Abstract
Cardiovascular magnetic resonance imaging (CVMRI) is of proven clinical value in the non-invasive imaging of cardiovascular diseases. CVMRI requires rapid image acquisition, but acquisition speed is fundamentally limited in conventional MRI. Parallel imaging provides a means for increasing acquisition speed and efficiency. However, signal-to-noise (SNR) limitations and the limited number of receiver channels available on most MR systems have in the past imposed practical constraints, which dictated the use of moderate accelerations in CVMRI. High levels of acceleration, which were unattainable previously, have become possible with many-receiver MR systems and many-element, cardiac-optimized RF-coil arrays. The resulting imaging speed improvements can be exploited in a number of ways, ranging from enhancement of spatial and temporal resolution to efficient whole heart coverage to streamlining of CVMRI work flow. In this review, examples of these strategies are provided, following an outline of the fundamentals of the highly accelerated imaging approaches employed in CVMRI. Topics discussed include basic principles of parallel imaging; key requirements for MR systems and RF-coil design; practical considerations of SNR management, supported by multi-dimensional accelerations, 3D noise averaging and high field imaging; highly accelerated clinical state-of-the art cardiovascular imaging applications spanning the range from SNR-rich to SNR-limited; and current trends and future directions.
Keywords: Cardiovascular MRI, Parallel imaging, Phased array technology, Many-element coil arrays, High field imaging
Introduction
Cardiovascular MR imaging (CVMRI) has become a valuable diagnostic imaging modality in the non-invasive detection of cardiovascular diseases [1–9]. The need for speed and efficiency dictated by physiological motion and flow has been a significant motivating force for the development of ever more rapid MR imaging techniques and tailored MR system hardware. Over the past several years numerous CVMRI techniques and their variants have been proposed and examined in early feasibility and clinical studies to address the need for balancing the competing constraints of spatial and temporal resolution, image quality and imaging speed [10–17].
Imaging speed is fundamentally limited in conventional MRI by technological and physiological limits to gradient switching rate and radio-frequency (RF) power deposition. The advent of parallel imaging [18, 19] introduced a new means of acceleration while still providing the full spatial information. In parallel MRI, RF detector coil sensitivities are used to encode simultaneous spatial information that complements the information obtained through sequential application of magnetic field gradients. Since the challenges and benefits of ultra-fast MRI are nowhere more apparent than in CVMRI, it is not surprising that some of the earliest parallel MRI applications were in the field of CVMRI [20–24]. With the broad clinical introduction of parallel MRI various means for increasing acquisition speed in CVMRI beyond previous limits have become available [25].
For conventional RF-coil array designs and typical imaging volumes, signal-to-noise ratio (SNR) losses associated with parallel MRI, particularly at comparatively high accelerations, have constituted a significant practical obstacle, as has the limited number of receiver channels available on most MR scanners. Previously clinically used net acceleration factors (R) generally have not exceeded moderate values of up to R=4. This constraint has prompted the development of many-element RF coil arrays in conjunction with many-receiver systems [26–28], which represent a key enabling factor for high accelerations.
Today, a move towards widespread availability of MR systems with 32 or more receiver channels equipped with many-element high density RF coil arrays is underway. The resulting gains in speed and efficiency promise to advance the capabilities of CVMRI [27–31] in various ways as outlined in Table 1. In the sections that follow, examples of these strategies will be provided including contrast-enhanced MR angiography, assessment of cardiac function, coronary arteriography, myocardial perfusion imaging, viability assessment and anatomical imaging. But first, this review addresses the key concepts, hardware requirements, and practical considerations of highly accelerated CVMRI. Finally, current trends-such as the trend towards high field imaging-and future directions in massively accelerated parallel CVMRI are considered. Of course, highly accelerated MRI is an area of vigorous ongoing basic and clinical research, and many potentially valuable developments will receive only brief mention here.
Table 1.
Summary of improvements in clinical CVMR capabilities facilitated by speed and efficiency gains of highly accelerated imaging
| Improved capabilities of highly accelerated CVMR | Contrast-enhanced 3D MRA | Cardiac function | Myocardial perfusion | Coronary artery imaging | Myocardial infarction & viability | Anatomical imaging & tissue characterization |
|---|---|---|---|---|---|---|
| Improve temporal resolution | x | x | x | |||
| Improve spatial resolution or coverage | x | x | x | x | x | x |
| Enable (short) breath-hold acquisitions | x | x (In place of lengthier free-breathing scans) | x | x | ||
| Increase patient comfort through shorter examinations | x | x | x | x | ||
| Replace 2D with 3D imaging strategies capable of efficient whole heart coverage | x | x | x | |||
| Overcome physiological and physical constraints | x | x | ||||
| Minimize the risk of slice mis-registration | x | x | x | |||
| Make extra bolus timing scans obsolete | x | |||||
| Bypass extra parameter calibration | x | x | ||||
| Uniform sensitivity to contrast enhancement | x | |||||
| Eliminate the need for time consuming localization | x | x | x | x |
Concepts of and practical considerations for highly parallel CVMRI
Basics of parallel MRI
Parallel imaging strategies use the spatial variation in RF detector coil sensitivities (Fig. 1) to encode simultaneous spatial information [18, 19]. Undersampled data are acquired using an RF coil array, with a reduced number of encoding gradient steps as compared with traditional unaccelerated acquisitions, and the missing information is reconstructed using knowledge of the coil sensitivities. Three intuitive pictures are helpful to understand the undersampled acquisition and image reconstruction approaches:
Fig. 1.
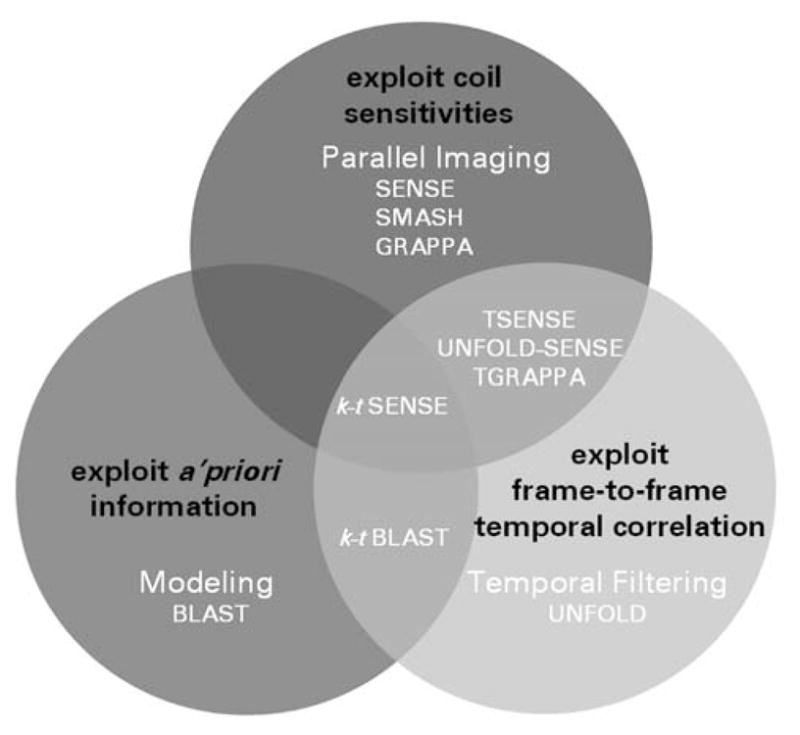
Synopsis of reconstruction strategies used for unaliasing of undersampled MR data to yield high net accelerations for cardiac imaging
The k-space picture, exemplified by the original SMASH technique and carried on in popular modern techniques such as GRAPPA [32], which involves the regeneration of missing k-space lines corresponding to omitted phase-encoding gradients [18].
The image-domain picture, as represented by the original Cartesian SENSE formulation, which involves the unfolding of aliased voxels that result from undersampling [19].
A generalized perspective, which connects the SMASH-like and SENSE-like pictures [33–36] and treats the encoding functions from each coil or gradient step as distinct projections of the imaged volume, and performs a generalized reconstruction from projections to generate images.
Recently, parallel imaging has been combined productively with techniques that use spatiotemporal correlations in dynamic imaging (Fig. 1) such as CINE or first pass bolus chase perfusion imaging. In these techniques, the availability of multiple time frames affords one the opportunity to vary acquisition trajectories as a function of time. This is the concept behind techniques such as UNFOLD [37] and k–t BLAST [38], which have been used primarily for CVMRI. In all cases, spatial information from coil arrays can be combined with temporal information to yield increased net accelerations for dynamic imaging. Examples of accelerated spatio-temporal hybrid techniques include UNFOLD-SENSE, TSESNE and k–t SENSE [38–40]. Table 2 surveys the basic principles, merits and limitations of parallel imaging techniques exploiting spatio-temporal correlations.
Table 2.
Survey of basic principles, merits and limitations of parallel imaging techniques exploiting spatio-temporal correlations
| Method | Basic principle | Merits | Limitations |
|---|---|---|---|
| UNFOLD | Exploits frame-to-frame temporal correlation (t-axis) of k–t space normally used to encode along the k-axes Change of the (decimated) sampling function from one time frame to the next (i.e., time interleaving of k-space in sequential images) results in aliasing along the temporal frequency spectrum Unaliasing is achieved by rejecting the aliased components in the temporal frequency spectrum by means of low-pass temporal filtering |
Does not necessarily require coil arrays No effect on SNR efficiency Helps to remove artifacts due to ambiguities in the partitioning of coil intensities among aliased positions in SENSE imaging without compromising spatial resolution No need for training data Can be combined with coil sensitivity encoding (UNFOLD-SENSE) |
Maximum acceleration factor shown so far is limited to 4 Assumes that the outer portion of the FOV is relatively static, which is not always the case in cardiac MRI due to chest wall motion |
| k–t BLAST | Based on the principle that images of an object acquired at different time points in a dynamic scan are inherently similar (large fractions of the image remain static or change in a predictable fashion) Consists of an undersampling and training stage Exploits a priori information from fully sampled, low spatial resolution training data, which carry full information on correlation between k-space and time Information from the training data is used to construct the k–t BLAST reconstruction filter Application of the filter to the aliased k–t space data recovers the unaliased signal |
Does not necessarily require coil arrays SNR reduction given by decimation factor only SNR reduction less than in coil sensitivity encoding-based techniques Can be combined with sensitivity encoding (k–t SENSE) |
Temporal fidelity must be viewed with caution in case of high accelerations (R=8) |
| TSENSE | Exploits frame-to-frame temporal correlation (UNFOLD) together with spatial information of coil sensitivity (SENSE) (Adaptive) spatio-temporal filtering method |
Ability to tolerate body motion or change in scan plane without reacquiring additional reference images | Noise amplification proportional to geometry-factor |
Tradeoffs for the increased speed and efficiency afforded by parallel imaging include the need to calibrate coil sensitivity patterns, the need to acquire training data in the case of k–t techniques, the possibility of image artifacts when calibration or training data are inaccurate, and reduced SNR compared with unaccelerated imaging using the same coil array. It should be noted that noise amplification, as characterized by the g-factor [19], is generally non-uniform in parallel imaging, which renders noise estimation using a region of interest (ROI) approach unsuitable for SNR measurements. Alternatively, SNR estimation using temporal filtering [41] or a general procedure for reconstruction in SNR units [42] is applicable to SNR measurements for certain parallel imaging techniques.
Integrated many-receiver MR system design
Early versions of many-receiver systems were constructed either by using custom data acquisition equipment [43, 44] or by integrating multiple sets of commercial MR system electronics [26–29]. The latter experimental prototype was used to demonstrate the clinical feasibility of order-of-magnitude accelerations [27, 28, 45, 46], which served, in part, to prompt further developments towards single cabinet many-receiver MR systems with 16 and more independent channels.
Today, commercial many-receiver MR systems operating at 1.5 T and 3.0 T are available, although the palette of hardware and software capabilities as well as scan options for highly accelerated CVMRI might vary from vendor to vendor (Table 3). As 32-receiver systems equipped with many-element, high density RF-coil arrays become readily available, experimental prototypes are expected to continue to evolve towards an even larger number of RF receive channels. Descriptions of such systems have begun to appear in early reports [43, 47].
Table 3.
Survey of vendor specific technologies and acronyms for highly accelerated imaging
| GE healthcare | Hitachi | Philips | Siemens | Toshiba | |
|---|---|---|---|---|---|
| Platform | Excite HDx | Achieva Freewave | Total imaging matrix (TIM) | Atlas | |
| Number of physical channels | Up to 32 channels | Up to 8 channels (support of up to 48 coil elements) | Up to 32 channels | Up to 32 channels (combination of up to 102 coil elements) | Up to 16 channels (combination of up to 128 coil elements) |
| Image-based reconstruction method | ASSET (Array Spatial Sensitivity Encoding Technique) | RAPID | SENSE | mSENSE | Speeder |
| Coil sensitivity reference | External reference scan | External reference scan | External reference scan Self-calibrating | External reference scan | |
| k-space based reconstruction method | GEM (Generalized Encoding Matrix) | GRAPPA | |||
| Coil sensitivity reference | Self-calibrating or external reference scan | Self-calibrating | |||
| Spatio-temporal correlation-based reconstruction method | UNFOLD (works in progress) | k–t BLAST k–t SENSE |
TSENSE |
Cardiac-optimized many-element RF-coil arrays
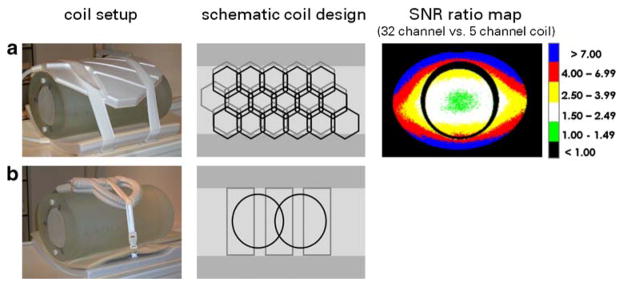
For many-element RF-coil array configurations customized for CVMRI, the coil elements located on the chest wall and towards the left side are of chief importance, due to the heart’s position in the chest cavity. Furthermore, the use of comparatively small, deep-lying fields of view with multiple subject-specific oblique image plane orientations, together with considerations of patient comfort, present a challenge for the design of robust and flexible many-element coil arrays. Early many-element coil arrays tended to be laid out on rigid frames and arranged in contoured 2D grids either with conventionally overlapped elements [26] or with element spacing carefully selected based on simulations of accelerated SNR [27]. A subsequent cardiac-optimized configuration used an asymmetric and nonuniform hexagonal lattice design with 21 anterior and 11 posterior circular coils [48]. Various cardiac-optimized coil arrays using honeycomb designs with overlapped elements are now becoming more readily available [49–51]. Figure 2 compares the baseline (unaccelerated) SNR of a prototype 32-element array designed for cardiac imaging at 1.5 T with that of a commercial 1.5-T cardiac array with five elements. In these phantom studies, the 32-element array clearly outperforms the 5-element array.
Fig. 2.
Signal-to-noise (SNR) comparison of two coil arrays designed for cardiac imaging at 1.5 T. Left: A 32-element array (Philips Research Laboratory, Hamburg, Germany) (a) and a 5-element array (Philips, Medical Systems, Best, The Netherlands) (b) in position around an elliptical loading phantom (L–R). Middle: Schematic coil geometry for the 32-element (a) and the 5-element (b) arrays. The central light-gray areas mark the superior-anterior extent of the region included in the SNR measurements. Right: Map of SNR ratio between the 32-element array and the 5-element array, obtained with unaccelerated imaging for a set of slices covering the region of interest indicated in light gray in the middle column (S–I coverage =16 cm). For the 32-element coil array, a baseline SNR improvement of up to a factor of 1.49 was found for the very central region, which mimics the position of the heart in the chest cavity. Very peripheral regions close to the coil elements revealed an SNR increase of up to a factor of >7
Commercial offerings are expected to continue to evolve towards arrays customized for highly accelerated clinical CVMRI, though the broad spectrum of cardiovascular MRI applications makes it challenging to identify an optimal many-element coil array design. However, the selected array should meet the following minimum requirements: (1) light weight design, (2) mechanical flexibility including the coil itself, cabling and coil positioning for common and rare anatomical variants, (3) ease of clinical use, (4) sufficiently high baseline SNR to offset anticipated SNR losses for high target acceleration factors, (5) large sensitive region that is sufficient to cover the cardiovascular anatomy of choice (for example heart + pulmonary vessels), (6) a desirable noise amplification profile for image planes of interest, (7) a multi-dimensional arrangement of coil elements to enable multi-dimensional accelerations, (8) uncompromised patient comfort and (9) principal directions of sensitivity variation in the array that match, as far as possible, the planned phase encoding directions to be accelerated. It is advisable to test the clinical performance of many-element coil array designs on site prior to purchase.
Practical considerations for highly accelerated CVMRI
2D vs. 3D imaging, and one-dimensional vs. multidimensional accelerations
In current clinical practice many CVMRI applications are confined to thin targeted slabs using free-breathing techniques or to a single slice per breath-hold. With eight to ten short axis slices required to cover the full cardiac anatomy, the conventional approach can result in prolonged examination times on the order of 10 min, with corresponding patient discomfort. Strategies for extensive anatomical coverage at the required spatial resolution within a single breath-hold have been elusive hitherto. For this reason, a strategy employing accelerated 2D techniques encompassing multiple slices per breath-hold is conceptually appealing for the pursuit of broad anatomic coverage in clinically acceptable examination times. For sufficiently high accelerations and sufficiently extended anatomic coverage, however, comprehensive breath-held 3D acquisitions become feasible, and the synergies between 3D sequences and parallel imaging come strongly into play. One particular synergy lies in the area of offsetting SNR losses through 3D noise averaging.
The use of many-element RF coil arrays can alleviate noise amplification to some extent, but electrodynamics dictate that a rapid degeneration of SNR at high one-dimensional accelerations is inevitable [52, 53]. Fortunately, a sufficient number of receivers allows the use of multi-dimensional RF coil array arrangements capable of multi-dimensional accelerations, which preserves SNR as compared to one-dimensional accelerations [52, 54]. Consequently, undersampling may be equally distributed to the ky and kz phase encoding directions in 3D imaging to afford higher net acceleration factors compared with 1D accelerations. However, conventional targeted slab CVMRI covers spatially limited volumes, over which it is difficult to obtain spatially distinct coil sensitivity information. Thus, parallel imaging is only practical in the slice direction if array elements are confined to a small coil diameter or if fairly thick slabs are used.
It should be mentioned that, for dynamic imaging applications, techniques that use spatio-temporal correlations may be a preferred means of achieving high accelerations, given their favourable SNR behaviour. Of course, these spatial-temporal techniques may be combined with parallel imaging for further improvements in imaging speed and/or coverage.
Whole heart coverage and the merits of simplification
For a number of reasons, traditional constraints on MR imaging speed have represented an obstacle to the widespread clinical application of CVMRI. One sometimes underappreciated consequence of scan-time constraints is an increase in the complexity of cardiac imaging examinations, which have tended to involve prescription of multiple targeted slices or slabs in a variety of patient-and anatomy-specific orientations. With the targeted-slab approach, some of the inherent advantages of MRI (e.g., flexibility of contrast mechanisms) are offset by the complexity of scan prescription and data acquisition. Highly accelerated parallel imaging can simplify existing CVMRI protocols by replacing multiple targeted scans with a single accelerated volumetric acquisition. When the imaged volume is sufficiently large, the need for precise targeted prescription is reduced, and in some cases time-consuming localization steps may even be bypassed entirely. For example, by simply prescribing large, judiciously centered axial or coronal volumes, one can achieve complete heart coverage in a single acquisition. With this approach, scan prescription is dramatically streamlined, allowing comprehensive anatomical or functional scanning of any anatomic variant at the press of a button. The frequently encountered difficulty of missed coil or image plane placement is avoided in this case. The volumetric approach also supports retrospective visualization of standard cardiac views or tortuous segments of large vessels and coronary arteries.
High field CVMRI
At low magnetic field strengths, SNR degradation associated with highly parallel CVMRI becomes increasingly challenging as the acceleration factor increases. In addition to higher baseline SNR, high field strengths offer the potential to reduce noise amplification in parallel imaging for B0>3.0 T [52, 53]. Meanwhile highly accelerated parallel imaging can help to address some of the practical limitations of high-field imaging, including RF power deposition constraints and susceptibility effects. Hence, high-field parallel imaging holds high promise for rapid comprehensive CVMRI examinations. Despite some appreciable tradeoffs associated with high-field imaging- e.g. the degradation in quality of steady-state free precession sequences-the promise of high-field parallel CVMRI has been recognized both as a driver and as a beneficiary of the current broad move towards clinical 3.0-T many-channel whole body MR systems.
Potential artifacts
Artifact galleries are available documenting parallel cardio-cascular MRI artifacts [55]. Artifacts are caused either by errors in any of the preparation stages of a parallel imaging study, by equipment malfunctions, or by “intrinsic” causes such as motion of the patient or the coil array [55]. Artifacts specific to highly accelerated parallel imaging can result either from the increase in the baseline SNR of many element RF-coil arrays, the acquisition (for example, 2D vs. 3D imaging) and reconstruction strategy (for example, coil sensitivity encoding vs. spatio-temporal correlations) used. Artifacts may be manifested as ghosting along the phase encoding direction, residual aliasing, slice wrapping, increased noise amplification and temporal blurring. Table 4 and Fig. 3 summarize potential artifacts specific to highly accelerated imaging together with workarounds. On the other hand, as will be discussed, the use of parallel imaging can reduce the incidence or severity of other common imaging artifacts, such as motional blurring, slice misregistration, Gibbs ringing and non-uniform suppression of healthy myocardium for all imaged sections. This balance should be considered in the planning and the interpretation of highly accelerated parallel CVMR studies.
Table 4.
Summary of potential artifacts specific to highly accelerated imaging using high-density RF-coil arrays together with workarounds
| Artifact | Root cause | Work around |
|---|---|---|
| Ghosting along the phase encoding direction for regions very close to the many-element coil array, even in the case of unaccelerated imaging (Fig. 3a) | Artifacts occur primarily in gradient echo imaging and are related to surviving transversal magnetization (higher coherences) caused by imperfect spoiling along the slice direction | Increase spoiler gradients along the slice direction to disturb any residual transversal magnetization (Fig. 3a) |
| Residual aliasing | Strong signal contributions of anatomy close to the RF-coil arrays (i.e., chest wall) | Use saturation slabs or fat suppression to suppress/reduce chest wall signal |
| Slice wrapping and aliasing along the slice direction in 3D imaging (Fig. 3b) | Imperfect slab excitation profile violates the need for the target “full” slab field of view (FOV) after reconstruction be free of aliasing along the slice direction | Improve slice profile of slab excitation Make the 3D RF-excitation slab thinner than phase encoded volume (slab correction factor =1.2–1.35) (Fig. 3b) Apply oversampling along the slab direction |
| Uncorrected aliasing (Fig. 3c) | Deficits in the estimation of the coil sensitivities due to limitations in the spatial resolution of the external reference scan | Improve spatial resolution of the external reference scan (Fig. 3c) Use self-calibrating techniques |
| Artifacts due to inappropriate fields of view such as unfolding along the phase encoding direction (Fig. 3d) | At least for image-domain SENSE reconstructions, overlap of structures in the target field of view leads to ambiguities in the partitioning of intensities among aliased positions resulting in aliasing artifacts | Use a more generous phase FOV, which serves to avoid phase wrap Use oversampling along the phase encoding direction together with larger acceleration factor to compensate for the speed penalty of oversampling (Fig. 3d) Use GRAPPA approach, which is more forgiving in case of overlap of structures Swap phase encoding and read out direction at the cost of lower net acceleration factors |
| Severe noise amplification for very high levels of acceleration | Artifacts due to excessive acceleration | Control background noise amplification by the use of an appropriate acceleration factor (maximum acceleration factor along any arbitrary direction is equal to the number of independent array elements) Do not attempt high accelerations with a target phase or slice FOV covering only a single or very few coil elements since it is difficult to obtain spatially distinct coil sensitivity information for spatially limited volumes Use parallel imaging techniques based on spatio-temporal correlations for dynamic imaging If possible use 2D vs. 1D accelerations |
| Noise truncation and flat contrast in 3D imaging | Dynamic range or digitization limitations in the analog-digital converter (ADC) due noise averaging in 3D imaging | Use 32-bit ADC resolution instead of 16 bit Use floating point format in reconstruction |
| Reduced border sharpness of moving structures in dynamic imaging (Fig. 3e) | Limited temporal fidelity of spatio-temporal correlations based techniques (k–t approach) | Reduce acceleration factor (Fig. 3e) Increase spatial resolution of training data Training data plug-in into the recon |
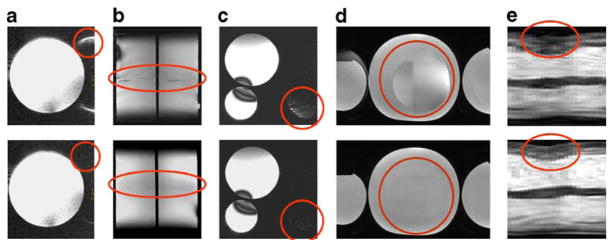
Fig. 3.
Gallery of artifacts, which might occur when using many-element coil arrays and highly accelerated MRI. a Ghosting along the phase encoding direction even in unaccelerated imaging (top). This artifact is related to surviving transversal magnetization caused by imperfect spoiling along the slice direction. If the spoiler gradients along the slice direction are increased (factor 4 in this example) to disturb any residual transversal magnetization the signal-to-artifact ratio can be significantly enhanced [from 3 (top) to 120 (bottom) in this example]. b Residual aliasing along the slice direction (top) caused by an imperfect slab excitation profile in accelerated 3D imaging. One approach to circumvent this issue is to make the 3D RF-excitation slab thinner than the phase encoded volume (bottom). The reformatted coronal slices shown here were taken from a two-fold accelerated (Rz=2) 3D data set covering the entire phantom. For the top image no slab correction factor was used while the bottom image was derived from a 3D data set using a slab correction factor of 1.35. c Uncorrected aliasing (top) induced by deficits in the coil sensitivity estimation due to limitations in the spatial resolution of the external reference scan. Solutions are available through the use of self-calibrating techniques or the improvement in the spatial resolution of the external reference scan. In this example, the spatial resolution of the reference scan was improved from (1.5×6.2×6.6) mm3 (top) to (1.5×1.5×2.2) mm3 (bottom) resulting in a signal-to-artifact-ratio of 10 (top) and 120 (bottom). d Unfolding along the phase encoding direction due to inappropriate fields of view (top). At least for image-domain SENSE reconstructions, overlap of structures in the target field of view leads to folding artifacts. Aliasing can be reduced by over-sampling along the phase encoding direction together with larger acceleration factor to compensate for the speed penalty (bottom). The following acquisition/reconstruction parameters were used: top) FOV=250 mm, Ry=2 (L–R), matrix size 256×256, (bottom) FOV=250 mm, Ry=4 (L–R), matrix size 256×512, over-sampling factor =2. e Reduced border sharpness of moving structures in dynamic imaging due to limited temporal fidelity of spatio-temporal correlations based techniques (top). In this example the border sharpness is illustrated using 1D-projections (m-mode like view) along a line through the short axis views covering the entire R-R interval, which were obtained from eight-fold accelerated 2D CINE SSFP using k–t BLAST (top). Image quality and border sharpness in the accelerated scan can be controlled by the use of an appropriate acceleration factor (bottom). For this reason the acceleration factor was reduced from 8 (top) to 5 (bottom)
One particular caveat about the use of highly accelerated cardiovascular MR is the requirement that the target “full” field of view (FOV) after reconstruction be free of aliasing along any direction to be accelerated. In SENSE-based imaging the overlap of structures in the target field of view leads to ambiguities in the partitioning of intensities among aliased positions resulting in image artifacts. Relaxation of the FOV constraint has been reported to be possible for GRAPPA reconstructions [56].
Clinical applications of highly accelerated CVMRI
Contrast-enhanced vascular imaging
First pass contrast-enhanced MR angiography (MRA) is a particularly appealing candidate for highly accelerated parallel imaging because it is comparatively rich in SNR. The relatively rapid passage of contrast agents through the vascular system requires fast imaging techniques for continuous bolus tracking or appropriately timed bolus chasing [57]. Highly accelerated parallel MRA may be used:
to eliminate extra bolus arrival time calibrations by enabling continuous volumetric acquisitions with previously unattainable temporal resolution or
to increase anatomic coverage or spatial resolution within a given imaging time, allowing comprehensive scanning of any target vessel territory, and the frequently-encountered difficulty of truncated anatomy is avoided, as illustrated in Fig. 4,
to improve temporal resolution of time-resolved MRA down to 2–4 s without substantially sacrificing the spatial resolution to clearly distinguish arterial from venous phases or to evaluate contrast dynamics [28].
Fig. 4.
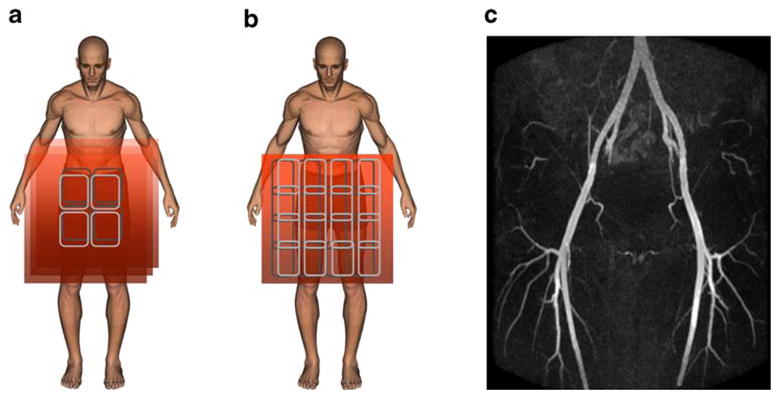
Schematic depiction of conventional MRA using multiple targeted slab acquisitions (a) versus highly accelerated parallel imaging with many-element coil arrays (b). Limitations in the breath-hold duration constrain the conventional approach to use of multiple tailored slabs. Maximum intensity projection (MIP) (c), was obtained from a first post-contrast phase, large volume coverage MRA study using a 32-element coil array. For this purpose accelerated data acquisition was timed for optimal arterial enhancement. The data were obtained in a 7-s breath-hold, reduced by an acceleration factor of 9 from an impractical 62 s
Twelve- to 16-fold accelerations have been reported for volumetric, contrast-enhanced MRA [27, 28], which enable comprehensive coverage of the vasculature with clinically useful spatial resolution. Substantial time-savings in image prescription and total examination time were noted in a feasibility study in which multiple targeted scans were replaced by a single accelerated scan covering a large volume [58]. The diagnostic feasibility of 2D accelerated renal MRA at 3.0 T was recently demonstrated [59], whereby the use of a 32-channel RF-coil array together with six-fold accelerations supported high image quality and extended anatomic coverage. The combination of speed, otherwise unattainable volume coverage, and simplification of scan prescription offers a versatile set of trade-offs to accommodate individuals with the most limited breath-hold capacities and is particularly useful for pediatric studies, as shown in Fig. 5.
Fig. 5.
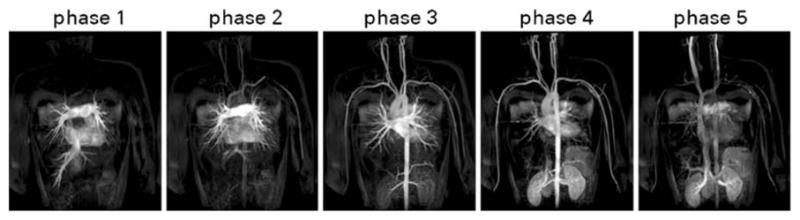
Coronal maximum intensity projections (MIPs) derived from a contrast-enhanced MRA of a pediatric patient with a congenital heart defect. The MIPs were selected from multiple dynamic volumetric acquisitions, reduced in duration by a factor of 8 from 36 s to 4.5 s. They are shown as successive time-resolved frames, obtained after contrast bolus injection. Note the extensive volume coverage of the vasculature from the carotid to the renal artery as well as the clear differentiation between the arterial and venous phase, achieved at this high temporal resolution. A 32-element coil array and a supporting 32-receiver imaging system were used for eight-fold accelerated data acquisition using two-dimensional parallel imaging (R=4×2). A matrix size of 416×263×65 and an FOVof 46×46×9.5 cm3 result in a high spatial resolution per unit acquisition time
It should be noted that parallel imaging techniques can be combined with the existing repertoire of traditional time- or data-sharing approaches such as half-fourier, key hole and time-resolved imaging of contrast kinetics (TRICKS) [60], resulting in net accelerations of up to 60 [61]. Multi-station and moving table MRI approaches have also been proposed to be combined with many-element receive coils and parallel imaging for situations in which extensive or multi-territory anatomic coverage is required to provide accurate diagnosis of systemic atherosclerotic disease [62, 63]. With the acquisition speed advantage of highly accelerated parallel imaging, cardiac gated large volume 3D scans also become possible to reduce cardiac motion-induced lumen blurring without exceeding clinically acceptable breath-hold times.
Global cardiac function assessment
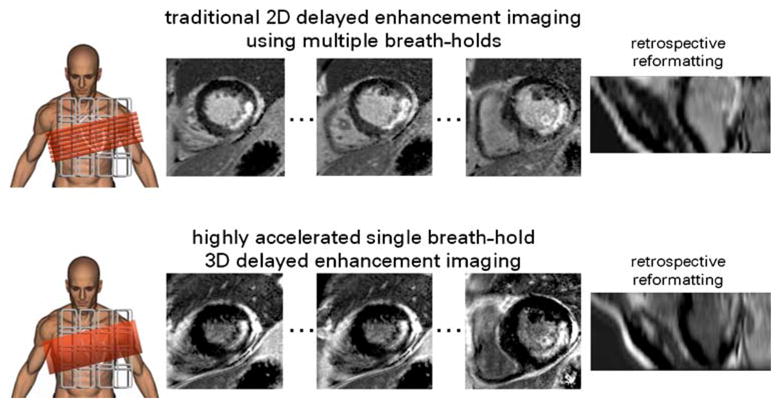
High SNR, high contrast-to-noise ratio (CNR) and high temporal resolution are essential for the precise assessment of global cardiac function using CINE imaging. To achieve apex-to-base coverage, the traditional approach results in total examination times of approximately 10–12 min may diminish patient compliance and may result in appreciable slice misregistration. Highly accelerated parallel imaging helps to overcome these difficulties by allowing 2D CINE techniques with multiple slices acquired per breath-hold, resulting in whole heart coverage within one to two breath-holds (Figs. 6 and 7) by using four-fold (SENSE, GRAPPA) or five-fold (k–t BLAST, TSENSE) 1D accelerations [64, 65]. Alternatively, single breath-hold, whole heart, 3D CINE acquisitions with acceptable spatial and temporal resolution may be achieved via six-fold (R=3×2) to eightfold (R=4×2) 2D accelerations (Fig. 8). Volumetric data sets are particularly desirable in part because they can be retrospectively reformatted to obtain multiple different cardiac views (Fig. 8). Accelerated 2D and 3D approaches offer means to accommodate individuals with the most limited breath hold capacities, including patients with compromised pulmonary function or congestive heart failure. To achieve the high accelerations required without incurring prohibitive SNR losses associated with sensitivity encoding, spatio-temporal correlations in dynamic CINE imaging can be exploited using k–t approaches [64, 66, 67], though the temporal fidelity of these approaches remains a concern [64]. A fairly rapid degeneration of myocardial border sharpness at accelerations R≥8 may be inevitable, which may lead to global cardiac function parameter statistically different from those obtained for the conventional approach (end-diastolic volume (EDV): 158±14 ml vs. 150±11 ml, end-systolic volume (ESV): 62±6 ml vs. 67±5 ml, ejection fraction (EF): 60±4 % vs. 54±3%) [64]. 2D CINE TSENSE yielded examination time reductions of approximately 80% at 1.5 T [65]. ESV and EF obtained from a five-fold accelerated patient study using 2D CINE TSENSE showed excellent correlation with the unaccelerated approach while EDV examination revealed a difference of (4.1±5.8 ml) between TSENSE accelerated 2D CINE and conventional 2D CINE [65].
Fig. 6.

Short axis CINE images of the heart obtained in systole using an unaccelerated (R=1) conventional 2D SSFP approach (a) and 2D SSFP in tandem with sensitivity encoding-based parallel imaging (b–e) using acceleration factors up to 8 (e). The increase in the acceleration factor together with a constant number of phase encoding steps per cardiac cycle resulted in a significant reduction in breath hold duration. Image quality is preserved in the accelerated scans. Images were acquired using a 32-element cardiac phased array coil. The speed gain of highly accelerated parallel imaging serves to cover the heart from the apex to the base in 1–2 short breath-holds. Over-sampling was applied along the phase encoding direction
Fig. 7.
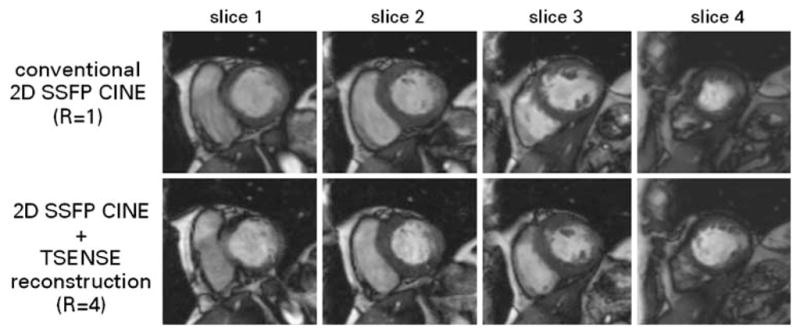
Short axis CINE images of the heart derived from unaccelerated conventional 2D SSFP imaging covering a single slice per breath-hold (top) and four-fold accelerated TSENSE 2D SSFP encompassing four slices per breath-hold (bottom). The latter supports dual breath-hold, wholeheart coverage acquisitions. The accelerated approach yielded examination time reductions of approximately 80% at 1.5 T and 3.0 T [65]. End-systolic volume and ejection fraction showed excellent correlation with the unaccelerated approach while the end-diastolic volume assessment revealed a minor difference of delta EDV =(4.1±5.8 ml) between TSENSE accelerated 2D CINE and conventional 2D CINE. (Images courtesy of Bernd J. Wintersperger, University of Munich Hospitals, Munich, Germany)
Fig. 8.
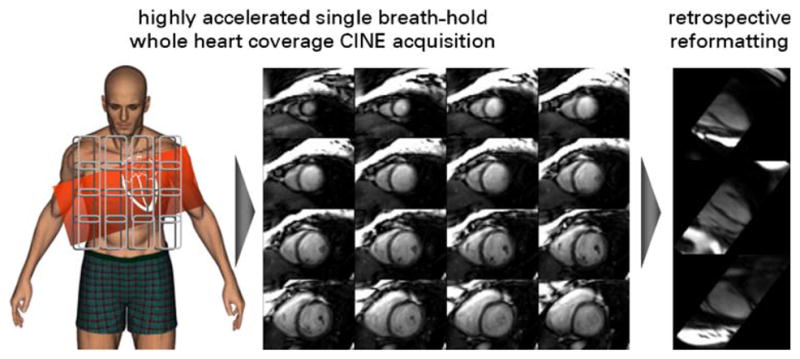
Middle: 16 out of a total of 25 short axis systolic views obtained from a single breath-hold, apex-to-base 3D CINE SSFP acquisition at 1.5 T using the k–t BLAST approach with an acceleration factor of R=12. Twenty cardiac phases were acquired. A 32-channel cardiac coil array was employed. Standard long axis cardiac views (top right: three-chamber view, middle right: four-chamber view, bottom right: two-chamber view) were reformatted retrospectively
Highly parallel CINE imaging also facilitates the capture of an increased number of cardiac phases without exceeding breath-hold constraints. The high temporal resolution of such approaches should allow accurate wall motion tracking and tracking of small rapidly moving structures such as valve cusps throughout the cardiac cycle-a capability expected to be beneficial for the examination of local contractile malfunctions and valvular disease.
First pass myocardial perfusion imaging
Remaining obstacles to a broader clinical acceptance of first-pass perfusion MRI are (1) the limited in-plane spatial resolution of approximately 3×3 mm2, which results in Gibbs ringing artifacts [68] and (2) the limited anatomic coverage achievable while accomplishing one- or two-heart-beat temporal resolution to track the contrast agent passage. To reach the goal of millimeter in-plane spatial resolution, while preserving single-heart-beat temporal resolution, k–t techniques can be exploited. A recent precursor to a broader clinical study [69] demonstrated that 8- to 16-fold accelerated k–t approach yielded image quality superior to that of the conventional approach, primarily as a result of the substantial suppression of Gibbs ringing artifacts as illustrated in Fig. 9. The ability to produce exquisite in-plane spatial resolution as demonstrated in Fig. 9 may offer greater diagnostic value for myocardial perfusion assessment and supports an extension of the perfusion assessment to the right ventricle, though the temporal fidelity of massively accelerated k–t techniques (R>8) must be viewed with caution. Hence its applicability for parametric analysis of the signal intensity time course requires further investigations. With sufficient accelerations, perfusion imaging should be feasible using 3D acquisitions for whole heart coverage.
Fig. 9.
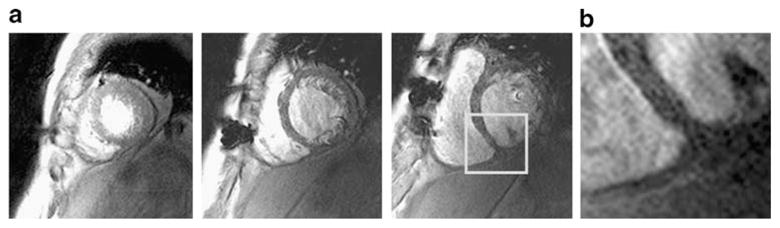
Selected short axis first-pass perfusion images (a) derived from a 3.0-T patient study after open chest surgery encompassing three slices per heartbeat using k–t BLAST reconstruction with 16-fold undersampling. The k–t BLAST approach enabled acceleration factors and image quality that are unattainable with one-dimensional SENSE. The image on the right (b) demonstrates the suppression of Gibbs ringing artifacts as well as a high spatial resolution of (1.0×1.0×8.0) mm3 in a magnified segment (marked by the white rectangle in the adjacent image). Images were acquired at 3.0 T with a 32-element cardiac coil array. Susceptibility artifacts were caused by surgical cerclages
Coronary MR angiography (CMRA)
Highly accelerated parallel imaging strategies provide several means of improving the quality of CMRA by minimizing the impact of physiological motion [29]. The time savings of accelerated CMRA have been translated into enhanced spatial resolution, which resulted in improved delineation of proximal and, most especially, distal segments of the coronary arteries [70].
Highly accelerated parallel imaging with accelerations as high as R=16 allow whole heart CMRA within a 1–2-min free breathing acquisition [71] or a single breath hold scan (Fig. 10), though SNR constraints currently limit clinical CMRA to a maximum acceleration factor of R=8. This constitutes a significant scan time reduction, which, in turn, may enable integration of CMRA into a short comprehensive CVMRI examination. Initial experience at 3.0 T suggests that higher field strengths in conjunction with tailored array designs may enable clinical CMRA with sub-millimeter spatial resolution, although the issue of the off-resonance sensitivity of SSFP techniques remains a concern.
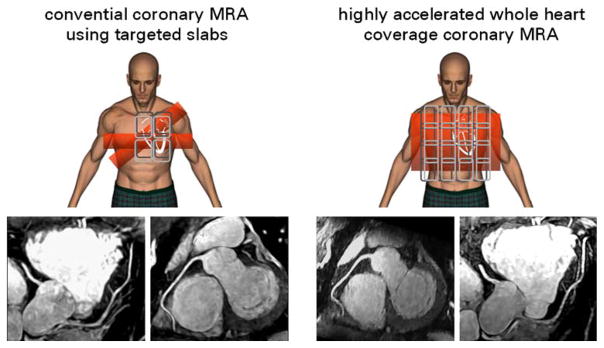
Fig. 10.
Conventional coronary MRA using targeted slabs (left) versus highly-accelerated parallel, whole-heart coverage coronary MR angiography (right). For the highly accelerated approach a 32-element array was used to achieve 8-fold (R=4×2) accelerations for an axial 3D SSFP image volume. Reformatted views of the left anterior descending and right coronary artery obtained from the targeted scans are shown at the bottom on the left hand side. The full complement of information was available following a highly accelerated, single breath-held scan with simple axial planning and whole heart coverage shown at the bottom on the right hand side
Detection of myocardial infarction and assessment of myocardial viability
The established CVMRI assessment of ischemic heart disease includes delayed contrast-enhanced imaging [72]. The resulting low SNR due to suppression of background and healthy myocardial signal presents a challenge for combinations with highly accelerated parallel imaging. However, the established unaccelerated approach exhibits long examination times of 10–15 minutes with corresponding patient discomfort and decay of contrast agent concentration over the course of the exam. Six- (R=3×2) to eight-fold (R=4×2) accelerated parallel imaging can produce whole-heart coverage in a single breath-hold (Fig. 11), increasing patient comfort and ensuring uniform suppression of healthy myocardium for all imaged sections. The inherently low SNR can be offset by the use of many-element coil arrays in synergy with high magnetic field strengths.
Fig. 11.
Top: Three representative short-axis views of the heart obtained from a 3.0-T study performed in a patient after open chest surgery using 2D segmented IR prepared gradient echo imaging covering a single slice per breath-hold together with phase sensitive reconstruction (PSIR); an approach that is used routinely in clinical assessment of myocardial infarction. Bottom: Three out of 20 short axis views obtained in the same examination using an eight-fold accelerated single breath-hold 3D segmented IR prepared gradient echo acquisition with apex-to-base coverage. Highly accelerated 3D imaging enabled acquisition of all data at the same time point of the contrast agent wash-in/wash-out kinetics, which helped to ensure uniform suppression of healthy myocardium for all imaged sections. The low SNR was offset by the use of high magnetic field strength (3.0 T) in conjunction with a 32-element cardiac-optimized coil array
Meanwhile, a phase sensitive reconstruction of inversion recovery (PSIR) technique has been shown to enhance the contrast between healthy and infarcted myocardial tissue [73, 74]. However, this approach requires two R-R intervals for full magnetization recovery and hence doubles the total scan time as compared with the conventional 1 R-R interval approach. This drawback can be compensated by using the time savings inherent to high accelerations while still preserving the ability of whole heart coverage 3D acquisitions.
Outlook
As high accelerations are accomplished more routinely, they should help to enable ever shorter comprehensive CVMRI examinations for the detection of heart disease, while improving volumetric coverage, operator convenience and patient comfort.
The extra diagnostic value afforded by highly accelerated parallel imaging is expected to drive future technological developments. As many-element, high density RF-coil arrays become more common, ergonomic requirements are likely to motivate innovative cabling methods, or even wireless coil connectivity. A move towards 64-bit reconstruction hardware and parallel computation has been recognized as an important step in overcoming current memory and performance constraints on image reconstruction for many-channel volumetric acquisitions. Another important development is the move towards commercial 3.0-T or even 7.0-T MR systems with 32 or more receiver channels. The requirements of CVMR at high- or ultra-high magnetic field strengths are also likely to pave the way for further advances in RF coil technology. For example, an encircling grid with a sufficient number of elements embedded in the whole body coil insert would allow arbitrary placement of encoding directions. Another exciting development is the extension of parallel MRI to the excitation phase to reduce RF power deposition and to improve B1 homogeneity at high field strengths [75, 76].
With appropriate hardware design and customized imaging techniques, one might envisage compressing a comprehensive CVMRI exam not merely into a span of 30–45 min, but even into a few short breath-holds or a short period of free breathing. While this is, for the moment, merely a vision, it continues to motivate new basic and clinical research, and offers the prospect of rapid volumetric imaging in the style of multi-detector CT while maintaining the full range of biochemical contrast options associated with MRI.
Acknowledgments
The authors gratefully acknowledge Marcus Katoh, Gabi Krombach, Harald Kuehl, Karl Ruhl, Elmar Spuentrup, Maral Tilbian, and Jane F. Utting (RWTH Aachen, Aachen, Germany); Christoph Leussler, (Philips Research Lab, Hamburg, Germany); Ruud de Boer and Marc Kouwenhoven (Philips Medical Systems, Best, The Netherlands); Randy O. Giaquinto and Christopher J. Hardy (GE Global Research, Niskayuna, New York, USA); James Akao, Randy Duensing, and Diana Spencer (IN VIVO Corp., Gainsville, Fl, USA); Bernd Kühn (Siemens Medical Solutions, Erlangen, Germany), Sebastian Kozerke (Institute for Biomedical Engineering, University and ETH Zurich, and Gyrotools, Zurich, Switzerland), Bernd J. Wintersperger (University of Munich Hospitals, Munich, Germany); Thea Marie Niendorf and Anna Tabea Niendorf, all of whom kindly contributed technical support or other valuable assistance. Portions of the presented work were supported by a grant from the START programme (46/06 RWTH Aachen, Aachen, Germany).
Contributor Information
Thoralf Niendorf, Email: niendorf@rad.rwth-aachen.de, Department of Diagnostic Radiology, RWTH Aachen, University Hospital, Pauwelsstrasse 30, 52057 Aachen, Germany, Tel.: +49-241-8080295, Fax: +49-241-803380295.
Daniel K. Sodickson, Email: Daniel.Sodickson@med.nyu.edu, Department of Radiology, Center for Biomedical Imaging, New York University, School of Medicine, 650 First Avenue, Suite 600-A, New York, NY, 10016, USA, Tel.: 212-263-4844, Fax: 212-263-4845
References
- 1.Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. Eur Heart J. 2004;25(21):1940–1965. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 2.Lima JA, Desai MY. Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol. 2004;44(6):1164–1171. doi: 10.1016/j.jacc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Spuentrup E, Botnar RM. Coronary magnetic resonance imaging: visualization of the vessel lumen and the vessel wall and molecular imaging of arteriothrombosis. Eur Radiol. 2006;16(1):1–14. doi: 10.1007/s00330-005-2886-7. [DOI] [PubMed] [Google Scholar]
- 4.Russo V, Renzulli M, La Palombara C, Fattori R. Congenital diseases of the thoracic aorta. Role of MRI and MRA. Eur Radiol. 2006;16(3):676–684. doi: 10.1007/s00330-005-0027-y. [DOI] [PubMed] [Google Scholar]
- 5.Gatehouse PD, Keegan J, Crowe LA, Masood S, Mohiaddin RH, Kreitner KF, Firmin DN. Applications of phase-contrast flow and velocity imaging in cardiovascular MRI. Eur Radiol. 2005;15(10):2172–2184. doi: 10.1007/s00330-005-2829-3. [DOI] [PubMed] [Google Scholar]
- 6.Bogaert J, Dymarkowski S. Delayed contrast-enhanced MRI: use in myocardial viability assessment and other cardiac pathology. Eur Radiol. 2005;15 (Suppl 2):B52–B58. doi: 10.1007/s10406-005-0093-x. [DOI] [PubMed] [Google Scholar]
- 7.Pons-Llado G. Assessment of cardiac function by CMR. Eur Radiol. 2005;15(Suppl 2):B23–B32. doi: 10.1007/s10406-005-0096-7. [DOI] [PubMed] [Google Scholar]
- 8.Croisille P, Revel D, Saeed M. Contrast agents and cardiac MR imaging of myocardial ischemia: from bench to bedside. Eur Radiol. 2006;16(9):1951–1963. doi: 10.1007/s00330-006-0244-z. [DOI] [PubMed] [Google Scholar]
- 9.Krombach GA, Niendorf T, Gunther RW, Mahnken AH. Characterization of myocardial viability using MR and CT imaging. Eur Radiol. 2007 doi: 10.1007/s00330-006-0531-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Czum JM, Prince MR. Emerging functional MR angiographic techniques. Magn Reson Imaging Clin N Am. 2005;13(1):181–188. doi: 10.1016/j.mric.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Stuber M, Botnar RM, Danias PG, Kissinger KV, Manning WJ. Submillimeter three-dimensional coronary MR angiography with real-time navigator correction: comparison of navigator locations. Radiology. 1999;212 (2):579–587. doi: 10.1148/radiology.212.2.r99au50579. [DOI] [PubMed] [Google Scholar]
- 12.Stehning C, Bornert P, Nehrke K, Eggers H, Stuber M. Free-breathing whole-heart coronary MRA with 3D radial SSFP and self-navigated image reconstruction. Magn Reson Med. 2005;54(2):476–480. doi: 10.1002/mrm.20557. [DOI] [PubMed] [Google Scholar]
- 13.Slavin GS, Wolff SD, Gupta SN, Foo TK. First-pass myocardial perfusion MR imaging with interleaved notched saturation: feasibility study. Radiology. 2001;219(1):258–263. doi: 10.1148/radiology.219.1.r01mr35258. [DOI] [PubMed] [Google Scholar]
- 14.Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199 (1):49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 15.Nayak KS, Cunningham CH, Santos JM, Pauly JM. Real-time cardiac MRI at 3 Tesla. Magn Reson Med. 2004;51 (4):655–660. doi: 10.1002/mrm.20053. [DOI] [PubMed] [Google Scholar]
- 16.McLeish K, Kozerke S, Crum WR, Hill DL. Free-breathing radial acquisitions of the heart. Magn Reson Med. 2004;52(5):1127–1135. doi: 10.1002/mrm.20252. [DOI] [PubMed] [Google Scholar]
- 17.Santos JM, Cunningham CH, Lustig M, Hargreaves BA, Hu BS, Nishimura DG, Pauly JM. Single breath-hold whole-heart MRA using variable-density spirals at 3T. Magn Reson Med. 2006;55(2):371–379. doi: 10.1002/mrm.20765. [DOI] [PubMed] [Google Scholar]
- 18.Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med. 1997;38(4):591–603. doi: 10.1002/mrm.1910380414. [DOI] [PubMed] [Google Scholar]
- 19.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42 (5):952–962. [PubMed] [Google Scholar]
- 20.Jakob PM, Griswold MA, Edelman RR, Manning WJ, Sodickson DK. Accelerated cardiac imaging using the SMASH technique. J Cardiovasc Magn Reson. 1999;1(2):153–157. doi: 10.3109/10976649909080844. [DOI] [PubMed] [Google Scholar]
- 21.Weiger M, Pruessmann KP, Boesiger P. Cardiac real-time imaging using SENSE. SENSitivity Encoding scheme. Magn Reson Med. 2000;43(2):177–184. doi: 10.1002/(sici)1522-2594(200002)43:2<177::aid-mrm3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Pruessmann KP, Weiger M, Boesiger P. Sensitivity encoded cardiac MRI. J Cardiovasc Magn Reson. 2001;3 (1):1–9. doi: 10.1081/jcmr-100000143. [DOI] [PubMed] [Google Scholar]
- 23.Sodickson DK, McKenzie CA, Li W, Wolff S, Manning WJ, Edelman RR. Contrast-enhanced 3D MR angiography with simultaneous acquisition of spatial harmonics: A pilot study. Radiology. 2000;217(1):284–289. doi: 10.1148/radiology.217.1.r00se47284. [DOI] [PubMed] [Google Scholar]
- 24.Weiger M, Pruessmann KP, Kassner A, Roditi G, Lawton T, Reid A, Boesiger P. Contrast-enhanced 3D MRA using SENSE. J Magn Reson Imaging. 2000;12(5):671–677. doi: 10.1002/1522-2586(200011)12:5<671::aid-jmri3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Niendorf T, Sodickson DK. Parallel imaging in cardiovascular MRI: methods and applications. NMR Biomed. 2006;19(3):325–341. doi: 10.1002/nbm.1051. [DOI] [PubMed] [Google Scholar]
- 26.Hardy CJ, Darrow RD, Saranathan M, Giaquinto RO, Zhu Y, Dumoulin CL, Bottomley PA. Large field-of-view real-time MRI with a 32-channel system. Magn Reson Med. 2004;52(4):878–884. doi: 10.1002/mrm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Hardy CJ, Sodickson DK, Giaquinto RO, Dumoulin CL, Kenwood G, Niendorf T, Lejay H, McKenzie CA, Ohliger MA, Rofsky NM. Highly parallel volumetric imaging with a 32-element RF coil array. Magn Reson Med. 2004;52(4):869–877. doi: 10.1002/mrm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodickson DK, Hardy CJ, Zhu Y, Giaquinto RO, Gross P, Kenwood G, Niendorf T, Lejay H, McKenzie CA, Ohliger MA, Grant AK, Rofsky NM. Rapid volumetric MRI using parallel imaging with order-of-magnitude accelerations and a 32-element RF coil array: feasibility and implications. Acad Radiol. 2005;12(5):626–635. doi: 10.1016/j.acra.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niendorf T, Hardy CJ, Giaquinto RO, Gross P, Cline HE, Zhu Y, Kenwood G, Cohen S, Grant AK, Joshi S, Rofsky NM, Sodickson DK. Toward single breath-hold whole-heart coverage coronary MRA using highly accelerated parallel imaging with a 32-channel MR system. Magn Reson Med. 2006;56(1):167–176. doi: 10.1002/mrm.20923. [DOI] [PubMed] [Google Scholar]
- 30.Wintersperger BJ, Reeder SB, Nikolaou K, Dietrich O, Huber A, Greiser A, Lanz T, Reiser MF, Schoenberg SO. Cardiac CINE MR imaging with a 32-channel cardiac coil and parallel imaging: impact of acceleration factors on image quality and volumetric accuracy. J Magn Reson Imaging. 2006;23(2):222–227. doi: 10.1002/jmri.20484. [DOI] [PubMed] [Google Scholar]
- 31.Kramer H, Schoenberg SO, Nikolaou K, Huber A, Struwe A, Winnik E, Wintersperger BJ, Dietrich O, Kiefer B, Reiser MF. Cardiovascular screening with parallel imaging techniques and a whole-body MR imager. Radiology. 2005;236(1):300–310. doi: 10.1148/radiol.2361040609. [DOI] [PubMed] [Google Scholar]
- 32.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized auto-calibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47 (6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 33.Kyriakos WE, Panych LP, Kacher DF, Westin CF, Bao SM, Mulkern RV, Jolesz FA. Sensitivity profiles from an array of coils for encoding and reconstruction in parallel (SPACE RIP) Magn Reson Med. 2000;44(2):301–308. doi: 10.1002/1522-2594(200008)44:2<301::aid-mrm18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 34.Sodickson DK, McKenzie CA. A generalized approach to parallel magnetic resonance imaging. Med Phys. 2001;28(8):1629–1643. doi: 10.1118/1.1386778. [DOI] [PubMed] [Google Scholar]
- 35.Yeh EN, McKenzie CA, Ohliger MA, Sodickson DK. Parallel magnetic resonance imaging with adaptive radius in k-space (PARS): constrained image reconstruction using k-space locality in radiofrequency coil encoded data. Magn Reson Med. 2005;53(6):1383–1392. doi: 10.1002/mrm.20490. [DOI] [PubMed] [Google Scholar]
- 36.Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46 (4):638–651. doi: 10.1002/mrm.1241. [DOI] [PubMed] [Google Scholar]
- 37.Madore B, Glover GH, Pelc NJ. Unaliasing by fourier-encoding the overlaps using the temporal dimension (UNFOLD), applied to cardiac imaging and fMRI. Magn Reson Med. 1999;42 (5):813–828. doi: 10.1002/(sici)1522-2594(199911)42:5<813::aid-mrm1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 38.Tsao J, Boesiger P, Pruessmann KP. k–t BLAST and k–t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med. 2003;50(5):1031–1042. doi: 10.1002/mrm.10611. [DOI] [PubMed] [Google Scholar]
- 39.Madore B. UNFOLD-SENSE: a parallel MRI method with self-calibration and artifact suppression. Magn Reson Med. 2004;52(2):310–320. doi: 10.1002/mrm.20133. [DOI] [PubMed] [Google Scholar]
- 40.Kellman P, Epstein FH, McVeigh ER. Adaptive sensitivity encoding incorporating temporal filtering (TSENSE) Magn Reson Med. 2001;45 (5):846–852. doi: 10.1002/mrm.1113. [DOI] [PubMed] [Google Scholar]
- 41.Reeder SB, Wintersperger BJ, Dietrich O, Lanz T, Greiser A, Reiser MF, Glazer GM, Schoenberg SO. Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med. 2005;54(3):748–754. doi: 10.1002/mrm.20636. [DOI] [PubMed] [Google Scholar]
- 42.Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005;54(6):1439–1447. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDougall MP, Wright SM. 64-channel array coil for single echo acquisition magnetic resonance imaging. Magn Reson Med. 2005;54(2):386–392. doi: 10.1002/mrm.20568. [DOI] [PubMed] [Google Scholar]
- 44.Bodurka J, Ledden PJ, van Gelderen P, Chu R, de Zwart JA, Morris D, Duyn JH. Scalable multichannel MRI data acquisition system. Magn Reson Med. 2004;51(1):165–171. doi: 10.1002/mrm.10693. [DOI] [PubMed] [Google Scholar]
- 45.Niendorf T, Sodickson DK, McKenzie CA, Farrar N, Hardy CJ, Zhu Y, Kenwood G, Harsh MJ, Rofsky NM. Massively accelerated comprehensive volumetric body imaging examinations with a 32-Channel MR-System. 12th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Kyoto, Japan. 2004. p. 2249. [Google Scholar]
- 46.Sodickson DK, Hardy CJ, Zhu Y, Giaquinto RA, Kenwood G, Niendorf T, Lejay H, McKenzie CA, Farrar N, Ohliger MA, Grant AK, Rofsky NM. Twelve- to sixteen-fold accelerations of contrast-enhanced MR angiography using highly parallel MRI with a 32-element array. 12th Scientific Meeting of the International Society of Magnetic Resonance in Medicine; Kyoto, Japan. 2004. p. 703. [Google Scholar]
- 47.Schmitt M, Potthast A, Sosnovik DE, Wiggins GC, Triantafyllou C, Wald LL. A 128 channel receive-only cardiac coil for 3T. Proceedings of the 15th Scientific Meeting of the International Society of Magnetic Resonance in Medicine; Berlin, Germany. 2007. p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardy CJ, Cline HE, Giaquinto RO, Niendorf T, Grant AK, Sodickson DK. 32-element receiver-coil array for cardiac imaging. Magn Reson Med. 2006;55(5):1142–1149. doi: 10.1002/mrm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spencer D, Akao J, Duensing R, Grant AK, Niendorf T, Sodickson DK. A 32-element cardiac receiver coil array parallel imaging. Proceedings of the 13th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Miami, Fl, USA. 2005. p. 911. [Google Scholar]
- 50.Lanz T, Kellman P, Nittka M, Greiser A, Griswold MA. A 32 channel cardiac array optimized for parallel imaging. Proceedings of the 14th Scientific Meeting of the International Society of Magnetic Resonance in Medicine; Seattle, Washington, USA. 2006. p. 2578. [Google Scholar]
- 51.Niendorf T, Schaeffter T, Kozerke S, Bhanniny R, Winkelmann R, Kouwenhoven M, Mazurkewitz P, Leussler C. 32-element coil array for highly accelerated clinical cardiac MR imaging. Proceedings of the 15th Scientific Meeting of the International Society of Magnetic Resonance in Medicine; Berlin, Germany. 2007. p. 3859. [Google Scholar]
- 52.Ohliger MA, Grant AK, Sodickson DK. Ultimate intrinsic signal-to-noise ratio for parallel MRI: electromagnetic field considerations. Magn Reson Med. 2003;50(5):1018–1030. doi: 10.1002/mrm.10597. [DOI] [PubMed] [Google Scholar]
- 53.Wiesinger F, Boesiger P, Pruessmann KP. Electrodynamics and ultimate SNR in parallel MR imaging. Magn Reson Med. 2004;52(2):376–390. doi: 10.1002/mrm.20183. [DOI] [PubMed] [Google Scholar]
- 54.Weiger M, Pruessmann KP, Boesiger P. 2D SENSE for faster 3D MRI. Magn Reson Mater Phy. 2002;14(1):10–19. doi: 10.1007/BF02668182. [DOI] [PubMed] [Google Scholar]
- 55.Niendorf T, Sodickson D. Acceleration of cardiovascular MRI using parallel imaging: basic principles, practical considerations, clinical applications and future directions. Rofo. 2006;178 (1):15–30. doi: 10.1055/s-2005-858686. [DOI] [PubMed] [Google Scholar]
- 56.Griswold MA, Kannengiesser S, Heidemann RM, Wang J, Jakob PM. Field-of-view limitations in parallel imaging. Magn Reson Med. 2004;52 (5):1118–1126. doi: 10.1002/mrm.20249. [DOI] [PubMed] [Google Scholar]
- 57.Carroll TJ, Grist TM. Technical developments in MR angiography. Ra-diol Clin North Am. 2002;40(4):921–951. doi: 10.1016/s0033-8389(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 58.Grant AK, Sodickson DK, Pedrosa I, Morrin M, Farrar N, Niendorf T, McKenzie CA, Hardy CJ, Giaquinto RA, Joshi S, Rofsky NM. Rapid volumetric body MRI: feasibility of assessment of highly accelerated parallel imaging for magnetic resonance urography. Proceedings of the 13th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Miami Beach, Florida, USA. 2005. p. 1907. [Google Scholar]
- 59.Fenchel M, Nael K, Deshpande VS, Finn JP, Kramer U, Miller S, Ruehm S, Laub G. Renal magnetic resonance angiography at 3.0 Tesla using a 32-element phased-array coil system and parallel imaging in 2 directions. Invest Radiol. 2006;41(9):697–703. doi: 10.1097/01.rli.0000233319.04760.a4. [DOI] [PubMed] [Google Scholar]
- 60.Korosec FR, Frayne R, Grist TM, Mistretta CA. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med. 1996;36(3):345–351. doi: 10.1002/mrm.1910360304. [DOI] [PubMed] [Google Scholar]
- 61.Hadizadeh DR, Gieseke J, Hoogeveen R, von Falkenhausen M, Meyer B, Urbach H, Schild HH, Willinek WA. 4D Time-resolved angiography with CENTRA Keyhole (4D-TRAK) and SENSE using a total acceleration factor of 60 as compared with catheter angiography in patients with cerebral arteriovenous malformations at 3.0T; Seattle, WA, USA. 2006. p. 807. [Google Scholar]
- 62.Fenchel M, Requardt M, Tomaschko K, Kramer U, Stauder NI, Naegele T, Schlemmer HP, Claussen CD, Miller S. Whole-body MR angiography using a novel 32-receiving-channel MR system with surface coil technology: first clinical experience. J Magn Reson Imaging. 2005;21(5):596–603. doi: 10.1002/jmri.20303. [DOI] [PubMed] [Google Scholar]
- 63.Zenge MO, Vogt FM, Brauck K, Jokel M, Barkhausen J, Kannengiesser S, Ladd ME, Quick HH. High-resolution continuously acquired peripheral MR angiography featuring partial parallel imaging GRAPPA. Magn Reson Med. 2006;56(4):859–865. doi: 10.1002/mrm.21033. [DOI] [PubMed] [Google Scholar]
- 64.Niendorf T, Katoh M, Kuehl HP, Grawe H, Bunke J, Kouwenhoven M, Guenther RW. Rapid assessment of cardiac function using 2D CINE SSFP imaging in conjunction with k–t BLAST and k–t SENSE. Proceedings of the 14th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Seattle, WA, USA. 2006. p. 3593. [Google Scholar]
- 65.Wintersperger BJ, Sincleair S, Runge VM, Dietrich O, Huber A, Reiser MF, Schoenberg SO. Dual breath-hold magnetic resonance cine evaluation of global and regional cardiac function. Eur Radiol. 2007;17(1):73–80. doi: 10.1007/s00330-006-0259-5. [DOI] [PubMed] [Google Scholar]
- 66.Katoh M, Spuentrup E, Buecker A, Kouwenhoven M, Bunke J, Guenther RW, Niendorf T. Single breath-hold whole-heart 3D CINE imaging using kt-BLASTand kt-SENSE. Proceedings of the 14th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Seattle, WA, USA. 2006. p. 1634. [Google Scholar]
- 67.Fenchel M, Deshpande VS, Nael K, Finn JP, Miller S, Ruehm S, Laub G. Cardiac cine imaging at 3 Tesla: initial experience with a 32-element body-array coil. Invest Radiol. 2006;41 (8):601–608. doi: 10.1097/01.rli.0000223896.70095.49. [DOI] [PubMed] [Google Scholar]
- 68.Di Bella EV, Parker DL, Sinusas AJ. On the dark rim artifact in dynamic contrast-enhanced MRI myo-cardial perfusion studies. Magn Reson Med. 2005;54(5):1295–1299. doi: 10.1002/mrm.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niendorf T, Utting JF, Tilbian M, Krombach GK, Ruhl K, Kouwenhoven M, Mazurkewitz P, Leussler C, Spencer D, Duensing R. Highly accelerated, millimeter in-plane resolution myocardial perfusion imaging using a 32-Channel 3.0 T System. Proceedings of the 15th Scientific Meeting of the International Society of Magnetic Resonance in Medicine; Berlin, Germany. 2007. p. 3618. [Google Scholar]
- 70.Niendorf T, Saranathan M, Lingamneni A, Pedrosa I, Spencer M, Cline H, Foo TK, Rofsky NM. Short breath-hold, volumetric coronary MR angiography employing steady-state free precession in conjunction with parallel imaging. Magn Reson Med. 2005;53 (4):885–894. doi: 10.1002/mrm.20446. [DOI] [PubMed] [Google Scholar]
- 71.Nehrke K, Bornert P, Mazurkewitz P, Winkelmann R, Grasslin I. Free-breathing whole-heart coronary MR angiography on a clinical scanner in four minutes. J Magn Reson Imaging. 2006;23(5):752–756. doi: 10.1002/jmri.20559. [DOI] [PubMed] [Google Scholar]
- 72.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 73.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myo-cardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47(2):372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huber A, Bauner K, Wintersperger BJ, Reeder SB, Stadie F, Mueller E, Schmidt M, Winnik E, Reiser MF, Schoenberg SO. Phase-sensitive inversion recovery (PSIR) single-shot TrueFISP for assessment of myocardial infarction at 3 tesla. Invest Radiol. 2006;41 (2):148–153. doi: 10.1097/01.rli.0000195843.97582.f4. [DOI] [PubMed] [Google Scholar]
- 75.Katscher U, Bornert P, Leussler C, van den Brink JS. Transmit SENSE. Magn Reson Med. 2003;49(1):144–150. doi: 10.1002/mrm.10353. [DOI] [PubMed] [Google Scholar]
- 76.Zhu Y. Parallel excitation with an array of transmit coils. Magn Reson Med. 2004;51(4):775–784. doi: 10.1002/mrm.20011. [DOI] [PubMed] [Google Scholar]