Abstract
Rationale
Nicotine infusions that are self-administered (contingent) or response-independent (noncontingent) increase lever pressing for a reinforcing nonpharmacological stimulus in rats, suggesting that in addition to primary reinforcement, nicotine self-administration may result from nicotine enhancing the reinforcement derived from nonnicotine stimuli.
Objectives
Based on our previous research, in this study, we tested the hypothesis that contingent and noncontingent nicotine would equally elevate responding for a moderately reinforcing visual stimulus, across a range of nicotine doses on both fixed ratio and progressive ratio reinforcement schedules.
Materials and methods
The rats lever pressed for a visual stimulus with contingent nicotine, noncontingent nicotine, or contingent saline. Separate groups responded for saline or nicotine without the visual stimulus. Three doses of nicotine (0.01, 0.03, and 0.09 mg/kg per infusion, free base) were tested in a between-groups design. After responding on an escalating fixed ratio reinforcement schedule, the rats were tested on a progressive ratio schedule.
Results
Compared to responding for the visual stimulus with saline, both contingent and noncontingent nicotine equally elevated lever pressing for the stimulus at each dose on fixed and progressive ratio schedules. In the absence of the stimulus, only the highest nicotine dose sustained self-administration.
Conclusions
The ability of noncontingent nicotine to elevate responding for a moderately reinforcing visual stimulus occurs across a range of doses, and both self-administered and noncontingent nicotine equally increase motivation to obtain the stimulus, as reflected by performance on a progressive ratio schedule. In the absence of a contingent stimulus, primary reinforcement from nicotine only supports self-administration at high nicotine doses in rats.
Keywords: Nicotine, Tobacco, Self-administration, Smoking, Nonpharmacological stimuli, Progressive ratio, Dose–response
Introduction
Current theories of tobacco dependence have advanced considerably from the initial hypothesis that smoking is sustained largely by the direct primary reinforcing effects of nicotine, the principal psychoactive ingredient in tobacco (US Department of Health and Human Services 1988). Recent data from behavioral models of nicotine reinforcement support the emerging hypothesis that primary reinforcement from nicotine only partially accounts for the robust levels of behavior generated by animals that self-administer nicotine and by human smokers. In addition to primary reinforcement, these outcomes may be attributed to the capacity of nicotine to nonassociatively facilitate behavior maintained by reinforcing nonnicotine stimuli (Donny et al. 2003; Chaudhri et al. 2005, 2006; Palmatier et al. 2006).
In support of the hypothesis that nicotine can enhance reinforced behavior, we have demonstrated that both self-administered (contingent) and response-independent (noncontingent) intravenous infusions of nicotine elevate responding for a moderately reinforcing, unconditioned visual stimulus (VS, 1-s onset of a white cue light, followed by the offset of a white houselight for 1 min) in rats (Donny et al. 2003). In this study, the rats that self-administered nicotine in conjunction with the VS also controlled the delivery of noncontingent nicotine or saline to animals that lever pressed for only the VS. Compared to responding for the VS with saline, both contingent and noncontingent nicotine equally and markedly elevated behavior maintained by the VS. This outcome strongly suggests that nicotine can increase the reinforcement derived from nonpharmacological stimuli, through a mechanism that does not require a temporal relationship between nicotine delivery, stimulus presentations, or behavior controlled by the stimulus.
These results were obtained using a unit dose of nicotine (0.03 mk/kg per infusion, free base) that maintains peak levels of operant responding on an escalating fixed ratio (FR) reinforcement schedule when contingent nicotine delivery is combined with a nonpharmacological stimulus (Corrigall and Coen 1989; Donny et al. 1998; Chaudhri et al. 2005). While the dose–response curve for self-administered nicotine has been well described, the impact of nicotine dose on the reinforcement-enhancing effect of noncontingent nicotine has not been characterized. Therefore, our first aim was to compare the dose–response functions for self-administered and noncontingent nicotine in rats that lever pressed for the VS (described above) on an escalating fixed ratio reinforcement schedule. We tested the specific hypothesis that both contingent and noncontingent nicotine would equally increase responding for the VS, particularly at low nicotine doses. This hypothesis is based on the observations that low nicotine doses are relatively ineffective in supporting the primary reinforcing effects of nicotine when nicotine alone is self-administered (Donny et al. 2003; Chaudhri et al. 2005) and that responding reinforced by the VS can be substantially enhanced by relatively small amounts of nicotine (Chaudhri et al. 2006; Palmatier et al. 2006). For example, a low dose of nicotine (0.01 mg/kg per infusion, free base) administered as a continuous noncontingent infusion throughout a 1-h test session markedly increases lever pressing for the VS (Donny et al. 2003). Furthermore, a single pre-session infusion of subcutaneous nicotine facilitates responding for both our unconditioned VS (Matteson et al. 2006) as well as a nonpharmacological conditioned reinforcer (Olausson et al. 2004).
Noncontingent nicotine does not elevate responding for a minimally reinforcing tone-light stimulus (Chaudhri 2005; Liu et al. 2005), suggesting that the capacity of nicotine to enhance behavior maintained by nonpharmacological stimuli reflects a nicotine-induced increase in the reinforcing effects of such stimuli: if the stimuli are not reinforcing, there is no effect of nicotine. This effect could be alternatively mediated by an enhancement in the sensory components of the stimulus (Terry et al. 2002), facilitation of the learning and memory of behavior driven by the stimulus (Levin 2002; Addy et al. 2003), or an increase in attention directed towards the stimulus (Grilly et al. 2000; Stolerman et al. 2000; Rezvani et al. 2002). In our second aim, we tested the hypothesis that nicotine increases the reinforcing strength of salient nonpharmacological stimuli by comparing the impact of contingent and noncontingent nicotine on responding maintained by the VS on a progressive ratio (PR) reinforcement schedule, across a range of nicotine doses. In progressive ratio reinforcement schedules, the response requirements for consecutive presentations of a reinforcer are increased within a single session, and the number of reinforcements achieved is interpreted as an index of the reinforcing strength and consequent motivational impact of that reinforcer on behavior (Risner and Goldberg 1983; Markou et al. 1993; Depoortere et al. 1993; Stafford et al. 1998; Donny et al. 1999; Barr and Phillips 1999; Nicola and Deadwyler 2000). In this study, we predicted that both contingent and noncontingent nicotine would equally enhance the motivation to obtain the VS, resulting in more VS presentations earned in the presence of nicotine compared to saline.
Materials and methods
Subjects
This experiment used two cohorts of male Sprague–Dawley rats (60 rats per cohort, Harlan Farms, 175–200 g on arrival) which were individually housed in a temperature-controlled environment (21°C) on a 12-h reversed light/dark cycle (lights off at 0700 hours). For 7 days before testing the rats were habituated to the colony room, where they had unrestricted access to food, and were weighed and handled daily. After habituation, the rats received 20 g rat chow per day for the remainder of the study. During self-administration, food was provided in their home cages after each daily session. The rats had unlimited access to water at all times.
Apparatus
Training and experimental sessions occurred in 25×31×28 cm3 operant conditioning chambers, which were outfitted with identical retractable levers, a food pellet trough directly in-between the levers, a white cue light above each lever, and an overhead houselight located directly above the pellet trough near the roof of the chamber. During self-administration sessions, the rats were connected to a drug-delivery swivel system that allowed nearly unrestricted movement in the chamber. The responses on the active lever, inactive lever, and reinforcements earned (VS presentations and/or number of infusions) were recorded using an interfaced computer software package (Med Associates, MED-PC IV). Exhaust fans within each sound-attenuating chamber produced a constant background noise (~75 db), which masked ambient noise as well as auditory cues associated with food and drug delivery.
Food training
Before testing, the animals were habituated to the operant conditioning chambers in a single 20-min session. After overnight food deprivation they were allowed to consume 75 food pellets (45 mg) in a single magazine training session during which both levers were retracted. Next, they were deprived with food overnight and hand-shaped to press the right (active) lever for 75 food pellets on a continuous reinforcement schedule. Rats that did not attain the criterion of responding for and consuming 50 pellets were re-shaped the following day. Responses made on the left (inactive) lever had no scheduled consequences. During magazine and shaping sessions, a dim red houselight, to which Sprague–Dawley rats are relatively insensitive, remained illuminated. No scheduled changes occurred in either visual or auditory stimuli.
Surgery
After training the rats were anesthetized with halothane, implanted with jugular catheters (Donny et al. 1999), and allowed at least 7 days to recover before the start of self-administration sessions. For 2 weeks after surgery the rats were treated with heparin and streptokinase to help maintain catheter patency, and with the antibiotic ticarcillan plus clavulanate (Timetin) to reduce post-surgical infections. Thereafter, the catheters were flushed once daily with 0.1 ml sterile heparinized saline containing Timetin (30 U/ml) on weekends, and both before (10 U/ml) and after (30 U/ml) each session on testing days. Catheter patency was determined on days 15 and 27 by infusing a small volume of chloral hydrate through the catheter to induce a temporary loss of muscle tone.
Self-administration sessions
Before self-administration rats were randomly divided into 11 groups. Animals in these groups responded on the active lever for a compound visual stimulus with contingent or noncontingent nicotine or lever pressed for nicotine in the absence of the VS. The delivery of noncontingent nicotine infusions was controlled by the self-administering rats, in a yoked design (Donny et al. 2003). Control rats responded for the VS combined with saline or for saline infusions without the VS. Three nicotine doses (0.01, 0.03, and 0.09 mg/kg per infusion, free base) were tested in a between-groups design. This range of nicotine doses supports robust self-administration when nicotine delivery is combined with a nonpharmacological stimulus (Corrigall and Coen 1989; Shoaib et al. 1997; Donny et al. 2000). All infusions were delivered in a volume of 0.1 ml/kg over approximately 1 s. The VS consisted of the onset of a white cue light located above the active lever for 1 s, followed by the offset of a white houselight for 1 min. The houselight offset signaled a time-out period during which responding on the active lever was recorded but not reinforced (Donny et al. 2003). The rats that lever pressed for nicotine or saline without the VS received an unsignaled 1-min time-out after each infusion (see Table 1).
Table 1.
Average responses on the active lever and inactive lever during the last 4 days of an FR5 schedule and PR schedule for each group
| Group | Fixed ratio scheduled (FR5) | Progressive ratio schedule | |||
|---|---|---|---|---|---|
| Active lever |
Inactive lever |
Time-out responding |
Active lever | Inactive lever | |
| Contingent NIC + VS (0.01 mg/kg per infusion), n=9 | 83.42±10.17 | 2.22±0.45 | 11.47±1.52 | 106.22±10.94 | 1.83±0.34 |
| Noncontingent NI + VS (0.01 mg/kg per infusion), n=11 | 78.39±8.97 | 2.50±0.38 | 13.50±2.00 | 95.34±6.12 | 2.34±0.32 |
| Contingent NIC + VS (0.03 mg/kg per infusion), n=8 | 57.66±7.41 | 3.50±0.76 | 10.78±1.49 | 131.66±14.55 | 2.88±0.59 |
| Noncontingent NIC + VS (0.03 mg/kg per infusion), n=11 | 62.93±4.82 | 3.68±0.50 | 7.80±0.77 | 98.60±10.90 | 3.38±0.39 |
| Contingent NIC + VS (0.09 mg/kg per infusion), n=10 | 54.23±4.19 | 1.20±0.29 | 5.85±0.90 | 122.78±14.79 | 4.43±0.88 |
| Noncontingent NIC + VS (0.09 mg/kg per infusion), n=11 | 67.75±6.73 | 2.82±0.51 | 9.34±1.07 | 158.91±15.46 | 4.39±0.62 |
| Contingent SAL + VS, n=8 | 24.88±3.30 | 1.38±0.30 | 5.19±0.75 | 42.59±6.75 | 2.81±0.54 |
| Contingent SAL + no VS, n=9 | 5.25±0.81 | 1.78±0.36 | 2.33±0.42 | 8.44±1.49 | 3.00±0.69 |
| Contingent NIC + no VS (0.01 mg/kg per infusion), n=6 | 3.79±0.66 | 1.25±0.53 | 2.13±0.36 | 8.63±2.41 | 2.33±0.51 |
| Contingent NIC + no VS (0.03 mg/kg per infusion), n=6 | 2.71±0.38 | 1.38±0.77 | 1.46±0.29 | 5.38±1.27 | 2.08±0.58 |
| Contingent NIC + no VS (0.09 mg/kg per infusion), n=6 | 38.67±7.91 | 4.63±0.97 | 13.04±2.85 | 127.13±33.10 | 17.25±5.27 |
The average responding during the 1-min time-out period after each reinforcer delivery is also indicated for the last 4 days of an FR5 schedule
Self-administration sessions began on a Monday and were conducted on weekdays during the dark phase of the light/dark cycle. Fixed ratio sessions (60 min) were run according to an escalating reinforcement schedule (FR1, days 1–5; FR2, days 6–8; and FR5, days 9–17). The same rats were then tested on a progressive ratio reinforcement schedule for 12 days. The PR sessions lasted for 220 min and used the formula 5 × EXP (0.2 × infusion number) − 5 (Depoortere et al. 1993; Donny et al. 1999), which results in the following sequence of required responses per reinforcement earned: 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 179, 219, 268, and 328.
Statistical analyses
Before statistical analyses, the data for 19 (out of 120) rats that failed in one or both of the chloral hydrate tests for catheter patency and for three additional rats that were identified as statistical outliers using SPSS (v11) were excluded. Outliers exhibited extreme values (>3 box plot lengths from the upper or lower edge of the interquartile range) on >60% of self-administration test days (Lehman 1995). The present study used two cohorts of rats, with each group represented approximately equally in both cohorts. In the absence of a significant main effect of cohort using analysis of variance (ANOVA), further analyses utilized data collapsed across both cohorts.
FR and PR schedules were analyzed separately to test the hypothesis that contingent and noncontingent nicotine would equally enhance lever pressing for the VS compared to saline, at each nicotine dose tested. Stable behavior on each schedule was characterized by data averaged across the last 2 days of FR5 (days 16–17) and the last 4 days of PR (days 26–29) (Chaudhri et al. 2005; Donny et al. 2003). The analyses were not conducted during early phases of acquisition, on FR1 and FR3 reinforcement schedules. Operant responding was analyzed with lever as the within-subject factor and group as the between-subjects factor, and the number of reinforcements earned (VS presentations or infusions) was analyzed for main effects of group or dose. Significant main effects were verified using targeted three- and four-way ANOVAs with Tukey honestly significant differences post hoc comparisons (α=0.05). With the exception of two groups [nicotine (NIC) + no VS, 0.01 mg/kg per infusion and NIC + no VS, 0.03 mg/kg per infusion], responding on the active lever was significantly higher than on the inactive lever (see Table 1). Therefore, only data on reinforcements earned are presented.
Results
Effects of contingent and noncontingent nicotine on responding for the VS
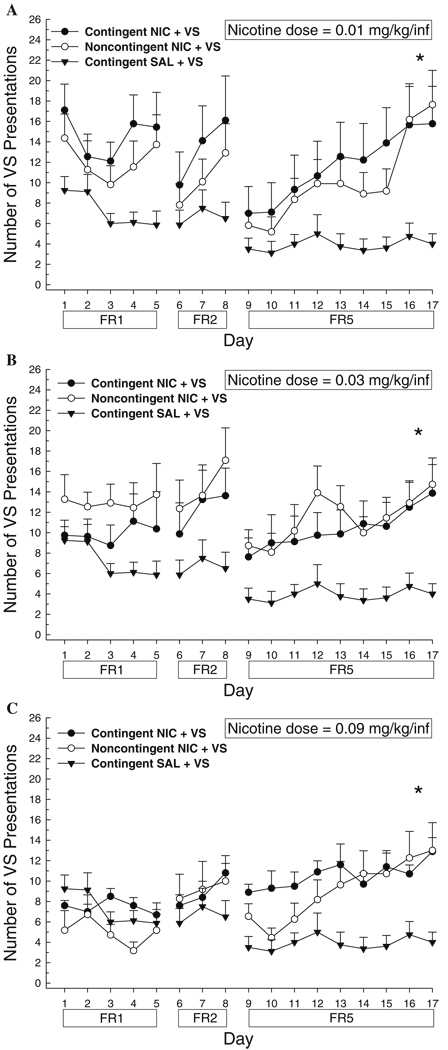
On an FR5 schedule, the number of VS presentations earned was significantly increased by contingent and noncontingent nicotine at each nicotine dose tested (Fig. 1a–c). Three-way comparisons between contingent NIC + VS, noncontingent NIC + VS, and contingent saline (SAL) + VS at each dose revealed a main effect of group (0.01 mg/kg per infusion, F2,25=4.66, p<0.05; 0.03 mg/kg per infusion, F2,24=5.68, p<0.05; and 0.09 mg/kg per infusion, F2,26=5.40, p<0.05), and Tukey post hoc comparisons confirmed that, at each dose, contingent and noncontingent nicotine were not different from each other, but both were significantly different from saline (p<0.05 for all latter comparisons). A separate analysis of dose and contingency for these six nicotine groups revealed no main effect of dose or contingency and no significant dose by contingency interaction.
Fig. 1.
Effects of nicotine (NIC) or saline (SAL) on responding for the visual stimulus (VS) on an escalating fixed ratio schedule. Data are mean (±SEM) VS presentations earned. a 0.01 mg/kg per infusion; b 0.03 mg/kg per infusion; c 0.09 mg/kg per infusion. The schedule of reinforcement is indicated below the abscissa. *p<0.05, contingent and noncontingent NIC > SAL on an FR5 schedule
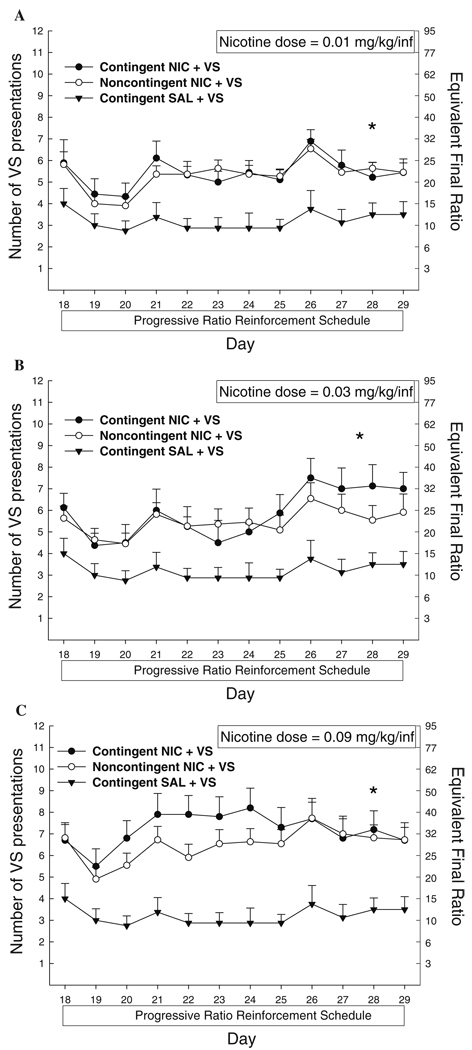
Contingent and noncontingent nicotine similarly increased the number of VS presentations earned on a PR schedule compared to saline (Fig. 2a–c). A significant main effect of group was indicated in three-way ANOVAs between contingent NIC + VS, noncontingent NIC + VS, and contingent SAL + VS at each dose (0.01 mg/kg per infusion, F2,25=7.77, p<0.05; 0.03 mg/kg per infusion, F2,24=5.65, p<0.05; and 0.09 mg/kg per infusion, F2,26=7.71, p<0.05). Tukey post hoc analyses confirmed no difference between nicotine groups at each dose but a significant enhancement in VS presentations earned in the presence of contingent or noncontingent nicotine compared to saline (p<0.05 for latter comparisons).
Fig. 2.
Mean (+SEM) number of VS presentations earned with NIC or SAL on a progressive ratio reinforcement schedule. Right axis indicates highest ratio of responding achieved. a 0.01 mg/kg per infusion; b 0.03 mg/kg per infusion; c 0.09 mg/kg per infusion. Significant between-groups comparisons at each day are indicated as follows: *p<0.05, contingent and noncontingent NIC > SAL
Using data averaged across the last 4 days of the PR schedule, there was no main effect of dose or contingency and no dose by contingency interaction. However, because behavior on a PR showed less variability across day compared to responding on an FR5 schedule, a separate analysis of dose was conducted for contingent NIC + VS across day (days 18–29) on a PR schedule. This analysis indicated a significant day by dose interaction for the comparison between 0.03 and 0.09 mg/kg per infusion (F11,176=3.27, p<0.05); on days 21–24, the number of reinforcements earned by rats self-administering 0.09 mg/kg per infusion was significantly greater compared to that with 0.03 mg/kg per infusion (Fig. 2b,c). No other dose comparisons were significant.
Nicotine self-administration in the absence of the VS
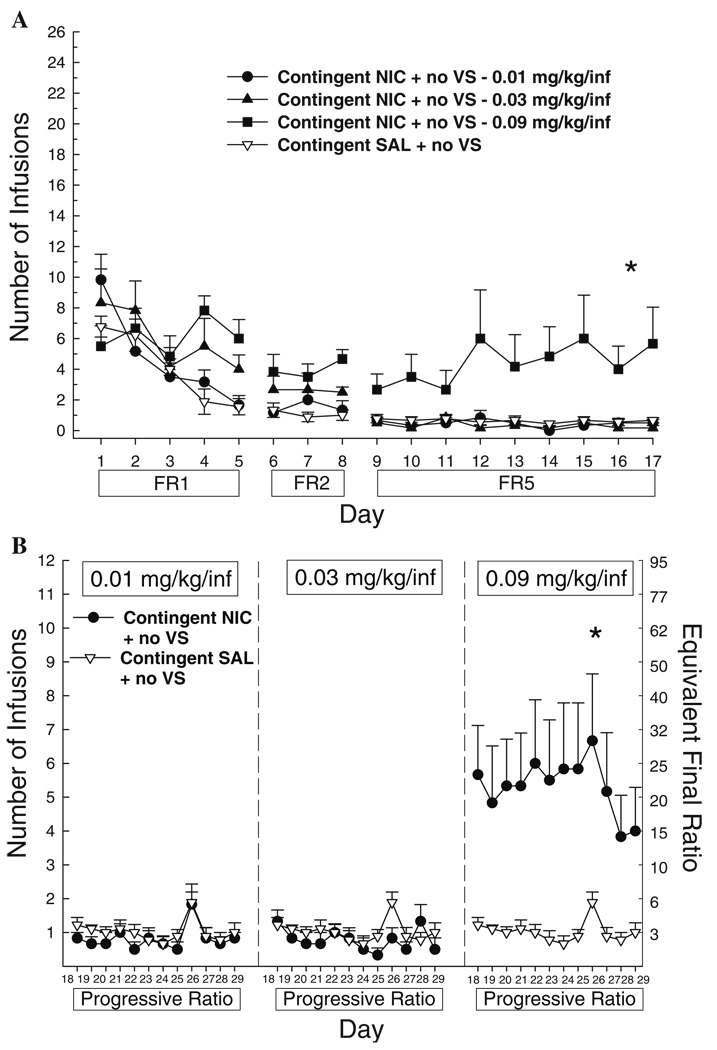
When lever pressing resulted in nicotine infusions without the VS, significant self-administration on both FR and PR schedules was only observed at 0.09 mg/kg per infusion (Fig. 3a,b). ANOVA conducted on contingent SAL + no VS and the three doses of contingent NIC + no VS indicated a significant main effect of group (FR5, F3,23=6.12, p<0.05; PR F3,23=7.01, p<0.05). Post hoc tests confirm that contingent NIC + no VS at 0.09 mg/kg per infusion supported more behavior than the two lower nicotine doses and saline on both reinforcement schedules (p<0.01 for all latter comparisons). The two lowest nicotine doses were no different from either each other or saline,
Fig. 3.
Mean (+SEM) number of infusions earned when responding is reinforced with NIC or SAL in the absence of a contingent VS on an escalating fixed ratio schedule (a) or a progressive ratio schedule (b, SAL + no VS group is replicated in each panel). The schedule of reinforcement is indicated below the abscissa. Significant between-groups comparisons at each day are indicated as follows: *p<0.05, all comparisons (0.09 mg/kg per infusion > saline, 0.01 and 0.03 mg/kg per infusion)
Impact of the contingent VS on lever pressing and nicotine self-administration
Response contingent delivery of the VS with saline produced greater reinforcement than saline without the VS, as indicated by a significant difference between contingent SAL + no VS and contingent SAL + VS (group: FR5, F1,15=12.67, p<0.01; PR, F1,15=13.36, p<0.01). In a separate analysis comparing contingent NIC + VS and contingent NIC + no VS groups across dose, combining nicotine delivery with the VS increased self-administration at 0.01 and 0.03 mg/kg per infusion (main effect of group: FR5, F5,39=7.97, p<0.001; PR, F5,39=11.13, p<0.001; p<0.01 in Tukey post hoc comparisons) but not at 0.09 mg/kg per infusion of nicotine.
Nicotine intake
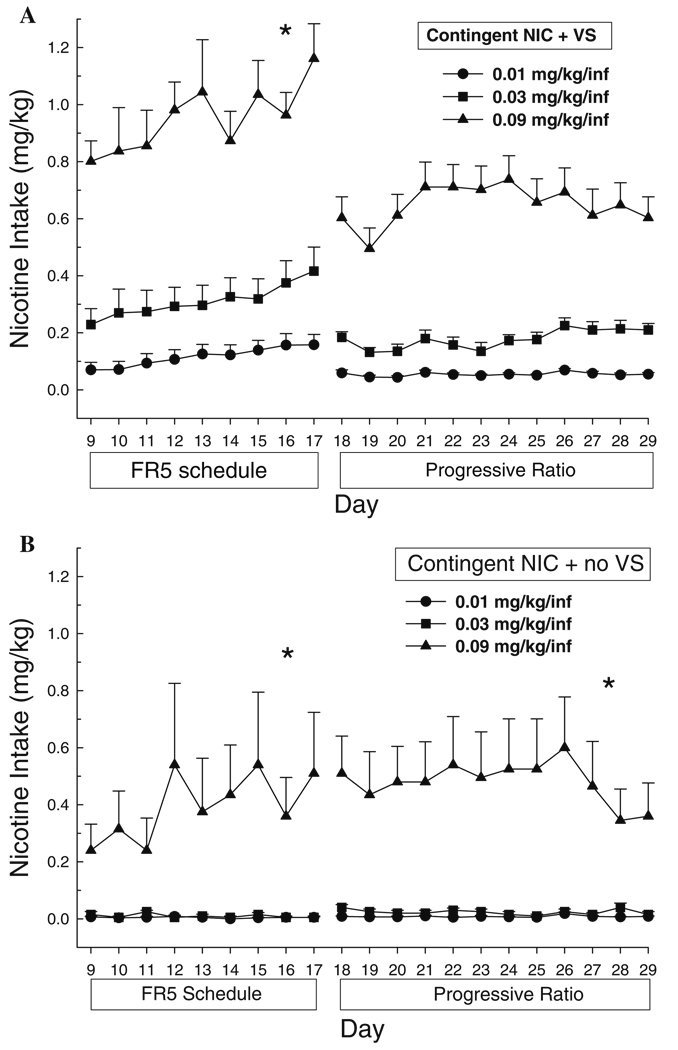
ANOVA conducted on the total amount of nicotine (mg/kg) self-administered by contingent NIC + VS and contingent NIC + no VS groups at each nicotine dose indicated a significant main effect of group (FR5, F5,39=24.76, p<0.001; PR, F5,39=20.83, p<0.001). When nicotine delivery was combined with the VS there was a dose-dependent difference in the total amount of nicotine self-administered (Fig. 4a). Post hoc Tukey tests indicate that nicotine intake was significantly higher at 0.09 mg/kg per infusion compared to the two lower doses on both reinforcement schedules (p<0.001 for each comparison). On a PR schedule, there was a trend for rats self-administering 0.03 mg/kg per infusion to achieve higher intake values compared to 0.01 mg/kg per infusion (p=0.056). In the absence of the VS, nicotine intake was greater at the highest dose tested, compared to 0.01 or 0.03 mg/kg per infusion (p<0.001 for each comparison).
Fig. 4.
Mean (+SEM) total NIC intake (mg/kg) for rats that lever pressed for a contingent NIC + VS or b contingent NIC + no VS on FR5 and progressive ratio reinforcement schedules. Significant between-groups comparisons at each day are indicated as follows: *p<0.05, all comparisons (0.09 mg/kg per infusion > saline, 0.01 and 0.03 mg/kg per infusion)
Discussion
In the present study, self-administered (contingent) and response-independent (noncontingent) nicotine administration increased lever pressing for a moderately reinforcing, unconditioned visual stimulus. The magnitude of this effect was equivalent for both nicotine conditions across dose, on both fixed and progressive ratio reinforcement schedules. In the absence of the contingent VS, only the highest nicotine dose (0.09 mg/kg per infusion) sustained infusion rates above saline.
These data extend our previous findings that lever pressing for the VS on an FR schedule is elevated by noncontingent nicotine administered either as discrete, pulsed infusions (0.03 mg/kg per infusion) or as a continuous infusion (0.01 mg/kg per infusion) delivered throughout a 1-h test session (Donny et al. 2003). We have also shown that in the absence of a contingent VS lever pressing with noncontingent nicotine is not different from the low levels of behavior obtained for contingent saline (Donny et al. 2003). These converging results demonstrate that nicotine can increase the behavior maintained by a moderately reinforcing nonnicotine stimulus across a range of nicotine doses, and strongly suggest that this effect occurs through a nonassociative mechanism.
The interpretation that an increase in operant responding for a contingent stimulus reflects an enhancement in the positive reinforcing value of that stimulus is prevalent in behavioral theories of reinforcement (Stewart 1960; Wise 1987). In the present study, noncontingent nicotine enhanced lever pressing for a VS using two distinct reinforcement schedules, which have been postulated to provide unique but complementary information about the processes that govern reinforcement. While FR schedules are thought to measure the hedonic impact of a drug, PR schedules have been used to index the motivational strength of pharmacological, natural, and nonpharmacological reinforcers (Risner and Goldberg 1983; Markou et al. 1993; Depoortere et al. 1993; Stafford et al. 1998; Donny et al. 1999; Barr and Phillips 1999; Nicola and Deadwyler 2000). The demonstration that nicotine, regardless of contingency, increased the number of VS presentations earned on a PR schedule may reflect a nicotine-induced enhancement in the incentive salience of that stimulus. Further experiments are needed to address the specific question of how the increase in reinforcing properties of the stimulus by nicotine is mediated.
Nicotine increased responding for the VS on a PR schedule even though overall nicotine intake was markedly lower on a PR schedule compared to the preceding FR5 schedule, suggesting that the capacity of nicotine to enhance reinforcement can occur with relatively small quantities of the substance. Additional support for this hypothesis comes from a study in which rats responded on one lever for nicotine (0.06 mg/kg per infusion) and on a second lever for the VS (Palmatier et al. 2006). The response levels on the nicotine lever were low and not different from those of control animals that only had access to contingent nicotine. However, responses for the VS were significantly higher than the behavior exhibited by control rats that only had access to the VS, and more closely approximated the behavior of rats that had access to an inactive lever and an active lever that controlled the delivery of nicotine combined with the VS (i.e., a standard self-administration condition). This study demonstrates that the robust ability of nicotine to increase responding for the VS can be achieved with relatively small amounts of self-administered nicotine.
The present data corroborate our previous hypothesis that nicotine and nonpharmacological stimuli interact to generate a substantial increase in self-administration, compared to nicotine self-administered in the absence of a contingent stimulus (Caggiula et al. 2002a,b; Donny et al. 2003; Chaudhri et al. 2005, 2006; Palmatier et al. 2006). They also illustrate a recurrent observation in our research that the interaction between nicotine and the VS appears to be more pronounced at lower compared to higher nicotine doses: combining the VS with nicotine has a greater impact on responding at 0.01 and 0.03 mg/kg per infusion, compared to 0.09 mg/kg per infusion (Chaudhri et al. 2005; present data). In contrast, the primary reinforcing effects of nicotine without a combined nonpharmacological stimulus support moderate levels of behavior at high but not low nicotine doses. These observations suggest that the impact of a contingent nonpharmacological stimulus on behavior may be greater at nicotine doses that demonstrate only weak primary reinforcing effects, compared to nicotine doses that sustain moderate responding on their own.
The dose–response function for contingent nicotine combined with a nonpharmacological stimulus is typically a shallow, inverted “U” shape, which peaks at low doses (0.01–0.03 mg/kg per infusion) and declines with increasing dose (Corrigall and Coen 1989; Shoaib et al. 1997; Donny et al. 1998; Rasmussen and Swedberg 1998; Chaudhri et al. 2005). The absence of a dose effect in the impact of contingent and noncontingent nicotine on responding for the VS in this experiment can be accounted for in at least two ways. First, in contrast to our previous research in which acquisition on an FR schedule is typically tested over 20–25 days (Donny et al. 1999, 2003), the rats in the present study had only 17 days of FR responding before the PR schedule. Dose effects may have conceivably emerged had responding been given longer to stabilize on an FR5 schedule, which may consequently have translated into dose-dependent behavior on a PR schedule. Second, the overall level of behavior obtained in the present experiment was somewhat lower than that in previous observations, as is evident in responding maintained on an FR5 schedule for the VS with saline (e.g., an average of 7.14±0.15 VS presentations in Chaudhri et al. 2005 vs 3.2±0.22 in the present study). This difference may be attributed to the specific shipments of animals used in this study or to external conditions (e.g., noise levels generated by ongoing construction) that were beyond the control of the experimenters. Regardless, the main effects in the present experiment are similar to pilot studies (unpublished data) and previously reported data (Caggiula et al. 2002a; Donny et al. 2003; Chaudhri et al. 2005).
A divergence in the dose–response curves for the two nicotine conditions would have implied that the primary reinforcing effects of contingent nicotine can be dissociated from the reinforcement-enhancing effects common to both contingent and noncontingent nicotine, based simply on nicotine dose. Although this conclusion cannot be drawn from the present findings, we have recently demonstrated that the primary reinforcing and reinforcement-enhancing effects of nicotine can be dissociated by varying the reinforcing strength of the contingent nonnicotine stimulus (see Chaudhri 2005 and Chaudhri et al. 2006, for a review). Contingent and Noncontingent nicotine both elevate responding for nonnicotine stimuli that are moderately or strongly reinforcing. However, if the stimulus is only weakly reinforcing, then behavior maintained by that stimulus is more effectively enhanced by contingent compared to noncontingent nicotine (Chaudhri 2005; Liu et al. 2005). This interaction has two important conclusions. First, it suggests that the primary reinforcing actions of contingent nicotine can enhance behavior maintained by weak nonpharmacological reinforcers, an effect that could result from the ability of contingent (but not noncontingent) nicotine to establish concurrent stimuli as conditioned reinforcers. Second, it suggests that the capacity of nicotine to enhance reinforcement is less robust if the stimulus itself is not a strong positive reinforcer.
Research on the neurobiology of nicotine suggests that the ventral tegmental area and its dopaminergic projection sites such as the nucleus accumbens may mediate the reinforcement-enhancing effect of nicotine. Nicotine can increase the responsiveness of the mesolimbic dopamine system when dopamine neuron firing switches from tonic to phasic, which could be in response to primary and/or conditioned reinforcers (Rice and Cragg 2004). Electrophysiological data suggest that nicotine can both disinhibit ventral tegmental area (VTA) dopamine neurons, and cause a glutamate-induced excitation of VTA dopamine neurons (Schilstrom et al. 1998; Grillner and Svensson 2000; Jones and Wonnacott 2004), creating a dopamine system that is hyper-excited and potentially more responsive to stimulation from incoming pharmacological and nonpharmacological reinforcers (Mansvelder and McGehee 2000; Dani et al. 2001; Dani and De Biasi 2001; Mansvelder and McGehee 2002; Mansvelder et al. 2002, 2003; Pidoplichko et al. 2004). Finally, the synchronized activation of VTA dopamine neurons by nicotine and by stimulation from incoming reward-related information could result in long-term potentiation, a cellular phenomenon implicated in learning and memory (Silva 2003) that could strengthen synapses that mediate the reinforcement conveyed by nonnicotine stimuli. Further research is needed to support the hypothesis that a nicotine-induced prolonged excitation of VTA dopamine neurons underlies the reinforcement-enhancing effects of nicotine, and to determine the localization and subunit composition of nicotinic acetylcholine receptors that may be involved.
In summary, we demonstrate in this study that the capacity of nicotine to enhance reinforced behavior is not limited by dose or reinforcement schedule. These findings corroborate the hypothesis that nicotine has dual roles in reinforcement: it can function as a weak primary reinforcer when administered contingently (Rose and Levin 1991; Cohen et al. 2005) but can also increase reinforced responding, an action that does not require response-dependent nicotine delivery (Donny et al. 2003; Olausson et al. 2004; Chaudhri et al. 2006). In this respect, nicotine parallels psychostimulant drugs such as cocaine and amphetamine, which have been shown to exhibit analogous dual effects in reinforcement (Robbins 1976; Taylor and Robbins 1984; Robbins et al. 1989; Phillips and Fibiger 1990). The insight that nicotine can enhance reinforcement through nonassociative mechanisms extends our evolving understanding of how nicotine, a relatively weak primary reinforcer, can mediate complex behaviors such as self-administration and smoking.
Acknowledgements
“Principles of laboratory animal care” (NIH No. 85-23, revised 1985) were followed throughout all experiments. The University of Pittsburgh Institutional Animal Care and Use Committee assurance number A3187-01 approved this research. The preparation of this manuscript was supported by National Institute on Drug Abuse research grants DA-010464 and DA-012655 and by a Howard Hughes pre-doctoral research fellowship awarded to N. Chaudhri.
Contributor Information
Nadia Chaudhri, Email: nchaudhri@egcrc.net, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA, USA; Ernest Gallo Clinic and Research Center, University of California at San Francisco, 5858 Horton Street, Suite 200, Emeryville, CA 94608, USA.
Anthony R. Caggiula, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA
Eric C. Donny, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA
Sheri Booth, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Maysa Gharib, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Laure Craven, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Matthew I. Palmatier, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA, USA Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Xiu Liu, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Alan F. Sved, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA, USA
References
- Addy NA, Nakijama A, Levin ED. Nicotinic mechanisms of memory: effects of acute local DHbetaE and MLA infusions in the basolateral amygdala. Brain Res Cogn Brain Res. 2003;16:51–57. doi: 10.1016/s0926-6410(02)00209-4. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002a;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002b;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Chaudhri N. Complex interactions between nicotine and nonpharmacological stimuli reveal a novel role for nicotine in reinforcement. Pittsburgh, PA: Department of Neuroscience, University of Pittsburgh; 2005. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–352. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Grillner P, Svensson TH. Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse. 2000;38:1–9. doi: 10.1002/1098-2396(200010)38:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Grilly DM, Simon BB, Levin ED. Nicotine enhances stimulus detection performance of middle-and old-aged rats: a longitudinal study. Pharmacol Biochem Behav. 2000;65:665–670. doi: 10.1016/s0091-3057(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman RS. A conceptual approach. Brooks/Cole; 1995. Statistics in the behavioral sciences. [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Chaudhri N, Sved AF. Reinforcement-enhancing effect of nicotine depends on the reinforcement valence of nondrug stimulus. Society for Neuroscience. 2005 abstract 1027.14. [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur J Pharmacol. 2003;480:117–123. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Matteson GL, Palmatier MI, Liu X, Chaudhri N, Caggiula AR, Donny EC, Sved AF. The reinforcement enhancing effect of nicotine depends on the incentive value of a nonpharmacological reinforcer. The 12th annual meeting of the Society for Research on Nicotine and Tobacco; February 15–18; Orlando, FL. 2006. [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci. 2000;20:5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny E, Liu X, Booth S, Gharib MA, Craven LA, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav Pharmacol. 1990;1:269–282. doi: 10.1097/00008877-199000140-00002. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Swedberg MD. Reinforcing effects of nicotinic compounds: intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1998;60:567–573. doi: 10.1016/s0091-3057(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Bushnell PJ, Levin ED. Effects of nicotine and mecamylamine on choice accuracy in an operant visual signal detection task in female rats. Psychopharmacology (Berl) 2002;164:369–375. doi: 10.1007/s00213-002-1221-0. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic–striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br J Addict. 1991;86:605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Nomikos GG, Nisell M, Hertel P, Svensson TH. N-Methyl-d-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience. 1998;82:781–789. doi: 10.1016/s0306-4522(97)00243-1. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol. 1960;53:187–193. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–154. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Risbrough VB, Buccafusco JJ, Menzaghi F. Effects of (+/−)-4-[[2-(1-methyl-2-pyrrolidinyl)ethyl]thio]phenol hydrochloride (SIB-1553A), a selective ligand for nicotinic acetylcholine receptors, in tests of visual attention and distractibility in rats and monkeys. J Pharmacol Exp Ther. 2002;301:284–292. doi: 10.1124/jpet.301.1.284. [DOI] [PubMed] [Google Scholar]
- USDHHS. Nicotine addiction: a report of the surgeon general. Rockville, MD: US Department of Health and Human Services, Office of the Assistant Secretary for Health, Office on Smoking and Health. 1988
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]