Abstract
Charles Darwin studied floral biology for over 40 years and wrote three major books on plant reproduction. These works have provided the conceptual foundation for understanding floral adaptations that promote cross-fertilization and the mechanisms responsible for evolutionary transitions in reproductive systems. Many of Darwin's insights, gained from careful observations and experiments on diverse angiosperm species, remain remarkably durable today and have stimulated much current research on floral function and the evolution of mating systems. Here I review Darwin's seminal contributions to reproductive biology and provide an overview of the current status of research on several of the main topics to which he devoted considerable effort, including the consequences to fitness of cross- versus self-fertilization, the evolution and function of stylar polymorphisms, the adaptive significance of heteranthery, the origins of dioecy and related gender polymorphisms, and the transition from animal pollination to wind pollination. Post-Darwinian perspectives on floral function now recognize the importance of pollen dispersal and male outcrossed siring success in shaping floral adaptation. This has helped to link work on pollination biology and mating systems, two subfields of reproductive biology that remained largely isolated during much of the twentieth century despite Darwin's efforts towards integration.
Keywords: adaptation, Charles Darwin, flowers, pollination, mating
1. Introduction
Why do flowers, the reproductive organs of angiosperms, exhibit such astonishing diversity in form when they have a single primary function—to ensure mating and reproductive success? The answer lies in the immobility of plants and their requirement for pollen vectors (animals, wind, water) to transport male gametes between individuals resulting in cross-pollination (Lloyd & Barrett 1996; Proctor et al. 1996; Harder & Barrett 2006; Waser & Ollerton 2006). Today the great structural variety of flowers is largely interpreted as the historical outcome of natural selection during interactions with diverse pollen vectors resulting in floral adaptation (Harder & Johnson 2009). Charles Darwin was the originator of this idea and the purpose of this article is to review his seminal contributions and demonstrate why he can be considered the founder of plant reproductive biology.
The adaptive diversification in form and function of flowers is also associated with an impressive variety of mating strategies and sexual systems that directly influence offspring quantity and quality (Richards 1997; Barrett 2002). The fundamental hermaphroditic condition of most angiosperm species results in opportunities for both cross- and self-pollination with mating patterns determined in part by morphological (Endress 1994) and physiological features of flowers (Franklin-Tong 2008). Spatial and temporal deployment of sex organs (pistils and stamens) within and among flowers play an important role in governing mating and fitness through female and male function. Hermaphroditic (perfect) and unisexual flowers occur in a bewildering array of combinations at the inflorescence, plant and population level, resulting in diverse sexual systems composed of different mixtures of hermaphroditic, female and male plants (Geber et al. 1999). Understanding the evolution and adaptive significance of this floral and sexual-system diversity in flowering plants is today a prominent research theme in reproductive biology.
Floral biology began in the seventeenth and eighteenth centuries when early naturalists, notably Christian Konrad Sprengel, Joseph Kölreuter and Thomas (Andrew) Knight, began to make controlled pollinations of flowers and attempted to understand their function (reviewed in Baker 1979). They observed that in many species insects were required for seed set. However, it was not until Charles Darwin fully turned his attention to plants after taking up residence at Down House in 1842 that the careful, but largely scattered, observations of the early naturalists began to be understood within a single framework, that of evolutionary biology, and specifically, the evolution of adaptation by natural selection. Darwin's studies on floral mechanisms that promote cross-pollination, the consequences of cross- versus self-fertilization on offspring performance, and the evolution and function of sexual polymorphisms were presented, respectively, in three influential books (Darwin 1862, 1876, 1877a). These works helped to provide the conceptual foundation for future research in plant reproductive biology and many of Darwin's ideas have remained resilient to modern scrutiny.
Here I briefly review the key contributions in each of Darwin's three major books on plant reproduction. I outline the approaches and methods he employed and consider why his work still influences research in this field. I then highlight selected topics that Darwin was especially interested in and wrote extensively about in his 1877a book The different forms of flowers on plants of the same species, specifically the evolution and function of floral polymorphisms, and the origins of dioecy and related gender strategies. In each case I review recent studies and evaluate the extent to which Darwin's legacy remains intact and where accommodation is necessary because of new discoveries. Finally, I consider some of Darwin's ideas on a neglected problem—the evolution of wind pollination from animal pollination—and discuss current work on this important evolutionary transition. Throughout I draw attention to problems on floral form and function that Darwin was not able to solve and discuss recent solutions.
2. Darwin and the foundations of plant reproductive biology
(a). The early years
On return from his voyage around the world on H.M.S. Beagle (1831–1836), Darwin spent a few years in London during which time he published The voyage of the beagle (Darwin 1839) and, in the same year, married his first cousin Emma Wedgwood. In 1842 ill-health forced him to move to the village of Downe in Kent where he would spend the rest of his life with his family working in near seclusion at Down House. The early years were taken up with various projects including a monograph on coral reefs, studies on barnacles and the accumulation of evidence culminating in the publication of the ‘Origin of species’ (Darwin 1859). Also, during this time Darwin began to work diligently on plants and his interests grew to the point where he dedicated much of his later life to botanical pursuits. Darwin was always a reluctant botanist, often pointedly referring to his inadequate knowledge of plants when compared with his ‘expert’ botanical colleagues and correspondents. Nevertheless, despite this modesty Darwin published numerous articles on plants and six botanical works. Indeed, it has been claimed that during his scientific career Darwin spent more time working on plants than any other group of organisms (Allen 1977). Today it is recognized that Darwin made original and influential contributions to the development of plant science (Ayres 2008).
Why did Darwin devote over 40 years of his life to working on plants, much of it on reproductive biology? A variety of influences were surely responsible. Foremost were Darwin's ill health, restricting travel and the types of work he could conduct, and an established family interest in growing plants. His grandfather Erasmus Darwin wrote an influential botanical text and his father Robert maintained a garden and tropical plant collection at his home (‘The Mount’) in Shrewsbury. The young Charles helped in the garden and was encouraged to record information on plants in his father's collection (Ayres 2008). Darwin's most influential mentors—Professor John Stevens Henslow at Cambridge and Sir Joseph Dalton Hooker at Kew—were botanists and they no doubt encouraged Darwin to consider plants as suitable subject material for evaluating his developing ideas on variation and evolution. Henslow in particular was especially interested in variation within species and this may have been influential. With a large garden and glasshouse at Down House, and the assistance of gardeners, plants were easy to grow and amenable to direct observation and experimentation. This no doubt satisfied Darwin's natural curiosity and his practical leanings. Beginning in 1841 he began publishing short articles in Gardener's Chronicle, the leading journal of gardeners and nurserymen. He later summarized much of his work on domesticated plants in the volume The variation of animals and plants under domestication (Darwin 1868). Darwin was also a prolific correspondent and because plant material, especially seeds, could easily be sent to him from many parts of the world he was able to grow many ‘foreign’ species at Down House, increasing his appreciation of plant diversity. Finally, as is evident from their increasing inclusion in later editions of the ‘Origin of species’, plants provided outstanding subjects for evaluating his ideas on the evolution of adaptation and the importance of outcrossing for maintaining variation. Indeed, in a letter to J. D. Hooker on 3 June 1857, he confessed that he found ‘any proposition more readily tested in botanical works… . than zoological’ (Darwin 1887).
(b). Orchid pollination
Darwin's first botanical book, ‘On the various contrivances by which British and foreign orchids are fertilised by insects and on the good effects of intercrossing’ (1862) challenged the widely held assumption that beautiful and complex flowers, such as those typical of orchids, were designed by God. Instead, Darwin viewed orchid floral diversification as evidence for his theory of evolution by natural selection. He envisioned this as a gradual process caused by incessant natural selection imposed by insect pollinators. Through numerous careful observations of the flowers of different species and some experimental manipulation, Darwin proposed that orchids possess ‘endless diversities of structure … for gaining the very same end, namely, the fertilization of one flower by pollen from another plant’ (Darwin 1877b, 2nd edn, p. 284). This work initiated his long-standing interest in the outcrossing mechanisms of flowers.
Although Darwin's orchid book largely concerns floral mechanisms promoting cross-pollination, he also described floral traits that promote self-pollination and reasoned that in Ophrys apifera (Bee Orchid) this condition was probably derived from outcrossing to ensure seed set in the absence of pollinators (Darwin 1877b, pp. 57–58). Indeed, it is in this book that Darwin first proposed what is now referred to as ‘the reproductive assurance hypothesis’ (Eckert et al. 2006) for the selection of self-fertilization stating ‘we are led to believe that the above-named self-fertile plants (Ophrys, Disa and Epidendrum) formerly depended on the visits of insects for their fertilization, and that from such visits failing they did not yield sufficient seed and were verging towards extinction. Under these circumstances it is probable that they were gradually modified, so as to become more or less completely self-fertile; for it would manifestly be more advantageous to a plant to produce self-fertilised seeds rather than none at all or extremely few seeds’ (Darwin 1877b, p. 292). Today the reproductive assurance hypothesis is the most widely accepted hypothesis for the evolution of self-fertilization.
Darwin's orchid book has a special place in the history of evolutionary biology. It not only represented the first comprehensive work on the form and function of flowers but it also identifies Darwin as perhaps the initiator of the ‘adaptationist programme’ that has come to dominate contemporary evolutionary biology, despite its detractors (Gould & Lewontin 1979). A recent review details evolutionary and functional evidence for floral adaptation based on studies of phenotypic selection and manipulative experiments (Harder & Johnson 2009). The authors conclude from these two sources of evidence that although natural selection is indeed the primary creative process driving adaptive floral diversification, as Darwin envisioned, the tempo with which adaptations arise varies over time, with periods of phenotypic stasis, because of weak and inconsistent selection, followed by bursts of diversification as a result of strong natural selection.
(c). Effects of cross and self-fertilization
From his work on orchids Darwin obtained considerable evidence that many aspects of floral form involved adaptations promoting cross-pollination. He did not simply accept that outcrossing created the variation on which natural selection acts. Instead in The effects of cross and self fertilisation in the vegetable kingdom (1876), Darwin asked what the direct advantages to offspring were that resulted from cross-fertilization rather than self-fertilization. ‘As plants are adapted by such diversified and effective means of cross-fertilization, it might have been inferred from this fact alone that they derived some great advantage from the process; and it is the object of the present work to show the nature and importance of the benefits thus derived’ (Darwin 1876, p. 2). Using nets to exclude potential insect pollinators, Darwin conducted controlled cross and self-pollinations on 57 species from 52 genera in 30 families of short-lived herbaceous angiosperm taxa. He then compared the performance of offspring by making numerous measurements of phenotypic traits. In some cases (e.g. with Ipomoea purpurea), these experiments were continued for up to 11 years including 10 generations of self- and cross-pollination. The principal conclusion from this painstaking work was that in the vast majority of cases cross-pollinated offspring outperformed self-pollinated offspring, thus providing the direct evidence that Darwin sought for the function of floral adaptations promoting cross-pollination.
Through his controlled pollinations Darwin documented for the first time widespread inbreeding depression in plant species, although he was not aware of its proximate genetic causes (deleterious genes). Today, inbreeding depression is a key parameter in models of mating-system evolution and there is considerable evidence that the magnitude of inbreeding depression plays an important role in explaining observed rates of cross- and self-fertilization in plant populations (Charlesworth & Charlesworth 1987; Husband & Schemske 1996). Over the past two decades there has been considerable progress in understanding the genetic architecture of inbreeding depression, and how fitness differences are influenced by environmental conditions. Darwin made efforts to control the influence of environmental effects in his experiments, at least within any given generation: ‘the cross and self-fertilised plants were subjected in the same generation to as nearly similar and uniform conditions as was possible. In the successive generations they were exposed to slightly different conditions as the seasons varied, and they were raised at different periods’ (Darwin 1876, p. 21). Environmental variation between years can be minimized by seed storage and growing all generations in a common environment as long as seeds remain viable (Barrett & Charlesworth 1991). Alternatively, some workers avoid the problems inherent in Darwin's experimental approach by using genetic markers to measure inbreeding depression under field conditions (Ritland 1990). The main finding from in situ field measurements is that inbreeding depression is considerably more intense in wild populations than under common garden or glasshouse conditions (Dudash 1990). This is probably because plants grown in the wild are subjected to a much wider range of environmental challenges particularly pest and disease pressures.
One of Darwin's regrets in his studies of cross- and self-fertilization of flowering plants is that he did not conduct controlled pollinations on the flowers of highly self-pollinating species. ‘It has been one of the greatest oversights in my work that I did not experimentise on such flowers, owing to the difficulty of fertilizing them, and to my not having seen the importance of the subjects’ (Darwin 1876, p. 387). Because Darwin specifically wanted to investigate the benefits of cross-pollination he selected species that he assumed were largely outcrossing. These were probably also chosen because their flowers were moderately large and easy to manipulate for controlled crosses. Subsequent workers have generally followed this tradition and have largely studied inbreeding depression in outcrossing species, or at least in those with mixed mating systems. Thus today we have relatively little data on the fitness effects of self- and cross-fertilization in predominantly selfing species and what information exists is contradictory. For example, Barrett & Charlesworth (1991) detected no inbreeding depression in a highly self-pollinating population of Eichhornia paniculata and suggested that purging of deleterious genes accounted for the absence of fitness differences between selfed and outcrossed progeny. In contrast, Ågren & Schemske (1993) found substantial inbreeding depression in two highly selfing species of Begonia and interpreted their findings as being consistent with a high mutation rate to mildly deleterious genes (and see Charlesworth et al. 1990). As Darwin recognized, it is unlikely that any flowering plant species is completely selfing so it remains an important issue to determine the fitness effects, if any, of occasional outcrossing in predominantly selfing species (and see Wright et al. 2008), and why complete selfing apparently does not evolve.
(d). Forms of flowers
Darwin's last book on plant reproductive biology The different forms of flowers on plants of the same species (1877a) is the widest ranging in scope and the remainder of this article will focus on several of its main themes. Although it has a particular focus on polymorphic sexual systems it integrates diverse sources of evidence from comparative morphology, compatibility studies, pollination biology, ecology and studies of inheritance. Six chapters are devoted to heterostylous floral polymorphisms, one to dioecy and related gender strategies, and the final chapter discusses species that are predominantly selfing through the formation of cleistogamous (closed) flowers. Darwin obtained immense pleasure from his studies of heterostyly on which he also published separate journal articles. In Darwin's autobiographical recollections (Darwin 1887) he states ‘I do not think anything in my scientific life has given me so much satisfaction as making out the meaning of the structure of heterostylous flowers’. Darwin provided the first functional interpretation of the adaptive significance of heterostyly and speculated on the evolutionary pathway leading to the evolution of distyly. As discussed in the next section, our understanding of stylar polymorphisms has broadened considerably to include a diversity of novel forms; however, all these can be accommodated within Darwin's general functional interpretation involving proficient animal-mediated cross-pollination.
A remarkable feature of ‘Forms of flowers’ is the extent to which Darwin identified general phenomena and concepts that form the basis of considerable contemporary research. Selected examples from his three books on reproductive biology are listed in table 1. Reasoned arguments based on diverse sources of evidence are the defining feature of Darwin's scientific approach. His three books involved careful observations, manipulative experiments, extensive data collection and thoughtful evolutionary inference. Few biologists of the time used these approaches in solving problems and most botanical studies relevant to ecology and evolutionary biology were descriptive in nature and remained so for some time after Darwin died in 1882. Indeed, it was not until the birth of population biology in the 1960s and 1970s that plant reproductive biology experienced a true renaissance, with theoretical models guiding experimental field studies of plant populations. David G. Lloyd played a prominent role in this renaissance as the founder of the modern theory of plant reproductive biology (Barrett & Harder 2006; Barrett & Charlesworth 2007). Darwin was Lloyd's intellectual hero and he used Darwin's seminal contributions as a foundation for many of his ideas.
Table 1.
Some examples of concepts and approaches that Darwin identified in his three books on reproductive biology that form the basis of contemporary research. Other examples largely concerned with floral adaptations are provided in Harder & Johnson (2009).
| concept or approach | Darwin book year, pages | relevant contemporary literature |
|---|---|---|
| reduced intensity of inbreeding depression with continued selfing | 1876, pp. 47–51 | Barrett & Charlesworth (1991) |
| self-incompatibility | 1876, pp. 329–347 | Franklin-Tong (2008) |
| function of synchronized dichogamy | 1876, pp. 390–391 | Harder et al. (2000) |
| floral display and geitonogamy | 1876, pp. 398–400 | Harder & Barrett (1995) |
| intra-specific variation in mating patterns | 1876, p. 441 | Barrett et al. (2009) |
| genetic markers and gametophytic competition | 1877a, p. 31, 241–242 | Cruzan & Barrett (1993) |
| environment-dependent inbreeding depression | 1877a, p. 234 | Dudash (1990) |
| reproductive compensation and sex allocation trade-offs | 1877a, pp. 7, 280, 309 | Ashman (1999) |
| mechanism of cross-promotion and evolution of heterostyly | 1877a, pp. 3, 260–268 | Lloyd & Webb (1992b) |
| adaptations for anemophily | 1877a, p. 94 | Culley et al. (2002) |
| evolutionary transitions from heterostyly to dioecy | 1877a, pp. 258, 284, 287 | Ganders (1979) |
| stressful environmental conditions promote evolution of gender dimorphism | 1877a, pp. 279–280, 344 | Ashman (2006) |
| redundancy of function: zygomorphy and heterostyly | 1877a, pp. 259, 340 | Barrett et al. (2000b) |
| evolution of selfing from outcrossing through reproductive assurance | 1877b, pp. 57–58, 292 | Eckert et al. (2006) |
| unsatisfactory pollinator service and pollen limitation | 1877b, p. 281 | Ashman et al. (2004) |
| efficiency of orchid pollen dispersal | 1877b, pp. 288–289 | Harder (2000) |
3. The diversity of stylar polymorphisms
Darwin's ‘Forms of flowers’ largely focused on plants with sexual polymorphisms in which populations are reproductively sub-divided into distinct mating groups. These may be morphologically indistinguishable, as in the different classes of homomorphic incompatibility (Franklin-Tong 2008), or, as with heterostyly, there are distinct morphological phenotypes or ‘morphs’. Darwin worked primarily on distyly and tristyly, but since then four additional stylar polymorphisms have been recognized: stigma-height dimorphism, enantiostyly, flexistyly and inversostyly, characterized by styles and stamens located in contrasting positions within and between flowers of the floral morphs (table 2; figure 1). Next I review what is known about the evolution and function of these stylar polymorphisms. Because a comprehensive review of heterostyly has recently been published (Barrett & Shore 2008), I highlight recent work on the less well-known examples of stylar polymorphism. I suggest that these contrasting floral designs represent alternative solutions for achieving precision in animal-mediated cross-pollination in different angiosperm lineages. More efficient cross-pollination is largely achieved by reducing lost mating opportunities caused by male gamete wastage because of interference between competing sex functions and self-pollination.
Table 2.
General features of the six classes of stylar polymorphism reported from flowering plants. The features listed are the most common condition(s) for each of the polymorphisms. S > L indicates S-morph dominant to L-morph; R > L right-handed dominant to left-handed; SI, self-incompatible; SC, self-compatible.
| stylar polymorphisms |
||||||
|---|---|---|---|---|---|---|
| general features | distyly | tristyly | stigma-height dimorphism | enantiostyly | flexistyly | inversostyly |
| taxonomic distribution | 28 families | 6 families | approx. 8 families | approx. 10 families | Alpinia and related genera Zingiberaceae | Hemimeris Scrophulariaceae |
| genetic system | 1 diallelic locus, S > L | 2 diallelic loci with epistasis S > M > L | 1 diallelic locus, S > L | 1 diallelic locus, R > L | unknown | unknown |
| compatibility systems and number of mating types | dimorphic SI, 2 | trimorphic SI, 3 | some late-acting SI, 2 | SC and SI, 2 | SC, 2 | SC, 2 |
| ancillary polymorphisms | pollen, stigma | pollen, stigma | absent | absent | absent | absent |
| floral design | actinomorphic tubular, two style lengths | actinomorphic tubular, three style lengths | actinomorphic tubular, two style lengths | zygomorphic non-tubular, heteranthery, style deflection horizontal | zygomorphic, hetero-dichogamy, movement herkogamy | zygomorphic, style deflection vertical |
| main floral reward | nectar | nectar | nectar | pollen | nectar | oil |
| pollinators | long-tongued bees, butterflies, moths, birds | long-tongued bees, butterflies | long-tongued bees, butterflies | pollen-collecting bees | large bees (Xylocopa) | oil-collecting bees (Rediviva) |
Figure 1.
Examples of species that exhibit the six reported stylar polymorphisms in flowering plants: (a) distyly—Primula beesiana (Primulaceae); (b) tristyly—Eichhornia azurea (Pontederiaceae); (c) stigma-height dimorphism—Narcissus gaditanus (Amaryllidaceae); (d) enantiostyly—Wachendorfia paniculata (Haemodoraceae); (e) flexistyly—Alpinia mutica (Zingiberaceae); (f) inversostyly—Hemimeris racemosa (Scrophulariaceae), image courtesy of Anton Pauw. General features of these polymorphisms are summarized in table 2.
(a). Heterostyly
Darwin reported the occurrence of heterostyly from 14 families and 38 genera. Today the number of families in which heterostyly is known has doubled, and the polymorphism has been used widely for addressing diverse questions in population genetics and reproductive ecology (Ganders 1979; Lloyd & Webb 1992a,b; Barrett 1992; Barrett et al. 2000a; Barrett & Shore 2008). Darwin was particularly intrigued by the evolution and function of heterostyly commenting that ‘The existence of plants which have been rendered heterostyled is a highly remarkable phenomenon’ (Darwin 1877a, p. 275). Although he was not confident about the evolutionary pathway(s) leading to the evolution of heterostyly, admitting that ‘This is a very obscure subject, on which I can throw little light, but which is worthy of discussion’ (Darwin 1877a, p. 260), he was more certain, based on his own experimental studies of Primula, Linum and Lythrum, about the functional significance of heterostyly. ‘We may feel sure that plants have been rendered heterostyled to ensure cross-fertilisation’ (Darwin 1877a, p. 258). Darwin interpreted the reciprocal positions of anthers and stigmas (reciprocal herkogamy) in the style morphs of heterostylous populations as a floral mechanism promoting animal-mediated cross-pollination through segregated pollen deposition on the bodies of pollinators.
The conspicuous pollen-size heteromorphism of many heterostylous species has enabled tests of the Darwinian hypothesis through studies of pollen deposition patterns on pollinators, and measurements of inter-morph pollen transfer based on the analysis of stigmatic pollen loads of naturally pollinated plants (reviewed in Lloyd & Webb 1992b). Interestingly, although Darwin documented pollen size differences between the morphs in numerous heterostylous species it does not appear that he ever considered testing his idea by examining the stigmatic pollen loads of wild populations, despite the common occurrence of Primula spp. in the vicinity of Down House. Subsequent experimental evidence from natural populations has generally supported Darwin's cross-promotion hypothesis, although marked asymmetries between morphs are evident in the total pollen captured and the amount that is compatible (Stone & Thomson 1994). For example, in distylous populations stigmas of the long-styled morph capture more total pollen, but the proportion of compatible pollen on stigmas of the short-styled morph is generally higher. This pattern is evident across diverse taxa differing in floral morphology and with diverse visitors and presumably reflects stereotypical entry and exit paths of pollinators during nectar feeding.
The evolutionary origins of heterostyly remain poorly understood. Phylogenetic studies combined with character mapping have provided only limited evidence on the evolutionary pathways by which distyly has evolved (reviewed in Barrett & Shore 2008). As Darwin surmised (1877a, p. 261), an early stage involving variation in stigma height (but not anther height) appears to precede the establishment of distyly. However, we are still some way from obtaining a clear picture of the stages in the build up of the polymorphism, especially the order in which the morphological polymorphisms and heteromorphic incompatibility are assembled. This problem is because of the difficulties of inferring ancestral states in phylogenies and the finding that heterostyly often appears to be basal in the lineages that have been examined (e.g. Mast et al. 2006) as Darwin also recognized. ‘In some of these families the heterostyled condition must have been acquired at a very remote period … and it is not probable that each species acquired its heterostyled structure independently of it close allies … [but] must have inherited their structure from a common progenitor’ (Darwin 1877a, p. 255). Another gap in our understanding is that the molecular basis of heterostyly has not been determined for any species. Although the pattern of inheritance of the style morphs is well established in diverse taxa, the identity, number and organization of genes controlling the heterostylous syndrome are unknown despite some recent progress (reviewed in Barrett & Shore 2008).
(b). Stigma-height dimorphism
Populations with stigma-height dimorphism are composed of two floral morphs that differ in the heights at which stigmas are located within flowers. In the L-morph, stigmas are positioned above the anthers, whereas in the S-morph stigmas are located below the anthers. Darwin (1877a) was aware of this condition in Linum grandiflorum and Cordia species and viewed the polymorphism as functioning in a manner similar to distyly (Darwin 1877a, p. 117, 253). Stigma-height dimorphism does bear some resemblance to distyly, and species with this condition have, following Darwin, been considered examples of ‘anomalous’ (Barrett & Richards 1990) or ‘atypical’ distyly (Dulberger 1992). However, the polymorphism differs from distyly because stamen levels in the floral morphs are of similar height and therefore sex-organ reciprocity is only weakly developed. Stigma-height dimorphism occurs in genera with distyly (e.g. Anchusa, Linum, Lithodora, Narcissus, Primula, reviewed in Barrett et al. 2000a; Ferrero et al. 2009), and in these cases the polymorphism may represent the transitional stage between stylar monomorphism and distyly envisioned by Darwin, and as predicted by models of the evolution of distyly (Charlesworth & Charlesworth 1979; Lloyd & Webb 1992a,b). In these cases the two stylar dimorphisms sometimes merge into one another and distinguishing them becomes somewhat arbitrary. However, stigma-height dimorphism also occurs sporadically in families with no heterostylous taxa (e.g. Liliaceae—Chlorogalum angustifolium, Jernstedt 1982; Epacridaceae—Epacris impressa, O'Brien & Calder 1989; Ericaceae—Kalmiopsis leachiana, Barrett et al. 2000a), indicating that the polymorphism is not always associated with the evolutionary build-up of distyly. Studies of the influence of stigma-height dimorphism on pollen export and receipt in both heterostylous and non-heterostylous groups would be valuable to determine the extent of inter-morph mating.
Stigma-height dimorphism is especially well represented in Narcissus, where it occurs in approximately 12 species and has been studied intensively in at least four (Dulberger 1964; Arroyo & Dafni 1995; Baker et al. 2000a,b; Arroyo et al. 2002; Cesaro et al. 2004). The inheritance of style length is the same as that in most distylous species (Lewis & Jones 1992) with the L-morph of genotype ss and the S-morph Ss (Dulberger 1967). Species are usually self-incompatible (e.g. N. assoanus, N. papyraceus, N. tazetta) or, less frequently, self-compatible (N. dubius). Unlike distylous populations in which morph ratios are commonly 1:1, Narcissus populations with stigma-height dimorphism usually exhibit L-morph-biased ratios. This appears to result from asymmetrical mating owing to intra-morph (assortative) mating in the L-morph (Barrett & Hodgins 2006). Assortative mating in Narcissus is permitted because heteromorphic incompatibility is absent in the genus. However, despite the occurrence of intra-morph mating, 1:1 morph ratios are reported from very large populations of N. assoanus (Baker et al. 2000a) and N. papyraceus (Arroyo et al. 2002), where they are associated with visitation by long-tongued hawkmoths. This indicates that in certain pollination environments the polymorphism is capable of functioning in a manner similar to distyly, as Darwin suggested (Darwin 1877a, p. 117). However, it remains unclear precisely how inter-morph pollen transfer is achieved in populations lacking balanced reciprocity of sex-organs. Several other self-incompatible genera with stigma-height dimorphism (e.g. Anchusa and Lithodora) exhibit L-morph-biased ratios and, at least in Anchusa, intra-morph mating is also permitted (Philipp & Schou 1981). Selection for balanced reciprocal herkogamy may be relaxed in these groups because, unlike species with heteromorphic incompatibility, the penalty of gamete wastage on incompatible stigmas resulting from intra-morph pollination is absent.
(c). Enantiostyly
Mirror-image flowers (enantiostyly) were first reported in the nineteenth century (Todd 1882; Wilson 1887) and involve the deflection of the style to the right or left side of the flower, usually with a single pollinating anther in the opposite direction. In what appears to be Darwin's last scientific correspondence on 10 April 1882 he wrote to J. E. Todd requesting seeds of enantiostylous Solanum rostratum so that ‘he may have the pleasure of experimenting with them’ (Darwin 1887), presumably to investigate form and function in this species (and see §4 below). This was not accomplished and it is only recently that attempts have been made to examine the functional significance of mirror-image flowers.
Enantiostyly has originated independently in at least 10 angiosperm families and unlike other stylar polymorphisms it is expressed at different levels of structural organization including the flower, inflorescence and plant levels (Barrett 2002; Jesson & Barrett 2003). In monomorphic enantiostyly the two flower forms occur within inflorescences either randomly located, or in fixed positions, or are segregated as right- or left-styled inflorescences (see fig. 2 in Barrett 2002). In contrast, populations of dimorphically enantiostylous species are composed of left- and right-styled plants, often in equal frequencies, as in Wachendorfia (Jesson & Barrett 2002a). Here the condition is a true genetic polymorphism and inheritance studies of stylar bending in Heteranthera multiflora indicate single-locus control with right deflection dominant to left (Jesson & Barrett 2002b).
Any adaptive explanation for the function of mirror-image flowers is complicated by the presence of both monomorphic and dimorphic enantiostyly. Most workers have assumed that enantiostyly promotes cross-pollination in a manner functionally similar to heterostyly. Bees visiting the two flower types pick up pollen on opposite sides of their bodies and segregated pollen deposition enhances pollination between the floral forms. Although this may be true for species with dimorphic enantiostyly, for those with monomorphic enantiostyly mixed flower types on a plant should increase geitonogamy (pollen transfer between flowers on a plant resulting in self-fertilization), potentially resulting in inbreeding depression and pollen discounting (Harder & Barrett 1995). Because of the potential costs associated with geitonogamy, the function of monomorphic enantiostyly remained enigmatic until recently.
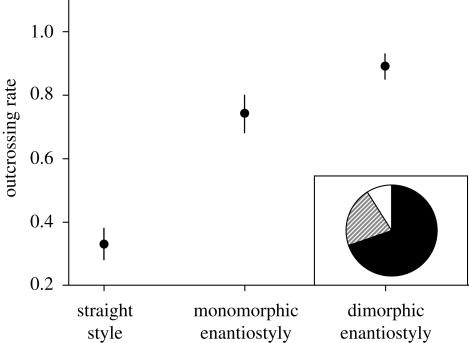
Comparative (Jesson & Barrett 2003), theoretical (Jesson et al. 2003) and experimental studies (Jesson & Barrett 2002c, 2005) have provided new insights into the evolution and function of enantiostyly. Phylogenetic evidence indicates evolutionary transitions from a straight-styled ancestor to monomorphic enantiostyly, with rare transitions to dimorphic enantiostyly. Experimental studies of S. rostratum, a monomorphically enantiostylous species, have evaluated the hypothesis that these transitions are driven by selection for proficient cross-pollination through a reduction in geitonogamous selfing (Jesson & Barrett 2002c, 2005). This was investigated by comparing mating patterns in arrays with three stylar conditions: plants with manipulated straight styles, monomorphic enantiostyly, and, through flower removal, dimorphic enantiostyly. Jesson and Barrett hypothesized that because of the absence of pollen segregation on pollinators, all flowers on straight-styled plants should receive and donate pollen to one another and, as a result, selfing rates would be higher than in enantiostylous arrays. They also predicted the lowest selfing rates in dimorphic arrays, because most pollen transfer should occur between plants of opposite stylar orientation because of pollen segregation on the right- and left-side of the pollinator's body. These predictions were confirmed using genetic markers (figure 2) and observations of pollen deposition on bees' bodies. Remarkably, through the simple removal of alternate flower forms from individual plants in dimorphic arrays, 75 per cent of all outcrossing events resulted from inter-morph (disassortative) pollination. This manipulation demonstrates the efficacy of the reciprocal floral morphologies in promoting pollen segregation and cross-pollen transfer between flowers of opposite stylar orientation.
Figure 2.
Enantiostyly promotes cross-pollination and reduces the incidence of self-fertilization in Solanum rostratum (Solanaceae). Outcrossing rates, estimated using allozyme markers, were compared in experimental arrays exhibiting three contrasting stylar conditions: plants with straight styles, plants with mixtures of right- and left-handed styles (monomorphic enantiostyly) and plants with either right- or left-handed styles (dimorphic enantiostyly). The pie diagram illustrates the proportion of matings in dimorphic enantiostylous arrays that resulted from self-fertilization (white), outcrossing between plants of the same style orientation (cross-hatched) and outcrossing between plants of opposite stylar orientation (black). After Jesson & Barrett (2002c), figure published with permission from Nature.
What are the evolutionary and functional relations between heterostyly and enantiostyly? Most heterostylous species possess heteromorphic incompatibility, whereas this type of incompatibility system is unknown in enantiostylous species, which are either self-compatible or possess homomorphic self-incompatibility. Flowers of the two stylar polymorphisms are quite distinct and, with the exception of Pontederiaceae (Graham & Barrett 1995) where they co-occur in different genera and have independent origins (Kohn et al. 1996), the polymorphisms are represented in different animal-pollinated families. Heterostyly is most commonly found in species with actinomorphic flowers in which nectar is concealed at the base of floral tubes and pollen is segregated longitudinally along the length of the pollinator during nectar feeding. In contrast, enantiostylous species are commonly buzz-pollinated, usually offering only pollen as a reward, and most possess anther dimorphism (heteranthery, discussed in detail below). The flowers are non-tubular and outward facing, with pollinators picking up pollen on the right and left sides of their bodies. Therefore, these two convergent stylar syndromes represent distinct functional solutions to the problem of achieving effective cross-pollination in species with alternate floral rewards (nectar versus pollen). Many interesting questions remain concerning the evolution of enantiostyly. Particularly perplexing is why transitions from monomorphic to dimorphic enantiostyly occur so infrequently, given the experimental evidence on the reproductive benefits of the polymorphic condition. Studies on the developmental and molecular genetics of dimorphic enantiostyly could shed light on this intriguing problem.
(d). Flexistyly
A new stylar polymorphism named flexistyly has recently been reported from the Zingiberaceae (Cui et al. 1996; Li et al. 2001, 2002; Zhang et al. 2003; Takano et al. 2005; Sun et al. 2007). It combines reciprocal herkogamy and dichogamy (temporal separation of female and male function) in a single floral strategy. Populations are composed of two self-compatible floral morphs—one that functions first as a female and later as a male (anaflexistylous morph), and the other with these sexual roles reversed (cataflexistylous morph). Styles of flowers that disperse pollen first curve upwards, so that the stigma is spatially separated from the anthers and cannot contact visiting bees. Around noon after male function is complete, the styles bend downwards into a position where stigmas contact pollinators. Style growth in the alternate morph is reversed, with stigmas receiving pollen in the morning and upward style curvature occurring in the afternoon. Thus anthers only shed pollen when styles are in the upward position and are located in the same position throughout the one-day anthesis period. Unlike heterostyly and enantiostyly, this polymorphism does not involve pollen segregation on pollinators' bodies to achieve inter-morph pollination. Remarkably, the stylar movements of the morphs tend to be synchronous and correlated with the foraging behaviour of floral visitors, mostly large-bodied bees, particularly Xylocopa (Zhang et al. 2003; Takano et al. 2005). The floral morphs in flexistylous populations are reported to occur in equal frequencies but extensive surveys have yet to be undertaken and the inheritance of the polymorphism is unknown.
Flexistyly is an example of heterodichogamy, a sex-phase polymorphism reported from 11 flowering plant families in which populations contain protandrous and protogynous morphs (Renner 2001). Flexistyly was first described as a floral mechanism that enhances outcrossing and reduces opportunities for selfing (Li et al. 2001, 2002). However, this interpretation is not sufficient if heterodichogamy also serves this role through the differential timing of female and male function. Recent experimental studies have demonstrated that the sex-phase synchrony does indeed reduce opportunities for self-pollination, obviating stylar bending as an anti-selfing mechanism (Sun et al. 2007). The style movement that characterizes flexistyly therefore probably functions in a manner similar to heterostyly and enantiostyly by limiting sexual interference between anthers and stigmas and promoting more effective pollen export between plants.
Flexistyly is reported from at least three clades and 24 species in Alpinia, Amomum and Etlingera (Kress et al. 2005). Phylogenetic reconstruction of the evolutionary history of Alpinia and related genera is currently equivocal regarding whether the polymorphism evolved once or on multiple occasions (Kress et al. 2005). Moreover, it is unclear how the polymorphism evolved, including the order of establishment of heterodichogamy and stylar bending, and which morph is likely to be closer to the ancestral condition in morphology and reproductive behaviour. The flowers of Alpinia are large in size, strongly zygomorphic and possess a single anther. These features would have probably precluded the origin of heterostyly and enantiostyly in Zingiberaceae and flexistyly likely represents a unique solution for achieving more effective cross-pollination in this tropical family.
(e). Inversostyly
Despite the key role that animal pollination plays in the evolution and maintenance of stylar polymorphisms, specialized pollinators are not generally involved in mediating cross-pollination. Inversostyly (a polymorphism in which the floral morphs display reciprocal vertical positioning of sexual organs), recently reported in Hemimeris racemosa (Scrophulariaceae) from the Cape region of South Africa, is an exception in this regard (Pauw 2005). The primary pollinators are female oil-collecting bees of the genus Rediviva, which carry pollen of the two morphs in discrete anterior and posterior locations on the underside of their bodies. This segregation probably facilitates cross-pollination in a manner functionally equivalent to heterostyly and enantiostyly. In most populations of H. racemosa there are two style morphs with styles deflected either upwards or downwards and two stamens located in the opposite position within flowers. The polymorphism resembles enantiostyly as both conditions involve flowers that differ in stylar orientation. However, inversostyly is distinct from enantiostyly because of the vertical rather than the horizontal positioning of sex organs and in the absence of heteranthery (discussed below).
Surveys of morph frequencies in 23 populations of H. racemosa revealed that 18 contained the two style morphs in similar frequencies, although there was a small bias in favour of the ‘style-down’ morph, perhaps because of selfing or improved pollen export (Pauw 2005). Significantly, five populations were composed of small-flowered, self-pollinating ‘homostylous’ morphs in which both anthers and stigma are positioned at the bottom of the flower. The evolution of self-pollination via homostyly is commonplace in species with stylar polymorphisms and usually results from selection for reproductive assurance when pollinator service is unreliable (e.g. distyly, Piper et al. 1984; tristyly, Ornduff 1972; enantiostyly, Jesson & Barrett 2002a). Hemimeris racemosa is an annual, self-compatible species that occurs in ephemeral habitats. These features are commonly associated with the evolution of selfing (e.g. Schoen et al. 1997), and related species of Hemimeris (e.g. H. sabulosa) are exclusively homostylous (Pauw 2005) and probably highly selfing.
The flowers of Hemimeris are zygomorphic, a relatively unusual association for species with heterostylous polymorphisms. Darwin was doubtful about the co-occurrence of zygomorphy and heterostyly. ‘Plants which are already well adapted by the structure of their flowers for cross-fertilisation by the aid of insects often possess an irregular corolla, which has been modelled in relation to their visits; and it would have been of little or no use to such plants to have become heterostyled. We can thus understand why it is that not a single species is heterostyled in such great families as the Leguminosae, Labiatae, Scrophulariaceae, Orchidaceae, &c., all of which have irregular flowers’ (Darwin 1877a, p. 259). Although Darwin's inference is generally true, exceptions do occur in some of the families he identified. Isolated occurrences of distyly have recently been reported from the Leguminosae (Caesalpinioideae, Hartley et al. 2002) and Lamiaceae (Barrett et al. 2000b). The zygomorphic and inversostylous flowers of Hemimeris represent another case of the apparent redundancy in function to which Darwin alludes. However, the style–stamen polymorphism in Hemimeris may serve to reduce interference between female and male function, whereas zygomorphy may primarily ensure stereotypical positioning of the specialized oil-collecting bees that visit flowers.
4. Function of heteranthery
Heteranthery is the occurrence of two or more distinct types of stamens within a flower. It has evolved in more than 20 families, and is particularly well represented in Caesalpinioideae, Pontederiaceae and Solanaceae (figure 3). The floral polymorphism is commonly associated with bee-pollinated, nectarless flowers, many of which are enantiostylous (Endress 1994; Jesson & Barrett 2003). Most heterantherous species possess two stamen types that differ in their location within a flower and in shape, size and colour (figure 3). Typically, one set of stamens is centrally located, has brightly coloured anthers (often yellow), and is easily accessible to visitors who collect pollen. The other stamen(s) is usually larger in size, often cryptically coloured, and is commonly displaced from the main floral axis to a position corresponding to the location of the style, as in most enantiostylous species. The repeated evolution of these similar patterns of anther differentiation in unrelated lineages implicates convergent evolution and raises the question of how heteranthery functions in the pollination process.
Figure 3.
Examples of species that exhibit heteranthery: (a) Cassia fistula (Caesalpinioideae) being visited by a Carpenter bee (Xylocopa spp.). FA indicates the feeding anthers and PA the pollinating anthers; (b) Solanum citrullifolium (Solanaceae), image courtesy of Mario Vallejo-Marín; (c) Heteranthera multiflora (Pontederiaceae) exhibits dimorphic enantiostyly, a left-handed flower is illustrated (see §3c); (d) Cyanella alba (Tecophilaeaceae) is reported to be dimorphically enantiostylous, a left-handed flower is illustrated. Image courtesy of Lawrence Harder.
Darwin was intrigued by heteranthery for over 20 years. Although he clearly suspected that the two anther types were likely to have different functions, writing in a letter to Asa Gray on 22 January 1862 that ‘I am now trying an experiment on one of the Melastomas; & I much suspect, that the two sets of anthers have different functions’ (Darwin 1887) he failed to provide an interpretation of their adaptive significance. In a letter to J. D. Hooker on 14 October 1862 he wrote ‘[Regarding plants] with two kinds of anthers … I am very low about them, and have wasted enormous labour over them, and cannot get a glimpse of the meaning of the parts’ (Darwin 1887). It was not until the German Naturalist Fritz Müller and his brother Hermann published a series of three papers in Nature (1881–1883, e.g. Müller 1883), and corresponded with Darwin on the topic, that he became aware of their novel explanation for the function of heteranthery. Based on observations of several heterantherous species in Brazil they proposed that anther differentiation involved specialization into ‘feeding’ and ‘pollinating’ functions and hence represented an example of ‘division of labour’ within flowers. The former anther type rewards pollinators, whereas the latter is primarily involved in promoting cross-pollination. Darwin appears to have accepted this explanation and he set about renewing his stalled investigations. In a letter to W. Thiselton-Dyer on 21 March 1881, he wrote ‘I have had a letter from Fritz Müller suggesting a novel and very curious explanation of certain plants producing two sets of anthers of different colour. This has set me on fire to renew the laborious experiments which I made on this subject, now 20 years ago’ (Darwin 1887). Indeed, Muller's hypothesis may well have prompted him to write to J. E. Todd the following year, as discussed earlier, to obtain seeds of S. rostraum which possesses conspicuous anther dimorphism and enantiostyly. In the preface to the reprint of the 1884 edition of ‘Forms of flowers’ Francis Darwin devotes a short section to heteranthery and includes a mention of papers by the Müller brothers and J. E. Todd in which the ‘division of labour hypothesis’, although not stated explicitly, is certainly implied. Since that time most workers have accepted the hypothesis as the most plausible explanation for the evolution of heteranthery but few experimental studies have evaluated the hypothesis (but see Bowers 1975; Tang & Huang 2007).
Muller's hypothesis predicts that pollinators should focus their pollen-collecting efforts on ‘feeding’ anthers rather than ‘pollinating’ anthers and as a consequence pollen from pollinating anthers is more likely to escape pollen collection and be transported to stigmas of other plants. Evidence to support both predictions has recently been obtained by experimental studies of S. rostratum and bumblebees foraging on floral arrays (Vallejo-Marín et al. 2009). By blocking with glue the small pore from which pollen is dispensed from the poricidal anthers, these workers were able to compare the two anther types with respect to the efficiency of pollen dispersal. They observed that bumblebees visiting flowers spent considerably more time extracting pollen from feeding anthers but, as predicted, pollinating anthers dispersed proportionately more pollen to stigmas of other plants. Using anther-removal experiments involving Melastoma malabatrichum, Luo et al. (2008) also obtained broadly similar results.
Why are pollinating anthers more effective than feeding anthers at pollen dispersal? Based on their studies of pollen deposition on the bodies of bees, grooming behaviour, and observations of anther and stigma positioning, Vallejo-Marín et al. (2009) suggest that more effective pollen transfer by pollinating anthers is the result of a more precise correspondence between pollen placement and stigma contact. Also, the reduced ability of pollinators to groom pollen deposited from pollinating anthers in comparison with feeding anthers plays an important role. Using evolutionary stability analysis of a model of the pollination process, Vallejo-Marín et al. (2009) also found that heteranthery was most likely to evolve when bees consume more pollen than should be optimally exchanged for pollination services, especially when pollinators adjust their visitation according to the amount of pollen collected. In essence the division of labour in anthers of heterantherous species arises because of the contrasting fates of pollen in nectarless flowers; pollen is the carrier of gametes for cross-pollination but is also food for pollinators. Heteranthery reduces this conflict by allowing different stamens to specialize in ‘pollinating’ and ‘feeding’ functions.
5. Evolution of dioecy and related gender strategies
In chapter seven of ‘Forms of flowers’ Darwin (1877a) focused his attention on the evolution of separate sexes (dioecy) and related gender strategies, particularly gynodioecy. It is clear that he considered that a neat division of sexual systems into Linnean categories masked considerable variability of potential evolutionary importance. Indeed, at the very beginning of the book Darwin drew attention to this problem ‘As far as the sexual relations of flowers are concerned, Linnaeus long ago divided them into hermaphrodite, monoecious, dioecious and polygamous species … but the classification is artificial, and the groups often pass into one another’ (Darwin 1877a, p. 1). Darwin may have considered the merging of sexual systems as evidence for his general thesis that most evolutionary change was gradual and involved small incremental steps resulting from natural selection. For example he noted that ‘various hermaphrodite plants have become or are becoming dioecious by many and excessively small steps’ (Darwin 1877a, p. 281) and ‘This case (Euonymus europaeus) appears to me very interesting, as showing how gradually an hermaphrodite plant may be converted into a dioecious one’ (Darwin 1877a, p. 292). In chapter seven Darwin tries to make sense of plant sexual diversity and clearly shows that in some groups sexual systems sometimes do not exhibit tidy boundaries. Today methods for measuring phenotypic gender (Lloyd 1980; Lloyd & Bawa 1984) provide ample support for the quantitative nature of plant sex.
Although dioecy is relatively infrequent in angiosperms, occurring in approximately 6 per cent of species (Renner & Rickelfs 1995), the polymorphism has evolved repeatedly from hermaphroditism, with at least 100 independent origins (Charlesworth 2002). Understanding how and why this transition occurs continues to attract attention from evolutionary biologists. Darwin considered the selective mechanisms responsible for the evolution of dioecy a particularly thorny problem stating that ‘There is much difficulty in understanding why hermaphrodite plants should ever have been rendered dioecious’ (Darwin 1877a, p. 279). Interestingly, given the main conclusion of his book on ‘Effects of cross and self-fertilization’ (1876), Darwin rejected the idea that dioecy is favoured because of the outbreeding advantage unisexuals may enjoy over hermaphrodites. ‘As we must assume that cross-fertilisation was assured before an hermaphrodite could be changed into a dioecious plant, we may conclude that the conversion has not been effected for the sake of gaining the great benefits which follow from cross-fertilisation’ (Darwin 1877a, p. 279). Instead, Darwin emphasized the resource costs of combined sex functions under stressful environmental conditions to explain why unisexual plants may be favoured. Theoretical and empirical work indicates that Darwin was only partially correct in his ideas. Both outbreeding advantage and resource allocation play important roles in the evolution of gender dimorphism (reviewed in Geber et al. 1999). Darwin's assumption that the hermaphroditic ancestors to gender dimorphic plants were outcrossing ignored the possibility that they may also produce self-fertilized seed (although see p. 303 in Darwin 1877a). This reduces the fitness of offspring compared with female variants, which benefit from outbreeding advantage. However, Darwin's ideas on resource allocation and the benefits of unisexuality in stress environments were prescient and there is now evidence that harsh conditions can play a role in the evolution of separate sexes, especially when dioecy evolves via the gynodioecy pathway (Case & Barrett 2004; Ashman 2006).
In some of the only ‘fieldwork’ on plant populations that Darwin mentions, he investigated sex-ratio variation in gynodioecious Thymus serpyllum near Torquay in Devon noting that ‘A very dry station apparently favours the presence of the female form’ (Darwin 1877a, p. 301). Recent studies have provided support for Darwin's field observations. In 14 gynodioecious species female frequencies are higher in environments with low resource availability such as reduced soil water and nutrients (reviewed in Ashman 2006). Potential mechanisms that could account for these patterns include resource limitation of seed production in hermaphrodites, intensified inbreeding depression in stress environments, and pollinator-mediated increases in rates of selfing. Because gynodioecy is frequently an intermediate stage in the evolution of dioecy, understanding the conditions favouring gender specialization can provide insights into the evolution of separate sexes.
Geographical variation in sex-phenotype frequencies represents the spatial template on which natural selection drives transitions among sexual systems (Barrett et al. 2001; Pannell et al. 2008). Patterns of sex-ratio variation can provide clues on the ecological mechanisms governing transitions. We have been investigating the geographical patterns of sex-ratio variation in Sagittaria latifolia, a clonal aquatic plant native to N. America with both monoecious and dioecious populations. The goal of our work is to understand the ecological and genetic mechanisms responsible for the maintenance of combined versus separate-sexed plants, and the forces driving transitions between sexual systems. Our investigations illustrate several of the themes that Darwin emphasized in ‘Forms of flowers’, especially his observations on the inter-gradation among sexual systems and the covariation of sex phenotypes and environmental conditions.
Throughout much of eastern N. America, populations of S. latifolia can usually be classified as either monoecious or dioecious. Monoecious populations commonly occupy ephemeral aquatic habitats such as ditches and farm ponds, whereas dioecious populations are found in more stable wetland habitats associated with large lakes and extensive river systems (Dorken et al. 2002). Common garden and transplant studies have demonstrated that populations of the two sexual systems are differentiated from one another, possessing a suite of life-history traits associated with adaptation to their contrasting wetland habitats (Dorken & Barrett 2003). Crossing studies indicate no barriers to inter-fertility between the sexual systems and patterns of genetic differentiation at neutral loci suggest that ecological differentiation limits extensive gene flow between the sexual systems (Dorken et al. 2002). The segregation of sex phenotypes in F1 and F2 crosses among plants from monoecious and dioecious populations is consistent with a model of sex determination involving two loci (Dorken & Barrett 2004a). In dioecious populations, sex is determined by Mendelian segregation of alleles, with males heterozygous at both the male- and female-sterility loci. In monoecious populations, plants are homozygous for alleles dominant to male sterility in females and recessive to female sterility in males. Several lines of evidence indicate that in S. latifolia monoecy is derived from dioecy, probably via the gynodioecy pathway (Dorken & Barrett 2004a,b).
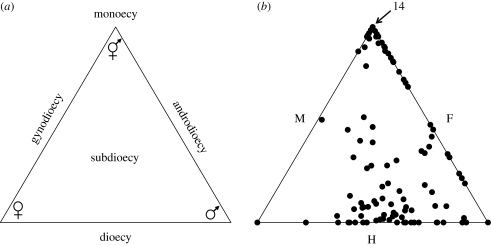
Current work at the northern range limit of dioecious populations indicates a more complicated picture of sexual diversity than a simple sub-division of populations into monoecy or dioecy implies. This is clearly evident from the results of a recent survey of the relative frequencies of the three sex phenotypes in 116 populations sampled in Ontario, Quebec and New York State (S. B. Yakimowski & S. C. H. Barrett 2009, unpublished data). First consider the sexual systems that could potentially arise from combining hermaphrodite, female and male plants in different combinations within a single population. Populations could be monoecious (hermaphrodites only), dioecious (females and males), gynodioecious (hermaphrodites and females), androdioecious (hermaphrodites and males) or sub-dioecious (hermaphrodites, females and males). Figure 4a identifies the location of these five sexual systems on a triangle of sex-phenotype space and figure 4b illustrates the observed patterns of sex-ratio variation revealed by our survey of S. latifolia populations. A significant percentage of the populations we sampled fall into the monoecious (12.1%) or dioecious (17.2%) categories. However, the majority of populations (70.7%) lie elsewhere in the triangle. Using functional criteria based on mating opportunities (see Pannell 2005) they could be classified as gynodioecious (4.3%), androdioecious (19.0%) or subdioecious (47.4%). Regardless of what names we wish to attach to the sexual condition of any given population, the observed patterns clearly demonstrate the enormous sexual diversity that can be maintained within a species.
Figure 4.
The diversity of sexual systems that can potentially occur in a species with three sex phenotypes: hermaphrodite, female and male. (a) Location of the five sexual systems resulting from plotting population sex ratios into a triangle of sex phenotype space. (b) The observed pattern of sex-ratio variation in a sample of 116 populations of the clonal aquatic Sagittaria latifolia from the northern portion of its range in eastern N. America. Each black dot represents a single population and is located on the triangle based on the frequencies of sex phenotypes within each population. Dots within the triangle are populations that contain all three phenotypes, populations with two sex phenotypes are on the axes, and populations containing a single sex phenotype are located on the apices of the triangle. Sampling of flowering ramets in each population followed methods detailed in Dorken & Barrett (2004b). Unpublished data of S. B. Yakimowsi & S. C. H. Barrett (2009).
What factors account for these complicated patterns of sexual variation? Several causes are evident involving both environmental and genetic factors. First, S. latifolia exhibits size-dependent gender modification with small individuals producing inflorescences with only staminate flowers before increasing in size and producing larger inflorescences with both staminate and pistillate flowers (Sarkissian et al. 2001). The populations distributed along the top-right axis of the triangle illustrate this phenomenon involving gender plasticity and contain hermaphrodites and low frequencies of male-functioning plants. However, further down this axis are populations in which there is a higher frequency of male-functioning plants and in these populations inflorescence sizes are equivalent to those of co-occurring hermaphrodites. These populations are genuinely androdioecious. Second, a common feature of dioecious populations, especially those that have originated via the gynodioecious pathway, is the occurrence of genetically based sex inconstancy in male plants (Lloyd 1976; Dorken & Barrett 2004a; Ehlers & Bataillon 2007). In populations of most dioecious species male sex inconstancy is expressed at a relatively low level (approx. 5%) and the few seeds produced by ‘leaky males’ probably have a relatively small impact on population sex ratios. In S. latifolia, a low level of male sex inconstancy is commonly observed in dioecious populations and this accounts for the cluster of populations close to, but not on, the horizontal axis of the triangle. Finally, a significant proportion of populations in our survey contained the three sex phenotypes and these cluster around the centre of the triangle. Using molecular markers we have demonstrated that these ‘sub-dioecious’ populations have originated in two distinct ways (S. B. Yakimowski & S. C. H. Barrett 2009, unpublished data). Most have arisen as a result of hybridization between monoecious and dioecious populations, with the remaining populations descended from dioecious populations in which inconstant males appear to have been favoured and have increased in frequency. We are currently investigating the potential ecological and evolutionary mechanisms responsible for these patterns and why they might occur at northern range limits. The patterns of sex ratio variation in S. latifolia demonstrate the enormous variation that can occur within and among populations of a single plant species. They also suggest that evolutionary transitions among sexual systems may not always be as gradual as Darwin generally envisioned. Major gene control of sexual traits may enable relatively rapid transitions to occur under the appropriate ecological conditions with implications for reproductive isolation and speciation.
6. Evolution of wind pollination
The evolution of wind pollination (anemophily) from animal pollination involves one of the most significant transformations in the form and function of flowers. Approximately 10 per cent of angiosperm species rely on wind for the transport of pollen between plants and anemophily has originated at least 65 times in diverse animal-pollinated lineages (Linder 1998). Unfortunately, our current understanding of this evolutionary transition is rudimentary at best (reviewed in Friedman & Barrett 2009). Few microevolutionary studies have investigated the ecological mechanisms involved, in part because intra-specific variation in pollination systems is rather uncommon (but see Goodwillie 1999; Culley et al. 2002). Darwin wrote sparingly about wind pollination in ‘Forms of flowers’ (1877a) but in a section on Anemophilous plants (Darwin 1876, pp. 400–414) in ch. 10 of ‘Effects of cross and self-fertilization’ he considered contrasts between animal and wind pollination. Darwin was puzzled by why wind pollination should evolve from animal pollination, a problem that has still not been answered satisfactorily. ‘As a large quantity of pollen is wasted by anemophilous plants, it is surprising that so many vigorous species of this kind abounding with individuals should still exist in any part of the world …. It seems at first sight a still more surprising fact that plants, after having been once rendered entomophilous, should ever again have become anemophilous’ (Darwin 1876, pp. 406–407). If anemophily is such an apparently wasteful process then why does it evolve so often from animal pollination?
The most frequent answer to this question is that anemophily evolves when animals become unreliable as pollen vectors because of inimical environmental conditions. This implies that when pollinator service is unsatisfactory and seed set pollen limited, anemophily is favoured because wind affords more reliable pollen dispersal. According to this scenario wind pollination provides reproductive assurance in much the same way that Darwin originally proposed for the evolution of self-pollination. Indeed, Darwin clearly viewed these two quite distinct reproductive transitions as resulting from the same selective mechanism. ‘If any entomophilous species ceased altogether to be visited by insects, it would probably perish unless it were rendered anemophilous, or acquired a full capacity for self-fertilisation’ (Darwin 1876, pp. 407–408). How could the same mechanism—reproductive assurance—result in such widely different morphological and functional outcomes? We have addressed this issue using two complementary approaches. The first involved comparative analyses to examine whether traits in ancestral lineages might influence whether wind pollination evolves (Friedman & Barrett 2008). The second uses field experiments to determine if the assumption of pollination inefficiency is as valid as many have suggested (Friedman & Barrett 2009).
We conducted a comparative analysis of 560 angiosperm species, including 68 wind-pollinated species, to investigate the correlated evolution of reproductive traits and the order in which they were acquired in lineages (Friedman & Barrett 2008). We found that wind pollination was significantly associated with dicliny (unisexual flowers), thus confirming an association that Darwin originally noted. ‘A remarkable fact with respect to anemophilous plants is that they are often diclinous, that is, they are either monoecious with their sexes separated on the same plant, or dioecious with their sexes on distinct plants’ (Darwin 1876, p. 408). Significantly, our analysis revealed that wind pollination evolves more often in animal-pollinated lineages that already possess unisexual flowers. This finding helps to explain how reproductive assurance could elicit such distinct reproductive outcomes as wind pollination and self-pollination. In animal-pollinated lineages with dicliny, autonomous intra-flower self-pollination is likely to be mechanically prevented in most species because perfect (hermaphroditic) flowers are generally required for this mode of self-pollination (but see Ågren & Schemske 1993). In these lineages only wind pollination can provide reproductive assurance. In contrast, in lineages with perfect flowers, genetic modifiers resulting in a loss of herkogamy (stigma–anther separation) are commonplace and cause within-flower autonomous self-pollination (e.g. Vallejo-Marín & Barrett 2009). This would relieve pollen limitation when pollinators are in short supply, leading to the evolution of selfing. Thus, according to this hypothesis the floral traits of ancestral populations are responsible for eliciting two quite different reproductive transitions.
How ‘inefficient’ is wind pollination and are wind-pollinated plants often pollen limited? Our field study of 19 wind-pollinated herbaceous species measured the amount of pollen produced by flowers and the fraction that was captured by stigmas (Friedman & Barrett 2009). Although the range in proportion of pollen captured (0.01–1.19%) was somewhat lower than comparable values for animal pollinated species (0.03–1.9%; Harder 2000), our data do not support the view that pollination in anemophilous species is much less efficient than in animal-pollinated species. High levels of ‘pollen wastage’ characterize both pollination systems. However, in our study pollen loads per stigma averaged 34.1 (standard error = 3.8 grains) and in only one of the 10 species that we investigated was there evidence of pollen limitation of seed set. Although Darwin commented on the pervasive pollen wastage of anemophilous plants he also noted that the vast majority of stigmas captured pollen, stating ‘it is not so surprising as it first appears that all, or nearly all the stigmas of anemophilous plants should receive pollen brought to them by mere chance by the wind’ (Darwin 1876, p. 406). If reproductive assurance does indeed turn out to be the primary mechanism responsible for the evolution of wind pollination, pollen limitation should be less common than in animal-pollinated plants, where it has been reported in 73 per cent of the studies in which it has been investigated (Ashman et al. 2004). Uniovulate flowers (Linder 1998; Friedman & Barrett 2008) and high population densities (Darwin 1876, p. 409) are both characteristic of anemophilous species and these features may help in reducing the incidence of pollen limitation in wind-pollinated species. Diverse reproductive solutions to pollen-limited seed set may be possible in addition to the evolution of self-fertilization (and see Harder & Aizen 2010).
7. Final remarks
Darwin's main contribution to plant reproductive biology was his recognition that much of the extraordinary diversity in floral form can be explained by natural selection of mechanisms promoting cross-pollination and reducing the incidence of self-fertilization and its harmful effects on offspring. In addition he also recognized that evolutionary history plays an important role in guiding future evolutionary change. ‘The wonderful diversity of the means for gaining the same end [cross-fertilisation] … depends on the nature of all the previous changes through which the species had passed, and on the more or less complete inheritance of the successive adaptations of each part to the surrounding conditions’ (Darwin 1877a, p. 258). Although current research on floral adaptation, mating systems and sexual-system evolution increasingly uses theoretical models (e.g. Morgan & Schoen 1997; Harder et al. 2008), genetic markers (e.g. Morgan & Conner 2001; Hodgins & Barrett 2008) and sophisticated genomic tools (e.g. Fishman et al. 2002; Wright et al. 2008; Foxe et al. 2009), the foundations that Darwin built in his three books on plant reproduction continue to provide the framework for many contemporary studies.
Not all of Darwin's ideas on plant reproductive biology are still valid today. For example, although Darwin was one of the first to recognize the prevalence and variation in the expression of self-incompatibility (which he termed self-sterility) in flowering plants, he rejected the notion that it had evolved to prevent self-fertilization and its harmful consequences (Darwin 1876, p. 345), a role that is now generally accepted. In ‘Forms of flowers’, Darwin often used group selection arguments, such as when discussing optimal sex ratios in dioecious populations (Darwin 1877a, p. 282), and he considered seed set (maternal fitness) as the sole target of fertility selection (Darwin 1877a, p. 260, 304, 345, 338). It was not until a century later that most workers in reproductive biology abandoned group selection thinking, came to appreciate that plants have both maternal and paternal fitness, and recognized that sexual selection plays a role in floral adaptation (Willson 1979; Lloyd 1984; Bell 1985; reviewed in Barrett & Charlesworth 2007). Today the importance of pollen dispersal and male outcrossed siring success in shaping floral evolution is generally appreciated (reviewed in Lloyd & Barrett 1996; Harder & Barrett 2006). This has helped to integrate work on pollination biology and mating systems, two subfields of reproductive biology that were largely isolated during much of the twentieth century despite Darwin's efforts to integrate them in his own work. Although Darwin failed to appreciate the importance of sexual selection and male fertility in plants, it is impressive that so much of what he wrote in his three books on plant reproductive biology has proven to be generally correct. It is also intriguing how close Darwin came in his work on heterostylous plants to obtaining the progeny ratios that enabled Gregor Mendel to first establish the principles of inheritance and initiate the science of genetics. The double crown was just beyond Darwin's reach.
Acknowledgements
I thank Peter Crane for the opportunity to participate in the Discussion Meeting on Darwin and the Evolution of Flowers, Sarah Yakimowski for permission to cite unpublished data, Jannice Friedman, Lawrence Harder, Stephen Wright and Mario Vallejo-Marín for assistance with references and discussion, Bill Cole for preparing the figures, Lawrence Harder, Anton Pauw and Mario Vallejo-Marín for permission to use their floral images, and NSERC and the Canada Research Chair's Programme for financial support.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Darwin and the evolution of flowers’.
References
- Ågren J., Schemske D. W.1993Outcrossing rate and inbreeding depression in two annual monoecious herbs, Begonia hirsuta and B. semiovata. Evolution 47, 125–135 (doi:10.2307/2410123) [DOI] [PubMed] [Google Scholar]
- Allen M.1977Darwin and his flowers London, UK: Faber and Faber [Google Scholar]
- Arroyo J., Dafni A.1995Variation in habitat, season, flower traits and pollinators in dimorphic Narcissus tazetta L. (Amaryllidaceae). New Phytol. 129, 135–145 (doi:10.1111/j.1469-8137.1995.tb03017.x) [DOI] [PubMed] [Google Scholar]
- Arroyo J., Barrett S. C. H., Hidalgo R., Cole W. W.2002Evolutionary maintenance of stigma-height dimorphism in Narcissus papyraceus (Amaryllidaceae). Am. J. Bot. 89, 1242–1249 (doi:10.3732/ajb.89.8.1242) [DOI] [PubMed] [Google Scholar]
- Ashman T.-L.1999Determinants of sex allocation in a gynodioecious strawberry. J. Evol. Biol. 12, 648–661 (doi:10.1046/j.1420-9101.1999.00059.x) [Google Scholar]
- Ashman T.-L.2006The evolution of separate sexes: a focus on the ecological context. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 204–222 Oxford, UK: Oxford University Press [Google Scholar]
- Ashman T.-L., et al. 2004Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408–2421 (doi:10.1890/03-8024) [Google Scholar]
- Ayres P.2008The aliveness of plants London, UK: Pickering & Chatto [Google Scholar]
- Baker H. G.1979Anthecology: old testament, new testament, apocrypha (Banquet Address, 8 February 1979). N.Z. J. Bot. 17, 431–440 [Google Scholar]
- Baker A. M., Thomson J. D., Barrett S. C. H.2000aEvolution and maintenance of stigma-height dimorphism in Narcissus I. Floral variation and style-morph ratios. Heredity 84, 502–513 (doi:10.1046/j.1365-2540.2000.00651.x) [DOI] [PubMed] [Google Scholar]
- Baker A. M., Thomson J. D., Barrett S. C. H.2000bEvolution and maintenance of stigma-height dimorphism in Narcissus II. Fitness comparisons between style morphs. Heredity 84, 514–524 (doi:10.1046/j.1365-2540.2000.00686.x) [DOI] [PubMed] [Google Scholar]
- Barrett S. C. H. (ed.)1992Evolution and function of heterostyly Berlin, Germany: Springer-Verlag [Google Scholar]
- Barrett S. C. H.2002The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–284 (doi:10.1038/nrg776) [DOI] [PubMed] [Google Scholar]
- Barrett S. C. H., Charlesworth D.1991Effects of a change in the level of inbreeding on the genetic load. Nature 352, 522–524 (doi:10.1038/352522a0) [DOI] [PubMed] [Google Scholar]
- Barrett S. C. H., Charlesworth D.2007David Graham Lloyd 20 June 1937–30 May 2006. Biogr. Mems. Fell. R. Soc. 53, 203–221 (doi:10.1098/rsbm.2007.0011) [Google Scholar]
- Barrett S. C. H., Harder L. D.2006David G. Lloyd and the evolution of floral biology: from natural history to strategic analysis. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 1–21 Oxford, UK: Oxford University Press [Google Scholar]
- Barrett S. C. H., Hodgins K. A.2006Floral design and the evolution of asymmetrical mating. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 239–255 Oxford, UK: Oxford University Press [Google Scholar]
- Barrett S. C. H., Richards J. H.1990Heterostyly in tropical plants. In Reproductive systems and speciation in tropical woody plants (eds G. Prance & G. Gottsberger). Memoir New York Bot. Gard. 55, 35–61 [Google Scholar]
- Barrett S. C. H., Shore J. S.2008New insights on heterostyly: comparative biology, ecology and genetics. In Self-incompatibility in flowering plants: evolution, diversity and mechanisms (ed. Franklin-Tong V.), pp. 3–32 Berlin, Germany: Springer-Verlag [Google Scholar]
- Barrett S. C. H., Jesson L. K., Baker A. M.2000aThe evolution of stylar polymorphisms in plants. Ann. Bot. 85(Suppl. A), 253–265 [Google Scholar]
- Barrett S. C. H., Wilken D. H., Cole W. W.2000bHeterostyly in the Lamiaceae: the case of Salvia brandegeei. Plant Syst. Evol. 223, 211–219 (doi:10.1007/BF00985280) [Google Scholar]
- Barrett S. C. H., Dorken M. E., Case A. L.2001A geographical context for the evolution of plant reproductive systems. In Integrating ecological and evolutionary processes in a spatial context (eds Silvertown J., Antonovics J.), pp. 341–363 Oxford, UK: Blackwell [Google Scholar]
- Barrett S. C. H., Ness R. W., Vallejo-Marín M.2009Evolutionary pathways to self-fertilization in a tristylous plant. New Phytol. 183, 546–556 (doi:10.1111/j.1469-8137.2009.02937.x) [DOI] [PubMed] [Google Scholar]
- Bell G.1985On the function of flowers. Proc. R. Soc. Lond. B 224, 223–265 (doi:10.1098/rspb.1985.0031) [Google Scholar]
- Bowers K. A. W.1975The pollination ecology of Solanum rostratum (Solanaceae). Am. J. Bot. 62, 633–638 (doi:10.2307/2441943) [Google Scholar]
- Case A. L., Barrett S. C. H.2004Environmental stress and the evolution of dioecy: Wurmbea dioica (Colchicaceae) in Western Australia. Evol. Ecol. 18, 145–164 (doi:10.1023/B:EVEC.0000021152.34483.77) [Google Scholar]
- Cesaro A. C., Barrett S. C. H., Maurice S., Vaissiere B. E., Thompson J. D.2004An experimental evaluation of self-interference in Narcissus assoanus: functional and evolutionary implications. J. Evol. Biol. 17, 1367–1376 (doi:10.1111/j.1420-9101.2004.00767.x) [DOI] [PubMed] [Google Scholar]
- Charlesworth D.2002Plant sex determination and sex chromosomes. Heredity 88, 94–101 (doi:10.1038/sj.hdy.6800016) [DOI] [PubMed] [Google Scholar]
- Charlesworth D., Charlesworth B.1979A model for the evolution of distyly. Am. Nat. 114, 467–498 (doi:10.1086/283496) [Google Scholar]
- Charlesworth D., Charlesworth B.1987Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18, 237–268 (doi:10.1146/annurev.es.18.110187.001321) [Google Scholar]
- Charlesworth B., Charlesworth D., Morgan M. T.1990Genetic loads and estimates of mutation rates in highly inbred plant populations. Nature 347, 380–382 (doi:10.1038/347380a0) [Google Scholar]
- Cruzan M. B., Barrett S. C. H.1993Contribution of cryptic incompatibility to the mating system of Eichhornia paniculata (Pontederiaceae). Evolution 47, 925–934 (doi:10.2307/2410195) [DOI] [PubMed] [Google Scholar]
- Cui X. L., Wei R. C., Huang R. F.1996A study on the breeding system of Amomum tsao-ko. In Proc. Second Symp. on the Family Zingiberaceae (eds Wu T. L., Wu Q. G., Chen Z. Y.), pp. 288–296 Guangzhou, China: South China Institute of Botany [Google Scholar]
- Culley T. M., Weller S. G., Sakai A. K.2002The evolution of wind pollination in angiosperms. Trends Ecol. Evol. 17, 361–369 (doi:10.1016/S0169-5347(02)02540-5) [Google Scholar]
- Darwin C.1839Journal of researches into the natural history and geology of the countries visited during the voyage of H.M.S. Beagle round the world London, UK: Henry Colburn [Google Scholar]
- Darwin C.1859On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- Darwin C.1862On the various contrivances by which British and foreign orchids are fertilised by insects, and on the good effects of intercrossing London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- Darwin C.1868The variation of animals and plants under domestication London, UK: John Murray [Google Scholar]
- Darwin C.1876The effects of cross and self fertilisation in the vegetable kingdom London, UK: John Murray [Google Scholar]
- Darwin C.1877aThe different forms of flowers on plants of the same species London, UK: John Murray [Google Scholar]
- Darwin C.1877bThe various contrivances by which orchids are fertilised by insects, 2nd edn London, UK: John Murray [Google Scholar]
- Darwin C.1887The life and letters of Charles Darwin including an autobiographical chapter, vol. 3 (ed. Darwin F.). London, UK: John Murray [Google Scholar]
- Dorken M. E., Barrett S. C. H.2003Life-history differentiation and the maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae). Evolution 57, 1973–1988 (doi:10.1111/j.0014-3820.2003.tb00378.x) [DOI] [PubMed] [Google Scholar]
- Dorken M. E., Barrett S. C. H.2004aSex determination and the evolution of dioecy from monoecy in Sagittaria latifolia (Alismataceae). Proc. R. Soc. Lond. B 271, 213–219 (doi:10.1098/rspb.2003.2580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorken M. E., Barrett S. C. H.2004bChloroplast haplotype variation among monoecious and dioecious populations of Sagittaria latifolia (Alismataceae) in eastern North America. Mol. Ecol. 13, 2699–2707 (doi:10.1111/j.1365-294X.2004.02246.x) [DOI] [PubMed] [Google Scholar]
- Dorken M. E., Friedman J., Barrett S. C. H.2002The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae). Evolution 56, 31–41 (doi:10.1111/j.0014-3820.2002.tb00847.x) [DOI] [PubMed] [Google Scholar]
- Dudash M. R.1990Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution 44, 1129–1139 (doi:10.2307/2409277) [DOI] [PubMed] [Google Scholar]
- Dulberger R.1964Flower dimorphism and self-incompatibility in Narcissus tazetta L. Evolution 18, 361–363 (doi:10.2307/2406347) [Google Scholar]
- Dulberger R.1967Pollination systems in plants in Israel. PhD thesis, Hebrew University, Jerusalem [Google Scholar]
- Dulberger R.1992Floral polymorphisms and their functional significance in the heterostylous syndrome. In Evolution and function of heterostyly (ed. Barrett S. C. H.), pp. 41–84 Berlin, Germany: Springer-Verlag [Google Scholar]
- Eckert C. G., Samis K. E., Dart S.2006Reproductive assurance and the evolution of uniparental reproduction in plants. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 183–203 Oxford, UK: Oxford University Press [Google Scholar]
- Ehlers B. K., Bataillon T.2007‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New. Phytol. 174, 194–211 (doi:10.1111/j.1469-8137.2007.01975.x) [DOI] [PubMed] [Google Scholar]
- Endress P. K.1994Diversity and evolutionary biology of tropical flowers Cambridge, UK: Cambridge University Press [Google Scholar]
- Ferrero V., Arroyo J., Vargas P., Thompson J. D., Navarro L.2009Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae). Persp. Plant Ecol. Evol. Syst. 11, 111–125 (doi:10.1016/j.ppees.2009.01.004) [Google Scholar]
- Fishman L., Kelly A. J., Willis J. H.2002Minor quantitative trait loci underlie floral traits associated with mating system divergence in Mimulus. Evolution 56, 2138–2155 (doi:10.1111/j.0014-3820.2002.tb00139.x) [DOI] [PubMed] [Google Scholar]
- Foxe J. P., Slotte T., Stahl E. A., Neuffer B., Hurka H., Wright S. I.2009Recent speciation associated with the evolution of selfing in Capsella. Proc. Natl Acad. Sci. USA 106, 5241–5245 (doi:10.1073/pnas.0807679106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong V. E. (ed.) 2008Self-incompatibility in flowering plants Berlin, Germany: Springer-Verlag [Google Scholar]
- Friedman J., Barrett S. C. H.2008A phylogenetic analysis of the evolution of wind pollination in the angiosperms. Int. J. Plant Sci. 169, 49–58 (doi:10.1086/523365) [Google Scholar]
- Friedman J., Barrett S. C. H.2009Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann. Bot. 103, 1515–1527 (doi:10.1093/aob/mcp035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganders F. R.1979The biology of heterostyly. N. Z. J. Bot. 17, 607–635 [Google Scholar]
- Geber M. A., Dawson T. E., Delph L. F. (ed.) 1999Gender and sexual dimorphism in flowering plants New York, NY: Springer-Verlag [Google Scholar]
- Goodwillie C.1999Wind pollination and reproductive assurance in Linanthus parviflorus (Polemoniaceae), a self-incompatible annual. Am. J. Bot. 86, 948–954 (doi:10.2307/2656611) [PubMed] [Google Scholar]
- Gould S. J., Lewontin R. C.1979The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B 205, 581–598 (doi:10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- Graham S. W., Barrett S. C. H.1995Phylogenetic systematics of Pontederiales: implications for breeding-system evolution. In Monocotyledons: systematics and evolution (eds Rudall P. J., Cribb P. J., Cutler D. F., Humphries C. J.), pp. 415–441 Kew, UK: Royal Botanic Gardens [Google Scholar]
- Harder L. D.2000Pollen dispersal and the floral diversity of monocotyledons. In Monocots (eds Wilson K. L., Morrison D. A.), pp. 243–257 Melbourne, Australia: CSIRO [Google Scholar]
- Harder L. D., Aizen M. A.2010Floral adaptation and diversification under pollen limitation. Phil. Trans. R. Soc. B 365, 529–543 (doi:10.1098/rstb.2009.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder L. D., Barrett S. C. H.1995Mating cost of large floral displays in hermaphrodite plants. Nature 373, 512–515 (doi:10.1038/373512a0) [Google Scholar]
- Harder L. D., Barrett S. C. H. (eds) 2006Ecology and evolution of flowers Oxford, UK: Oxford University Press [Google Scholar]
- Harder L. D., Johnson S.2009Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytol. 183, 530–545 (doi:10.1111/j.1469-8137.2009.02914.x) [DOI] [PubMed] [Google Scholar]
- Harder L. D., Barrett S. C. H., Cole W.2000The mating consequences of sexual segregation within inflorescences of flowering plants. Proc. R. Soc. Lond. B 267, 315–320 (doi:10.1098/rspb.2000.1002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder L. D., Richards S. A., Routley M. B.2008Effects of reproductive compensation, gamete discounting and reproductive assurance on mating-system diversity in hermaphrodites. Evolution 62, 157–172 (doi:10.1111/j.1558-5646.2007.00272.x) [DOI] [PubMed] [Google Scholar]
- Hartley M. L., Tshamekeng E., Thomas S. M.2002Functional heterostyly in Tylosema esculentum (Caesalpinioideae). Ann. Bot. 89, 67–76 (doi:10.1093/aob/mcf006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins K. A., Barrett S. C. H.2008Natural selection on floral traits through male and female function in wild populations of the heterostylous daffodil Narcissus triandrus. Evolution 62, 1751–1763 (doi:10.1111/j.1558-5646.2008.00404.x) [DOI] [PubMed] [Google Scholar]
- Husband B. C., Schemske D. W.1996Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50, 54–70 (doi:10.2307/2410780) [DOI] [PubMed] [Google Scholar]
- Jernstedt J. A.1982Floral variation in Chlorogalum angustifolium (Liliaceae). Madroño 29, 87–94 [Google Scholar]
- Jesson L. K., Barrett S. C. H.2002aEnantiostyly in Wachendorfia (Haemodoraceae); the influence of reproductive systems on the maintenance of the polymorphism. Am. J. Bot. 89, 253–263 (doi:10.3732/ajb.89.2.253) [DOI] [PubMed] [Google Scholar]
- Jesson L. K., Barrett S. C. H.2002bThe genetics of mirror-image flowers. Proc. R. Soc. Lond. B 269, 1835–1839 (doi:10.1098/rspb.2002.2068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesson L. K., Barrett S. C. H.2002cSolving the puzzle of mirror-image flowers. Nature 417, 707 (doi:10.1038/417707a) [DOI] [PubMed] [Google Scholar]
- Jesson L. K., Barrett S. C. H.2003The comparative biology of mirror-image flowers. Int. J. Plant Sci. 164, S237–S249 (doi:10.1086/378537) [Google Scholar]
- Jesson L. K., Barrett S. C. H.2005Experimental tests of the function of mirror-image flowers. Biol. J. Linn. Soc. 85, 167–179 (doi:10.1111/j.1095-8312.2005.00480.x) [Google Scholar]
- Jesson L. K., Barrett S. C. H., Day T.2003A theoretical investigation of the evolution and maintenance of mirror-image flowers. Am. Nat. 161, 916–930 (doi:10.1086/375176) [DOI] [PubMed] [Google Scholar]
- Kohn J. R., Graham S. W., Morton B., Doyle J. J., Barrett S. C. H.1996Reconstruction of the evolution of reproductive characters in Pontederiaceae using phylogenetic evidence from chloroplast DNA restriction-site variation. Evolution 50, 1454–1469 (doi:10.2307/2410883) [DOI] [PubMed] [Google Scholar]
- Kress W. J., Liu A. Z., Newman M., Li Q. J.2005The molecular phylogeny of Alpinia (Zingiberaceae): a complex and polyphyletic genus of gingers. Am. J. Bot. 92, 167–178 (doi:10.3732/ajb.92.1.167) [DOI] [PubMed] [Google Scholar]
- Lewis D., Jones D. A.1992The genetics of heterostyly. In Evolution and function of heterostyly (ed. Barrett S. C. H.), pp. 129–150 Berlin, Germany: Springer-Verlag [Google Scholar]
- Li Q. J., Xu Z. F., Xia Y. M., Zhang L., Deng X. B., Gao J. Y.2001Study on the flexistyly pollination mechanism in Alpinia plants (Zingiberaceae). Acta Bot. Sin. 43, 364–369 [Google Scholar]
- Li Q. J., Kress W. J., Xu Z. F., Mia Y. M., Zhang L., Deng X. B., Gao J. Y.2002Mating system and stigmatic behaviour during flowering of Alpinia kwangsiensis (Zingiberaceae). Plant Syst. Evol. 232, 123–132 (doi:10.1007/s006060200031) [Google Scholar]
- Linder H. P.1998Morphology and the evolution of wind pollination. In Reproductive biology in systematics, conservation and economic botany (eds Owens S. J., Rudall P. J.), pp. 123–135 Royal Botanic Gardens: Kew [Google Scholar]
- Lloyd D. G.1976The transmission of genes via pollen and ovules in gynodioecious angiosperms. Theoret. Popul. Biol. 9, 299–316 (doi:10.1016/0040-5809(76)90050-2) [DOI] [PubMed] [Google Scholar]
- Lloyd D. G.1980Sexual strategies in plants III. A quantitative method for describing the gender of plants. N. Z. J. Bot. 18, 103–108 [Google Scholar]
- Lloyd D. G.1984Gender allocations in outcrossing cosexual plants. In Perspectives on plant population ecology (eds Dirzo R., Sarukhan J.), pp. 277–300 Sunderland, MA: Sinauer [Google Scholar]
- Lloyd D. G., Barrett S. C. H. (eds) 1996Floral biology: studies on floral evolution in animal-pollinated plants New York, NY: Chapman & Hall [Google Scholar]
- Lloyd D. G., Bawa K. S.1984Modification of the gender of seed plants in varying conditions. Evol. Biol. 17, 255–388 [Google Scholar]
- Lloyd D. G., Webb C. J.1992aThe evolution of heterostyly. In Evolution and function of heterostyly (ed. Barrett S. C. H.), pp. 151–178 Berlin, Germany: Springer-Verlag [Google Scholar]
- Lloyd D. G., Webb C. J.1992bThe selection of heterostyly. In Evolution and function of heterostyly (ed. Barrett S. C. H.), pp. 179–208 Berlin, Germany: Springer-Verlag [Google Scholar]
- Luo Z., Zhang D., Renner S. S.2008Why two kinds of stamens in buzz-pollinated flowers? Experimental support for Darwin's division-of-labour hypothesis. Funct. Ecol. 22, 794–800 (doi:10.1111/j.1365-2435.2008.01444.x) [Google Scholar]
- Mast A. R., Kelso S., Conti E.2006Are any primroses (Primula) primitively monomorphic? New Phytol. 171, 605–616 (doi:10.1111/j.1469-8137.2006.01700.x) [DOI] [PubMed] [Google Scholar]
- Morgan M. T., Conner J. K.2001Using genetic markers to directly estimate male selection gradients. Evolution 55, 272–281 (doi:10.1111/j.0014-3820.2001.tb01292.x) [DOI] [PubMed] [Google Scholar]
- Morgan M. T., Schoen D. J.1997The role of theory in an emerging new plant reproductive biology. TREE 12, 231–234 (doi:10.1016/S0169-5347(97)01045-8) [DOI] [PubMed] [Google Scholar]
- Müller F.1883Two kinds of stamens with different functions in the same flower. Nature 27, 364–365 (doi:10.1038/027364b0) [Google Scholar]
- O'Brien S. P., Calder D. M.1989The breeding biology of Epacris impressa: is this species heterostylous? Aust. J. Bot. 37, 43–54 (doi:10.1071/BT9890043) [Google Scholar]
- Ornduff R.1972The breakdown of trimorphic incompatibility in Oxalis section Corniculatae. Evolution 26, 52–65 (doi:10.2307/2406982) [DOI] [PubMed] [Google Scholar]
- Pannell J. R.2005Phenotypic plasticity and a functional vs. genetic perspective on plant gender. New Phytol. 168, 506–510 (doi:10.1111/j.1469-8137.2005.01579.x) [DOI] [PubMed] [Google Scholar]
- Pannell J. R., Dorken M. E., Pujol B., Berjano R.2008Gender variation and transitions between sexual systems in Mercurialis annua (Euphorbiaceae). Int. J. Plant Sci. 169, 129–139 (doi:10.1086/523360) [Google Scholar]
- Pauw A.2005Inversostyly: a new stylar polymorphism in an oil-secreting plant, Hemimeris racemosa (Scrophulariaceae). Am. J. Bot. 92, 1878–1886 (doi:10.3732/ajb.92.11.1878) [DOI] [PubMed] [Google Scholar]
- Philipp M., Schou O.1981An unusual heteromorphic incompatibility system: distyly, self-incompatibility, pollen load and fecundity in Anchusa officinalis (Boraginaceae). New Phytol. 89, 693–703 (doi:10.1111/j.1469-8137.1981.tb02348.x) [DOI] [PubMed] [Google Scholar]
- Piper J. G., Charlesworth B., Charlesworth D.1984A high-rate of self-fertilization and increased seed fertility of homostyle primroses. Nature 310, 50–51 (doi:10.1038/310050a0) [Google Scholar]
- Proctor M., Yeo P., Lack A.1996The natural history of pollination Portland, OR: Timber Press [Google Scholar]
- Renner S. S.2001How common is heterodichogamy? TREE 16, 595–597 (doi:10.1016/S0169-5347(01)02280-7) [Google Scholar]
- Renner S. S., Rickelfs R. E.1995Dioecy and its correlates in the angiosperms. Am. J. Bot. 82, 596–606 (doi:10.2307/2445418) [Google Scholar]
- Richards A. J.1997Plant breeding systems London, UK: Chapman & Hall [Google Scholar]
- Ritland K.1990Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution 44, 1230–1241 (doi:10.2307/2409284) [DOI] [PubMed] [Google Scholar]
- Sarkissian T. S., Barrett S. C. H., Harder L. D.2001Gender variation in Sagittaria latifolia (Alismataceae): is size all that matters? Ecology 82, 360–373 (doi:10.1890/0012-9658(2001)082[0360:GVISLA]2.0.CO;2) [Google Scholar]
- Schoen D. J., Johnston M. O., L'Heureux A. M., Marsoais J. V.1997Evolutionary history of the mating system in Amsinckia (Boraginaceae). Evolution 51, 1090–1099 (doi:10.2307/2411038) [DOI] [PubMed] [Google Scholar]
- Stone J. L., Thomson J. D.1994The evolution of distyly: pollen transfer in artificial flowers. Evolution 48, 1595–1606 (doi:10.2307/2410250) [DOI] [PubMed] [Google Scholar]
- Sun S., Gao J. Y., Liao W. J., Li Q. J., Zhang D. Y.2007Adaptive significance of flexistyly in Alpinia blepharocalyx (Zingiberaceae): a hand-pollination experiment. Ann. Bot. 99, 661–666 (doi:10.1093/aob/mcl292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A., Gisil J., Yusoff M., Tachi T.2005Floral and pollinator behaviour of flexistylous Bornean ginger, Alpinia nieuwenhuizii (Zingiberaceae). Plant Syst. Evol. 252, 167–173 (doi:10.1007/s00606-004-0258-4) [Google Scholar]
- Tang L. L., Huang S. Q.2007Evidence for reductions in floral attractants with increased selfing rates in two heterandrous species. New Phytol. 175, 588–595 (doi:10.1111/j.1469-8137.2007.02115.x) [DOI] [PubMed] [Google Scholar]
- Todd J. E.1882On the flowers of Solanum rostratum and Cassia chamaecrista. Am. Nat. 16, 281–287 (doi:10.1086/273056) [Google Scholar]
- Vallejo-Marín M., Barrett S. C. H.2009Modification of flower architecture during early stages in the evolution of self-fertilization. Ann. Bot. 103, 951–962 (doi:10.1093/aob/mcp015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo-Marín M., Manson J. S., Thomson J. D., Barrett S. C. H.2009Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. J. Evol. Biol. 22, 828–839 (doi:10.1111/j.1420-9101.2009.01693.x) [DOI] [PubMed] [Google Scholar]
- Waser N. M., Ollerton J. (eds) 2006Plant-pollinator interactions from specialization to generalization Chicago, IL: University of Chicago Press [Google Scholar]
- Willson M. F.1979Sexual selection in plants. Am. Nat. 113, 777–790 (doi:10.1086/283437) [Google Scholar]
- Wilson J.1887On the dimorphism of the flowers of Wachendorfia paniculata. Trans. Proc. Bot. Soc. Edin. 17, 73–77 [Google Scholar]
- Wright S. I., Ness R. W., Foxe J. P., Barrett S. C. H.2008Genomic consequences of outcrossing and selfing in plants. Int. J. Plant Sci. 169, 105–118 (doi:10.1086/523366) [Google Scholar]
- Zhang L., Li Q. J., Deng X. B., Ren P. Y., Gao J. Y.2003Reproductive biology of Alpinia blepharocalyx (Zingiberaceae): another example of flexistyly. Plant Syst. Evol. 241, 67–76 (doi:10.1007/s00606-003-0021-2) [Google Scholar]