Abstract
Glucose and tumor necrosis factor-α (TNFα) concentrations are elevated in diabetes. Both of these factors correlate with diabetic vasculopathy and endothelial cell apoptosis, yet their combined effects have not been measured. We have previously shown that the angiogenic growth factor fibroblast growth factor-2 (FGF-2), which is generally protective against endothelial cell death, is similarly elevated in high glucose conditions. We therefore investigated the effect of TNFα on endothelial cell death under normal and elevated glucose conditions, with a particular focus on FGF-2. Porcine aortic endothelial cells were cultured in 5 and 30 mM glucose and stimulated with TNFα, together with FGF-2 or a neutralizing FGF-2 antibody. Cell death was measured via cell counts or an annexin apoptotic assay, and cell cycle phase was determined by propidium iodide labeling. TNFα-induced endothelial cell death increased for cells in high glucose, and cell death was enhanced with increasing FGF-2 exposure and negated by a neutralizing FGF-2 antibody. Endothelial cells were most susceptible to TNFα-induced cell death when stimulated with FGF-2 18 h prior to TNFα, corresponding to cell entry into S phase of the proliferative cycle. The FGF-2 associated increase in TNFα-induced cell death was negated by blocking cell entry into S phase. Endothelial cell release of FGF-2 in high glucose leads to cell cycle progression, which makes cells more susceptible to TNFα-induced cell death. These data suggest that growth factor outcomes in high glucose depend on secondary mediators such as cytokines and stimulation cell cycle timing.
Endothelial cells are critical regulators of vascular homeostasis, maintaining balance in a system that constantly adapts to environmental stimuli. In health, the endothelial monolayer controls thrombosis through regulated release of prostacyclin and tissue plasminogen activator, vasomotor tone through nitric oxide, and proliferation through heparan sulfate proteoglycans and growth factors (Michiels, 2003). When tissue damage occurs, endothelial cells quickly adapt to promote thrombosis, signal vasoconstriction, and begin the healing process of angiogenesis (Li et al., 2003; Werner and Grose, 2003).
This delicate endothelial control of vascular homeostasis is upset by excess glucose, even at levels commonly observed in persons with diabetes (Martin et al., 2003). Hyperglycemia is toxic to vascular tissue and endothelial cells in particular. Endothelial cell dysfunction contributes to diabetic vascular morbidity and mortality from diseases such as atherosclerosis, retinopathy, nephropathy, and impaired wound healing (Tsilibary, 2003; Brem and Tomic-Canic, 2007; Beckman et al., 2002). In high glucose, endothelial cells overexpress membrane cell adhesion molecules and become hyperpermeable to proteins and cells (Hempel et al., 1997; Kado et al., 2001). Endothelial-dependent vascular relaxation is impaired, with diminished activity of the vasodilator nitric oxide and increased activity of vasoconstrictors such as angiotensin II and endothelin-1 (Calver et al., 1992; Cai and Harrison, 2000; Cooper et al., 2001; Cardillo et al., 2002). Angiogenesis becomes dysregulated, resulting in excess or inadequate blood vessel growth in different tissue beds (Martin et al., 2003).
Diabetes is a complex disease, with many biochemical alterations beyond glucose. Tumor necrosis factor-α (TNFα), an inflammatory cytokine that also affects endothelial cell function, is chronically elevated in persons with diabetes. In fact, increased plasma TNFα levels have been directly linked to hyperglycemia in humans (Esposito et al., 2002). High levels of systemic and local TNFα correlate with diabetic complications, including retinopathy, neuropathy, and nephropathy (Hasegawa et al., 1991; Satoh et al., 2003; Krady et al., 2005). On a cellular level, TNFα increases endothelial cell adhesion molecule expression and permeability, down regulates endothelial nitric oxide synthase to decrease nitric oxide production, and disrupts regulated formation of new blood vessels through inappropriate growth factor release or endothelial cell apoptosis (Pober et al., 1986; Royall et al., 1989; Robaye et al., 1991; Yoshida et al., 1997; Anderson et al., 2004).
Both hyperglycemia and TNFα increase endothelial cell apoptosis, but their combined effects have not been examined. We previously demonstrated that availability of fibroblast growth factor-2 (FGF-2), a potent angiogenic growth factor, is increased in high glucose conditions (Morss and Edelman, 2007). FGF-2 enhances endothelial cell proliferation, stimulates angiogenesis, and protects endothelial cells from cell death (Nugent and Iozzo, 2000). We therefore sought to determine how hyperglycemia affects TNFα-induced endothelial cell death, and whether glucose-induced FGF-2 release might have a protective effect.
Materials and Methods
Cell culture
Endothelial cells were isolated from porcine aortae by the collagenase dispersion method and were used between passages 4 and 9. Cells were maintained in supplemented media consisting of Dulbecco’s Modified Eagle’s Medium (DMEM, low glucose) with 5% fetal bovine serum, 1% penicillin-streptomycin, and 2% glutamine (Invitrogen, Carlsbad, CA). For high glucose media, d-glucose was added to supplemented low glucose media (5 mM, 90 mg/dl) to a final concentration of 30 mM (540 mg/dl). Mannitol was the osmotic control. Culture media was changed every 48 h. All experiments were performed on post-confluent cells 4 days after endothelial cells were seeded at a concentration of 1 × 105 cells/cm2. Preliminary experiments showed a significant difference between cells cultured in low and high glucose media at this time point.
Porcine TNFα was from R&D Systems (Minneapolis, MN/Rocky Hill, NJ). Recombinant human FGF-2 was obtained from Peprotech. Aphidicolin was purchased from Sigma (St. Louis, MO). Blocking antibodies for FGF-2 and TNFα were from Upstate Biotechnology (Lake Placid, NY) and Serotec (Raleigh, NC), respectively. The blocking antibodies specifically neutralized FGF-2 and TNFα as measured through enzyme-linked immunosorbent assay (ELISA) and apoptosis assays.
FGF-2 quantification
Endothelial cell released FGF-2 was measured in conditioned media. Media samples were collected without disturbing cells and centrifuged to pellet any dead floating cells. The supernatant was either used immediately or stored for up to 3 days at −20°C. FGF-2 levels in conditioned media samples were quantified via FGF-2 ELISA (R&D Systems) as per manufacturer instructions.
Cell death measurement
Dead cells were quantified by collecting conditioned media and counting floating cells with a Coulter counter (Beckman Coulter, Fullerton, CA). Live attached cells were trypsinized, counted and compared to dead cell numbers. Floating cells were confirmed as dead using Trypan Blue exclusion.
Endothelial cell apoptosis was measured via annexin V-propidium iodide labeling and confirmed by terminal deoxynucleotidyltransferase dUTP nick end labeling (TUNEL). Annexin V binds phosphatidylserine translocated from the inner to the outer cell membrane. Cells in early apoptosis are identified as annexin V-positive and negative for the vital dye propidium iodide, which is membrane impermeant and excluded from viable cells. Endothelial cells were prepared for the annexin V-propidium iodide assay by combining floating and trypsin-released attached cells. Samples were centrifuged to pellet cells, washed thoroughly, resuspended in annexin binding buffer, and labeled with annexin V-fluorescein and propidium iodide as per kit instructions (BD Pharmingen, San Jose, CA). Samples were analyzed immediately by flow cytometry (BD FACScan).
Cell cycle analysis
Endothelial cell progression through the cell cycle was analyzed by measuring DNA content with propidium iodide (Molecular Probes, Carlsbad, CA). Propidium iodide binds to DNA by intercalating between base pairs. DNA binding enhances propidium iodide fluorescence 20–30 times, therefore cell cycle can be determined by measured the fluorescence of cells exposed to propidium iodide. Cells with unreplicated DNA are in G0/G1 phase, cells with replicated DNA (twice the amount of unreplicated DNA) are in G2/M phase, and cells with an intermediate amount of DNA are in S phase.
Cells were prepared for propidium iodide cell cycle analysis by detachment with trypsin. Cells were centrifuged for 10 min at low speed, and the remaining supernatant was aspirated. Cells were resuspended in stain solution (3% w/v polyethylene glycol 6000, 50 µg/ml propidium iodide, 180 U/ml RNAse, 0.1% Triton X-100 in 4 mM citrate buffer, pH 7.2) at a concentration of 2 × 106 cells/ml. RNAse was added to ensure that only DNA was labeled, since propidium iodide also binds RNA. After 20 min of incubation at 37°C, salt solution (3% w/v polyethylene glycol 6000, 50 µg/ml propidium iodide, 0.1% Triton X-100 in 0.4 M salt buffer, pH 7.2) was added for a final concentration of 1 × 106 cells/ml. Cells were stored overnight at 4°C and analyzed by flow cytometry the following morning.
Statistical analysis
Prism software (Graphpad) was used for statistical analyses. Each figure is presented as one representative of three experiments, with each experiment having n = 3. Data were normally distributed and expressed as mean ± standard deviation. Comparisons between two groups were analyzed by Student’s t-test, and comparisons among more than two groups were analyzed by ANOVA. P ≤ 0.05 was considered statistically significant and is indicated with a pound sign (#). P ≤ 0.01 is indicated with an asterisk (*).
Results
Endothelial cell TNFα-induced apoptosis in high glucose
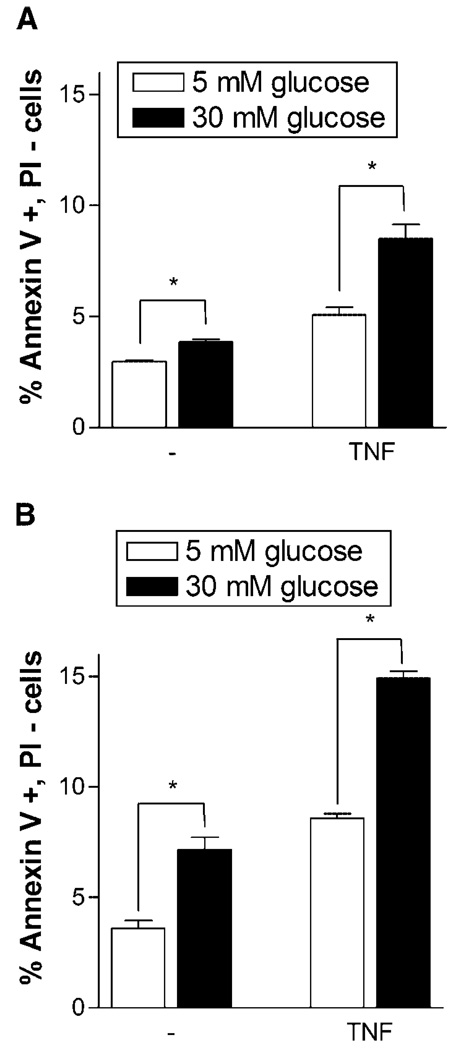
Both hyperglycemia and TNFα independently lead to increased endothelial cell apoptosis. We now show that endothelial cells are more susceptible to TNFα-induced apoptosis in a high glucose environment. Endothelial cells cultured in 5 and 30 mM glucose media were exposed to TNFα for 12 or 24 h. At 12 h, high glucose culture alone increased endothelial cell apoptosis by 30%. While TNFα stimulation increased apoptosis for both 5 and 30 mM glucose cells, the increase for 30 mM glucose cells was more than two times that of 5 mM glucose cells (Fig. 1A). At 24 h, high glucose culture alone increased endothelial cell apoptosis 100% (Fig. 1B). Upon TNFα stimulation, apoptosis more than doubled in both 5 and 30 mM glucose culture, yet the apoptosis increase for 30 mM glucose cells was 155% greater than that of 5 mM glucose cells.
Fig. 1.
TNFα induces increased apoptosis in endothelial cells cultured in 30 mM glucose. Endothelial cells cultured in 5 or 30 mM glucose supplemented media were given fresh media or fresh media with 5 ng/ml TNFα. After (A) 12 h or (B) 24 h cells were harvested and prepared for annexin V—propidium iodide flow cytometry. Data shown is one representative experiment of three, n = 3. *P < 0.01.
Role of cell-released FGF-2 in TNFα-induced cell death
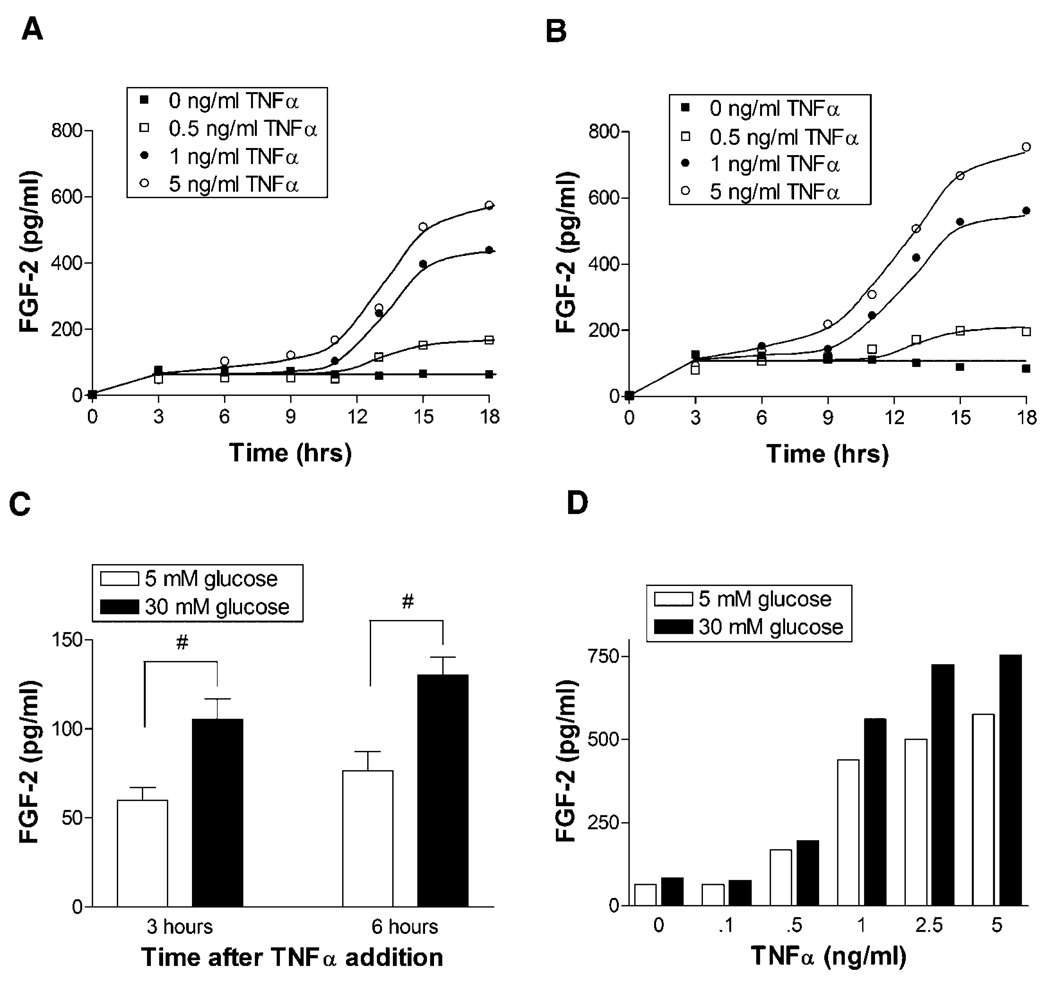
We next investigated the role of endothelial cell released FGF-2 in low and high glucose culture. FGF-2 can be protective against endothelial cell apoptosis, and we have previously published that endothelial cells release more FGF-2 into the media when grown in 30 mM glucose culture (Morss and Edelman, 2007). We now show that FGF-2 release in response to TNFα was enhanced in high glucose culture (Fig. 2A,B). FGF-2 release began as early as 6–9 h after TNFα stimulation and increased up to 18 h after TNFα stimulation. On average, across all levels of TNFα stimulation, FGF-2 release was 75% greater in high glucose than low glucose cells, even at time points as early as 3 and 6 h after TNFα addition (Fig. 2C). At 18 h, released FGF-2 increased with TNFα in a dose dependent manner and was consistently higher for 30 mM glucose cells (Fig. 2D). Data collected beyond 18 h was not included as there were extensive floating dead cells in the media, which limited data integrity.
Fig. 2.
FGF-2 release in response toTNFα is greater in 30 mM glucose culture. Endothelial cells cultured in (A) 5 mM glucose or (B) 30 mM glucose supplemented media were given fresh media with 0, 0.5, 1, or 5 ng/ml TNFα. At time points from 3 to 18 h, a 100 µl sample of media was taken from the cells. Media FGF-2 level was quantified via FGF ELISA. C: Highlights early FGF-2 concentration differences in 5 and 30 mM glucose cells for pooled data across all TNFα levels. D: Highlights the FGF-2 release dose response to TNFα for 5 and 30 mM glucose cells 18 h after TNFα stimulation. Data shown is one representative experiment of three. # P < 0.05.
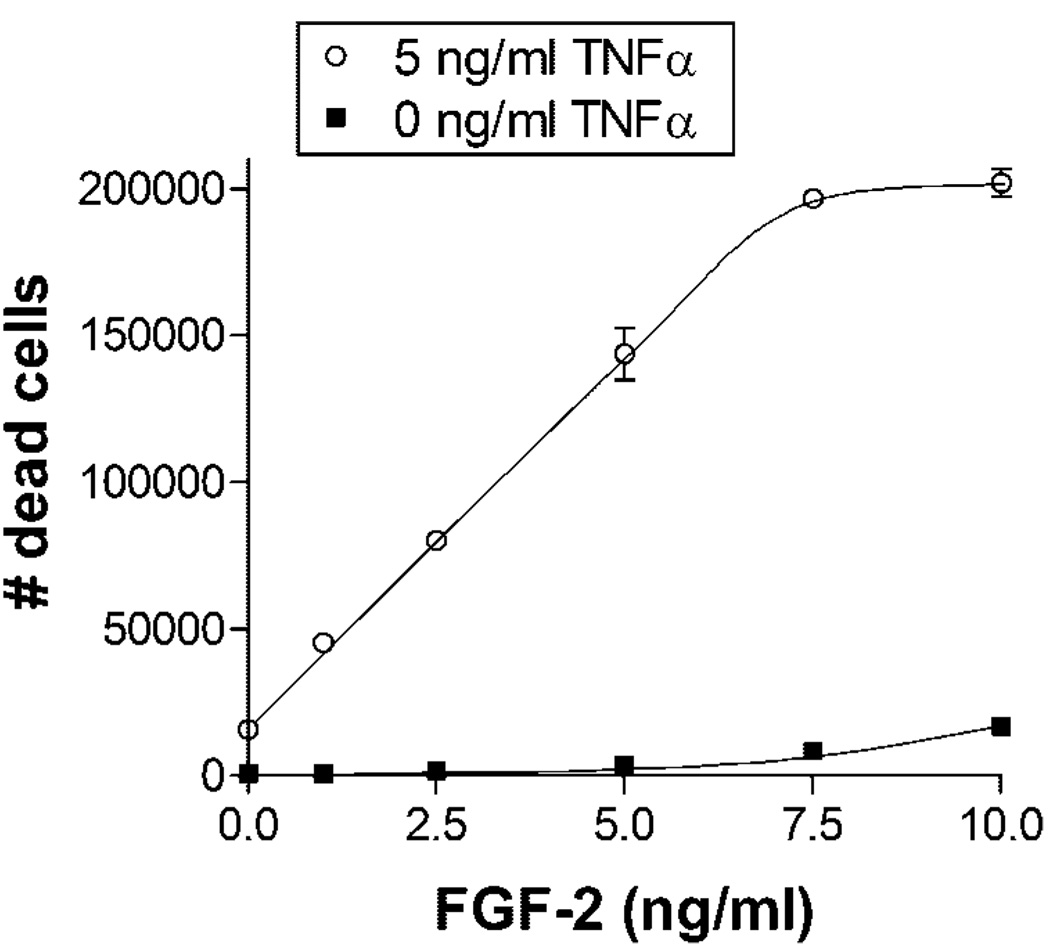
FGF-2 was added to endothelial cells grown in 5 mM glucose to independently examine the role of FGF-2. FGF-2 was added 48 h prior to TNFα stimulation to ensure complete saturation and simulate the chronically elevated FGF-2 environment experienced by cells in high glucose culture. TNFα-induced endothelial cell death increased linearly with FGF-2 concentration up to a saturating FGF-2 dose of 7.5–10 ng/ml (Fig. 3). TNFα-induced cell death at 10 ng/ml FGF-2 was nearly 13 times higher than without exogenous FGF-2.
Fig. 3.
FGF-2 increases endothelial cell TNFα-induced cell death in a dose dependent manner. Endothelial cells cultured in 5 mM glucose supplemented media were given fresh media with 0, 2.5, 5, 7.5, or 10 ng/ml FGF-2. After 48 h, 5 ng/ml TNFα was added. Floating dead cells were counted 24 h after TNFα addition. Data shown is one representative experiment of three, n = 3. P < 0.0001 by ANOVA.
Cell cycle dependence of TNFα-induced cell death
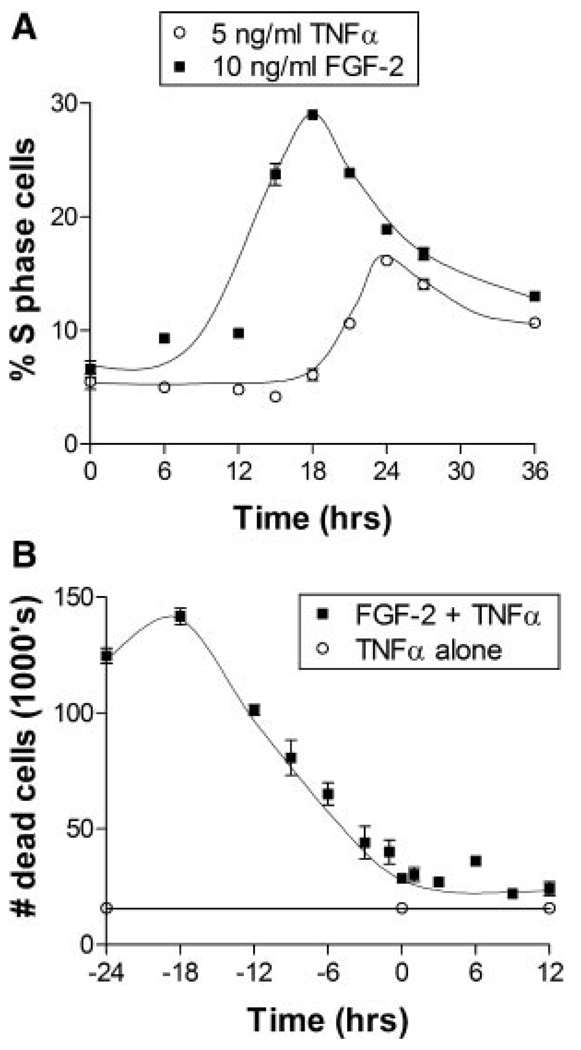
As cells are more susceptible to death at certain phases of the cell cycle, we explored whether the increase in TNFα-induced death with FGF-2 was related to cell cycle progression. FGF-2 was added to endothelial cells in 5 mM glucose culture at times ranging from 24 h before to 12 h after TNFα stimulation. FGF-2 had no statistically significant increase or decrease in cell death when added concurrently or after TNFα. TNFα-induced cell death was greater when FGF-2 was added at any time before TNFα; however peak cell death was achieved when FGF-2 was added 18 h before TNFα (Fig. 4B). The incubation period required for maximum FGF-2 effect on TNFα cell death correlated with cell cycle entry into S phase of the cell cycle. At roughly 18 h after FGF-2 stimulation, endothelial cells showed a peak in S phase cells (Fig. 4A). Cells stimulated with TNFα progressed similarly through the cell cycle but showed a 6 h delay, which may correspond to the timing of FGF-2 release from TNFα stimulation.
Fig. 4.
Endothelial cell TNFα-induced cell death correlates with FGF-2 induced entry into S phase. A: Endothelial cells cultured in 5 mM glucose with 0.5% FBS were given 10 ng/ml FGF-2 or 5 ng/ml TNFα from 0 to 36 h before they were harvested and prepared for cell cycle flow cytometry. B: Endothelial cells cultured in 5 mM glucose with 0.5% FBS were given 10 ng/ml FGF-2 from 24 h before to 12 h after addition of 5 ng/ml TNFα. 24 h after TNFα addition, floating dead cells were collected and counted using a Coulter counter. Data shown is one representative experiment of three, n = 3.
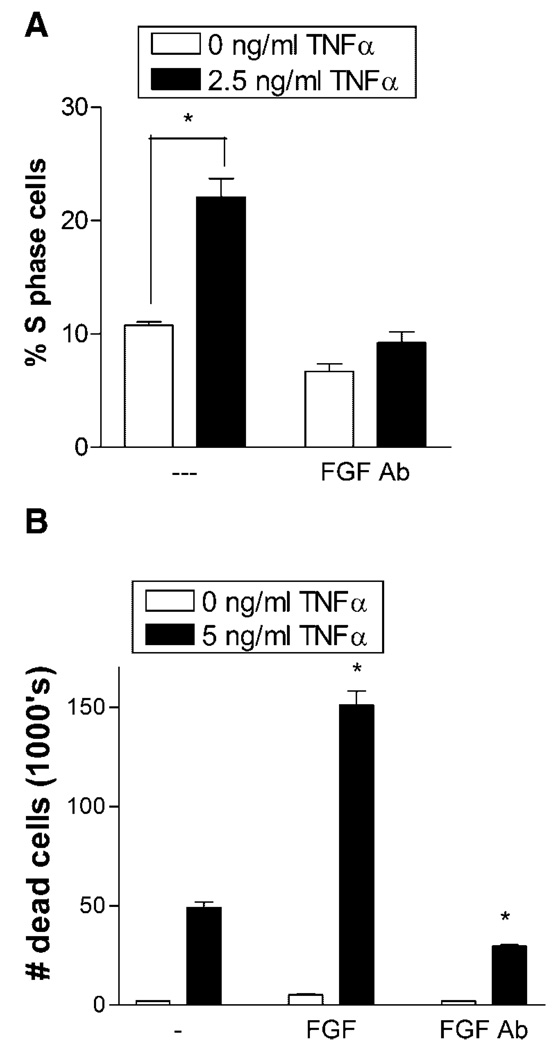
FGF-2 released from cells stimulated by TNFα progressed cells into S phase, but an FGF-2 neutralizing antibody eliminated this effect. Conditioned media from cells stimulated with TNFα was applied to unstimulated cells, and the cell cycle phase was measured. Conditioned media doubled the percentage of TNFα naive cells that entered S phase (Fig. 5A). When an FGF-2 neutralizing antibody was added to the TNFα conditioned media, cell progression into S phase dropped below the control level.
Fig. 5.
An FGF-2 neutralizing antibody blocked TNFα induced cell entry into S phase and decreases TNFα apoptosis. A: Endothelial cells cultured in 5 mM glucose media with 0.5% FBS were given 2.5 ng/ml TNFα. After 24 h, conditioned media was collected from these cells, concentrated by centrifugation, and 50 µl added to subconfluent, starved endothelial cells for 24 h. Cells were then harvested and prepared for cell cycle flow cytometry. B: Endothelial cells cultured in 5 mM glucose were given 10 ng/ml FGF-2 or 1 µg/ml neutralizing FGF-2 antibody. After 48 h, cells were given 5 ng/ml TNFα. Floating dead cells were counted 24 h after TNFα addition. Data shown is one representative experiment of three, n = 3. *P < 0.01.
To investigate whether FGF-2-enhanced TNFα cell death is related to cell cycle S phase, we blocked FGF-2 induced cell cycle progression using a neutralizing FGF-2 antibody. Endothelial cells were preconditioned for 48 h in FGF-2 or in FGF-2 antibody to ensure saturation. Cell death increased more than 2 times when TNFα was added to FGF-2 stimulated cells over when TNFα was added alone (Fig. 5B). In contrast, cells preconditioned with the neutralizing FGF-2 antibody demonstrated a 39% decrease in TNFα-induced cell death from the control.
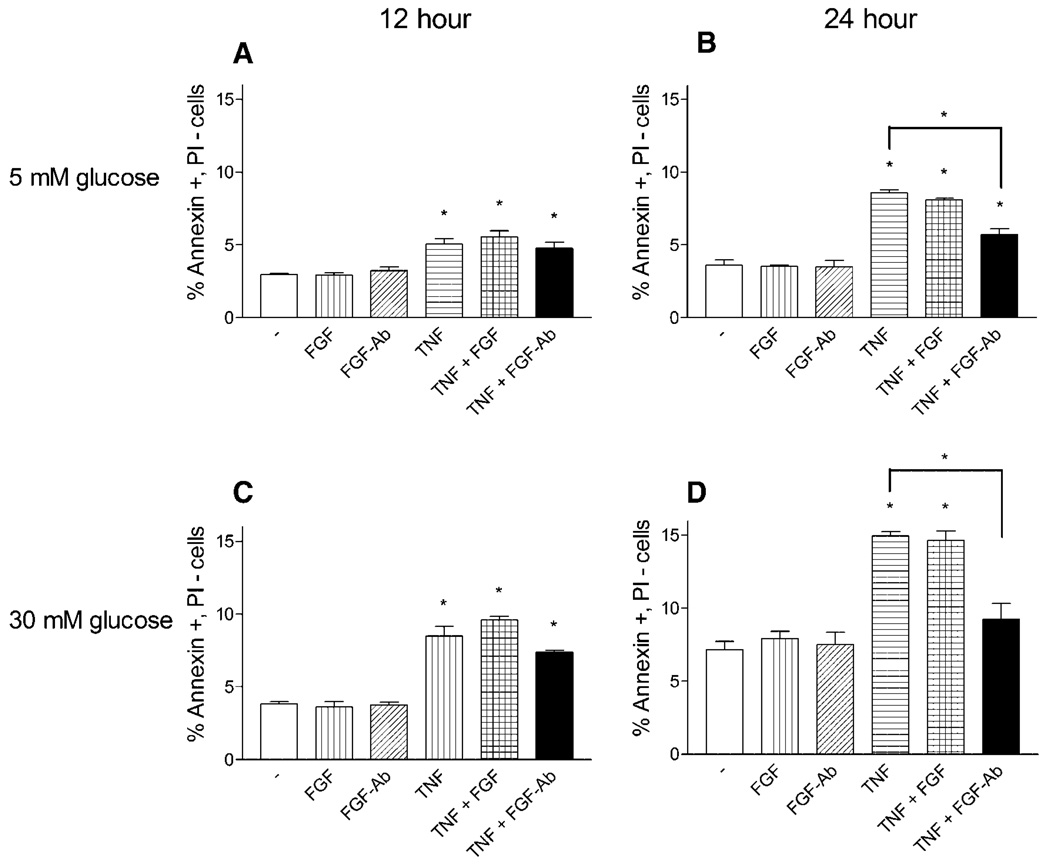
We next determined the effect of exogenous FGF-2 or neutralization of cell released FGF-2 specifically on apoptosis. After 12 h of TNFα exposure, neither the addition of FGF-2 nor neutralizing FGF-2 antibody concurrent with TNFα affected the rise in apoptosis (Fig. 6A,C). At 24 h, exogenous FGF-2 still did not increase apoptosis. However, a neutralizing FGF-2 antibody decreased TNFα apoptosis for both 5 and 30 mM glucose cells by 51% and 61%, respectively (Fig. 6B,D).
Fig. 6.
FGF-2 neutralization reduces TNFα endothelial cell apoptosis. Endothelial cells cultured in 5 or 30 mM glucose supplemented media were given fresh media or fresh media with 10 ng/ml FGF-2, 1 µg/ml FGF-2 neutralizing antibody, 5 ng/ml TNFα, TNFα with FGF-2, or TNFα with FGF-2 antibody. After (A,C) 12 h or (B,D) 24 h cells were harvested and prepared for annexin V—propidium iodide flow cytometry. Data shown is one representative experiment of three, n = 3. *P < 0.01.
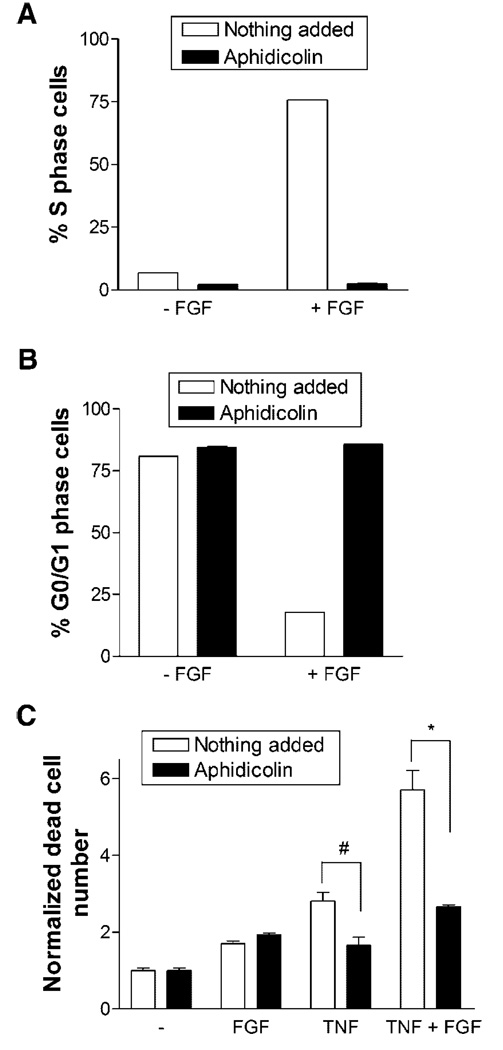
The FGF-2 effect could have been independent of cell cycle progression. We therefore used the cell cycle blocker, aphidicolin, to elucidate the specific role of cell cycle. Aphidicolin effectively prevented cells from entering S phase when stimulated with FGF-2 (Fig. 7A,B). Whereas over 75% of cells entered S phase when exposed to FGF-2, with aphidicolin less than 3% of cells entered S phase and over 80% of cells were arrested in G0/G1 phase. When aphidicolin was added with TNFα, cell death was reduced by two times (Fig. 7C). A similar reduction in cell death with aphidicolin was observed when cells were stimulated with both TNFα and FGF-2.
Fig. 7.
Blocking cell cycle progression eliminates FGF-2 effect on TNFα-induced apoptosis. A,B: Endothelial cells cultured in 5 mM glucose media with 0.5% FBS were given serum free media with 0 or 10 ng/ml FGF-2, and 2 µg/ml of the cell cycle blocker aphidicolin was added to some samples. After 24 h, cells were harvested and prepared for cell cycle flow cytometry. C: Endothelial cells cultured in 5 mM glucose were given 10 ng/ml FGF-2, 5 ng/ml TNFα, or FGF-2 and TNFα with or without 2 µg/ml aphidicolin. After 24 h, floating dead cells were counted using a Coulter counter. Data shown is one representative experiment of three, n = 3. *P < 0.01; # P < 0.05.
Discussion
Endothelial cell dysfunction is a critical step in the accelerated arteriopathies and diminished wound repair of diabetes mellitus. Hyperglycemia has been clinically demonstrated to further exacerbate cell and tissue effects (Lorenzi et al., 1985; Martin et al., 2003; Beckman et al., 2002). We now show that glucose concentration, elevated to values often seen in persons with diabetes, increases endothelial cell death in response to TNFα, and that this effect can be abrogated through either a neutralizing FGF-2 antibody or by preventing endothelial cell progression through S phase of the cell cycle.
Enhancement of cell death by TNFα in the presence of FGF-2 highlights the importance of secondary mediators in growth factor biology and diabetic complications. High systemic FGF-2 levels have been demonstrated in persons with diabetes, yet diabetic vascular disease can take varied forms in different tissue beds (Zimering and Eng, 1996). Since FGF-2 is most often considered as promoting cell proliferation, repair and survival in the face of environmental stress, reduction of endothelial cell viability in specific tissues under elevated FGF-2 is perplexing (Nugent and Iozzo, 2000). Our data now implicate secondary mediators in determining the local effect of systemic growth factor elevation. TNFα can modify FGF-2’s effects to the extent of inducing cell death, rather than survival. Other factors may exist that similarly alter growth factor function, contributing to challenges in clinical applications of growth factor therapy.
FGF-2 action depends not only on secondary mediators but also on stimulation timing relative to TNFα, in particular with respect to the cell’s phase in the proliferative cycle. When FGF-2 was administered at the same time as TNFα, it had no effect on endothelial cell death (Fig. 1). However, cell death was greatly increased if an 18 h delay was imposed between FGF-2 and TNFα exposure (Fig. 4 and Fig. 5). This value coincides with the peak in FGF-2 induction of S phase and implies that potential toxicity is greatest after cell commitment to proliferation and mitosis. These data propose that timing of growth factor and anti-inflammatory therapies may be critical to their success. Our previously published work suggests that additional FGF-2 is stored in the diabetic extracellular matrix (Morss and Edelman, 2007). In diabetes, chronically heightened circulating TNFα coupled with increased FGF-2 reservoirs may lead to recurrent injury and inflammation rather than healing and resolution (Esposito et al., 2002). For example, in an acute wound that progresses through the repair process, the TNFα signal may have dissipated before the FGF-2 signal progresses cells through S phase. However, in a chronic wound, TNFα and FGF-2 may form a vicious cycle. TNFα induces release of FGF-2, and if TNFα is still present during cell S phase progression in response to FGF-2 release, then cell death is induced rather than early angiogenesis and healing. This cycle could extend beyond endothelial cells to fibroblasts, which show similar reactivity to TNFα and FGF-2 and are critical to wound repair (Liu et al., 2006). In treating diabetic wounds, healing might be accelerated by blocking FGF-2 during periods of elevated TNFα, or by administering growth factor therapy in conjunction with TNFα neutralizing antibodies.
Tissue homeostasis depends on a closely regulated balance between cell proliferation and cell death. In fact, programmed cell death via apoptosis provides an important control for mutation prevention in the proliferative mitotic process. During mitosis, cell cycle checkpoints ensure that critical events of the previous phase have been successfully completed prior to progression to the next phase (King and Cidlowski, 1998). If DNA damage is detected at a checkpoint, the transcription factor p53 is phosphorylated. p53 then activates the DNA repair process, arrests the cell cycle until the DNA is repaired, or initiates apoptosis if DNA damage proves irreparable. Many apoptotic agents, including both TNFα and Fas ligand, lead to DNA damage and have been associated with translocation of p53 from the cytoplasm to the nucleus (Beletskaya et al., 1997; Donato and Perez, 1998). This provides a possible mechanism for the increased efficacy of certain apoptotic agents in specific phases of the mitotic cell cycle.
TNFα has been previously linked to cell cycle effects, and our data further support that claim (Shih and Stutman, 1996; Faraco et al., 1999). While the cell cycle blocker, aphidicolin, was itself damaging to cells, it did effectively block both cell progression into S phase and increased cell apoptosis from the combination of TNFα and FGF-2. Thus the death promoting effect of FGF-2 in the presence of TNFα appears to be cell cycle related. The addition of FGF-2 alone, in low glucose media without TNFα stimulation, increased both cell proliferation and cell death in subconfluent endothelial cells (Fig. 3 and Fig. 5B). However, this death did not appear to follow apoptotic pathways, since there was no significant increase in Annexin V positive cells (Fig. 6). We additionally show a greater effect of FGF-2 on nonspecific cell death than on apoptotic cell death with TNFα stimulation, as indicated by measuring more floating dead cells than annexin V positive apoptotic cells. This is likely related to the unique ability of TNFα to induce both necrosis and apoptosis (Fiers et al., 1996). The type of TNFα-induced cell death depends on the cell cycle, with cells arrested in G0/G1 dying by necrosis, and those progressing through S phase dying by apoptosis (Faraco et al., 1999).
The question remains why FGF-2 had no measurable protective influence once the cell cycle effect was blocked. Just as TNFα-induced cell death is cell cycle phase dependent, FGF-2 promotion of cell survival could also be specific to a particular phase of the cell cycle. FGF-2 could promote proliferation when the cell is in a quiescent state but promote survival when the cell is already committed to mitosis. Cell cycle dependence could help explain how a single growth factor can induce varied end results through distinct pathways. Alternatively, the effect of FGF-2 on cell survival could be cell type specific. FGF-2 protects against TNFα-induced apoptosis in fibroblasts but promotes apoptosis in glomerular endothelial cells (Gardner and Johnson, 1996; Messmer et al., 2000). Alternatively, intracellular signaling pathways could be involved. FGF-2 enhances cell survival by signaling down the Akt pathway (Eswarakumar et al., 2005). It is possible that the Akt survival pathway is not protective against TNFα induced apoptosis, or that TNFα blocks FGF-2 signal progression down this pathway. TNFα increases intracellular reactive oxygen species, which can decrease phosphorylation and activation of Akt. Since both FGF-2 and TNFα signal through NFκB and JNK, dysregulation of these pathways may contribute to the pro-apoptotic FGF-2 effect with TNFα (Aggarwal, 2003; Varfolomeev and Ashkenazi, 2004; Eswarakumar et al., 2005). Further investigation is needed to understand the intracellular signaling pathways in different cell types.
Acknowledgments
Elazer R. Edelman was supported in part by a grant from the USA National Institutes of Health (HL 49039).
Contract grant sponsor: NIH;
Contract grant numbers: HL, 49039.
Literature Cited
- Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nature Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Anderson HDI, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004;279:963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis - Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Beletskaya IV, Nikonova LV, Beletsky IP. Cell cycle specificity of Fas-mediated apoptosis in WIL-2 cells. FEBS Lett. 1997;412:91–93. doi: 10.1016/s0014-5793(97)00740-0. [DOI] [PubMed] [Google Scholar]
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases—The role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric-oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation. 2002;106:1783–1787. doi: 10.1161/01.cir.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]
- Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K. Mechanisms of diabetic vasculopathy: An overview. Am J Hypertens. 2001;14:475–486. doi: 10.1016/s0895-7061(00)01323-6. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Perez M. Tumor necrosis factor-induced apoptosis stimulates p53 accumulation and p21WAF1 proteolysis in ME-180 cells. J Biol Chem. 1998;273:5067–5072. doi: 10.1074/jbc.273.9.5067. [DOI] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans—Role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Faraco PR, Ledgerwood EC, Vandenabeele P, Prins JB, Bradley JR. Tumor necrosis factor induces distinct patterns of caspase activation in WEHI-164 cells associated with apoptosis or necrosis depending on cell cycle stage. Biochem Biophys Res Commun. 1999;261:385–392. doi: 10.1006/bbrc.1999.1042. [DOI] [PubMed] [Google Scholar]
- Fiers W, Beyaert R, Boone E, Cornelis S, Declercq W, Decoster E, Denecker G, Depuydt B, DeValck D, DeWilde G, Goossens V, Grooten J, Haegeman G, Heyninck K, Penning L, Plaisance S, Vancompernolle K, VanCriekinge W, Vandenabeele P, VandenBerghe W, VandeCraen M, Vandevoorde V, Vercammen D. TNF-induced intracellular signaling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J Inflamm. 1996;47:67–75. [PubMed] [Google Scholar]
- Gardner AM, Johnson GL. Fibroblast growth factor-2 suppression of tumor necrosis factor alpha-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M. Possible role of tumor-necrosis-factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991;40:1007–1012. doi: 10.1038/ki.1991.308. [DOI] [PubMed] [Google Scholar]
- Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, Luft FC, Haller H. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res. 1997;81:363–371. doi: 10.1161/01.res.81.3.363. [DOI] [PubMed] [Google Scholar]
- Kado S, Wakatsuki T, Yamamoto M, Nagata N. Expression of intercellular adhesion molecule-1 induced by high glucose concentrations in human aortic endothelial cells. Life Sci. 2001;68:727–737. doi: 10.1016/s0024-3205(00)00968-1. [DOI] [PubMed] [Google Scholar]
- King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–617. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- Krady JK, Basu A, Allen CM, Xu YP, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- Liu R, Bal HS, Desta T, Behl Y, Graves DT. Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am J Pathol. 2006;168:757–764. doi: 10.2353/ajpath.2006.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi M, Cagliero E, Toledo S. Glucose toxicity for human-endothelial cells in culture—Delayed replication, disturbed cell-cycle, and accelerated death. Diabetes. 1985;34:621–627. doi: 10.2337/diab.34.7.621. [DOI] [PubMed] [Google Scholar]
- Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- Messmer UK, Briner VA, Pfeilschifter J. Basic fibroblast growth factor selectively enhances TNF-alpha-induced apoptotic cell death in glomerular endothelial cells: Effects on apoptotic signaling pathways. J Am Soc Nephrol. 2000;11:2199–2211. doi: 10.1681/ASN.V11122199. [DOI] [PubMed] [Google Scholar]
- Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196:430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- Morss AS, Edelman ER. Glucose modulates basement membrane fibroblast growth factor-2 via alterations in endothelial cell permeability. J Biol Chem. 2007;282:14635–14644. doi: 10.1074/jbc.M608565200. [DOI] [PubMed] [Google Scholar]
- Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- Pober JS, Gimbrone MA, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human-endothelial cells by interleukin-1, tumor-necrosis-factor, and immune interferon. J Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- Robaye B, Mosselmans R, Fiers W, Dumont JE, Galand P. Tumor-necrosis-factor induces apoptosis (programmed cell-death) in normal endothelial-cells in vitro. Am J Pathol. 1991;138:447–453. [PMC free article] [PubMed] [Google Scholar]
- Royall JA, Berkow RL, Beckman JS, Cunningham MK, Matalon S, Freeman BA. Tumor necrosis factor and interleukin 1-alpha-increase vascular endothelial permeability. Am J Physiol. 1989;257:L399–L410. doi: 10.1152/ajplung.1989.257.6.L399. [DOI] [PubMed] [Google Scholar]
- Satoh J, Yagihashi S, Toyota T. The possible role of tumor necrosis factor-alpha in diabetic polyneuropathy. Exp Diabesity Res. 2003;4:65–71. doi: 10.1155/EDR.2003.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih SC, Stutman O. Cell cycle-dependent tumor necrosis factor apoptosis. Cancer Res. 1996;56:1591–1598. [PubMed] [Google Scholar]
- Tsilibary E. Microvascular basement membranes in diabetes mellitus. J Pathol. 2003;200:537–547. doi: 10.1002/path.1439. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: An apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimering MB, Eng J. Increased basic fibroblast growth factor-like substance in plasma from a subset of middle-aged or elderly male diabetic patients with microalbuminuria or proteinuria. J Clin Endocrinol Metab. 1996;81:4446–4452. doi: 10.1210/jcem.81.12.8954057. [DOI] [PubMed] [Google Scholar]