strategies for preventing, diagnosing and treating cancer. The development of molecular imaging technologies and supercomputer drug screening are just two of the newer technologies that will be key in the development of effective anticancer agents with identified molecular targets.
A great deal of emphasis has been placed on the role of diet and cancer risk. However, relying on epidemiologic data to identify specific dietary agents obviously has not worked very well, especially in studies that focus on the generalized effects of diet. Determining individual relevant components in a diet high in fruits and vegetables does not seem feasible because of the complexity of food and the evident limitations of questionnaire-based surveys. The limited success of effective, nontoxic anticancer agent development indicates that strategies need to be developed that can effectively differentiate between promising candidate compounds and those that are much less likely to exhibit efficacy. We must be able to identify the correct molecular targets and then be able to successfully transition preclinical results into the clinical situation. Failure of candidate compounds is often associated with a lack of efficacy, suboptimal formulation, unknown toxicity, or poor bioavailability. Developing nontoxic highly effective agents against a known molecular target is critical (for example nontoxic COX2 inhibitors). One approach to developing these kinds of agents might involve the testing of the agent in animals under conditions that mimic definitive clinical trial endpoints, such as administering the compound(s) when preneoplastic lesions are present. This approach would appear to be a better indicator of the human situation, and if a compound was effective under these conditions, this might indicate that it has a greater likelihood for success in Phase II or Phase III trials.
Another strategy is the implementation of Phase 0 trials, which are designed to evaluate targeted anticancer agents in small, early-phase human clinical trials 120. These trials are intended to expedite the clinical evaluation of new molecules by allowing less restrictive requirements for manufacturing and toxicologic assessment 121, 122. Goals for Phase 0 trials include replication of preclinical mechanism(s) of action in a human intervention trial; characterization of initial pharmacokinetic and pharmacodynamic profiles; and evaluation of biodistribution patterns, based on imaging technologies. A phase 0 trial should be conducted before the traditional phase I dose-limiting toxicity trial, comprise only a limited number of subjects, have no therapeutic or diagnostic intent, and be relatively short in duration. Success in a Phase 0 trial might permit an improved selection of effective compounds for further development. Methods to identify compounds, which have good bioavailability and are highly active, earlier in the drug development cycle would offer clear advantages.
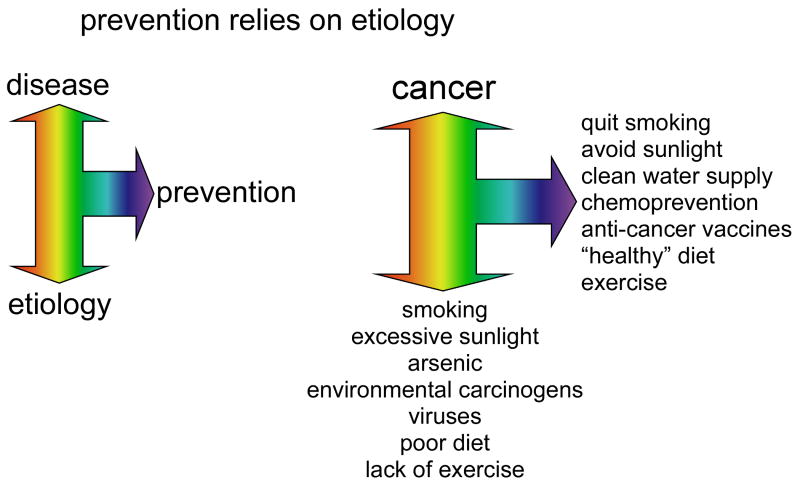
Prevention always relies on etiology and therefore, education of the general public regarding risk factors associated with developing cancer is a must. The World Health Organization presents 9 leading risk factors that could reduce cancer incidence (Box 2). Based on these, prevention strategies should include the avoidance of these risk factors; vaccination against human papilloma virus (HPV) and hepatitis B virus (HBV); control of occupational hazards; and reduction of exposure to sunlight (http://www.who.int/mediacentre/factsheets/fs297/en/index.html) (Figure 2).
Box 2. WHO defined risk factors for cancer development.
(http://www.who.int/mediacentre/factsheets/fs297/en/index.html)
The World Health Organization (WHO) provides leadership on global health matters and one of its agenda items is to harness research, information and evidence. WHO works with leading experts to set standards based on the most reliable research evidence. They have concluded that cancer is the leading cause of death worldwide and more than 30% of cancer deaths can be prevented by modifying or avoiding key risk factors. 1.
Tobacco use
Overweight or obese
Low fruit and vegetable intake
Physical inactivity
Alcohol use
Sexually transmitted HPV-infection
Urban air pollution
Indoor smoke from household use of solid fuels
Exposure to UV
Figure 2. Prevention relies on etiology.
An observation (etiology) is associated with a disease and then steps are taken to prevent that disease. In cancer, smoking, sunlight, arsenic, environmental carcinogens, viruses, poor diet and lack of exercise are all etiological factors. Many cancers can be prevented by avoiding these factors.
Summary and Conclusions
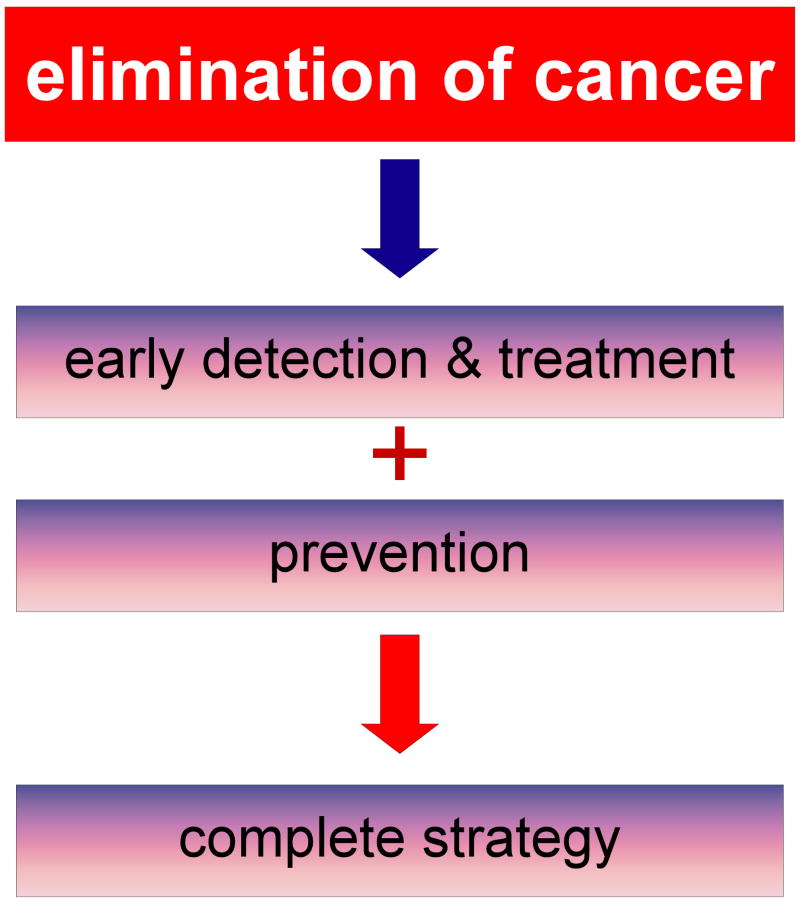
Historically, the persistent focus on only early detection and treatment of cancer with less emphasis on prevention has not been effective in eliminating this devastating disease. The three elements must be melded together to create a complete strategy for cancer control (Figure 3). Effectual future total anticancer strategies are critically needed and must meet specific criteria to succeed. These strategies must be practical and must focus on high-risk populations, such as smokers, carriers of known genetic risk factors, specific ethnic groups, sunbathers, and those in high-risk occupational or environmental positions. The total strategy must consider that cancer types vary in different countries and regions throughout the world. For example, Bangladesh is known to have very high levels of arsenic-induced cancers and both China and Korea have a high rate of smoking- and diet-induced cancers. The complete strategy will very likely evolve into more personalized prevention based on our different genetic backgrounds and varied sensitivity to environmental carcinogens. Genomics will be used more and more to identify the high-risk individual and accurate end-point biomarker development will be crucial. The complete strategy to cancer elimination will require an allocation of additional resources and commitment by science, industry, and government.
Figure 3. Strategy for eliminating cancer.
The complete strategy for the elimination of cancer requires the blending of early detection, treatment, and prevention. None can be effective independently of the others.
Timeline
168 B.C. –unhealthy diet associated with cancer 2
1713 –absence of cervical cancer and high incidence of breast cancer in nuns 3
1761 –recognition of dangers of tobacco use 4
1775 –occupation-related scrotal cancer risk in chimney sweeps 5
1902 –ionizing radiation (X-rays) discovered to induce tumors 9,10
1911 –viruses shown to cause cancers in chickens 11
1913 –Ladies Home Journal publishes first popular article about warning signs for cancer 16
1915 –cancer induced in laboratory animals by application of coal tar 12
1928 –vaginal cell smears (the Pap smear) can reveal the presence of cervical cancer 43
1937 –National Cancer Institute Act (http://www.cancer.gov/aboutnci/)
1939 –first research on smoking and lung cancer link initiated (http://www.cancer.gov/aboutnci/)
1943 –Pap smear introduced 44
1950 –cigarette smoking-cancer link confirmed 20,23
1951 –early version of mammogram 37
1954 –barrier contraceptives associated with lower risk of cervical cancer 45
–Hammond-Horn Study (U.S.) 24-26 and British Doctors' Study (U.K) 27-29 confirm link between smoking and lung cancer
1955 –NCI forms Cancer Chemotherapy National Service Center to screen potential anticancer natural and synthetic compounds (http://www.cancer.gov/aboutnci/)
1956 –recognition of relationship between age at menopause and risk of breast cancer 40
1959 –ACS Cancer Prevention Study I (CPS I) on cigarette smoking and lung cancer death 30,31
1962 –Royal College of Physicians report links smoking and lung cancer 32
1963 –first randomized controlled trial of periodic breast cancer screening with mammography 38,39
1964 –U.S. Surgeon General report links smoking to lung cancer 33
1967 –fecal occult blood test (FOBT) introduced to screen for colorectal cancer 72
1969 –first colonoscopy of entire colon 73
–American Health Foundation (Institute for Cancer Prevention) established (http://www.sourcewatch.org/index.php?title=American_Health_Foundation)
1971 –President Nixon signs National Cancer Act of 1971 (http://www.cancer.gov/aboutnci/national-cancer-act-1971/allpages)
–asbestos exposure linked to risk of rare lung cancer, mesothelioma 34
1974 –cervical cancer linked to a virus 46
–tamoxifen prevents breast cancer in rats 41
1976 –term chemoprevention is coined by Michael Sporn 80,81 (initially called “chemoprophylaxis of carcinogenesis” by Lee Wattenberg in 1966 79)
1978 –tamoxifen approved by FDA to treat estrogen receptor positive breast cancer
1981 –first hepatitis B virus vaccine for liver cancer prevention 48
1982 –ACS Cancer Prevention Study II (CPS II) initiated to analyze risk and preventive factors involved in cancer (1.2 million men and women) (http://www.cancer.org/docroot/RES/content/RES_6_2_Study_Overviews.asp)
–U.S. National Research Council Committee publishes interim guidelines on diet and cancer 76
1987 –HPV identified as risk factor for cervical cancer 49,50
1988 –Surgeon General's Report on Nutrition and Health: most important personal habits to affect health are smoking, alcohol consumption, and diet 78
1991 –5 A Day for Better Health Program initiated (http://apps.nccd.cdc.gov/5ADaySurveillance/)
–ACS CPS II data demonstrate a decreased risk of colon cancer in people who take aspirin regularly 89
1994 –Alpha-Tocopherol Beta-Carotene Cancer Prevention Study finds increased lung cancer risk with beta-carotene supplements in smokers and no effect of alpha-tocopherol 96
–BRCA1 and BRAC2 genes identified as risk factors for breast cancer 42
1996 –CARET results in higher risk of lung cancer in smokers compared with placebo 97, 100, 102
1997 –NCI establishes NCI Division of Cancer Prevention (http://prevention.cancer.gov/about/mission)
1998 –Breast Cancer Prevention Trial shows tamoxifen reduces breast cancer by 50% in high-risk women 84
–FDA approves tamoxifen for prevention of breast cancer
1999 – MORE trial show raloxifene reduces the risk of breast cancer by 76% in postmenopausal women with osteoporosis 87
–Hybrid Capture II HPV DNA test approved by FDA as test used with Pap smear 51,52
2000 –celecoxib shown to prevent colon cancer polyps in individuals carrying the APC gene for familial adenomatous polyposis 90
2002 –HPV clinical trial shows vaccine is effective against cervical cancer 40
–second-hand smoke is classified as carcinogenic to humans 35
2003 –Prostate Cancer Prevention Trial shows finasteride reduces the risk of prostate cancer 83
2006 –STAR trial reveals raloxifene as effective as tamoxifen to reduce recurrence of invasive breast cancer 86, confirmed by RUTH trial 88
– APC trial confirms effectiveness of celecoxib to prevent colon polyps but use is associated with increased adverse cardiovascular events 91
–FDA approves the vaccine Gardasil to prevent HPV-induced cervical cancer 54,55
–The U.S. Surgeon General releases a report on the harmful health consequences of secondhand smoke 36
–results of WHI trial indicate no effect of low-fat diet on invasive breast cancer risk and no effect of calcium and vitamin D on colorectal cancer risk 116,117
2007 –FDA approves raloxifene to reduce the risk of invasive breast cancer
–HPV clinical trial confirms effectiveness of vaccination against HPV, widespread vaccination recommended 53,56
–WHEL study indicate that diets high in vegetables, fruit, and fiber and low in fat do not prevent breast cancer recurrence 108
–WINS shows that a low-fat diet may help prevent breast cancer recurrence in postmenopausal women 109,110
–results of WHI trial indicate low-fat diet may reduce ovarian cancer incidence 115
2008 –Phase II trial shows that combination of DFMO and sulindac prevents colon polyp recurrence 92
2009 –SELECT shows that vitamin E and selenium do not prevent prostate cancer 106
– “Fruits & Veggies–More Matters™” replaces the “5 A Day” national program in the US (www.pbhfoundation.org)
References
- 1.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–93. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 2.Gurunluoglu R, Gurunluoglu A. Paul of Aegina: landmark in surgical progress. World J Surg. 2003;27:18–25. doi: 10.1007/s00268-002-6464-8. [DOI] [PubMed] [Google Scholar]
- 3.Ramazzini B. In: De Morbis Artificium Diatriba. Wright WC, translator. University of Chicago Press; Chicago, IL: 1940. [Google Scholar]
- 4.Hill J. In: Cautions against the immoderate use of snuff. Baldwin R, Jackson J, editors. London: 1761. [Google Scholar]
- 5.Pott P. Chirurgical observations relative to the cataract, the polypus of the nose, the cancer of the scrotum, the different kinds of ruptures and the mortifications of the toes and feet. Haves, Clarke and Collins; London: 1775. [Google Scholar]
- 6.Hutchinson J. On some examples of arsenic-keratosis of the skin and of arsenic-cancer. Trans Path Soc London. 1888;39:352–393. [Google Scholar]
- 7.Rehn L. Bladder tumours in fuchsin workers. Arch Fuer Klin Chirurgie. 1895;50:588–600. [Google Scholar]
- 8.Huff J. In: Carcinogenicity Testing, Predicting, and Interpreting Chemical Effects. Kitchin KT, editor. Marcel Dekker; New York: 1999. pp. 21–123. [Google Scholar]
- 9.Frieben A. Demonstration eines Cancroid der rechten Handdruckens das sich nach langdauerneder Einwirkung von Roentgenstrahlen entwickelt hat. Fortschr Roentgenstr. 1902;6:106–111. [Google Scholar]
- 10.Sick H. Karzinom der Haut das auf dem Boden eines Roentgenulcus enstanden its. Muench Med Wochenschr. 1902;50:1445. [Google Scholar]
- 11.Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exper Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamagiwa K, Ichikawa K. Experimental study of the pathogenesis of carcinoma. CA Cancer J Clin. 1977;27:174–81. doi: 10.3322/canjclin.27.3.174. [DOI] [PubMed] [Google Scholar]
- 13.Hill MJ. Changes and developments in cancer prevention. J R Soc Health. 2001;121:94–7. doi: 10.1177/146642400112100209. [DOI] [PubMed] [Google Scholar]
- 14.Gofman JW, O'Connor E. Cancer in the family: Does each case require more than one cause? The likelihood of co-action. Committee for Nuclear Responsibility (CNR); San Francisco, CA: 1999. [Google Scholar]
- 15.Creech HJ. Historical review of the American Association of Cancer Research, Inc., 1941--1978. Cancer Res. 1979;39:1863–90. [PubMed] [Google Scholar]
- 16.Adams SH. What Can We Do About Cancer? The Most Vital and Insistent Question in the Medical World. Ladies Home Journal. 1913;30:21–22. [Google Scholar]
- 17.Cantor D. Introduction: cancer control and prevention in the twentieth century. Bull Hist Med. 2007;81:1–38. doi: 10.1353/bhm.2007.0001. [DOI] [PubMed] [Google Scholar]
- 18.Cantor D. Cancer, quackery and the vernacular meanings of hope in 1950s America. J Hist Med Allied Sci. 2006;61:324–68. doi: 10.1093/jhmas/jrj048. [DOI] [PubMed] [Google Scholar]
- 19.Campbell TC, Campbell TM. The China Study. Benbella Books; Dallas, TX: 2004. [Google Scholar]
- 20.Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J Am Med Assoc. 1950;143:329–36. doi: 10.1001/jama.1950.02910390001001. [DOI] [PubMed] [Google Scholar]
- 21.Research, World Cancer Research Fund/American Institute for Cancer Research, editor. AICR. Food, Nutrition and the Prevention of Cancer: a global perspective. AICR; Washington D.C.: 1997. [Google Scholar]
- 22.World Cancer Research Fund/American Institute for Cancer Research, editor. AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington D.C.: 2007. [Google Scholar]
- 23.Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2:739–48. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond EC, Horn D. The relationship between human smoking habits and death rates: a follow-up study of 187,766 men. J Am Med Assoc. 1954;155:1316–28. doi: 10.1001/jama.1954.03690330020006. [DOI] [PubMed] [Google Scholar]
- 25.Hammond EC, Horn D. Smoking and death rates; report on forty-four monghs of follow-up of 187,783 men. II. Death rates by cause. J Am Med Assoc. 1958;166:1294–308. doi: 10.1001/jama.1958.02990110030007. [DOI] [PubMed] [Google Scholar]
- 26.Hammond EC, Horn D. Smoking and death rates; report on forty-four months of follow-up of 187,783 men. I. Total mortality. J Am Med Assoc. 1958;166:1159–72. doi: 10.1001/jama.1958.02990100047009. [DOI] [PubMed] [Google Scholar]
- 27.Doll R, Hill AB. The mortality of doctors in relation to their smoking habits; a preliminary report. Br Med J. 1954;1:1451–5. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doll R, Hill AB. Lung cancer and other causes of death in relation to smoking; a second report on the mortality of British doctors. Br Med J. 1956;2:1071–81. doi: 10.1136/bmj.2.5001.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond EC. Cancer etiology: new prospective epidemiological study. CA Cancer J Clin. 1959;9:177–8. doi: 10.3322/canjclin.9.5.177. [DOI] [PubMed] [Google Scholar]
- 31.Hammond EC. Smoking in Relation to Mortality and Morbidity. Findings in First Thirty-Four Months of Follow-up in a Prospective Study Started in 1959. J Natl Cancer Inst. 1964;32:1161–88. [PubMed] [Google Scholar]
- 32.Royal College of Physicians. Smoking and Health. London: 1962. [Google Scholar]
- 33.U.S Department of Health and Human Services, editor. Surgeon General. Smoking and Health: The 1964 Surgeon General's Report as a Turning Point in the Anti-Smoking Movement. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Washington D.C.: 1964. [Google Scholar]
- 34.Selikoff IJ, Hammond EC. Asbestos exposure to coke oven operators. J Occup Med. 1971;13:496–7. doi: 10.1097/00043764-197110000-00010. [DOI] [PubMed] [Google Scholar]
- 35.IARC, editor. Tobacco Smoke and Involuntary Smoking. World Health Organization; International Agency for Research on Cancer; Lyon Cedex 08: 2002. [Google Scholar]
- 36.U.S Department of Health and Human Services, editor. Surgeon General. The Health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Washington D.C.: 2006. [Google Scholar]
- 37.Leborgne R. Diagnosis of tumors of the breast by simple roentgenography; calcifications in carcinomas. Am J Roentgenol Radium Ther. 1951;65:1–11. [PubMed] [Google Scholar]
- 38.Sam S. Evidence on screening for breast cancer from a randomized trial. Cancer. 1977;39:2772–2782. doi: 10.1002/1097-0142(197706)39:6<2772::aid-cncr2820390665>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro S. Periodic screening for breast cancer: the HIP Randomized Controlled Trial. Health Insurance Plan. J Natl Cancer Inst Monogr. 1997:27–30. doi: 10.1093/jncimono/1997.22.27. [DOI] [PubMed] [Google Scholar]
- 40.Koutsky LA, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 41.Jordan VC. Antitumour activity of the antioestrogen ICI 46,474 (tamoxifen) in the dimethylbenzanthracene (DMBA)-induced rat mammary carcinoma model. J Steroid Biochem. 1974;5:354. [Google Scholar]
- 42.Wooster R, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994;265:2088–90. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- 43.Papanicolaou G. Proceedings of the Third Race Betterment Conference; 1928. pp. 528–534. [Google Scholar]
- 44.Papanicolaou GN, Traut H. The diagnostic value of vaginal smears in carcinoma of the uterus. American Journal of Obstetrics and Gynecology. 1941;42:193–206. [Google Scholar]
- 45.Wynder EL, Cornfield J, Schroff PD, Doraiswami KR. A study of environmental factors in carcinoma of the cervix. Am J Obstet Gynecol. 1954;68:1016–47. doi: 10.1016/s0002-9378(16)38400-9. discussion, 1048-52. [DOI] [PubMed] [Google Scholar]
- 46.Beral V. Cancer of the cervix: a sexually transmitted infection? Lancet. 1974;1:1037–40. doi: 10.1016/s0140-6736(74)90432-2. [DOI] [PubMed] [Google Scholar]
- 47.Hewitt H. The vast investment in research into the role of viruses in human cancer has largely been wasted because there is still little firm evidence for such a role. Eur J Cancer Prev. 1992;1:187–9. doi: 10.1097/00008469-199202000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Blumberg BS, London WT. Hepatitis B virus and the prevention of primary hepatocellular carcinoma. N Engl J Med. 1981;304:782–4. doi: 10.1056/NEJM198103263041312. [DOI] [PubMed] [Google Scholar]
- 49.Barrasso R, De Brux J, Croissant O, Orth G. High prevalence of papillomavirus-associated penile intraepithelial neoplasia in sexual partners of women with cervical intraepithelial neoplasia. N Engl J Med. 1987;317:916–23. doi: 10.1056/NEJM198710083171502. [DOI] [PubMed] [Google Scholar]
- 50.Schiffman MH, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993;85:958–64. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 51.Manos MM, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605–10. doi: 10.1001/jama.281.17.1605. [DOI] [PubMed] [Google Scholar]
- 52.Callaghan J, Karim S, Mortlock S, Wintert M, Woodward N. Hybrid capture as a means of detecting human papillomavirus DNA from liquid-based cytology specimens: a preliminary evaluation. Br J Biomed Sci. 2001;58:184–9. [PubMed] [Google Scholar]
- 53.Paavonen J, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 54.New vaccine prevents cervical cancer. FDA Consum. 2006;40:37. [PubMed] [Google Scholar]
- 55.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 56.Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–8. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 57.Hockberger PE. A history of ultraviolet photobiology for humans, animals and microorganisms. Photochem Photobiol. 2002;76:561–79. doi: 10.1562/0031-8655(2002)0760561AHOUPF2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 58.Findlay GM. Ultra-violet light and skin cancer. The Lancet. 1928:1070–1073. [Google Scholar]
- 59.Bode AM, Dong Z. Mitogen-Activated Protein Kinase Activation in UV-Induced Signal Transduction. Sci STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 60.Epstein JH. Photocarcinogenesis, skin cancer, and aging. J Am Acad Dermatol. 1983;9:487–502. doi: 10.1016/s0190-9622(83)70160-x. [DOI] [PubMed] [Google Scholar]
- 61.Fitzpatrick TB, Sober AJ, Pearson BJ, Lew R. In: Research in Photobiology. Castellani A, editor. Plenum Publishing Corp; New York: 1976. pp. 485–490. [Google Scholar]
- 62.Forbes PD, Davis RJ, Urbach F. In: Research in Photobiology. Castellani A, editor. Plenum Publishing Corp; New York: 1976. pp. 469–478. [Google Scholar]
- 63.IARC. Solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1–316. [PMC free article] [PubMed] [Google Scholar]
- 64.Kusewitt DF, Budge CL, Anderson MM, Ryan SL, Ley RD. Frequency of ultraviolet radiation-induced mutation at the hprt locus in repair-proficient murine fibroblasts transfected with the denV gene of bacteriophage T4. Photochem Photobiol. 1993;58:450–4. doi: 10.1111/j.1751-1097.1993.tb09589.x. [DOI] [PubMed] [Google Scholar]
- 65.Ley RD. Photoreactivation in humans. Proc Natl Acad Sci U S A. 1993;90:4337. doi: 10.1073/pnas.90.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Setlow RB. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci U S A. 1974;71:3363–6. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Laat A, van der Leun JC, de Gruijl FR. Carcinogenesis induced by UVA (365-nm) radiation: the dose-time dependence of tumor formation in hairless mice. Carcinogenesis. 1997;18:1013–20. doi: 10.1093/carcin/18.5.1013. [DOI] [PubMed] [Google Scholar]
- 68.de Laat JM, de Gruijl FR. The role of UVA in the aetiology of non-melanoma skin cancer. Cancer Surv. 1996;26:173–91. [PubMed] [Google Scholar]
- 69.Gasparro FP. Sunscreens, skin photobiology, and skin cancer: the need for UVA protection and evaluation of efficacy. Environ Health Perspect. 2000;108 1:71–8. doi: 10.1289/ehp.00108s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Runger TM. Role of UVA in the pathogenesis of melanoma and non-melanoma skin cancer. A short review. Photodermatol Photoimmunol Photomed. 1999;15:212–6. doi: 10.1111/j.1600-0781.1999.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 71.Setlow RB. Spectral regions contributing to melanoma: a personal view. J Investig Dermatol Symp Proc. 1999;4:46–9. doi: 10.1038/sj.jidsp.5640180. [DOI] [PubMed] [Google Scholar]
- 72.Greegor DH. Diagnosis of large-bowel cancer in the asymptomatic patient. JAMA. 1967;201:943–5. [PubMed] [Google Scholar]
- 73.Wolff WI. Colonoscopy: history and development. Am J Gastroenterol. 1989;84:1017–25. [PubMed] [Google Scholar]
- 74.Wynder EL, Gori GB. Contribution of the environment to cancer incidence: an epidemiologic exercise. J Natl Cancer Inst. 1977;58:825–32. doi: 10.1093/jnci/58.4.825. [DOI] [PubMed] [Google Scholar]
- 75.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 76.Committee on Diet, N, and Cancer. National Research Council; Washington, D.C.: 1982. pp. 1–478. [Google Scholar]
- 77.Reddy BS, et al. Nutrition and its relationship to cancer. Adv Cancer Res. 1980;32:237–345. doi: 10.1016/s0065-230x(08)60363-2. [DOI] [PubMed] [Google Scholar]
- 78.U.S Department of Health and Human Services, editor. Surgeon General. The Surgeon General's Report on Nutrition and Health. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Washington D.C.: 1988. [Google Scholar]
- 79.Wattenberg LW. Chemoprophylaxis of carcinogenesis: a review. Cancer Res. 1966;26:1520–6. [PubMed] [Google Scholar]
- 80.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36:2699–702. [PubMed] [Google Scholar]
- 81.Wattenberg LW. Chemoprevention of cancer. Cancer Res. 1985;45:1–8. [PubMed] [Google Scholar]
- 82.Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–7. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 83.Thompson IM, Klein EA, Lippman SM, Coltman CA, Djavan B. Prevention of prostate cancer with finasteride: US/European perspective. Eur Urol. 2003;44:650–5. doi: 10.1016/j.eururo.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Fisher B, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 85.Fisher B, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 86.Vogel VG, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 87.Cummings SR, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 88.Barrett-Connor E, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 89.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–6. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 90.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 91.Bertagnolli MM, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 92.Meyskens FL, Jr, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sporn MB, Hong WK. Concomitant DFMO and sulindac chemoprevention of colorectal adenomas: a major clinical advance. Nat Clin Pract Oncol. 2008;5:628–9. doi: 10.1038/ncponc1221. [DOI] [PubMed] [Google Scholar]
- 94.Williams RW. The Natural History of Cancer With Special Reference to Its Causation and Prevention. W. Heinemann; London: 1908. [Google Scholar]
- 95.Lambe W. Water and vegetable diet in consumption, scrofulla, cancer, asthma, and other chronic diseases. Fowlers and Wells; New York: 1854. [Google Scholar]
- 96.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 97.Omenn GS, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 98.Albanes D, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 99.Virtamo J, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. Jama. 2003;290:476–85. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 100.Omenn GS, et al. The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res. 1994;54:2038s–2043s. [PubMed] [Google Scholar]
- 101.Neuhouser ML, et al. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET) Cancer Epidemiol Biomarkers Prev. 2003;12:350–8. [PubMed] [Google Scholar]
- 102.Goodman GE, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96:1743–50. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 103.Taylor PR, Albanes D. Selenium, vitamin E, and prostate cancer--ready for prime time? J Natl Cancer Inst. 1998;90:1184–5. doi: 10.1093/jnci/90.16.1184. [DOI] [PubMed] [Google Scholar]
- 104.Brawley OW, Parnes H. Prostate cancer prevention trials in the USA. Eur J Cancer. 2000;36:1312–5. doi: 10.1016/s0959-8049(00)00105-2. [DOI] [PubMed] [Google Scholar]
- 105.Kumar NB, Besterman-Dahan K. Nutrients in the Chemoprevention of Prostate Cancer: Current and Future Prospects. Cancer Control. 1999;6:580–586. doi: 10.1177/107327489900600604. [DOI] [PubMed] [Google Scholar]
- 106.Lippman SM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pierce JP, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23:728–56. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 108.Pierce JP, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blackburn GL, Wang KA. Dietary fat reduction and breast cancer outcome: results from the Women's Intervention Nutrition Study (WINS) Am J Clin Nutr. 2007;86:s878–81. doi: 10.1093/ajcn/86.3.878S. [DOI] [PubMed] [Google Scholar]
- 110.Chlebowski RT, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–76. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 111.Pierce JP. Diet and breast cancer prognosis: making sense of the Women's Healthy Eating and Living and Women's Intervention Nutrition Study trials. Curr Opin Obstet Gynecol. 2009;21:86–91. doi: 10.1097/gco.0b013e32831da7f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cummings NB. Women's health and nutrition research: US governmental concerns. J Am Coll Nutr. 1993;12:329–36. doi: 10.1080/07315724.1993.10718318. [DOI] [PubMed] [Google Scholar]
- 113.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 114.Prentice RL, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–42. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 115.Prentice RL, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99:1534–43. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rohan TE, et al. Low-fat dietary pattern and risk of benign proliferative breast disease: a randomized, controlled dietary modification trial. Cancer Prev Res (Phila Pa) 2008;1:275–84. doi: 10.1158/1940-6207.CAPR-08-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 118.Tomatis L, Huff J. Evolution of cancer etiology and primary prevention. Environ Health Perspect. 2001;109:A458–60. doi: 10.1289/ehp.109-a458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–45. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 120.Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for industry, investigators, and reviewers: exploratory IND studies. U.S. Department of Health and Human Services; 2006. [Google Scholar]
- 121.Kummar S, et al. Phase 0 clinical trials: conceptions and misconceptions. Cancer J. 2008;14:133–7. doi: 10.1097/PPO.0b013e318172d6f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Doroshow JH, Parchment RE. Oncologic phase 0 trials incorporating clinical pharmacodynamics: from concept to patient. Clin Cancer Res. 2008;14:3658–63. doi: 10.1158/1078-0432.CCR-07-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van den Brandt PA, Botterweck AA, Goldbohm RA. Salt intake, cured meat consumption, refrigerator use and stomach cancer incidence: a prospective cohort study (Netherlands) Cancer Causes Control. 2003;14:427–38. doi: 10.1023/a:1024979314124. [DOI] [PubMed] [Google Scholar]
- 124.La Vecchia C, Franceschi S, Levi F. Epidemiological research on cancer with a focus on Europe. Eur J Cancer Prev. 2003;12:5–14. doi: 10.1097/00008469-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 125.Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1–6. doi: 10.1111/j.1349-7006.2005.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Archer VE. Latitudinal variation of digestive tract cancers in the US and China. Nutr Cancer. 1989;12:213–23. doi: 10.1080/01635588909514021. [DOI] [PubMed] [Google Scholar]
- 127.Coggon D, Barker DJ, Cole RB, Nelson M. Stomach cancer and food storage. J Natl Cancer Inst. 1989;81:1178–82. doi: 10.1093/jnci/81.15.1178. [DOI] [PubMed] [Google Scholar]
- 128.Boeing H. Epidemiological research in stomach cancer: progress over the last ten years. J Cancer Res Clin Oncol. 1991;117:133–43. doi: 10.1007/BF01613137. [DOI] [PubMed] [Google Scholar]
- 129.Cohen AJ, Roe FJ. Evaluation of the aetiological role of dietary salt exposure in gastric and other cancers in humans. Food Chem Toxicol. 1997;35:271–93. doi: 10.1016/s0278-6915(96)00114-7. [DOI] [PubMed] [Google Scholar]